Abstract
Asymmetrical dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase, is increasingly recognized as a putative biomarker in cardiovascular and renal disease. Elevated plasma levels of ADMA are the consequence of increased synthesis, reduced renal clearance or reduced enzymatic degradation. Based upon the metabolic fate the highest plasma concentrations of ADMA have been reported in patients with renal failure in whom this molecule accumulates. However, the range of published ADMA levels in patients with chronic renal failure as well as in patients with end-stage renal failure undergoing maintenance hemodialysis, peritoneal dialysis or kidney transplant recipients is widely scattered and overlaps with the levels reported in healthy individuals. This wide distribution can in part be explained by different bioanalytical techniques and the lack of standardization of such assays. This review summarizes available literature on ADMA in patients with kidney disease and stresses the urgent need for a consensus regarding reference values for different analytical methods in order to appreciate the prognostic significance of elevated ADMA levels. At present, one cannot advocate this molecule for risk assessment or individual patient prognosis in the clinical work-up of patients with renal impairment.
Key Words: Asymmetrical dimethylarginine, Symmetric dimethylarginine
Background
Since the seminal observation by Vallance et al. [1] in 1992 there has been considerable interest in the pathophysiological relevance of asymmetrical dimethylarginine (ADMA), an endogenous inhibitor of all three isoforms of nitric oxide synthase (NOS), especially in the field of nephrology where its role in chronic kidney disease (CKD) and end-stage renal disease (ESRD) has been extensively investigated.
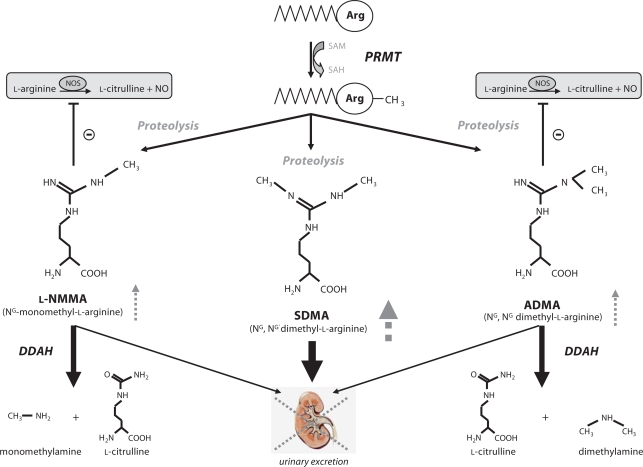
The dimethylarginines ADMA and symmetric dimethylarginine (SDMA) were first isolated from human urine by Kakimoto and Akazawa [2] in 1970 by ion exchange chromatography. Methylarginines derive from the posttranslational methylation of L-arginine residues within proteins catalyzed by a family of enzymes called protein arginine methyltransferases (PRMT) [3]. To date, nine PRMT genes have been cloned (PRMT1–9), based on substrate and product specificity PRMTs are divided into enzymes with type I or type II activity. Both types form monomethyl L-arginine (L-NMMA), but differ in that type I enzymes produce ADMA whereas type II enzymes generate symmetrical dimethylarginine (SDMA). Monomethylation seems to be an intermediate step in type I or type II PRMT reactions. Upon proteolysis, methylarginines are released into the circulation where ADMA competes with L-arginine for NOS (fig. 1). Initially, it was believed that methylarginines are cleared from the circulation by renal excretion and do not undergo metabolism.
Fig. 1.
Generation and metabolism of methylarginines. SAM = S-adenosyl-methionine; SAH = S-adenosyl-homocysteine; PRMT = protein arginine N-methyltransferase; DDAH = dimethylarginine dimethylaminohydrolase. Dashed lines and arrows in gray indicate expected changes of methylarginines in renal failure.
This was supported by the notion that these molecules accumulate in patients with renal failure [1]. However, McDermott [4] noted that the urinary recovery of methylarginines following intravenous injection in rabbits was 0.14% for L-NMMA, 5.1% for ADMA and 66% for SDMA, indicating that especially L-NMMA and ADMA must undergo extensive metabolism. Later, Ogawa et al. [5] identified and purified an enzyme termed dimethylarginine dimethylaminohydrolase (DDAH) that catalyzes the hydrolysis of L-NMMA and ADMA into L-citrulline and mono- or dimethylamine (fig. 1). In 1999 a second isoform with distinct tissue distribution was identified by Leiper et al. [6]. It was estimated that the daily generation of ADMA is ∼300 μmol. Approximately 50 μmol are excreted via the urine, and therefore, 80–85% of ADMA is estimated to be degraded by DDAH [7]. Notably, SDMA is not metabolized by DDAH and appears to be solely eliminated via the urine (fig. 1).
Based on the generation and metabolism of ADMA, elevated levels are the consequence of increased synthesis (enhanced activity or expression of PRMTs), reduced renal clearance or reduced enzymatic degradation (decreased activity or expression of DDAH). The latter two mechanisms have been shown to contribute to elevations of ADMA in renal disease whereas the role of PRMT under this condition remains unknown.
In the last two decades, a variety of studies investigated the role of ADMA in patients with different stages of CKD, patients with ESRD undergoing hemodialysis (HD) or peritoneal dialysis (PD) as well as kidney transplant recipients. All studies demonstrated a marked increase of ADMA levels in patients with renal failure; however, the range of reported ADMA levels varies considerably (up to 10-fold). In addition, there is an overlap between ADMA levels in patients with ESRD and healthy control individuals between different studies despite the fact that similar detection methods were used. Furthermore, the ratio of SDMA/ADMA which should rise in renal failure based on the fact that SDMA appears to be solely cleared from the circulation via the urine also shows great variation. To make things even more complicated, the role of HD to lower ADMA levels remains all but clear with clearance rates ranging anywhere between 0 and 80%.
Thus, although there is accumulating body of evidence that ADMA has the potential to become a novel cardiovascular biomarker, a critical appraisal of ADMA in renal disease seems mandatory. The aim of this review was to summarize and compare available literature on ADMA in patients with CKD, ESRD (HD or PD) and kidney transplant recipients. In addition, this review briefly focuses on therapeutic interventions to modulate ADMA levels in humans as well as on the role of ADMA as a potential biomarker in renal disease. Finally, we comment on some of the pitfalls associated with ADMA measurement.
Study Design and Selection Criteria for Studies
We performed a comprehensive search using Medline database (January 1970–August 2007). The following search criteria were used: asymmetric dimethylarginine, symmetric dimethylarginine, ADMA, SDMA, renal failure, CKD, ESRD, HD, PD, transplantation, creatinine, GFR. Exclusion criteria were reviews, case reports and studies investigating the role of ADMA in acute renal failure or in pediatric patients. Except for one Chinese article, there was no restriction to language.
Based on the fact that many studies were underpowered with respect to sample size and given the wide range of ADMA levels using different analytical methods that would cause statistical heterogeneity, we deliberately refrained from performing a meta-analysis but rather decided to summarize available literature. Apart from selection bias the relationship with patient averages across trials may not be the same as the relationship for patients within trials. Thus, results from meta-analyses can be misleading and hard to interpret.
Serum creatinine levels that were indicated as mg/dl were converted to μmol/l by multiplication with the factor 88.4. Since the ratio of L-arginine/ADMA, which is crucial with regard to NOS enzyme inhibition, was not specified by all authors it was estimated by using the mean values of L-arginine and ADMA.
In cases of missing information the abbreviation n.i. (not indicated) was used. To calculate mean values of variables that were expressed as subgroup analyses the mean from these groups were multiplied with the number of subjects, subsequent values of different subgroups were added and the sum was divided through the total number of subjects to obtain the mean of the entire sample. Under such circumstances and in studies in which mean values had to be extrapolated from bar graph figures, an asterisk (*) was added behind the estimated mean.
Pearson correlation coefficients were calculated using SPSS software package (Version 14.0).
Results and Discussion
Plasma ADMA Levels in CKD
We identified 17 studies in which plasma ADMA levels were determined in patients with CKD [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. These studies included >500 control individuals as well as >1,500 patients with renal impairment spanning the entire spectrum of CKD1-5 (table 1). In studies with control groups, plasma ADMA levels were significantly elevated in CKD patients, the increase versus controls ranged from 1.13- to 3.36-fold. Compared to HD patients (table 2), ADMA levels were considerably lower in patients with CKD. ADMA levels were in the range of 0.36–1.40 in controls and 0.46–4.20 in patients with CKD (table 1). In 4 studies, ADMA levels were considerably higher both in controls as well as in patients [14, 21, 22, 24] whereas in all the remaining studies ADMA levels were rather low. SDMA levels were also higher in patients versus controls in all studies and ranged from 0.14 to 0.90 in controls and from 0.59 to 3.20 in patients with CKD. Except for 3 studies [11, 14, 16] the ratio of SDMA/ADMA was in the range of ∼1 or greater in patients with CKD (table 1). The ratio ranged from 0.35 to 1.12 in controls and from 0.20 to 4.16 in patients with CKD (table 1).
Table 1.
