Abstract
Objective
To investigate the clinical features and natural history of mal de debarquement (MdD).
Design
Retrospective case review with follow-up questionnaire and telephone interviews.
Setting
University Neurotology Clinic.
Patients
Patients seen between 1980 and 2006 who developed a persistent sensation of rocking or swaying for at least 3 days after exposure to passive motion.
Main outcome measure
Clinical features, diagnostic testing, and questionnaire responses.
Results
Of 64 patients (75 % women) identified with MdD, 34 completed follow-up questionnaires and interviews in 2006. Most patients had normal neurological exams, ENGs and brain MRIs. The average age of the first MdD episode was 39 ± 13 years. A total of 206 episodes were experienced by 64 patients. Of these, 104 episodes (51 %) lasted > 1 month; 18 %, > 1 year; 15 %, > 2 years; 12 %, > 4 years, and 11 %, > 5 years. Eighteen patients (28 %) subsequently developed spontaneous episodes of MdD-like symptoms after the initial MdD episode. There was a much higher rate of migraine in patients who went on to develop spontaneous episodes (73 %) than in those who did not (22 %). Subsequent episodes were longer than earlier ones in most patients who had multiple episodes. Re-exposure to passive motion temporarily decreased symptoms in most patients (66 %). Subjective intolerance to visual motion increased (10 % to 66 %) but self-motion sensitivity did not (37 % to 50 %) with onset of MdD.
Conclusion
The majority of MdD episodes lasting longer than 3 days resolve in less than one year but the probability of resolution declines each year. Many patients experience multiple MdD episodes. Some patients develop spontaneous episodes after the initial motion-triggered episode with migraine being a risk factor.
Keywords: mal de debarquement, vestibular adaptation, motion sensitivity, visual motion, migraine
Introduction
In 1881, J.A. Irwin described a condition in which vestibular adaptation to sea conditions led to unsteady gait in the first few hours after returning to land [9]. This phenomenon has been termed mal de debarquement (MdD) and is usually described as an internal sense of rocking, bobbing or swaying that appears after disembarking from a vessel in which one has been passively moved [3, 14]. Brief episodes of MdD are quite common in healthy individuals. Gordon’s study of navy crewmen after sea voyage showed that 72 % of 116 subjects experienced transient MdD immediately after disembarking from a ship, which resolved spontaneously in less than 6 hours in 88 % of subjects and in less than 2 days in all subjects [5]. However, there are scattered reports of MdD symptoms lasting months or years, a phenomenon termed persistent mal de debarquement [3]. Most cases of MdD reported occurred following cruises, but exposure to other forms of passive motion has also been described [12, 14]. Hain reported the largest case series to date in which 27 individuals with symptoms longer than one month underwent a questionnaire survey [7]. These patients did not undergo a neurological exam or vestibular testing; however other reports indicate that neurological exams and vestibular function testing is generally normal in patients with MdD [3, 14]. Most studies also report a female predominance and frequent symptom duration of longer than one year [3, 7, 14].
We present our experience with MdD in a University setting in which all patients underwent a detailed neurological examination and most underwent diagnostic testing. Based on Gordon’s study of MdD duration in normal individuals, we used 3 or more days of MdD symptoms occurring after passive movement as the inclusion criteria. Though patients were seen over a span of 26 years, many were able to be contacted for follow-up interviews in the year of the study. We report interesting new features of MdD including the emergence of spontaneous MdD-like episodes after the initial motion-triggered episode, the lengthening of subsequent MdD episodes, the development of visual motion sensitivity with the onset of MdD, and the association of spontaneous MdD-like episodes with migraine.
Methods
The study design was approved by the Institutional Review Board at the UCLA School of Medicine. Records from a University Neurotology database were obtained for patients given a diagnosis of mal de debarquement by YHC, GI or RWB between 1980 and 2006. Patients who described an internal sensation of motion such as rocking, swaying or bobbing lasting at least three days after exposure to passive motion were included. Sixty-four charts were reviewed. Basic clinical features assessed included sex, age of onset, age at last follow-up, the type of motion exposure (e.g., boat, car, airplane, etc.), duration of episodes, number of episodes, neurological exam findings, ENG abnormalities and brain imaging reports. Follow-up questionnaire packets were sent to the patient at the most recent address on file for them.
