SUMMARY
Although it is firmly established neuronal activity is a prime determinant of animal behavior, relationships between astrocytic excitation and animal behavior have remained opaque. Cerebellar Bergmann glia are radial astrocytes that are implicated in motor behavior and exhibit Ca2+-excitation. However, Ca2+-excitation in these cells has not previously been studied in behaving animals. Using two-photon microscopy we found that Bergmann glia exhibit three forms of Ca2+-excitation in awake behaving mice. Two of these are ongoing within the cerebellar vermis. During locomotor performance concerted Ca2+-excitation arises in networks of at least hundreds of Bergmann glia extending across several hundred microns or more. Concerted Ca2+-excitation was abolished by anesthesia or blockade of either neural activity or glutamatergic transmission. Thus, large networks of Bergmann glia can be activated by specific animal behaviors and undergo excitation of sufficient magnitude to potentially initiate macroscopic changes in brain dynamics or blood flow.
INTRODUCTION
Neurons have long been studied in live animals by electrophysiological techniques, but astrocytes’ lack of electrical excitability has made these cells’ relationships to animal behavior more challenging to uncover. Nonetheless, since the discovery of astrocytic Ca2+-excitation in vitro, multiple processes that involve astrocytic Ca2+-signaling have emerged. In adults these include modulation of neuronal activity and regulation of cerebral blood flow (Fiacco and McCarthy, 2006; Iadecola and Nedergaard, 2007). However, the current in vivo data generally share two limitations.
First, results have generally been obtained in anesthetized animals, and with the exception of one paper that noted Ca2+ transients in neocortical astrocytes in behaving mice (Dombeck et al., 2007), it is largely unexplored whether there are specific behaviors that elicit astrocytic Ca2+-excitation. Anesthesia precludes animal behavior, and neuronal dynamics can be quite distinct in the anesthetized and awake brain (Greenberg et al., 2008; Rinberg et al., 2006). Since astrocytic dynamics may reflect aspects of neuronal activity (Fiacco and McCarthy, 2006), the strong possibility exists astrocytes will show equally divergent dynamics across brain states.
A second issue is that in vivo studies to date have mainly examined neocortical protoplasmic astrocytes. Like neurons, astrocytes display considerable non-uniformity, so astrocytic dynamics might vary substantially between brain areas and layers (Fiacco and McCarthy, 2006; Takata and Hirase, 2008). In the adult brain, a basic distinction exists between the radial astrocytes, which include the Bergmann glia of the cerebellar cortex and Müller cells of the retina, and the protoplasmic ‘star-shaped’ astrocytes of the neocortex and other areas. These two classes exhibit markedly different morphologies and in vitro patterns of Ca2+-excitation (Fiacco and McCarthy, 2006). Thus, the patterns of Ca2+-excitation seen in protoplasmic astrocytes and Bergmann glia of behaving animals may be expected to differ substantially.
Motivated both by the recent imaging progress and the above limitations, we studied Ca2+-excitation within Bergmann glial networks in awake behaving mice. We focused on a basic behavior, locomotion, towards ascertaining whether Bergmann glia have behaviorally triggered dynamics. These cells lie in areas crucial for motor coordination, are implicated in motor behavior (Shibuki et al., 1996), form gap-junction coupled networks, and can be identified by the palisade arrangement of their fibers and status as the sole astrocytes in the cerebellar molecular and Purkinje cell layers. We developed methods for two-photon imaging in the cerebellum of awake, head-restrained mice allowed to move on an exercise ball and studied how wakefulness and motor behavior affect Ca2+-excitation as it normally occurs in Bergmann glial networks.
We found that Bergmann glia of the cerebellar vermis, an area implicated in locomotor coordination, exhibit three forms of Ca2+-transients in awake mice. One of these, which we named ‘Ca2+ flares’, was triggered during locomotion and extended over macroscopic domains at least hundreds of microns across. Flares involved large networks of astrocytes, with at minimum hundreds of Bergmann glial fibers, and arose with onset kinetics similar to those of local increases in blood perfusion accompanying locomotion. The other two forms of Ca2+-excitation, which we named ‘sparkles’ and ‘bursts’, were ongoing in awake resting animals. Sparkles appeared restricted to individual fibers. Bursts were radial waves expanding in three dimensions across the fibers of ~10-40 Bergmann glia. The dependence of flares on locomotion, as well as the absence of flares and near abolition of sparkles under anesthesia, emphasize that studies in awake animals are essential.