ADMA levels in patients with CKD
| Subjects |
Stages of CKD |
Creatinine or GFR |
L-Arginine μM |
ADMA μM |
L-Arginine/ADMA |
SDMA μM |
SDMA/ADMA |
Detection method |
|
|---|---|---|---|---|---|---|---|---|---|
| MacAllister (1996) | |||||||||
| Control | 9 | – | n.i. | 75 | 0.36 | 208.3 | 0.39 | 1.08 | HPLC |
| CKD | 4 | CKD5 | >500 μmol/l | 35 | 0.77 | 45.5 | 3.20∗ | 4.16 | |
| Marescau (1997) | |||||||||
| Control | 66 | – | 73.0 μmol/l∗ | 110 | 0.41 | 268.3 | 0.38 | 0.93 | HPLC |
| CKD | 135 | CKD 2–5, 16% diab. | 426 μmol/l∗ | 122∗ | 0.76∗ | 160.5 | 2.08∗ | 2.74 | |
| Al Banchaabouchi (2000) | |||||||||
| Control | 37 | – | 68 μmol/l | n.i. | 0.41 | n.i. | 0.38 | 0.93 | HPLC |
| CKD | 135 | CKD 2–5 | 426 μmol/l∗ | n.i. | 0.76∗ | n.i. | 2.08∗ | 2.74 | |
| Schmidt (2000) | |||||||||
| Control | 9 | – | 71 μmol/l | 68 | 0.40 | 170.0 | 0.14 | 0.35 | HPLC |
| CKD | 13 | CKD n.i., 38% diab. | 274 μmol/l | 67 | 1.26 | 53.2 | 0.60 | 0.48 | |
| Fleck (2001) | |||||||||
| Control | 22 | – | 88 μmol/l | 75∗ | 0.73 | 102.7 | 0.50 | 0.68 | HPLC |
| CKD | 111 | CKD n.i. | 256 μmol/l | 55∗ | 0.85 | 64.7 | 2.05 | 2.41 | |
| Wahbi (2001) | |||||||||
| Control | 9 | – | n.i. | 118 | 0.61 | 193.4 | 0.41 | 0.67 | HPLC |
| CKD | 13 | CKD 3–5 | 553 μmol/l | 125 | 1.04 | 120.2 | 2.47 | 2.37 | |
| Kielstein (2002) | |||||||||
| Control | 16 | – | 88 μmol/l | 59.0 | 1.40 | 54.3 | 0.64 | 0.46 | HPLC |
| CKD | 44 | CKD 1–5, non-diab. | 199 μmol/l∗ | 52.0∗ | 4.20 | 13.1 | 0.83∗ | 0.20 | |
| Schiel (2003) | |||||||||
| Control | 51 | – | n.i. | n.i. | 0.7∗ | n.i. | 0.5∗ | 0.71 | HPLC |
| CKD | 99 | CKD n.i. | 381 μmol/l | n.i. | 0.9∗ | n.i. | 2.1∗ | 2.33 | |
| Saran (2003) | |||||||||
| Control | 6 | – | <88 μmol/l | n.i. | 0.55 | n.i. | 0.40 | 0.73 | HPLC |
| CKD | 8 | CKD 4–5, non-diab. | 354 μmol/l | n.i. | 1.85 | n.i. | 1.18 | 0.64 | |
| Tarnow (2004) | |||||||||
| Control | 192 | type 1 diab. normoalb. | 76 μmol/l | 62.3 | 0.40 | 156 | 0.41 | 1.02 | HPLC |
| CKD | 408 | CKD 1–5, type 1 diab. | 102 μmol/l | 72.8 | 0.46 | 160 | 0.59 | 1.28 | |
| Fliser (2005) | |||||||||
| CKD | 227 | CKD 1–4, non-diab. | 179 μmol/l | n.i. | 0.46 | n.i. | 0.91 | 1.98 | LC-MS |
| Ravani (2005) | |||||||||
| Control | 22 | – | n.i. | n.i. | 0.69 | n.i. | n.i. | n.i. | ELISA |
| CKD | 131 | CKD 2–5, 24% diab. | 212 μmol/l | n.i. | 0.78 | n.i. | n.i. | n.i. | |
| Nanayakkara (2005) | |||||||||
| Control | 53 | – | n.i. | 94.0 | 0.42 | 223.8 | 0.47 | 1.12 | HPLC |
| CKD | 93 | CKD 2–4, non-diab. | 205 μmol/l | 96.4 | 0.52 | 185.4 | 1.12 | 2.15 | |
| Yilmaz (2006)1 | |||||||||
| Control | 30 | – | GFR ∼109 ml/min | n.i. | 1.07 | n.i. | n.i. | n.i. | HPLC |
| CKD | 123 | CKD 1–4, non-diab. | GFR ∼59 ml/min∗ | n.i. | 2.34∗ | n.i. | n.i. | n.i. | |
| Caglar (2006) | |||||||||
| Control | 38 | – | 76 μmol/l | n.i. | 1.08 | n.i. | 0.90 | 0.83 | HPLC |
| CKD | 78 | CKD 1, non-diab. | 70 μmol/l | n.i. | 1.92 | n.i. | 1.81 | 0.94 | |
| Busch (2006) | |||||||||
| Control | 22 | – | n.i. | 74.8 | 0.73 | 102.5 | 0.50 | 0.68 | HPLC |
| CKD | 82 | CKD 2–5, 29% diab. | 385 μmol/l | 55.6 | 0.88 | 63.2 | 2.00 | 2.27 | |
| Yilmaz (2007) | |||||||||
| Control | 36 | – | GFR ∼112 ml/min | 94.1 | 0.88 | 105.8 | 0.82 | 0.93 | HPLC |
| CKD | 66 | CKD 1–4, non-diab. | GFR ∼43 ml/min | 81.5 | 1.55 | 55.6 | 1.64 | 1.06 |
HPLC = High-performance liquid chromatography; LC-MS = liquid chromatography mass spectrometry; ELISA = enzyme-linked immunosorbent assay. An asterisk indicates the estimated mean.
Patients with CKD5 were excluded since they were on hemodialysis.
Table 2.
ADMA levels in patients on hemodialysis
| Subjects |
Duration of HD, months |
L-Arginine μM |
ADMA μM |
L-Arginine/ADMA |
SDMA μM |
SDMA/ADMA |
Detection method |
||
|---|---|---|---|---|---|---|---|---|---|
| Vallance (1992) | Control | 6 | – | ∼971 | 0.571 | 170.21 | 0.571 | 1.01 | HPLC |
| HD | 9 | n.i. | ∼591 | 4.351 | 13.61 | 4.351 | 1.01 | ||
| MacAllister (1996) | Control | 9 | – | 75.3 | 0.36 | 209.2 | 0.39 | 1.08 | HPLC |
| HD | 6 | ∼24 | 35.4 | 0.99 | 35.7 | 3.78 | 3.82 | ||
| Anderstam (1997) | Control | 7 | – | n.i. | 0.36 | n.i | 0.37 | 1.03 | HPLC |
| HD | 19 | n.i. | n.i. | 0.59 | n.i. | 2.85 | 4.83 | ||
| Kielstein (1999) | Control | 37 | – | 75.5 | 1.0 | 79.2 | 0.80 | 0.80 | HPLC |
| HD | 43 | ∼38 | 75.9 | 6.0 | 13.7 | 5.20 | 0.87 | ||
| Schmidt (1999) | Control | 13 | – | 84 | 0.40 | 210.0 | 0.12 | 0.30 | HPLC |
| HD | 17 | n.i. | 77 | 4.14 | 18.6 | 1.41 | 0.34 | ||
| Fleck (2001) | Control | 22 | – | 75∗ | 0.73 | 102.7 | 0.50 | 0.68 | HPLC |
| HD | 85 | n.i. | 60∗ | 1.05 | 57.1 | 2.68 | 2.55 | ||
| Wahbi (2001) | Control | 9 | – | 118 | 0.61 | 193.4 | 0.41 | 0.67 | HPLC |
| HD | 17 | n.i. | 116 | 0.99 | 117.2 | 4.53 | 4.58 | ||
| Raj (2002) | Control | 6 | – | 93.7 | 0.90 | 105.8 | n.i. | n.i. | HPLC |
| HD | 27 | n.i. | 105.3 | 4.00 | 33.9 | n.i. | n.i. | ||
| Osanai (2002) | Control | 13 | – | n.i. | 0.80 | n.i. | n.i. | n.i. | HPLC |
| HD | 51 | n.i. | n.i. | 3.04 | n.i. | n.i. | n.i. | ||
| Schiel (2003) | Control | 51 | – | n.i. | 0.7∗ | n.i. | 0.5∗ | 0.71 | HPLC |
| HD | 84 | n.i. | n.i. | 1.0∗ | n.i. | 2.5∗ | 2.50 | ||
| Bergamini (2004) | Control | 10 | – | 104.8 | 1.41 | 74.3 | 0.68 | 0.54 | HPLC |
| HD | 11 | ∼35 | 126.9 | 3.62 | 35.1 | 6.30 | 2.38 | ||
| Martens-Lobenhoffer (2004) | Control | 47 | – | 63.9 | 0.35 | 182.6 | 0.46 | 1.31 | LC-MS |
| HD | 30 | n.i. | 48.1 | 0.67 | 71.8 | 3.16 | 4.72 | ||
| Mochizuki (2005) | Control | 9 | – | 102.9 | 0.30 | 461.5 | n.i. | n.i. | HPLC |
| HD | 10 | ∼155 | 66.2 | 2.20 | 32.6 | n.i. | n.i. | ||
| Morimoto (2005) | Control | 20 | – | n.i. | 0.44 | n.i. | n.i. | n.i. | HPLC |
| HD | 31 | ∼76 | n.i. | 0.72 | n.i. | n.i. | n.i. | ||
| Siroka (2005) | Control | 31 | – | n.i. | 0.82 | n.i. | n.i. | n.i. | ELISA |
| HD | 8 | n.i. | n.i. | 1.81 | n.i. | n.i. | n.i. | ||
| Yilmaz (2006) | Control | 30 | – | n.i. | 1.07 | n.i. | n.i. | n.i. | HPLC |
| HD | 36 | >12 | n.i. | 4.63 | n.i. | n.i. | n.i. | ||
| Aslam (2006) | Control | 19 | – | 95.3 | 0.59 | 161.5 | 0.65 | 1.10 | HPLC |
| HD | 19 | ∼48 | 86.7 | 1.94 | 44.7 | 3.20 | 1.65 |
HPLC = High-performance liquid chromatography; LC-MS = liquid chromatography mass spectrometry; ELISA = enzyme-linked immunosorbent assay. An asterisk indicates the estimated mean.