Each questionnaire packet contained a “do not wish to participate” card with a stamped envelope and a consent form. If no response was received in two months, a second packet was sent. The patients were called at the most recent telephone number available if there was no response one month after the second mailing. Thirty-four patients returned questionnaires and could be contacted for follow-up interviews by phone or a clinic visit to validate their responses. Six “do not wish to participate” cards were returned. Twenty-four patients could not be contacted either by phone or by mail.
Responses were scored if they were clear and unambiguous. Therefore, a different number of responses was available for several of the clinical features. All percentages are rounded to the nearest whole number; therefore totals are not always 100 %. Basic clinical features are reported for all 64 patients diagnosed with MdD. Specific features additionally assessed in the questionnaire such as a past history of migraine headaches, aura, vertigo, self-motion sensitivity, visual motion sensitivity and effect of treatment are reported for the 34 patients who returned questionnaires.
Eighteen of the 64 MdD patients also reported experiencing spontaneous MdD-like episodes after their initial motion triggered episode. Because these spells were different from but related to pure motion-triggered MdD episodes, we present the data for pure MdD (only motion triggered), mixed MdD (both motion-triggered and spontaneous MdD), and all MdD separately.
Criteria for migraine headache and aura were according to International Classification of Headache Disorders 2nd ed guidelines [8]. Self-motion sensitivity was defined as the development of nausea during passive motion with the minimum criterion being sickness as a passenger in a car. Visual motion sensitivity was defined as the development of nausea when exposed to motion of the visual world (e. g., watching movies or freeway traffic) or exposure to virtual reality scenarios (e.g., video games).
Statistical analysis
Of the 15 variables evaluated, 11 were qualitative (sex, exam, ENG, MRI, migraine headache, visual aura, vertigo, motion sensitivity, visual motion sensitivity, motion effect on symptoms and trigger type) and 4 were quantitative (age at first episode, age at last follow-up, number of episodes, duration of episodes). Comparisons between groups using qualitative and quantitative variables were done with Fisher’s Exact Tests or Kruskal-Wallis rank sum tests and t-tests, respectively. A Bonferroni correction was used to adjust for multiple hypothesis testing. p values are reported after correction with a 5 % level to determine significance. Survival curves are presented using the Kaplan-Meier method to examine the proportion of episodes that continued at each time period of follow-up.
Results
Of the 64 patients with MdD, 46 patients experienced “pure” MdD while 18 patients experienced “mixed” MdD (Table 1). The motion triggered MdD episodes occurred before the spontaneous MdD-like episodes in patients with mixed MdD. There were more women affected by MdD than men in our series (75 %), especially in the group that went on to develop mixed MdD (89 %). The age of onset was significantly earlier in patients with mixed MdD than pure MdD (33 ± 10 years vs 43 ± 13 years, p value < 0.01, Bonferroni correction = 2). Neurological examinations were generally normal, but also showed some inconsistent abnormalities (Table 1). One patient showed poor ambulation secondary to amyotrophic lateral sclerosis and two patients had isolated difficulty with tandem gait. One patient showed positional torsional/downbeat nystagmus on examination and midline cerebellar atrophy on brain MRI. Coordination tests in this patient were completely normal, however, including gait. She had never experienced balance problems until she developed MdD after a 9-day cruise. Her symptoms significantly improved with driving and she developed severe visual motion sensitivity only after the onset of her MdD. Therefore, the MRI findings appeared to be incidental.
Table 1.