RESULTS
Imaging Bergmann Glia in Awake Mice During Rest and Locomotion
Our first goal was to visualize glial Ca2+ transients in behaving mice without undue levels of motion artifact. To achieve this we used two-photon microscopy in awake, head-restrained mice allowed to run voluntarily on an exercise ball that rotates about one axis (Figure 1). We monitored rotation of the exercise ball using an optical encoder that reports rotations as slight as 0.5 deg, corresponding to 0.5 mm movements along the running surface (Figure 1A). This provides a sensitive measure of locomotion that does not rely on electromyography, which can suffer from cross talk between muscles in mice (Koekkoek et al., 2002).
Figure 1. Two-Photon Microscopy in Cerebellar Cortex of Behaving Mice.
(A) Two-photon microscopy in awake, head-restrained mice using an exercise ball. Mouse locomotion was tracked by an optical encoder on the ball, which provided a sensitive, quantitative, and repeatable readout of running onset/offset, speed and direction. In addition, mouse behavior was recorded on video (Supplemental Data).
(B) Areas of lobules V and VI of the cerebellar vermis examined in this study by Ca2+-imaging (pink). Scale bar: 1 mm.
(C) Probability densities of image displacements in dorso-ventral, z (blue), medio-lateral, x (green), and rostro-caudal, y (red), dimensions from a set of 7 example experiments. Insets: Colored traces show x (green) and y (red) image displacements (left inset), or x (green) and z (blue) image displacements (right inset), during two examples of mouse locomotion (black trace). x and y displacements were corrected by image registration prior to data analysis. Scale bars: Upper, 50 mm/s; Lower, 10 s and 5 μm.
After habituating a mouse to periods of head-restraint, we created an optical window in the cranium, injected the membrane-permeant fluorescent Ca2+-indicator Oregon-Green-488-BAPTA-1-acetoxymethyl (OGB-1-AM) into cerebellar cortex, and performed Ca2+-imaging across areas 200-500 μm wide with the cranium held secure. With this approach we studied lobules V and VI of the vermis (Figure 1B), areas implicated in coordinating hind and forelimbs (Andersson and Armstrong, 1987; Leicht and Schmidt, 1977), particularly the ipsilateral forelimb in rodent (Jorntell et al., 2000).
Within OGB-1-AM fluorescence images Bergmann glia could be identified by the distinct palisade arrangement of their fibers in the molecular layer and the size and location of their somata in the Purkinje cell layer (De Blas, 1984; Reichenbach et al., 1995) (Figure 2). However, since OGB-1-AM also labels neurons, we sought a way to identify Bergmann glia more readily by using an established astrocyte marker of a different color, the red fluorescent dye sulforhodamine101 (SR101) (Nimmerjahn et al., 2004). To confirm that SR101 marks Bergmann glia, we applied SR101 to the cerebellar cortex of S100β-EGFP Kosmos mice, which express green fluorescent protein (GFP) in astrocytes under the control of the S100β promoter (Zuo et al., 2004). The spatial distributions of GFP expression and SR101 labeling were virtually identical, distinguishable only by their intensity variations (Figure 2A, Figure S1). SR101 stained Bergmann glial end-feet contacting blood vessels, glial limitans at the pial surface, rows of palisade fibers extending in the dorsal-ventral dimension, and densely packed somata surrounding Purkinje cell bodies (Figures S1 and S2). The root mean square radii that we estimated from both high-magnification SR101 images of palisade fibers in cross-section (0.95 ± 0.6 μm; n = 1374 palisade fibers, 10 mice) and SR101 images acquired at lower magnifications used for Ca2+-imaging (1.8 ± 0.6 μm; n = 15613 palisade fibers, 17 mice) were consistent with the prior estimate of ~1.5 μm radius for palisade fibers examined by electron microscopy (Grosche et al., 1999). Thus, as in prior studies of Ca2+-dynamics in neocortical astrocytes using OGB-1-AM and SR101 (Nimmerjahn et al., 2004) the SR101 counter stain allowed Bergmann glia to be identified unambiguously (Figure 2B; Movie S2).
Figure 2. Palisades and Somata of Bergmann Glia Were Identified Based on Anatomical Features and Sulforhodamine 101 (SR101) Co-staining.