Plasma dimethylarginine levels (= ADMA+SDMA) indicated by Vallance et al. were 1.15 μm in controls and 8.7 μm in dialysis patients, plasma ADMA/SDMA ratio ∼1:1 both for controls and patients; L-arginine/dimethylarginine ratio was 84.3 in controls and 6.8 in dialysis patients.
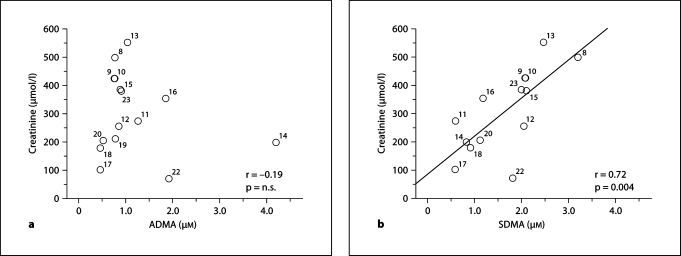
In agreement with a recent meta-analysis [25], we observed a strong correlation between serum creatinine and SDMA but not ADMA levels in patients with CKD (fig. 2).
Fig. 2.
Scatterplots showing the correlation between serum creatinine and ADMA (a) or SDMA (b) levels in patients with CKD. Creatinine as well as ADMA and SDMA values are depicted in table 1; numbers next to each circle reflect the corresponding reference. The studies by Yilmaz et al. [21, 24] are not included since they lack information on serum creatinine levels.
Plasma ADMA Levels in Patients with ESRD
Published ADMA levels in dialysis patients vary anywhere between non-detectable [26] to 8.0 μM[27]. Even after excluding these extremes, levels are widely scattered between 0.59 and 6.0 μM (table 2). Due to different renal replacement therapies we separately summarized plasma ADMA levels in patients undergoing HD, PD and in transplant recipients. Studies performed in patients on HD were further subdivided into those that included a control group and those that did not. Studies lacking important information for controls were added to the latter group.
ADMA Levels in HD Patients (Studies with Control Groups)
We identified a total of 17 studies in which plasma ADMA levels were measured in patients on HD as well as in control individuals [1, 8, 12, 13, 15, 21,28,29,30,31,32,33,34,35,36,37,38]. These studies included a total of 339 control subjects and 533 dialysis patients. In all studies, plasma ADMA levels were elevated in patients versus controls (table 2). However, there was a wide distribution of ADMA both in controls as well as in dialysis patients between different studies. In control groups, plasma ADMA levels ranged from 0.30 to 1.41 μM, in dialysis patients levels between 0.59 and 6.0 μM were reported (table 2). Compared to control groups a 1.4- to 10.3-fold increase of ADMA levels was observed in dialysis patients. Similarly, plasma SDMA levels that were reported in 11 out of 17 studies ranged from 0.12 to 0.80 μM in controls and from 1.41 to 6.30 μM in dialysis patients. Compared to control groups a 5.0- to 11.7-fold increase of SDMA levels was observed. The ratio of SDMA/ADMA ranged from 0.30 to 1.31 in controls and from 0.34 to 4.83 in dialysis patients (table 2). Plasma L-arginine levels that were analyzed in 10 studies ranged from 75 to 118 μM in controls and from 35 to 127 μM in dialysis patients. Given the wide distribution of ADMA levels, the estimated L-arginine/ADMA ratio showed a wide range from 74 to 461 in controls and from 13 to 117 in dialysis patients (table 2).
ADMA Levels in HD Patients (Studies without Control Groups)
A variety of other investigators analyzed plasma ADMA levels in HD patients [27,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Since the results are almost identical to those studies that included a control group we did not summarize these studies in a separate table. In these studies ADMA levels range from anywhere between 0.8 and 8.0 μM.
Plasma ADMA Levels in PD Patients
We identified 7 studies in which plasma ADMA levels were measured in patients undergoing PD, these studies included 124 control individuals and 162 patients that were either treated with continuous ambulatory PD (CAPD) or different modes of automated PD (APD) [13, 28, 29,56,57,58,59]. All but one study included a control group. Again, plasma ADMA levels were markedly elevated in PD patients versus controls, however, similarly to studies in patients with CKD or patients on HD an overlap between plasma ADMA levels in controls and patients was observed between different studies. Plasma ADMA levels ranged from 0.36 to 1.0 μM in controls and from 0.49 to 2.16 in PD patients, compared to controls ADMA levels were 1.4- to 5.4-fold higher in PD patients. Thus, compared to patients on HD, plasma ADMA levels appear to be lower in PD patients which may in part be explained by more preserved residual renal function and continuous versus intermittent renal replacement therapy. Plasma SDMA levels that were measured in 5 studies ranged from 0.12 to 0.80 in controls and from 0.75 to 5.10 in patients on PD, compared to controls SDMA levels were 5.4- to 9.8-fold higher in PD patients. Except for one study [56], a marked increase of SDMA/ADMA ratio was observed in patients on PD (0.35–4.18).
Plasma ADMA Levels in Kidney Transplant Recipients
The role of ADMA in patients after kidney transplantation has received little attention so far. We only identified 5 studies in which plasma ADMA levels were measured in renal transplant recipients [12, 15, 23, 60, 61]. Fleck et al. [12], Schiel et al. [15] and Busch et al. [23] studied patients with similar renal function (serum creatinine ∼160 μmol/l) and reported ADMA levels of ∼1.0 μM and SDMA levels of ∼1.1 μM. In these 3 studies, ADMA levels were similar to those measured in patients on HD (∼1 μM), however SDMA levels were twice as low as in dialysis patients (∼1.2 vs. ∼2.4 μM). Recently, Yilmaz et al. [60] analyzed 27 patients that received kidney transplants from a living donor. ADMA levels and serum creatinine were markedly elevated before transplantation (4.42 μM and ∼775 μmol/l, respectively); both parameters rapidly declined over 28 days (1.08 μM and ∼100 μmol/l, respectively). The authors also studied flow-mediated vasodilation which markedly improved after transplantation and showed a strong negative correlation with ADMA levels. Cupisti et al. [61] recently observed an improvement in flow-mediated vasodilation in 20 renal transplant patients after a 5-week treatment with a diet rich in soy protein. The amelioration of endothelial function was associated with an improved L-arginine/ADMA ratio.
Given the limited data available, further studies are needed to confirm the role of ADMA in renal transplantation.
Impact of a Single Dialysis Session on Plasma ADMA Levels
We identified 19 studies in which the impact of a single HD session on the clearance of ADMA was analyzed [1, 8, 13,27,28,29,30,31,33,34,35,39,40,41,42,43, 59, 62, 63]. The studies included 245 patients; in 10 studies reduction of SDMA levels was also analyzed. Again, there was a wide distribution regarding the clearances of ADMA and SDMA with clearance rates ranging from 0 to 80% and from 0 to 70%, respectively (table 3).
Table 3.