Clinical features of MdD patients
| Clinical features | all MdD total n = 64 (%) | pure MdD total n = 46 (%) | mixed MdD total n = 18 (%) |
|---|---|---|---|
| Sex | |||
| Female | 48 (75) | 32 (70) | 16 (89) |
| Male | 16 (25) | 14 (30) | 2 (11) |
| Age of first attack | |||
| Average (SD) | 39 (13) | 43 (13) | 33 (10) |
| Median | 38 | 42 | 36 |
| Age at last follow-up | |||
| Average | 47 (14) | 49 (15) | 45 (7) |
| Median | 46 | 47 | 47 |
| Exam | |||
| Normal | 58 (91) | 40 (87) | 18 (100) |
| Abnormal | 6a (9) | 6 (13) | 0 (0) |
| total n = 43 (%) | total n = 32 (%) | total n = 11 (%) | |
| ENG | |||
| Normal | 43 (100) | 32 (100) | 11 (100) |
| Abnormal | 0 (0) | 0 (0) | 0 (0) |
| total n = 53 (%) | total n = 38 (%) | total n = 15 (%) | |
| MRI | |||
| Normal | 50 (94) | 36 (95) | 14 (93) |
| Abnormal | 3b (6) | 2 (5) | 1 (7) |
positional torsional/downbeat nystagmus (1); facial twitch (1); head tilt (1); poor ambulation (3)
midline cerebellar atrophy (1); left cerebellar venous angioma (1); cholesterol cyst, temporal bone (1)
Over half of the patients experienced multiple episodes of MdD triggered by different exposures. Percentages for each trigger were calculated out of the total number of patients in each MdD group who had ever had an episode from the trigger indicated. Thus, 52 of 64 patients had ever had an episode of MdD triggered by a boat ride or cruise, 26 by an airplane flight and 10 by either a car or a train ride. The majority of patients with multiple episodes experienced less than 5 lifetime episodes (Table 2). Four of the 18 patients in the mixed MdD group experienced more than one motion-triggered episode. Therefore, the number of episodes reported for this group mostly reflects additional spontaneous episodes.
Table 2.
MdD episode triggers and duration
| Episode Characteristics | all MdD total n = 64 (%) | pure MdD total n = 46 (%) | mixed MdD total n = 18 (%) |
|---|---|---|---|
| Triggers | |||
| Boat/Cruise | 52 (81) | 34 (74) | 18 (100) |
| Airplane | 26 (41) | 22 (48) | 4 (22) |
| Car/Train | 10 (16) | 6 (13) | 4 (22) |
| Number of episodes | |||
| 1 | 23 (36) | 23 (50) | 0 (0) |
| 2–5 | 26 (41) | 16 (35) | 10a (56) |
| 6–20 | 10 (16) | 5 (11) | 5 (28) |
| > 20 | 5 (8) | 2 (4) | 3 (17) |
| totalb = 41 (%) | total = 23 (%) | total = 18 (%) | |
| Relative duration of the first versus the last episode | |||
| First > Last | 5 (12) | 4 (17) | 1 (6) |
| Last > First | 25 (61) | 13 (57) | 12 (67) |
| Equal | 3 (7) | 1 (4) | 2 (11) |
| Undeterminable | 8 (20) | 5 (22) | 3 (17) |
reflects both motion-triggered and spontaneous episode
based on the total number of patients who had more than one episode
The majority (61 %) of patients with multiple episodes experienced progressively longer symptoms with each subsequent episode. The median duration of the first episode was 4 months and the last episode was 12 months in patients who had had at least two episodes. In 8 patients (20 %), the relative duration of the first and last episode could not be determined because the patient was still experiencing an MdD episode at the time of follow-up and the duration had not yet exceeded that of the first episode (Table 2).