(A) The red fluorescent astrocyte marker SR101 stains Bergmann glia in the cerebellar cortex of adult mice. Localization of SR101 to Bergmann glia was verified in the Kosmos mouse line expressing EGFP under the control of an S100β promoter, an established marker of astrocytes. Left, EGFP expression in Bergmann glia of the Kosmos line. Center, SR101 staining pattern in the same mouse. Right, Image overlays showing that SR101 stains Bergmann glia somata and palisade fibers (yellow, open arrow heads) in the Purkinje cell (bottom) and molecular layers (top), respectively. Molecular layer interneurons and Purkinje cells (closed arrow heads) are labeled neither in the Kosmos line nor by SR101.
(B) SR101 co-staining was used to identify Bergmann glial palisades and somata in OGB-1-AM fluorescence images. Left, OGB-1-AM staining pattern in the molecular (top) and Purkinje cell layer (bottom). Center, SR101 staining in the same mouse. Right, Overlay images, in which Bergmann glia fibers, end foot processes and somata are indicated (open arrow heads) (See also Figures S1 and S2). Molecular layer interneurons and Purkinje cells (closed arrowheads in top and bottom rows, respectively) are stained by OGB-1-AM but not SR101.
Scale bars: 15 μm.
Since SR101 is a non-functional morphological marker, we also used it to characterize the motion artifacts in our imaging data from awake behaving mice. When the mice were not driving the exercise ball, motion artifacts in both lateral (medio-lateral (x): ± 0.4 μm s.d.; rostro-caudal (y): ± 0.6 μm s.d.; n = 74 events, 3 mice) and axial (dorso-ventral (z), ± 1.5 μm s.d., n = 19 events; 4 mice) dimensions were essentially negligible. During mouse locomotion there were small, transient in-plane displacements (x: ± 0.7 μm s.d.; y: ± 0.8 μm s.d.; n = 74 events; 3 mice; Figure 1C) that were correctible by offline image registration. Artifacts due to axial displacements (z: ± 3.4 μm s.d; n = 19 events; 4 mice; Figure 1C) were largely mitigated by the coinciding alignments of the optical axis and Bergmann glial palisade fibers in the dorsal-ventral direction. Crucially, within SR101 images we saw no characteristic phenomena resembling the forms of Ca2+-excitation seen repeatedly in OGB-1-AM images, from all 33 alert mice studied.
Having achieved a suitable imaging assay, we next characterized these forms of Ca2+-excitation in detail. As elaborated below, all the Ca2+ transients we describe occurred within, but were not strictly limited to, image regions of interest (ROIs) demarcating SR101-labeled palisade fibers. These transients exhibited time courses that evolved on the seconds time scale, consistent with these occurring in non-neuronal cells lacking fast Ca2+ dynamics, and were quite distinct from the rapid dendritic Ca2+ spikes (~10 ms rise time) we regularly observed in Purkinje neurons.
Localized Ca2+ Sparkles are Ongoing in Awake Mice
When alert mice were on the exercise ball but voluntarily sitting still, we observed two forms of spontaneous Ca2+ excitation in Bergmann glial fibers. The first of these forms was markedly sensitive to isoflurane anesthesia, and we termed these events Ca2+ sparkles due to their highly localized appearance.
Sparkles occurred within individual palisade fiber ROIs (Figures 3A-D), which had been identified by SR101 labeling and collectively exhibited arrangements and sizes consistent with those of Bergmann glial main fibers (Figures 2A and S1). At higher optical magnifications we saw that sparkles sometimes included regions surrounding the palisade fibers (Figure 3C). However, the resolution of our imaging data did not permit us to determine conclusively whether individual finer Bergmann glial processes, which are very dense outside the palisades and closely entwined with neuronal processes, also undergo Ca2+ excitation.
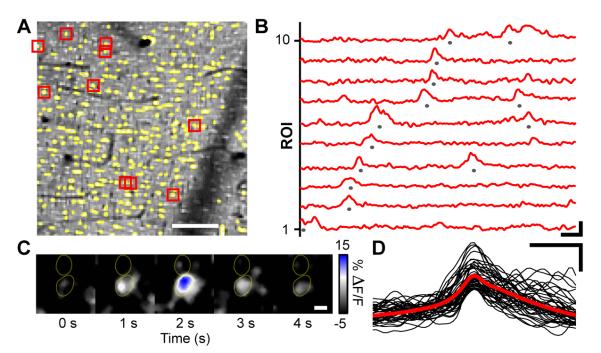
Figure 3. Sparkles Are Ongoing Ca2+ Transients That Appear in Individual Palisade Fibers.