Impact of a single hemodialysis session on ADMA and SDMA levels
| Subjects |
Dialysis filter DM/TT |
ADMA pre, μM |
ADMA post, μM |
% reduction ADMA |
SDMA pre, μM |
SDMA post, μM |
% reduction SDMA |
|
|---|---|---|---|---|---|---|---|---|
| Vallance (1992) |
3 |
n.i. |
4.0 |
2.1 |
>40% |
n.i. |
n.i. |
n.i. |
| Arese (1995) | 5 | CU/PS | ∼1.35∗ | ∼0.52∗ | ∼61%∗ | ∼2.93∗ | ∼1.25∗ | ∼57%∗ |
| PFD/HD/HDF/4 h |
||||||||
| MacAllister (1996) | 6 | PS | 0.99 | 0.77 | ∼22% | 3.78 | 2.23 | ∼41% |
| HD/4 h |
||||||||
| Anderstam (1997) | 12 | n.i. | ∼0.59∗ | ∼0.45∗ | ∼23% | ∼2.85∗ | ∼1.71∗ | ∼40% |
| HD/n.i. |
||||||||
| Kielstein (1999) | 8 | HP | ∼5.0∗ | ∼6.0∗ | 0% | 3.97 | 6.25 | 0% |
| HD/4.5 h |
∼50% 5 h post HD∗ vs. pre-dialysis |
0% 5 h post HD vs. pre-dialysis |
||||||
| Schmidt (1999) | 11 | n.i. | 4.14 | 1.45 | ∼65% | 1.41 | 0.62 | ∼56% |
| HD/3.5–4 h |
||||||||
| Kang (1999) | 10 | PS | 8.0 | 5.0 | ∼37% | n.i. | n.i. | n.i. |
| HD/4 h |
||||||||
| Gomez-Fernandez (2000) | 6 | CU/PAN/SPAN | 5.8 CU | 0.9 CU | ∼84% CU | n.i. | n.i. | n.i. |
| HD/4 h | 5.0 PAN | 2.4 PAN | ∼52% PAN | |||||
| 4.9 SPAN |
1.5 SPAN |
∼69% SPAN |
||||||
| Wahbi (2001) | 17 | n.i. | 0.99 | 0.63 | ∼36% | 4.53 | 2.95 | ∼35% |
| HD/n.i. |
||||||||
| Cross (2001) | 16 | HP | 0.68 | 0.43 | ∼37% | n.i. | n.i. | n.i. |
| HD/4 h |
||||||||
| Schroder (2001) | 6 | CE/PA | n.i. | n.i. | ∼40–65%∗ | n.i. | n.i. | ∼50–70%∗ |
| HD/HDF/5 h |
||||||||
| Raj (2002) | 27 | PS | 4.0 | 1.6 | ∼60% | n.i. | n.i. | n.i. |
| HD/4 h |
||||||||
| Kielstein (2004) | 30 | PS | 4.35 | 4.76 | 0% | n.i. | n.i. | n.i. |
| Genius-HD/∼4 h |
||||||||
| Bergamini (2004) | 11 | PS | 3.62 | 2.31 | ∼36% | 6.30 | 3.61 | ∼43% |
| HD/4 h |
||||||||
| Mochizuki (2005) | 10 | PS | 2.2 | 2.4 | 0% | n.i. | n.i. | n.i. |
| HD/4 h |
45% 4 h post HD |
|||||||
| Morimoto (2005) | 10 | PS/PSE | 0.69 PS | 0.41 PS | ∼41% PS | n.i. | n.i. | n.i. |
| HD/4 h |
0.74 PSE |
0.41 PSE |
∼45% PSE |
|||||
| Kalousova (2006) | 20 | PS | 0.81 HD | 0.52 HD | ∼36% HD | ∼3.2 HD∗ | ∼1.8 HD∗ | 44% HD |
| HD/HDF/4 h |
0.79 HDF |
0.48 HDF |
∼39% HDF |
∼3.1 HDF∗ |
∼1.6 HDF∗ |
48% HDF |
||
| Soveri (2007) | 22 | n.i. | 0.99 | 0.73 | ∼26% | n.i. | n.i. | n.i. |
| HD or HDF/4 h |
||||||||
| Grooteman (2007) | 15 | CU/PS/PES | 0.62 | 0.43 | ∼32% | 2.55 | 1.42 | ∼44% |
| HD/4–5 h | no differences in dialyzer clearance | no differences in dialyzer clearance |
DM = Dialysis mode; TT = treatment time; CE = cellulose; CU = cuprophane; HP = hemophane; PS = polysulphone; PSE = vitamin E-coated poly-sulphone; PES = polyethersulfone; PA = polyamide; PAN = polyacrylnitrile; SPAN = special polyacrylnitrile; HD = bicarbonate hemodialysis; HDF = hemodiafiltration; PFD = paired filtration dialysis. An asterisk indicates the estimated mean.
Lack of effectiveness of dialysis to lower ADMA has been in part attributed to protein binding as well as possible redistribution of this molecule during HD [42]. In this context, red blood cells are capable to either buffer or release ADMA [64]. Such a mechanism may be influenced by the degree of renal anemia as well as the artificial circulation during dialysis. Kielstein et al. [29] and Mochizuki et al. [34] observed no immediate decline of ADMA levels following a regular dialysis session, however 4–5 h after the end of HD a marked decline by 45–50% was observed. Intriguingly, SDMA levels remained elevated at this timepoint (no reduction after 5 h versus pre-dialysis levels). Based on the low molecular weight of ADMA (202 Da), which is in the range of urea (60 Da), HD should be very effective in reducing the blood levels of this endogenous NOS inhibitor. However, this may not be the case as pointed out by Kielstein et al. [42] who assessed the dialyzer clearance of ADMA. Thus, opposed to the dialyzer clearances of urea (∼161 ml/min) and creatinine (∼173 ml/min), that of ADMA was only 92 ml/min. Approximately 37 μmol ADMA were recovered in the spent dialysate [42]. This amount roughly averages ∼15% of daily produced ADMA. Interestingly, the daily urinary excretion of ADMA in healthy humans is ∼50 μmol; accordingly one would not assume that dialysis should be more effective than native functioning kidneys.
There is also no evidence that the use of different dialysis membrane filters (i.e. biocompatible high-flux filters) or different dialysis treatment modalities (i.e. hemodiafiltration) can improve the clearance of ADMA [43, 63]. Thus, high-flux dialyzers or hemodiafiltration have no benefit over low-flux HD with respect to lowering ADMA [43, 63].
Increase of dialysis dose and frequency was also shown to be ineffective to lower ADMA levels [46]. In their recently published randomized cross-over study in which the impact of four different dialyzers on ADMA and SDMA clearance was analyzed in 15 patients on regular HD, Grooteman et al. [63] could nicely demonstrate that patient-related factors appear to be far more predictive regarding ADMA levels rather than different modes of HD treatment. Thus, interindividual variability of ADMA levels was ∼40% whereas intraindividual variability during the entire study period was less than 1% [63].
In a recent review of uremic toxins, Vanholder et al. [65] pointed out that ADMA belongs to one of the view solutes for which a substantial gap between mean and maximum uremic concentrations has been reported. Accordingly, further well-designed studies are necessary to determine the clearance rate of ADMA in patients with ESRD.
Overall, available studies summarized in this review (table 3) suggest ADMA clearance rates between 20 and 40% during a regular HD.
Therapeutic Strategies to Modulate ADMA in Renal Disease
Based on the generation and metabolism of ADMA, specific strategies to modulate its plasma concentrations should either focus on ways to reduce the synthesis or improve the clearance or enzymatic degradation. The design of PRMT inhibitors to target ADMA synthesis appears an unlikely option since methylation of arginine residues isa highly regulated, most likely obligatory step in protein turnover. As shown in table 3, the ability and effectiveness of dialysis to lower ADMA levels in patients with ESRD remains controversial but clearly appears to be lower than expected due to protein binding.
Thus, most investigators have focused on DDAH as a likely target to modulate ADMA levels since dysregulation of this enzyme appears to be the key mechanism by which ADMA levels are elevated in various disorders. DDAH is a highly oxidative sensitive enzyme; oxidation of a critical sulfhydryl group (CYS249) reduces DDAH enzyme activity. Accordingly, antioxidants have been shown to lower ADMA levels by increasing DDAH activity [66]. Furthermore, recent studies indicate that all-trans retinoic acid and the farnesoid X receptor (FXR) agonist GW4064 modulate ADMA levels by regulating DDAH [67, 68]. Possible explanations are the presence of a PPAR/RXR binding site within the DDAH2 gene and a FXR response element within the DDAH1 gene.
A less specific approach to counterbalance the detrimental effects of ADMA is to overcome NOS antagonism by excess substrate supply with the precursor amino acid L-arginine since the L-arginine/ADMA ratio should theoretically determine the degree of enzyme inhibition. However, even in patients with ESRD plasma concentrations of L-arginine are at least 10-fold higher than that of ADMA and far exceed the Km of NOS which is in the low micromolar range. Accordingly, the concept that substrate bioavailability should be a rate-limiting factor for NO production seems illogical. Nevertheless, some investigators have shown that L-arginine can improve endothelial function in humans [69, 70]. Based on these observations, the term ‘L-arginine paradox’ has been coined.
In animal models of acute or chronic kidney disease, modification of dietary L-arginine intake has been associated both with beneficial as well as deleterious effects [71]. Studies in humans indicate that L-arginine supplementation does not modify the course of renal disease in humans with chronic glomerular diseases [71].
Other, non-specific pharmacologic interventions that have been investigated in humans include the use of statins and blockade of the renin-angiotensin system (RAS) with either angiotensin-converting enzyme inhibitors (ACE inhibitors) or AT1 receptor blockers (ARBs). With respect to statins, all but one study failed to observe significant reductions in plasma ADMA levels despite marked improvement in lipid profiles [66]. The impact of RAS-blocking agents on ADMA metabolism appears to be more complex. While some investigators observed significant reductions of plasma ADMA levels with these agents [24, 38,72,73,74,75,76], more recent studies could not confirm this observation [77, 78]. Except for one study in dialysis patients that showed a ∼40% reduction of ADMA levels following a 6-week treatment with the ARB valsartan [38], the remaining trials in patients with CKD or normal renal function revealed reductions of ADMA levels in the range of 15–25% [24,72,73,74,75,76]. Interpretation of available studies is complicated due to differences in study design, study cohorts, the possible confounding role of underlying cardiovascular co-morbidities, treatment regimen and duration, the use of different bioanalytical methods to determine ADMA levels and, more importantly, the fact that most studies were simply underpowered to address this issue. However, it is noteworthy that the two negative studies [77, 78] were the only trials in which ADMA was measured by mass spectrometry, the gold standard for determination of ADMA. The observed discrepancies highlight the need for further research in this field, in especially well-designed, adequately powered, prospective clinical trials using state-of-the-art techniques. An ongoing trial in more than 800 patients with diabetic nephropathy treated with either the ARB telmisartan or valsartan may help to decipher the role of these drugs on ADMA metabolism [79].
ADMA: A Biomarker in Renal Disease?