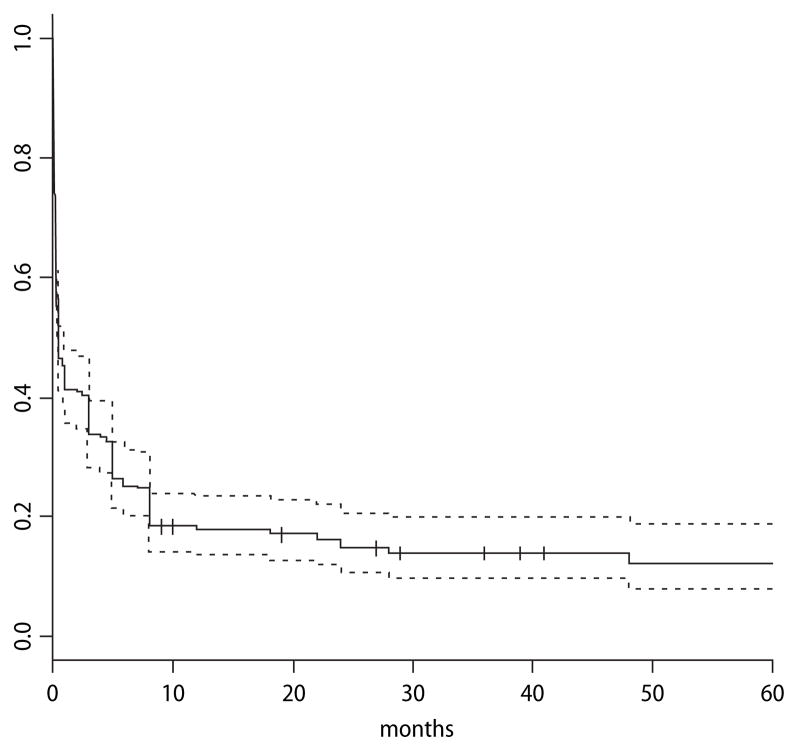
Because all patients had different durations of follow-up, we estimated the probability of resolution of MdD episodes in time epochs of 1 month according to whether the episode had resolved by the time of follow-up. We present the data in a Kaplan Meier survival curve format for 206 motion triggered episodes. If the patient was not able to give a precise number for the number of episodes they had experienced but only a range, then the average of the range was used to contribute to the total.
One-hundred-sixty-nine of 206 episodes had resolved by the time of follow-up. One hundred-four (51 %) episodes lasted longer than one month; 18 % of episodes lasted longer than 1 year; 15 % longer than 2 years, 12 % longer than 4 years, and 11 % longer than 5 years (Fig. 1).
Fig. 1.
Kaplan Meier Survival Curve for episode duration. The proportion of 206 MdD episodes that continued past each month is shown here. Crosses represent individual episodes that have been followed for the duration indicated and have not yet resolved. Dashed lines = 95 % confidence interval
Fifty-four of 64 (84 %) patients had experienced at least one lifetime episode of MdD lasting longer than one month and 25 patients (39 %) had already experienced at least one MdD episode lasting longer than one year before presenting to our clinic. Of the 16 patients who had ever experienced an MdD episode lasting longer than two years, 7 were men.
The overall prevalence of migraine headache in our MdD patient group (38 %) was higher than population baseline (17.1 % in women, 5.6 % in men [13]) but separating this group into the pure MdD group and the mixed MdD group showed that most of this risk came from the mixed MdD group (mixed = 73 % vs pure = 22 %) (Table 3). The rates of aura and vertigo did not differ between the pure and mixed groups. The prevalence of migraine was similar between patients who had a single motion triggered episode and multiple motion triggered episodes. Therefore, migraine only appeared to be a risk factor for the development of spontaneous MdD-like episodes.
Table 3.