(A) OGB-1-AM image showing cellular structures in a superficial region of the cerebellar molecular layer. Ca2+ dynamics were analyzed in regions of interest (ROI) where palisade fibers (yellow) had been identified by SR101 co-staining. Example traces of relative increases in OGB-1-AM fluorescence, ΔF(t)/F, from 10 ROIs marked red are shown in (B). Scale bar: 50 μm.
(B) Traces of Ca2+ signals from individual palisade fiber ROIs show examples of sparkles, defined as events of >10% ΔF(t)/F (marked by gray dots) for purposes of analysis. Traces are arranged by sparkle onset time. Scale bars: 5 s, 10% ΔF(t)/F.
(C) High magnification OGB-1-AM ΔF(t)/F images showing a Ca2+ sparkle in one out of two neighboring palisade fibers, whose perimeters (yellow outlines) were determined from the simultaneously acquired SR101 images. Scale bar: 3 μm.
(D) Overlay of 45 individual sparkle time courses (black) and an averaged trace (red) for sparkles that occurred within the yellow ROIs in (A). Scale bars: 5 s, 10% ΔF(t)/F.
Sparkles were modest in amplitude, with relative fluorescence increases (ΔF(t)/F) sometimes just above background fluctuations (see Table S1 for an overview of sparkle properties). To study sparkles’ time evolution, we examined the subset of sparkles that passed a threshold criterion of having a >10% increase in ΔF(t)/F. This approach is conservative, in that the selected events can be readily identified above background noise, but does lead to an underestimate of event rates. Within this event subset (peak ΔF(t)/F; 13.2 ± 4.1 %, mean ± s.d.; range, 10.1-134.3%; n = 4285 events; 15 mice), sparkles had variable duration (3.3 ± 3.9 s; range, 0.5-35.4 s; n = 4285 events; 15 mice) (Figures 3B and 3D). The estimated lower bound on the frequency of sparkles was 4.5 ± 2.6 Hz/mm2 (range, 1.1-11.0 Hz/mm2, n = 4285 events; 15 mice) (Figure 3B). By considering the density of glial fibers and the proportion of the molecular layer sampled in our recordings, we estimated that this bound corresponds to a mean rate of sparkles of ~0.01-0.1 min−1 per individual glial fiber. This is comparable to spontaneous Ca2+-excitation rates for neocortical astrocytes (Hirase et al., 2004; Takata and Hirase, 2008).
Sparkle rates were reduced but not abolished by local blockade of either neuronal activity using the Na+-channel blocker tetrodotoxin (TTX) (1.5 ± 0.5 Hz/mm2; range, 1.0-2.2 Hz/mm2; likelihood ratio test, p < 10−6; n = 277 events; 5 mice) or glutamatergic transmission using the broad-spectrum glutamate receptor antagonist γ-D-glutamylglycine (γDGG) (3.6 ± 2.3 Hz/mm2; range, 0.5-5.7 Hz/mm2; likelihood ratio test, p < 0.002; n = 614 events; 4 mice). Pyridoxalphosphate-6-azephenyl-2′,4′-disulfonic acid (PPADS), a non-selective P2 purinergic blocker, failed to reduce sparkle rates (5.7 ± 2.5 Hz/mm2; range, 3.3-8.4 Hz/mm2; likelihood ratio test, p > 0.2; n = 974 events; 3 mice). However, in mice anesthetized by isoflurane, sparkle rates fell markedly (0.2 ± 0.1 Hz/mm2; range, 0.0-0.3 Hz/mm2; likelihood ratio test, p < 10−5; n = 14 events; 4 mice). In summary, as reported for other types of astrocytes (Nett et al., 2002; Takata and Hirase, 2008) there appears to be an ‘intrinsic’ sub-population of Ca2+ transients that persists under blockade of neuronal activity and another sub-population that does not.
In contrast to the sparkles we regularly saw in palisade fibers, we only occasionally saw spontaneous Ca2+ transients in Bergmann glial somata. The latter events were almost three orders of magnitude less frequent (~8.9 ± 3.7 mHz/mm2; n = 24 recordings; 14 mice), and involved large ΔF(t)/F increases (22 ± 12 % ; n = 12 events; 14 mice) that were localized to one or a few neighboring somata.