Several investigators have proposed ADMA as a potentially useful biomarker in cardiovascular disease. In order to appreciate this proposal for patients with kidney disease, one has to address the following questions:
(1) Is ADMA simply a marker of renal disease or does it participate in pathobiology, in other words is ADMA only an innocent bystander associated with renal disease or is there any causal relationship?
(2) Is ADMA a useful tool to diagnose severity of disease?
(3) Is ADMA a prognostic marker of complications?
(4) Is ADMA a useful tool to monitor response to therapy?
The prerequisite to answer all these questions is the availability of standardized bioanalytical assays capable to measure trace amounts of ADMA with sufficient sensitivity and specificity as well as reproducibility. As pointed out in this review, although a variety of investigators used the same analytical methods the range of reported ADMA levels in patients with renal disease is wide. Given the obvious limitations regarding the detection of ADMA, one has to cast doubts that ADMA fulfils the requirements of a useful biomarker in renal disease, nevertheless we want to try to answer the questions raised above.
Until recently, there was no compelling evidence that ADMA plays a causal role in the pathophysiology of vascular disease. Thus, although elevated ADMA levels have been frequently associated with various cardiovascular risk factors it remained elusive whether this molecule is simply a marker or also a ‘maker’ of vascular disease. The strongest evidence pointing to the crucial role of ADMA on vascular biology stems from the generation of DDAH1 knockout mice [80]. Homozygous disruption of the DDAH1 gene leads to an embryonic lethal phenotype whereas heterozygous mutants are viable. These mice have higher plasma ADMA levels and an enhanced systemic blood pressure thereby contrasting the phenotype of DDAH transgenic mice overexpressing human DDAH1 [81]. Further characterization of these mice is necessary to confirm the role of ADMA on vascular biology.
Despite the wide range of reported ADMA levels in patients with renal disease there is some evidence that ADMA may be a useful marker to assess severity of kidney disease. Most studies are of limited value since they were cross-sectional in nature and simply show an association of elevated ADMA levels with renal disease. Relatively few prospective studies have been conducted [18, 19, 23, 47, 49]. These are, however, further limited since ADMA levels were only determined at baseline. To the best of our knowledge, there is no large study that has performed serial measurements of ADMA levels over time in patients with advancing renal failure. Such intraindividual dynamics are essential to unequivocally demonstrate a tight relationship between ADMA levels and progression or severity of renal disease. Thus far, two large clinical trials by Tarnow et al. [17] and Fliser et al. [18] that included patients with diabetic and non-diabetic CKD have demonstrated a gradual increase of ADMA levels with declining renal function after stratification of patients according to glomerular filtration rate. These studies support the potential role of ADMA to predict severity of renal disease. Overall, serum creatinine is not well correlated with ADMA levels (fig. 2) whereas SDMA shows a close correlation. Accordingly, SDMA appears to be a better marker of renal function, most likely because this molecule does not undergo enzymatic degradation.
The role of ADMA as a prognostic biomarker in patients with ESRD undergoing dialysis remains somewhat controversial. In 2001, Zoccali et al. [47] reported the results of a prospective cohort study in which 225 HD patients were followed over approximately 3 years. In this study, elevated plasma ADMA levels ranked as the second most important factor predicting overall mortality and cardiovascular events outweighing established risk factors such as diabetes, hypercholesterolemia or elevated levels of CRP, to name but a few [47]. Surprisingly, in a prospective cohort study in 200 patients with CKD (n = 81 on dialysis), Busch et al. [23] observed an opposite effect. Lower ADMA levels emerged as an independent risk factor for cardiovascular events and the authors proposed that ADMA might represent a candidate for the phenomenon of paradoxical or reverse epidemiology that has been observed in dialysis patients [23]. Accordingly, ADMA alone seems to be an inadequate marker for risk assessment in dialysis patients and should be viewed in light of other factors such as malnutrition (albumin levels) or inflammation (CRP levels) that have been associated with poor survival in dialysis patients. These underlying co-morbidities may in part explain why low ADMA levels have been associated with poor outcome.
The prognostic value of ADMA in patients with CKD has been evaluated by Fliser et al. [18] in the ‘Mild to Moderate Kidney Disease Study’. In this study, 177 patients with non-diabetic CKD were followed over a mean follow-up of 54 months during which time predefined study endpoints consisting of doubling of serum creatinine and/or need for renal replacement therapy were monitored. A total of 65 patients reached the specified endpoint, these individuals had significantly higher baseline ADMA levels compared to the remaining patients (0.42 vs. 0.55, p < 0.01). Most intriguingly, plasma ADMA levels were the only independent predictor of progression of renal disease. Similar findings were made by Ravani et al. [19] in 131 patients with CKD (mean follow-up of 27 months) in which ADMA emerged as an independent predictor of progression to dialysis and death.
Whether measurement of ADMA levels may be a useful tool to monitor response to therapy remains to be proven. As pointed out above, there are no specific pharmacologic agents available that can modulate plasma levels of ADMA. Non-specific therapies have either failed to reduce ADMA levels, yielded indefinite results or need confirmation in large prospective clinical trials.
Determination of Plasma ADMA Levels in Humans
A variety of techniques have been used for the quantification of ADMA in human plasma samples [82]. Most of these are based on solid-phase extraction of basic plasma components followed by derivatization and liquid chromatography with fluorimetric or mass spectrometric detection [82]. Despite the precision of these devices the major limitation lies in the time-consuming, cumbersome and expensive procedure that does not allow high-throughput analysis which is necessary to make this marker readily available for the clinical routine. The wide range of ADMA levels reported in control individuals and patients with renal failure can only in part be explained by the different use of bioanalytical methods that lack standardization. If one accepts differences in absolute levels of dimethylarginines the scatter of the SDMA/ADMA ratio is still noteworthy and lacks explanation. Overall, it seems difficult to compare results obtained in different studies. Despite these limitations the role of ADMA in renal disease has to remain undisputed. Indeed, all investigators have unanimously observed elevated levels of this molecule in patients with renal disease.
Given the obvious pitfalls in the detection of ADMA an ELISA assay has recently become available that allows rapid determination of ADMA in human plasma samples [83]. Whether this assay will allow better comparison of results between different trials needs to be determined. The ELISA assay has recently been applied in two large clinical trials, the CARDIAC and AtheroGene study, in which the impact of ADMA in patients with coronary heart disease was analyzed [84, 85]. These studies demonstrate that ADMA is an independent cardiovascular risk factor and that baseline ADMA levels independently predict future cardiovascular risk. However, the major limitation of both studies is that the absolute differences between cases and controls (0.7 vs. 0.6 μM) or patients with or without cardiovascular event (0.7 vs. 0.63 μM) is rather low. Notably, the reference value for this ELISA assay determined in 500 healthy subjects was reported as 0.69 μM[86].
Overall, plasma ADMA has a very narrow concentration distribution, with an interindividual coefficient of variation of approximately 12% [82] which undermines that one cannot be satisfied with the wide range that has been reported in healthy individuals as well as in patients with renal failure. Normal reference values of 0.50–2.20 μM have been indicated in the literature [52]. Such a wide range has to be questioned since small differences in ADMA levels have been associated with increased cardiovascular risk.
A recent study has attempted to define reference values for ADMA in healthy individuals [87]. In this study, ADMA was measured by HPLC method with pre-column derivatization and fluorescence detection in 292 males aged 20–75 years. The median ADMA levels varied from 0.58 μM (<35 years) to 0.64 μM (>64 years), indicating only small differences between different age groups. Based upon the variation of ADMA levels in healthy control individuals summarized in this review that were determined with similar HPLC techniques, the study by Meinitzer et al. [87] highlights the need for further critical validation of analytical methods. Reference values for patients with CKD or ESRD will be hard to determine since multiple factors (residual renal function, mode of renal replacement therapy, degree of proteinuria and anemia, malnutrition, underlying co-morbidities) may have a profound impact on ADMA levels in these subjects.
The variability of ADMA levels currently precludes useful application of this putative biomarker for risk assessment in patients with renal disease. At present, it remains more than questionable whether this molecule will ever reach the stage of a useful laboratory parameter that will influence the guidance of patients. Above all, a consensus regarding reference values and analytical methods seems necessary.
Conclusions
All clinical studies unanimously demonstrate that plasma ADMA levels are elevated in patients with renal failure, however the range of reported ADMA and SDMA levels is substantial. Overall, simple, standardized analytical techniques are warranted to allow better comparison of this putative cardiovascular risk marker in clinical trials. Furthermore, SDMA and L-arginine levels and subsequent ratios with ADMA (L-arginine/ADMA and SDMA/ADMA) should be reported in future clinical trials in order to determine whether combined analysis of these three parameters may help to unravel the role of ADMA in renal disease.
At present, ADMA cannot be advocated as a useful clinical laboratory parameter or biomarker in patients with renal disease.
Acknowledgements
This work was supported by the ‘Interdisziplinäres Zentrum für Klinische Forschung’ (IZKF) at the Hospital of the University of Erlangen-Nuremberg, funded by the ‘Bundesministerium für Bildung und Forschung’ (01KS0002) as well as support from the National Institutes of Health (RO1 HL073084).