Association of MdD with migraine, aura, vertigo, and motion sensitivity
| Associated Syndromes | all MdD total n = 34 (%) | pure MdD total n = 23 (%) | mixed MdD total n = 11 (%) |
|---|---|---|---|
| Migraine headache (all) | 13 of 34 (38) | 5 of 23 (22) | 8 of 11 (73) |
| with aura | 7 of 34 (21) | 3 of 23 (13) | 4 of 11 (36) |
| Vertigo | 4a of 34 (12) | 1 of 23 (4) | 3 of 11 (27) |
| Self-motion sensitivityb | |||
| Prior | 11 of 30 (37) | 6 of 21 (29) | 5 of 9 (56) |
| Post | 16 of 32 (50) | 7 of 21 (33) | 9 of 11 (82) |
| Prior vs. post | p = NS | ||
| Visual motion sensitivityb | |||
| Prior | 3 of 30 (10) | 1 of 21 (5) | 2 of 9 (22) |
| Post | 21 of 32 (66) | 11 of 21 (52) | 9 of 11 (82) |
| Prior vs. post | p < 0.001 | ||
| Re-exposure to passive motion effect on symptomsb | |||
| Decrease | 21 of 32 (66) | 17 of 22 (77) | 4 of 10 (40) |
| Increase | 1 of 32 (3) | 0 of 22 (0) | 1 of 10 (10) |
| No consistent effect | 10 of 32 (31) | 5 of 22 (23) | 5 of 10 (50) |
| Decrease vs. increase vs. no effect | p < 0.001 | ||
benign paroxysmal positional vertigo (3); benign recurrent vertigo (1)
based on clear known responses
The baseline rate of self-motion sensitivity was higher in the mixed MdD group (56 %) than in the pure MdD group (29 %) but this difference was not significant and neither group reported a significant increase in their sensitivity to self-motion after the onset of MdD. In contrast, both groups developed intolerance to visual motion (5 % to 52 % in pure MdD; 22 % to 82 % in mixed MdD, p < 0.001, Bonferroni correction = 9). The majority (66 %) of patients with MdD reported significant but temporary relief of their symptoms when re-exposed to passive motion (such as driving, sailing or rocking in a chair). Thirty-one percent did not experience consistent improvement with motion while only 3 % (1 person) reported worsening of symptoms with motion. All patients reported the effect of motion on their last episode, which was always a spontaneous episode in the mixed group (Table 3).
Since most patients had usually tried some kind of treatment for MdD prior to presenting to our clinic, we inquired about their experience with the most commonly used treatments. They were asked to report their responses according to the following scale:
1 = Greatly relieved symptoms (i.e., allowed return to work or socializing);
2 = Moderately improved symptoms;
3 = Small but noticeable improvement;
4 = Minimal improvement, if any;
5 = No improvement at all;
6 = Made symptoms worse.
Benzodiazepines were reported to be the most helpful, with stress reduction techniques, selective serotonin reuptake inhibitors, and physical/vestibular therapy also reported to be beneficial in some patients. Anti-emetics, tricyclic antidepressants, diuretics and dietary modifications were generally considered unhelpful. There were too few responses for beta blockers, calcium channel blockers and anticonvulsants to note a real effect but the few responses reported were unfavorable (Table 4).
Table 4.
Therapeutic responses
| Treatment | |||
|---|---|---|---|
| Benzodiazepine | Anticonvulsant | ||
| Number scoreda | 12 | Number scored | 3 |
| Average | 2.6 | Average | 5.3 |
| Median | 2 | Median | 5 |
| Antiemetic | Acetazolamide or other diuretic | ||
| Number scored | 14 | Number scored | 5 |
| Average | 4.6 | Average | 4.4 |
| Median | 5 | Median | 5 |
| Selective serotonin reuptake inhibitor | Decreased salt or diet modification | ||
| Number scored | 13 | Number scored | 6 |
| Average | 2.9 | Average | 3.8 |
| Median | 3 | Median | 4.5 |
| Tricyclic antidepressant | Stress reduction | ||
| Number scored | 9 | Number scored | 9 |
| Average | 4.3 | Average | 2.8 |
| Median | 5 | Median | 3 |
| Beta blocker or calcium channel blocker | Physical or vestibular therapy | ||
| Number scored | 2 | Number scored | 15 |
| Average | 5 | Average | 3 |
| Median | 5 | Median | 4 |
number scored = number of patients who had tried indicated therapy
In the free response section of the questionnaire, seven patients reported worsening of their symptoms with hormonal fluctuations, such as ovulation and menses. Thirteen patients indicated developing anxiety and/or depression from the lack of a diagnosis and resolution of their symptoms. Emotional and physical stress (in particular, sleep deprivation) were subjectively perceived to exacerbate MdD symptoms.
Discussion
Though several case reports detail many of the relevant features of MdD, we present a comprehensive review of the largest series of MdD patients to date with information on episode duration, number and associated features. Our study supports conclusions in prior case reports that the typical MdD patient is an otherwise healthy individual who develops a perception of rocking or swaying after a period of passive movement, obtains relief with re-exposure to passive movement, and has normal diagnostic testing including vestibular function testing and brain imaging. Because of the larger sample size and longer period of follow-up, we also noted some new findings including a higher proportion of men affected with MdD, the development of spontaneous MdD-like episodes after initial motion triggered episodes, the association of these MdD-like episodes with migraine, and the development of intolerance to visual motion with MdD.