Ca2+ Bursts Involve up to ~40 Bergmann Glial Cells in Alert Mice
Like sparkles, bursts occurred spontaneously in mice resting voluntarily on the exercise ball. Bursts appeared as radially expanding waves that spread over approximately ellipsoidal regions with greater medio-lateral (55 ± 37 μm; n = 95; 17 mice; Wilcoxon signed rank test, p < 0.001) than rostro-caudal extent (44 ± 33 μm; n = 95; 17 mice; Wilcoxon signed rank test, p < 0.001) (Figures 4D and 4F). These waves swept over both palisade regions, where the primary Bergmann glial fibers could clearly be seen in the movie data to undergo Ca2+ activation, and non-palisade regions where we could not resolve the individual fibers or anatomical processes involved. Peak ΔF(t)/F increases for bursts (13 ± 4.8 %; range, 2.8 −25%; n = 96 events; 11 mice) were similar to those for sparkles, but the two phenomena were unmistakably distinct since bursts occupied much larger volumes (Figure 4, Movie S1). Like sparkles, bursts were infrequent, but rates varied substantially between mice (168 ± 187 mHz/mm2; range, 36.7-566 mHz/mm2; n = 161 events; 8 mice).
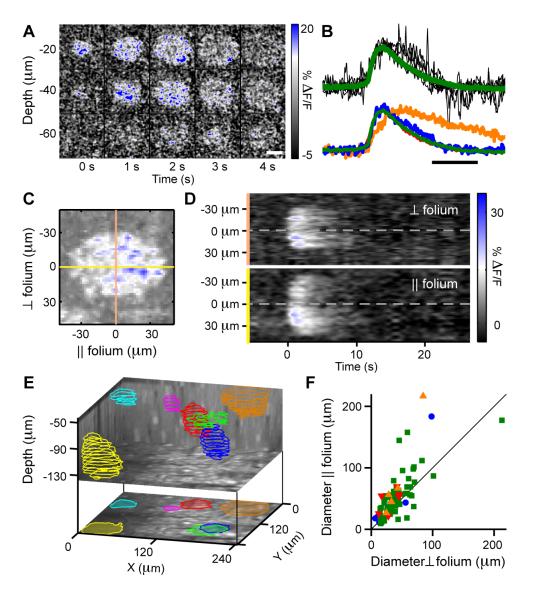
Figure 4. Bursts Are Ongoing Ca2+ Transients Spreading as 3D Radial Waves Over Multiple Bergmann Glia.
(A) Example 3D imaging data showing ΔF(t)/F optical cross-sections of OGB-1-AM fluorescence at five time points. Indicated depth values are relative to the pial surface. Scale bar: 50 μm.
(B) Top, 6 individual (black) ΔF(t)/F time traces for bursts and the mean time course (green) averaged over 96 bursts in 11 animals, measured over fixed areas that covered the bursts’ centers in awake resting mice. Bottom, averaged and normalized ΔF(t)/F traces under four conditions: isoflurane-anesthetized (blue; n = 8 events, 5 mice), awake resting (green; n = 96 events, 11 mice), TTX- (red; n = 9 events, 5 mice) and γDGG-treated (orange; n = 7 events, 2 mice). Different animals were used for different drugs. Scale bar: 5 s.
(C) Cross-sectional ΔF(t)/F fluorescence image of a burst at a fixed depth and time. Yellow and peach lines respectively indicate directions parallel and perpendicular to the cerebellar folium.
(D) ΔF(t)/F time courses along the yellow and peach-colored lines in (C). Note that bursts have larger diameter in the direction parallel to the folium and spread outwards from a central initiation site.
(E) Top, locations and volumetric profiles of 7 bursts detected by 3D imaging during an 8.2 min period. Colored outlines show extent of Ca2+ spread for each burst at various depths. Bottom, z-projection image.
(F) Population data showing bursts have greater extent parallel to the long axis of the cerebellar folium, which lies perpendicular to the parasagittal planes of cerebellar cortex. Colored data points denote individual bursts recorded under the four conditions noted in (B).