References
- 1.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 2.Kakimoto Y, Akazawa S. Isolation and identification of N-G,N-G- and N-G,N′-G-dimethyl-arginine, N-∊-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-δ-hydroxylysine from human urine. J Biol Chem. 1970;245:5751–5758. [PubMed] [Google Scholar]
- 3.Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 4.McDermott JR. Studies on the catabolism of Ng-methylarginine, Ng, Ng-dimethylarginine and Ng, Ng-dimethylarginine in the rabbit. Biochem J. 1976;154:179–184. doi: 10.1042/bj1540179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogawa T, Kimoto M, Sasaoka K. Occurrence of a new enzyme catalyzing the direct conversion of NG, NG-dimethyl-L-arginine to L-citrulline in rats. Biochem Biophys Res Commun. 1987;148:671–677. doi: 10.1016/0006-291x(87)90929-6. [DOI] [PubMed] [Google Scholar]
- 6.Leiper JM, Santa Maria J, Chubb A, MacAllister RJ, Charles IG, Whitley GS, Vallance P. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J. 1999;343:209–214. [PMC free article] [PubMed] [Google Scholar]
- 7.Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23:1455–1459. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- 8.MacAllister RJ, Rambausek MH, Vallance P, Williams D, Hoffmann KH, Ritz E. Concentration of dimethyl-L-arginine in the plasma of patients with end-stage renal failure. Nephrol Dial Transplant. 1996;11:2449–2452. doi: 10.1093/oxfordjournals.ndt.a027213. [DOI] [PubMed] [Google Scholar]
- 9.Marescau B, Nagels G, Possemiers I, De Broe ME, Becaus I, Billiouw JM, Lornoy W, De Deyn PP. Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metabolism. 1997;46:1024–1031. doi: 10.1016/s0026-0495(97)90273-0. [DOI] [PubMed] [Google Scholar]
- 10.Al Banchaabouchi M, Marescau B, Possemiers I, D'Hooge R, Levillain O, De Deyn PP. NG, NG-dimethylarginine and NG, NG-dimethylarginine in renal insufficiency. Pflügers Arch. 2000;439:524–531. doi: 10.1007/s004249900220. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt RJ, Baylis C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int. 2000;58:1261–1266. doi: 10.1046/j.1523-1755.2000.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleck C, Janz A, Schweitzer F, Karge E, Schwertfeger M, Stein G. Serum concentrations of asymmetric (ADMA) and symmetric (SDMA) dimethylarginine in renal failure patients. Kidney Int. 2001;78(suppl):S14–S18. doi: 10.1046/j.1523-1755.2001.59780014.x. [DOI] [PubMed] [Google Scholar]
- 13.Wahbi N, Dalton RN, Turner C, Denton M, Abbs I, Swaminathan R. Dimethylarginines in chronic renal failure. J Clin Pathol. 2001;54:470–473. doi: 10.1136/jcp.54.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kielstein JT, Boger RH, Bode-Boger SM, Frolich JC, Haller H, Ritz E, Fliser D. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J Am Soc Nephrol. 2002;13:170–176. doi: 10.1681/ASN.V131170. [DOI] [PubMed] [Google Scholar]
- 15.Schiel R, Franke S, Busch M, Muller A, Fleck C, Muller UA, Braun A, Stein G. Effect of smoking on risk factors for cardiovascular disease in patients with diabetes mellitus and renal insufficiency. Eur J Med Res. 2003;8:283–291. [PubMed] [Google Scholar]
- 16.Saran R, Novak JE, Desai A, Abdulhayoglu E, Warren JS, Bustami R, Handelman GJ, Barbato D, Weitzel W, D'Alecy LG, Rajagopalan S. Impact of vitamin E on plasma asymmetric dimethylarginine in chronic kidney disease: a pilot study. Nephrol Dial Transplant. 2003;18:2415–2420. doi: 10.1093/ndt/gfg406. [DOI] [PubMed] [Google Scholar]
- 17.Tarnow L, Hovind P, Teerlink T, Stehouwer CD, Parving HH. Elevated plasma asymmetric dimethylarginine as a marker of cardiovascular morbidity in early diabetic nephropathy in type 1 diabetes. Diabetes Care. 2004;27:765–769. doi: 10.2337/diacare.27.3.765. [DOI] [PubMed] [Google Scholar]
- 18.Fliser D, Kronenberg F, Kielstein JT, Morath C, Bode-Boger SM, Haller H, Ritz E. Asymmetric dimethylarginine and progression of chronic kidney disease: the mild to moderate kidney disease study. J Am Soc Nephrol. 2005;16:2456–2461. doi: 10.1681/ASN.2005020179. [DOI] [PubMed] [Google Scholar]
- 19.Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: a competing risks modeling approach. J Am Soc Nephrol. 2005;16:2449–2455. doi: 10.1681/ASN.2005010076. [DOI] [PubMed] [Google Scholar]
- 20.Nanayakkara PW, Teerlink T, Stehouwer CD, Allajar D, Spijkerman A, Schalkwijk C, ter Wee PM, van Guldener C. Plasma asymmetric dimethylarginine concentration is independently associated with carotid intima-media thickness and plasma soluble vascular cell adhesion molecule-1 concentration in patients with mild-to-moderate renal failure. Kidney Int. 2005;68:2230–2236. doi: 10.1111/j.1523-1755.2005.00680.x. [DOI] [PubMed] [Google Scholar]
- 21.Yilmaz MI, Saglam M, Caglar K, Cakir E, Sonmez A, Ozgurtas T, Aydin A, Eyileten T, Ozcan O, Acikel C, Tasar M, Genctoy G, Erbil K, Vural A, Zoccali C. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis. 2006;47:42–50. doi: 10.1053/j.ajkd.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 22.Caglar K, Yilmaz MI, Sonmez A, Cakir E, Kaya A, Acikel C, Eyileten T, Yenicesu M, Oguz Y, Bilgi C, Oktenli C, Vural A, Zoccali C. ADMA, proteinuria, and insulin resistance in non-diabetic stage I chronic kidney disease. Kidney Int. 2006;70:781–787. doi: 10.1038/sj.ki.5001632. [DOI] [PubMed] [Google Scholar]
- 23.Busch M, Fleck C, Wolf G, Stein G. Asymmetrical (ADMA) and symmetrical dimethylarginine (SDMA) as potential risk factors for cardiovascular and renal outcome in chronic kidney disease – possible candidates for paradoxical epidemiology? Amino Acids. 2006;30:225–232. doi: 10.1007/s00726-005-0268-8. [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz MI, Saglam M, Sonmez A, Caglar K, Cakir E, Kurt Y, Eyileten T, Tasar M, Acikel C, Oguz Y, Vural A, Yenicesu M. Improving proteinuria, endothelial functions and asymmetric dimethylarginine levels in chronic kidney disease: ramipril versus valsartan. Blood Purif. 2007;25:327–335. doi: 10.1159/000107410. [DOI] [PubMed] [Google Scholar]
- 25.Kielstein JT, Salpeter SR, Bode-Boeger SM, Cooke JP, Fliser D. Symmetric dimethylarginine as endogenous marker of renal function – a meta-analysis. Nephrol Dial Transplant. 2006;21:2446–2451. doi: 10.1093/ndt/gfl292. [DOI] [PubMed] [Google Scholar]
- 26.Mendes Ribeiro AC, Roberts NB, Lane C, Yaqoob M, Ellory JC. Accumulation of the endogenous L-arginine analogue NG-monomethyl-L-arginine in human end-stage renal failure patients on regular haemodialysis. Exp Physiol. 1996;81:475–481. doi: 10.1113/expphysiol.1996.sp003950. [DOI] [PubMed] [Google Scholar]
- 27.Kang ES, Tevlin MT, Wang YB, Chiang TM, Cardenas R, Myers LK, Acchiardo SR. Hemodialysis hypotension: interaction of inhibitors, iNOS, and the interdialytic period. Am J Med Sci. 1999;317:9–21. doi: 10.1097/00000441-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Anderstam B, Katzarski K, Bergstrom J. Serum levels of NG, NG-dimethyl-L-arginine, a potential endogenous nitric oxide inhibitor in dialysis patients. J Am Soc Nephrol. 1997;8:1437–1442. doi: 10.1681/ASN.V891437. [DOI] [PubMed] [Google Scholar]
- 29.Kielstein JT, Boger RH, Bode-Boger SM, Schaffer J, Barbey M, Koch KM, Frolich JC. Asymmetric dimethylarginine plasma concentrations differ in patients with end-stage renal disease: relationship to treatment method and atherosclerotic disease. J Am Soc Nephrol. 1999;10:594–600. doi: 10.1681/ASN.V103594. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt RJ, Domico J, Samsell LS, Yokota S, Tracy TS, Sorkin MI, Engels K, Baylis C. Indices of activity of the nitric oxide system in hemodialysis patients. Am J Kidney Dis. 1999;34:228–234. doi: 10.1053/AJKD03400228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raj DS, Vincent B, Simpson K, Sato E, Jones KL, Welbourne TC, Levi M, Shah V, Blandon P, Zager P, Robbins RA. Hemodynamic changes during hemodialysis: role of nitric oxide and endothelin. Kidney Int. 2002;61:697–704. doi: 10.1046/j.1523-1755.2002.00150.x. [DOI] [PubMed] [Google Scholar]