The sex distribution of MdD patients in our study did not support prior reports that MdD is largely a disorder of middle-aged women. Only 1 of 27 responders to Hain’s [7] study and none of Mair’s 10 patients were men [14], compared to 16 of 64 (25 %) patients in our series. This proportion was even higher in those with MdD episodes lasting more than 2 years (44 %). This difference is most likely due to changes in men’s attitudes toward seeking healthcare but may also be affected by our referral base. Nevertheless, many women with MdD reported that their symptoms were worsened by hormonal changes, indicating that the higher proportion of women in ours as well as in all other studies of MdD is not likely to be simply a matter of ascertainment bias.
The visual motion sensitivity that developed in our MdD patients may indicate that there is differential weighting of visual versus vestibular information during the initial motion exposure. Maintenance of stable posture relies on interactions between visual, vestibular, proprioceptive and somatosensory input [2, 10, 11, 17]. The relative weighting of these inputs changes in disease processes and in situations where one input may be less reliable than others [1, 15]. On a cruise ship (the most common trigger for MdD) the vestibular system may be activated by the rhythmic fore-aft movement of the vessel, but visual input indicates that the environment is staying still, as everything in the environment is perceived to move together. Somatosensory and proprioceptive input is generally minimal in this situation because vessel movement does not change joint angles or elicit pressure changes. In this context, some implicit decision must be made about the reliability of these various systems in order to release postural control from consciousness and to minimize the consequences of sensory conflict – motion sickness [4, 18]. This period of recalibration is well-known among sailors and is known as getting one’s “sea-legs.”
Peripheral vestibular function testing is normal and there are no consistent structural brain imaging abnormalities in patients with MdD. Current evidence, therefore points to MdD being a disorder of multisensory adaptation. Memory for an internal representation of external passive movement such as occurs on a cruise ship may underlie one mechanism for MdD [16], a theory which may explain why some MdD patients subsequently developed spontaneous episodes of very similar MdD-like symptoms. Such spontaneous spells may reflect a kind of flashback memory. The lengthening of MdD spells after repeated exposures as seen in our study would fit a model of reinforcement in which subsequent episodes reactivate and enhance latent patterned activity.
We used 3 days as inclusion criteria based on Gordon’s two studies of 116 [5] and 234 [6] navy crewmen which found that MdD symptoms resolved within 2 days in all these presumably normal individuals. Our study confirmed our clinical impression that many spells that last more than three days resolve in less than one month and that the majority of spells that last longer than a month still resolve in less than one year. This was seen despite a possible confounder in our study that our patient population represents a more severely affected group than the general population of people who develop MdD.
Based on the probability of symptom resolution with time, we propose the term “prolonged MdD” for motion triggered symptoms that last between 2 days to 1 month and the term “persistent MdD” for symptoms that last longer than 1 month. There are currently not enough data to determine when MdD becomes permanent but, in our study, there were no MdD episodes that resolved after 5 years. Future studies using a prospective design, such as evaluating all patients disembarking from a cruise, are needed to obtain the true natural history of MdD.
Though all patients reported in this study had true motion-triggered MdD, we often see patients who experience the spontaneous onset of MdD-like symptoms without ever having had a motion-triggered episode. Patients with isolated spontaneous MdD-like symptoms represent a heterogeneous population for which no etiology has been determined though, in our experience, there is a high prevalence of migraine in this population. The mixed MdD group found in this study supports a connection between true motion triggered MdD and spontaneous MdD-like spells as the mixed group was also over-represented with migraine patients.