By using three-dimensional (3D) two-photon imaging to monitor multiple tissue depths with near simultaneity (Gobel et al., 2007), we found bursts originated across all depths of the molecular layer and had axial extents of 56 ± 23 μm (n = 10 events; 5 mice) centered around the point of initiation (Figures 4E; Movie S1). Typical burst volumes (86,000 ± 61,000 μm3; n = 10 events; 5 mice) obtained by 3D imaging implicate the involvement of many fine fibers and several main fibers from several neighboring Bergmann glia. Based on these cells’ somatic density and the projection pattern of their ~2-5 main fibers per cell (De Blas, 1984; Reichenbach et al., 1995), we estimate that a burst encompasses on average ~10 but up to ~36 Bergmann glia. However, after having characterized bursts’ volumes, we used only 2D-imaging for examination of bursts’ temporal profiles, due to the superior time resolution (~12-20 Hz frame rate) of 2D- as compared to 3D-imaging (1-4 Hz).
We examined closely the time course of fluorescence rise and decline within bursts’ central regions, where the signal was sufficiently high above background levels to permit detailed analysis. Bursts displayed time courses (Figure 4B) similar to the time evolution of Bergmann glial Ca2+-transients in vitro, as previously recorded from fixed locations within cerebellar slices in response to parallel fiber stimulation (Beierlein and Regehr, 2006; Piet and Jahr, 2007). The similarity suggests both phenomena might involve ATP-triggered release of Ca2+ from intracellular stores, as found in vitro. Consistent with this, bursts were virtually eliminated by PPADS (1.5 ± 3.0 mHz/mm2; n = 1 event; 4 mice; likelihood ratio test, p < 0.01). However, bursts persisted in vivo after application of either TTX (41 ± 48 mHz/mm2; n = 46 events; likelihood ratio test, p > 0.01 in 7 out of 8 mice), γDGG (28 ± 23 mHz/mm2; n = 9 events; likelihood ratio test, p > 0.1 in 2 out of 4 mice), or isoflurane anesthesia (28 ± 51 mHz/mm2; n = 23 event, 18 mice; likelihood ratio test, p > 0.01) (Table S2).
We found the burst time course was well modeled kinetically by a simple biochemical cascade of two reaction events in sequence, . k1 and k2 respectively denote the rate constants for transitions out of an initial state of resting [Ca2+] (state 0), and a transient state in which [Ca2+] is elevated (state 1). This reaction scheme leads to a time course that is the convolution of two exponentials, and bursts’ time courses conformed closely to this mathematical form with 1/k1 = 1.5 ± 0.7 s and 1/k2 = 1.8 ± 1.2 s (r = 0.9 ± 0.1; n = 96 events; 11 mice). The two rate constants for each burst were highly correlated, with 81% of events showing a negligible (<1%) difference between 1/k1 and 1/k2, which suggests both rate constants might depend on a common factor.
In the presence of γDGG, 1/k1 values (2.2 ± 1.2 s; range, 0.7 - 3.6 s; n = 7 events; 2 mice; Wilcoxon rank sum test, p < 0.1) did not change significantly. However, 1/k2 values lengthened (9.6 ± 10 s; range, 3.3 - 30 s; n = 7 events; 2 mice; Wilcoxon rank sum test, p < 0.001) as compared to all other pharmacological conditions (Figure 4B). Since bursts persist under γDGG, glutamate reception might play a role in bursts’ termination but perhaps not in their initiation (Discussion).
Given that bursts expand outwards from a central initiation point, Ca2+ excitation might be initiated by the diffusion of a small molecule trigger, such as ATP. Since bursts only propagated over tens of microns, we could only estimate an approximate 3D diffusion constant (~150 μm2/s; n = 13 events, 4 mice) and propagation speed (~10 μm/s; n = 11 events, 4 mice) (Supplemental Discussion).
Motor Behavior Activates Ca2+ Flares Across Bergmann Glial Networks
When mice ran voluntarily on the exercise ball, concerted Ca2+-excitation arose in large networks of Bergmann glia in the vermis. These events, which we termed flares, occurred at the onset of locomotion and appeared to be composed of a large number of sparkles, combined with Ca2+-excitation in areas surrounding the palisade fibers (Figure 5; Movie S2). Flares often covered our entire field of view (200-500 μm) and thus typically involved the hundreds of Bergmann glial palisade fibers we could see, as well as adjoining areas between the palisades.