- 32.Osanai T, Fujiwara N, Saitoh M, Sasaki S, Tomita H, Nakamura M, Osawa H, Yamabe H, Okumura K. Relationship between salt intake, nitric oxide and asymmetric dimethylarginine and its relevance to patients with end-stage renal disease. Blood Purif. 2002;20:466–468. doi: 10.1159/000063555. [DOI] [PubMed] [Google Scholar]
- 33.Bergamini S, Vandelli L, Bellei E, Rota C, Manfredini P, Tomasi A, Albertazzi A, Iannone A. Relationship of asymmetric dimethylarginine to haemodialysis hypotension. Nitric Oxide. 2004;11:273–278. doi: 10.1016/j.niox.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Mochizuki S, Ono J, Yada T, Ogasawara Y, Miyasaka T, Kimoto M, Kashihara N, Kajiya F. Systemic nitric oxide production rate during hemodialysis and its relationship with nitric oxide-related factors. Blood Purif. 2005;23:317–324. doi: 10.1159/000087769. [DOI] [PubMed] [Google Scholar]
- 35.Morimoto H, Nakao K, Fukuoka K, Sarai A, Yano A, Kihara T, Fukuda S, Wada J, Makino H. Long-term use of vitamin E-coated polysulfone membrane reduces oxidative stress markers in haemodialysis patients. Nephrol Dial Transplant. 2005;20:2775–2782. doi: 10.1093/ndt/gfi121. [DOI] [PubMed] [Google Scholar]
- 36.Siroka R, Trefil L, Rajdl D, Racek J, Rusnakova H, Cibulka R, Eiselt J, Filipovsky J. Asymmetric dimethylarginine, homocysteine and renal function – is there a relation? Clin Chem Lab Med. 2005;43:1147–1150. doi: 10.1515/CCLM.2005.199. [DOI] [PubMed] [Google Scholar]
- 37.Martens-Lobenhoffer J, Krug O, Bode-Boger SM. Determination of arginine and asymmetric dimethylarginine in human plasma by liquid chromatography/mass spectrometry with the isotope dilution technique. J Mass Spectrom. 2004;39:1287–1294. doi: 10.1002/jms.684. [DOI] [PubMed] [Google Scholar]
- 38.Aslam S, Santha T, Leone A, Wilcox C. Effects of amlodipine and valsartan on oxidative stress and plasma methylarginines in end-stage renal disease patients on hemodialysis. Kidney Int. 2006;70:2109–2115. doi: 10.1038/sj.ki.5001983. [DOI] [PubMed] [Google Scholar]
- 39.Arese M, Strasly M, Ruva C, Costamagna C, Ghigo D, MacAllister R, Verzetti G, Tetta C, Bosia A, Bussolino F. Regulation of nitric oxide synthesis in uraemia. Nephrol Dial Transplant. 1995;10:1386–1397. [PubMed] [Google Scholar]
- 40.Gomez-Fernandez P, Velasco G, Esteban J, Moreno VG, Guillen DA, Garcia Barroso C, Almaraz M. L-Arginine-nitric oxide pathway in hemodialysis (in Spanish) Nefrologia. 2000;20:262–268. [PubMed] [Google Scholar]
- 41.Schroder M, Riedel E, Beck W, Deppisch RM, Pommer W. Increased reduction of dimethylarginines and lowered interdialytic blood pressure by the use of biocompatible membranes. Kidney Int. 2001;78(suppl):S19–S24. doi: 10.1046/j.1523-1755.2001.59780019.x. [DOI] [PubMed] [Google Scholar]
- 42.Kielstein JT, Boger RH, Bode-Boger SM, Martens-Lobenhoffer J, Lonnemann G, Frolich JC, Haller H, Fliser D. Low dialysance of asymmetric dimethylarginine – in vivo and in vitro evidence of significant protein binding. Clin Nephrol. 2004;62:295–300. doi: 10.5414/cnp62295. [DOI] [PubMed] [Google Scholar]
- 43.Kalousova M, Kielstein JT, Hodkova M, Zima T, Dusilova-Sulkova S, Martens-Lobenhoffer J, Bode-Boger SM. No benefit of hemodiafiltration over hemodialysis in lowering elevated levels of asymmetric dimethylarginine in ESRD patients. Blood Purif. 2006;24:439–444. doi: 10.1159/000095360. [DOI] [PubMed] [Google Scholar]
- 44.Osanai T, Nakamura M, Sasaki S, Tomita H, Saitoh M, Osawa H, Yamabe H, Murakami S, Magota K, Okumura K. Plasma concentration of coupling factor 6 and cardiovascular events in patients with end-stage renal disease. Kidney Int. 2003;64:2291–2297. doi: 10.1046/j.1523-1755.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- 45.Beerenhout CH, Luik AJ, Jeuken-Mertens SG, Bekers O, Menheere P, Hover L, Klaassen L, van der Sande FM, Cheriex EC, Meert N, Leunissen KM, Kooman JP. Pre-dilution on-line haemofiltration vs. low-flux haemodialysis: a randomized prospective study. Nephrol Dial Transplant. 2005;20:1155–1163. doi: 10.1093/ndt/gfh775. [DOI] [PubMed] [Google Scholar]
- 46.Chan CT, Harvey PJ, Boger R, Pierratos A, Floras JS. Dissociation between the short-term effects of nocturnal hemodialysis on endothelium dependent vasodilation and plasma ADMA. Arterioscler Thromb Vasc Biol. 2005;25:2685–2686. doi: 10.1161/01.ATV.0000193890.94720.1a. [DOI] [PubMed] [Google Scholar]
- 47.Zoccali C, Bode-Boger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frolich J, Boger R. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 48.Mallamaci F, Tripepi G, Maas R, Malatino L, Boger R, Zoccali C. Analysis of the relationship between norepinephrine and asymmetric dimethyl arginine levels among patients with end-stage renal disease. J Am Soc Nephrol. 2004;15:435–441. doi: 10.1097/01.asn.0000106717.58091.f6. [DOI] [PubMed] [Google Scholar]
- 49.Mallamaci F, Tripepi G, Cutrupi S, Malatino LS, Zoccali C. Prognostic value of combined use of biomarkers of inflammation, endothelial dysfunction, and myocardiopathy in patients with ESRD. Kidney Int. 2005;67:2330–2337. doi: 10.1111/j.1523-1755.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 50.Testa A, Spoto B, Tripepi G, Mallamaci F, Malatino L, Fatuzzo P, Maas R, Boeger R, Zoccali C. The GLU298ASP variant of nitric oxide synthase interacts with asymmetric dimethyl arginine in determining cardiovascular mortality in patients with end-stage renal disease. J Hypertens. 2005;23:1825–1830. doi: 10.1097/01.hjh.0000182528.59687.d1. [DOI] [PubMed] [Google Scholar]
- 51.Spoto B, Benedetto FA, Testa A, Tripepi G, Mallamaci F, Maas R, Boeger RH, Zoccali C, Parlongo RM, Pisano A. Atherosclerosis and the Glu298Asp polymorphism of the eNOS gene in white patients with end-stage renal disease. Am J Hypertens. 2005;18:1549–1555. doi: 10.1016/j.amjhyper.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Mallamaci F, Cutrupi S, Pizzini P, Tripepi G, Zoccali C. Urotensin II and biomarkers of endothelial activation and atherosclerosis in end-stage renal disease. Am J Hypertens. 2006;19:505–510. doi: 10.1016/j.amjhyper.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 53.Zoccali C, Benedetto FA, Maas R, Mallamaci F, Tripepi G, Malatino LS, Boger R. Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J Am Soc Nephrol. 2002;13:490–496. doi: 10.1681/ASN.V132490. [DOI] [PubMed] [Google Scholar]
- 54.Zoccali C, Mallamaci F, Maas R, Benedetto FA, Tripepi G, Malatino LS, Cataliotti A, Bellanuova I, Boger R. Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine in hemodialysis patients. Kidney Int. 2002;62:339–345. doi: 10.1046/j.1523-1755.2002.00437.x. [DOI] [PubMed] [Google Scholar]
- 55.Kumagai H, Sakurai M, Takita T, Maruyama Y, Uno S, Ikegaya N, Kato A, Hishida A. Association of homocysteine and asymmetric dimethylarginine with atherosclerosis and cardiovascular events in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48:797–805. doi: 10.1053/j.ajkd.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt RJ, Yokota S, Tracy TS, Sorkin MI, Baylis C. Nitric oxide production is low in end-stage renal disease patients on peritoneal dialysis. Am J Physiol. 1999;276:F794–F797. doi: 10.1152/ajprenal.1999.276.5.F794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mittermayer F, Schaller G, Pleiner J, Vychytil A, Sunder-Plassmann G, Horl WH, Wolzt M. Asymmetrical dimethylarginine plasma concentrations are related to basal nitric oxide release but not endothelium-dependent vasodilation of resistance arteries in peritoneal dialysis patients. J Am Soc Nephrol. 2005;16:1832–1838. doi: 10.1681/ASN.2004121109. [DOI] [PubMed] [Google Scholar]
- 58.Yano A, Nakao K, Sarai A, Akagi S, Kihara T, Morimoto H, Nakamura A, Hiramatsu M, Nagake Y, Makino H. Elevated serum interleukin-18 levels might reflect the high risk of hospitalization in patients on peritoneal dialysis. Nephrology (Carlton) 2005;10:576–582. doi: 10.1111/j.1440-1797.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 59.Cross JM, Donald A, Vallance PJ, Deanfield JE, Woolfson RG, MacAllister RJ. Dialysis improves endothelial function in humans. Nephrol Dial Transplant. 2001;16:1823–1829. doi: 10.1093/ndt/16.9.1823. [DOI] [PubMed] [Google Scholar]