Physicians who care for patients with MdD know that this syndrome can be extremely debilitating cognitively and psychologically. The perception of rocking is a distraction for most people, and the extra attention paid to balance control is perceived as a constant mental strain. Many patients report that one of the most difficult aspects of their experience with MdD is the lack of recognition of their symptoms as a true disorder and the misattribution of the anxiety and depression they subsequently develop as the underlying cause for their symptoms. Earlier recognition of this disorder will allow patients to avoid unnecessary tests and to begin lifestyle changes such as stress reduction, regular sleep and regular exercise to better manage their symptoms.
Acknowledgments
This study was funded by NIH grant P50 DC 05224 from the NIDCD, NIH grant 5 U54 RR019482, and the Clinical Research Training Fellowship from the American Academy of Neurology.
Footnotes
Statistical analysis: Performed by Dr. Chiara Sabatti, Department of Human Genetics, UCLA and Jae Brodsky, Department of Statistics, UCLA.
References
- 1.Bles W, Vianney de Jong JM, de Wit G. Compensation for labyrinthine defects examined by use of a tilting room. Acta Otolaryngol. 1983;95:576–579. doi: 10.3109/00016488309139445. [DOI] [PubMed] [Google Scholar]
- 2.Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- 3.Brown JJ, Baloh RW. Persistent mal de debarquement: a motion induced subjective disorder of balance. Am J Otolaryngol. 1987;8:219–222. doi: 10.1016/s0196-0709(87)80007-8. [DOI] [PubMed] [Google Scholar]
- 4.Flanagan MB, May JG, Dobie TG. The role of vection, eye movements and postural instability in the etiology of motion sickness. J Vest Res. 2004;14:335–346. [PubMed] [Google Scholar]
- 5.Gordon CR, Spitzer O, Doweck I, Melamed Y, Shupak A. Clinical features of mal de debarquement: adaptation and habituation to sea conditions. J Vest Res. 1995;5:363–369. [PubMed] [Google Scholar]
- 6.Gordon CR, Spitzer O, Shupak A, Doweck I. Survey of mal de debarquement. BMJ. 1992;304:544. doi: 10.1136/bmj.304.6826.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hain TC, Hanna PA, Rheinberger MA. Mal de Debarquement. Arch Otolaryngol Head Neck Surg. 1999;125:615–620. doi: 10.1001/archotol.125.6.615. [DOI] [PubMed] [Google Scholar]
- 8.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders 2nd edition. Cephalalgia. 2004;24(Suppl 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 9.Irwin JA. The pathology of seasickness. Lancet. 1881;2:907–909. [Google Scholar]
- 10.Jeka J, Oie K, Schoner G, Dijkstra T, Henson E. Position and velocity coupling of postural sway to somatosensory drive. J Neurophysiol. 1998;79:1661–1674. doi: 10.1152/jn.1998.79.4.1661. [DOI] [PubMed] [Google Scholar]
- 11.Kavounoudias A, Roll R, Roll JP. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol. 2001;532:869–878. doi: 10.1111/j.1469-7793.2001.0869e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis RF. Frequency-specific mal de debarquement. Neurology. 2004;63:1983–1984. doi: 10.1212/01.wnl.0000144701.94530.6a. [DOI] [PubMed] [Google Scholar]
- 13.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 14.Mair IWS. The mal de debarquement syndrome. J Audiol Med. 1996;5:21–25. [Google Scholar]
- 15.Mergner T, Maurer C, Peterka RJ. A multisensory posture control model of human upright stance. Prog Brain Res. 2003;142:189–201. doi: 10.1016/S0079-6123(03)42014-1. [DOI] [PubMed] [Google Scholar]
- 16.Moeller L, Lempert T. Mal de debarquement: pseudo-hallucinations from vestibular memory? J Neurol. 2007;254:813–815. doi: 10.1007/s00415-006-0440-4. [DOI] [PubMed] [Google Scholar]
- 17.Peterka RJ, Benolken MS. Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Exp Brain Res. 1995;105:101–110. doi: 10.1007/BF00242186. [DOI] [PubMed] [Google Scholar]
- 18.Reason JT. Motion sickness adaptation: a neural mismatch model. Journal of the Royal Society of Medicine. 1978;71:819–829. doi: 10.1177/014107687807101109. [DOI] [PMC free article] [PubMed] [Google Scholar]