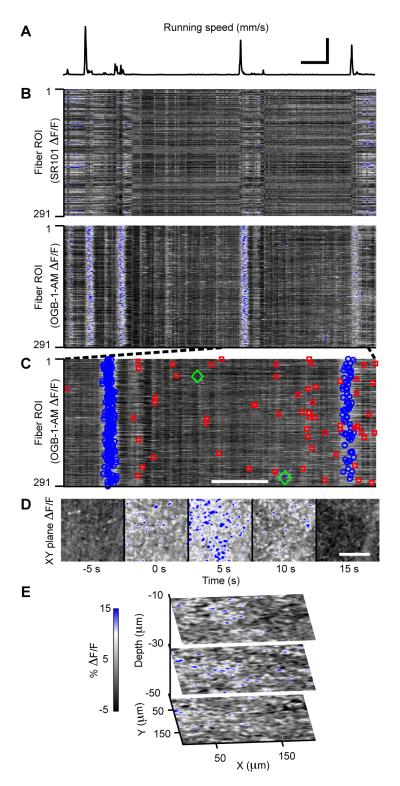
Figure 5. Motor Behavior Evokes Concerted Ca2+-Excitation Across Bergmann Glial Networks.
(A) Encoder trace of the exercise ball’s rotational speed, showing multiple running bouts during an example 9.8 min period. Scale bars: 50 s and 20 mm/s; the former also applies to (B).
(B) Top, SR101 ΔF(t)/F traces from 291 palisade fiber regions of interest (ROI) during the same time interval as in (A). Bottom, the same palisade fiber ROIs in OGB-1-AM images reliably exhibit concerted ΔF(t)/F increases, flares, during running. Signals of similar magnitude and time course are absent in ΔF(t)/F traces of non-functional indicator SR101. ΔF(t)/F scale bar in E also applies to B-D. ROIs were sorted by x coordinate.
(C) OGB-1-AM ΔF(t)/F traces over the interval indicated in (B) by dashed lines. Flares are marked blue and are interleaved in time with sparkles (red) and bursts (green). Scale bar: 50 s.
(D) Image frames of OGB-1-AM ΔF(t)/F increases relative to movement onset during the first flare in (C) illustrating the broad spatial extent of flares. Scale bar: 100 μm.
(E) Images from a 3D recording of ΔF(t)/F increases during a flare. Indicated depth values were measured relative to the pial surface.
Because astrocytes have been reported to show Ca2+ responses to mechanical stress, we were initially concerned flares might be artifacts due to transient mechanical perturbation to the brain at running onset. However, multiple lines of evidence and control experiments were inconsistent with this possibility, leading us to conclude flares are part of normal brain physiology. First, flares show a completely different time course (Figure 5 and 6) and are an order of magnitude more rapid than the Ca2+ signals reported to occur in response to mechanical stress (Charles et al., 1991; Chen et al., 1999). Second, flares occurred in our movie data even when there was no perceptible movement of the brain (Figure S4A). Third, in cases when there were measurable, micron-scale brain displacements at motor onset, we found no correlation between the magnitude of tissue displacements and either the occurrence or amplitude of Ca2+ flares (Figure S4B). Fourth, the occurrences of flares were independent of mechanical factors such as the craniotomy size (e.g. 3.0, 5.0, and 7.5 mm2 for the recordings of Figures S4A and S3, and Figure 5A-D, respectively), whether the dura mater was removed, or the craniotomy covered with agarose and a cover slip. Fifth, we never saw flares in control experiments with awake mice in which we applied pressure on the animal’s head holder or optical window (n = 30 stimulations, 3 mice; Figure S3). This was so even when the applied pressure caused far greater tissue displacements than those occurring during running-evoked flares in the same mice. Sixth, there were many instances of substantial brain displacements when the mice were not running and these did not produce flares (Figure S4B). Seventh, flares were abolished by blockade of neuronal Na+ channels or glutamatergic transmission (Figure 6), showing that flares require neuronal activity and do not arise from direct mechanical activation of individual Bergmann glia. Eighth, we saw during flares no signs of spreading neuronal depression, a known effect of mechanical injury to the brain (Charles et al., 1991; Chen et al., 1999), as assessed by imaging neuronal Ca2+ activity in Purkinje cell dendrites and molecular layer interneurons (data not shown). Thus, we found no support for the idea flares were mechanically induced and proceeded to characterize flares in more detail (Table S3).