- 60.Yilmaz MI, Saglam M, Caglar K, Cakir E, Ozgurtas T, Sonmez A, Eyileten T, Yenicesu M, Acikel C, Oguz Y, Ozcan O, Bozlar U, Erbil K, Aslan I, Vural A. Endothelial functions improve with decrease in asymmetric dimethylarginine levels after renal transplantation. Transplantation. 2005;80:1660–1666. doi: 10.1097/01.tp.0000183750.22675.be. [DOI] [PubMed] [Google Scholar]
- 61.Cupisti A, Ghiadoni L, D'Alessandro C, Kardasz I, Morelli E, Panichi V, Locati D, Morandi S, Saba A, Barsotti G, Taddei S, Arnoldi A, Salvetti A. Soy protein diet improves endothelial dysfunction in renal transplant patients. Nephrol Dial Transplant. 2007;22:229–234. doi: 10.1093/ndt/gfl553. [DOI] [PubMed] [Google Scholar]
- 62.Soveri I, Lind L, Wikstrom B, Zilmer M, Zilmer K, Fellstrom B. Improvement in central arterial pressure waveform during hemodialysis is related to a reduction in asymmetric dimethylarginine levels. Nephron Clin Pract. 2007;106:c180–c186. doi: 10.1159/000104429. [DOI] [PubMed] [Google Scholar]
- 63.Grooteman MP, Wauters IM, Teerlink T, Twisk JW, Nube MJ. Plasma Dimethylarginine levels in chronic hemodialysis patients are independent of the type of dialyzer applied. Blood Purif. 2007;25:281–289. doi: 10.1159/000104868. [DOI] [PubMed] [Google Scholar]
- 64.Billecke SS, Kitzmiller LA, Northrup JJ, Whitesall SE, Kimoto M, Hinz AV, D'Alecy LG. Contribution of whole blood to the control of plasma asymmetrical dimethylarginine. Am J Physiol. 2006;291:H1788–H1796. doi: 10.1152/ajpheart.00066.2006. [DOI] [PubMed] [Google Scholar]
- 65.Vanholder R, De Smet R, Glorieux G, Argiles A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jorres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 66.Maas R. Pharmacotherapies and their influence on asymmetric dimethylarginine. Vasc Med. 2005;10(suppl 1):S49–S57. doi: 10.1191/1358863x05vm605oa. [DOI] [PubMed] [Google Scholar]
- 67.Achan V, Tran CT, Arrigoni F, Whitley GS, Leiper JM, Vallance P. all-Trans-retinoic acid increases nitric oxide synthesis by endothelial cells: a role for the induction of dimethylarginine dimethylaminohydrolase. Circ Res. 2002;90:764–769. doi: 10.1161/01.res.0000014450.40853.2b. [DOI] [PubMed] [Google Scholar]
- 68.Hu T, Chouinard M, Cox AL, Sipes P, Marcelo M, Ficorilli J, Li S, Gao H, Ryan TP, Michael MD, Michael LF. Farnesoid X receptor agonist reduces serum asymmetric dimethylarginine levels through hepatic dimethylarginine dimethylaminohydrolase-1 gene regulation. J Biol Chem. 2006;281:39831–39838. doi: 10.1074/jbc.M606779200. [DOI] [PubMed] [Google Scholar]
- 69.Boger RH, Bode-Boger SM, Thiele W, Creutzig A, Alexander K, Frolich JC. Restoring vascular nitric oxide formation by L-arginine improves the symptoms of intermittent claudication in patients with peripheral arterial occlusive disease. J Am Coll Cardiol. 1998;32:1336–1344. doi: 10.1016/s0735-1097(98)00375-1. [DOI] [PubMed] [Google Scholar]
- 70.Drexler H, Zeiher AM, Meinzer K, Just H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by L-arginine. Lancet. 1991;338:1546–1550. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- 71.Cherla G, Jaimes EA. Role of L-arginine in the pathogenesis and treatment of renal disease. J Nutr. 2004;134:2801S–2806S. doi: 10.1093/jn/134.10.2801S. 2818S–2819S. [DOI] [PubMed] [Google Scholar]
- 72.Chen JW, Hsu NW, Wu TC, Lin SJ, Chang MS. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol. 2002;90:974–982. doi: 10.1016/s0002-9149(02)02664-4. [DOI] [PubMed] [Google Scholar]
- 73.Delles C, Schneider MP, John S, Gekle M, Schmieder RE. Angiotensin converting enzyme inhibition and angiotensin II AT1-receptor blockade reduce the levels of asymmetrical NG,NG-dimethylarginine in human essential hypertension. Am J Hypertens. 2002;15:590–593. doi: 10.1016/s0895-7061(02)02278-1. [DOI] [PubMed] [Google Scholar]
- 74.Ito A, Egashira K, Narishige T, Muramatsu K, Takeshita A. Renin-angiotensin system is involved in the mechanism of increased serum asymmetric dimethylarginine in essential hypertension. Jpn Circ J. 2001;65:775–778. doi: 10.1253/jcj.65.775. [DOI] [PubMed] [Google Scholar]
- 75.Ito A, Egashira K, Narishige T, Muramatsu K, Takeshita A. Angiotensin-converting enzyme activity is involved in the mechanism of increased endogenous nitric oxide synthase inhibitor in patients with type 2 diabetes mellitus. Circ J. 2002;66:811–815. doi: 10.1253/circj.66.811. [DOI] [PubMed] [Google Scholar]
- 76.Napoli C, Sica V, de Nigris F, Pignalosa O, Condorelli M, Ignarro LJ, Liguori A. Sulfhydryl angiotensin-converting enzyme inhibition induces sustained reduction of systemic oxidative stress and improves the nitric oxide pathway in patients with essential hypertension. Am Heart J. 2004;148:e5. doi: 10.1016/j.ahj.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 77.Fliser D, Wagner KK, Loos A, Tsikas D, Haller H. Chronic angiotensin II receptor blockade reduces (intra)renal vascular resistance in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16:1135–1140. doi: 10.1681/ASN.2004100852. [DOI] [PubMed] [Google Scholar]
- 78.Warnholtz A, Ostad MA, Heitzer T, Thuneke F, Frohlich M, Tschentscher P, Schwedhelm E, Boger R, Meinertz T, Munzel T: AT1-receptor blockade with irbesartan improves peripheral but not coronary endothelial dysfunction in patients with stable coronary artery disease. Atherosclerosis 2006; epub ahead of print. [DOI] [PubMed]
- 79.Boger RH, Schwedhelm E, Maas R, Quispe-Bravo S, Skamira C. ADMA and oxidative stress may relate to the progression of renal disease: rationale and design of the VIVALDI study. Vasc Med. 2005;10(suppl 1):S97–S102. doi: 10.1191/1358863x05vm608oa. [DOI] [PubMed] [Google Scholar]
- 80.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O'Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- 81.Dayoub H, Achan V, Adimoolam S, Jacobi J, Stuehlinger MC, Wang BY, Tsao PS, Kimoto M, Vallance P, Patterson AJ, Cooke JP. Dimethylarginine dimethylaminohydrolase regulates nitric oxide synthesis: genetic and physiological evidence. Circulation. 2003;108:3042–3047. doi: 10.1161/01.CIR.0000101924.04515.2E. [DOI] [PubMed] [Google Scholar]
- 82.Teerlink T. Measurement of asymmetric dimethylarginine in plasma: methodological considerations and clinical relevance. Clin Chem Lab Med. 2005;43:1130–1138. doi: 10.1515/CCLM.2005.197. [DOI] [PubMed] [Google Scholar]
- 83.Schulze F, Wesemann R, Schwedhelm E, Sydow K, Albsmeier J, Cooke JP, Boger RH. Determination of asymmetric dimethylarginine using a novel ELISA assay. Clin Chem Lab Med. 2004;42:1377–1383. doi: 10.1515/CCLM.2004.257. [DOI] [PubMed] [Google Scholar]
- 84.Schulze F, Lenzen H, Hanefeld C, Bartling A, Osterziel KJ, Goudeva L, Schmidt-Lucke C, Kusus M, Maas R, Schwedhelm E, Strodter D, Simon BC, Mugge A, Daniel WG, Tillmanns H, Maisch B, Streichert T, Boger RH. Asymmetric dimethylarginine is an independent risk factor for coronary heart disease: results from the multicenter Coronary Artery Risk Determination investigating the Influence of ADMA Concentration (CARDIAC) study. Am Heart J. 2006;152:493e1–e8. doi: 10.1016/j.ahj.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 85.Schnabel R, Blankenberg S, Lubos E, Lackner KJ, Rupprecht HJ, Espinola-Klein C, Jachmann N, Post F, Peetz D, Bickel C, Cambien F, Tiret L, Munzel T. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res. 2005;97:e53–e59. doi: 10.1161/01.RES.0000181286.44222.61. [DOI] [PubMed] [Google Scholar]
- 86.Schulze F, Maas R, Freese R, Schwedhelm E, Silberhorn E, Boger RH. Determination of a reference value for NG,NG-dimethyl-L-arginine in 500 subjects. Eur J Clin Invest. 2005;35:622–626. doi: 10.1111/j.1365-2362.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 87.Meinitzer A, Puchinger M, Winklhofer-Roob BM, Rock E, Ribalta J, Roob JM, Sundl I, Halwachs-Baumann G, Marz W. Reference values for plasma concentrations of asymmetrical dimethylarginine and other arginine metabolites in men after validation of a chromatographic method. Clin Chim Acta. 2007;384:141–148. doi: 10.1016/j.cca.2007.07.006. [DOI] [PubMed] [Google Scholar]