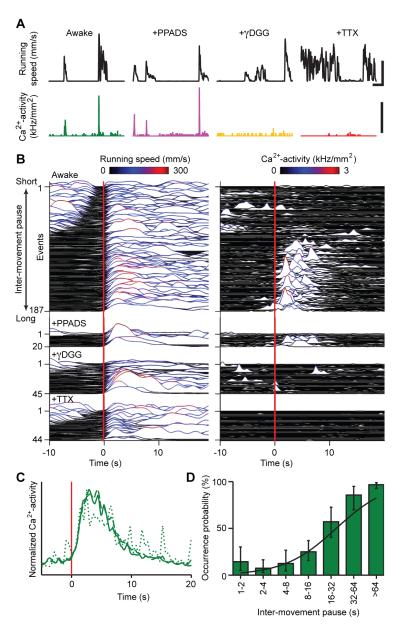
Figure 6. Locomotor Evoked Ca2+ Flares Depend on Neuronal Activity, Have Limited Duration, and Exhibit a Refractory Period.
(A) Example traces revealing Ca2+ flares (bottom) as a function of locomotor activity (black), under four conditions: awake-untreated (green; averaged signal from 194 ROIs), PPADS- (purple; 231 ROIs), γDGG- (orange; 192 ROIs) and TTX-treated (red; 191 ROIs). Data for each drug condition are from a different individual mouse. Concerted Ca2+ activity was quantified by the rate of palisade fiber activation per unit area using a threshold criterion of 10% ΔF(t)/F for each individual fiber. Upper scale bars, 50 s and 150 mm/s. Lower scale bars, 1 kHz/mm2.
(B) Population data showing paired traces of running speed (left) and concurrent Ca2+-activity (right) in untreated (n = 187; 15 mice), PPADS- (n = 20; 3 mice), γDGG- (n = 45; 4 mice), and TTX-treated mice (n = 44; 5 mice). Red lines indicate movement onset. For each condition, traces are ordered by the pause duration after the prior movement.
(C) Normalized mean time courses of flare activation triggered on movement onset (red line), for all (solid), brief (dotted), and extended movements (dashed) showing that flares have limited duration. Flares in panel B with peak amplitudes of at least 20 Hz/mm2 were included for averaging.
(D) The probability of observing a flare at locomotor initiation versus the pause duration between movements, computed across the 187 movements shown in B, showing flares have a refractory period (compare to B).
Among flares’ interesting properties was their mode of initiation. Flares did not seem to involve a stereotyped manner of propagation, but usually arose similarly across multiple locomotor events within individual fields of view (n = 187 running bouts; 15 mice). At flare onset we typically saw a smaller number of fibers within the palisades exhibiting Ca2+ increases that resembled sparkles (Movie S2). Adjoining areas between palisade fibers also underwent Ca2+ activation. The number of sparkling fibers rose over 4.2 ± 1.4 s (range, 1.2 – 8.2 s; n = 93 events; 15 mice) until reaching peak activation (Figure 5D).
We initially wondered if isolated sparkles might be spontaneously occurring, ‘miniature’ flares, perhaps analogous to the miniature post-synaptic potentials that represent spontaneous neurotransmission. Flares showed a similar pharmacological profile as sparkles and were eliminated by TTX (n = 44 running bouts; 5 mice) or γDGG (n = 45 running bouts; 4 mice) (Figure 6A and 6B) even while locomotor activity persisted. In isoflurane-anesthetized mice we never saw flares (14 recordings; 4 mice), even if we moved or electrically stimulated the animals’ limbs (n = 13 stimulations, 3 mice; Figure S5). PPADS did not significantly affect flares (n = 20 running bouts; 3 mice; Figures 6A and 6B; Table S3). However, by comparing the SR101 and OGB-1-AM data channels we saw that Ca2+ increases during flares always occurred both in the palisades and the surrounding areas where finer Bergmann glial fibers lie (Figures 5D and 5E). This does not eliminate the possibility flares might be seeded by or partly composed of sparkles, but does show flares are not simply composites of multiple sparkles (Discussion).
We next characterized the spatial magnitude of flares, because events involving widespread activation might be more likely to have a role in animal behavior or large-scale brain dynamics. 3D imaging showed flares occurred over at least ~0.5 × 0.5 × 0.1 mm3 (our maximum sample volume), reached significant depths of the molecular layer, and activated palisade fibers along large portions of their axial extent (Figure 5E), as compared to sparkles that had more limited axial size. Flares tapered dramatically towards the Purkinje cell layer, but sometimes involved and spread between a few Bergmann glial cell bodies. Thus, flares are macroscopic and cover several cerebellar microzones, divisions of the cerebellar cortex that map to parts of the body (Apps and Garwicz, 2005).
Ca2+ Flares Have Refractory Dynamics That Depend on Running History
As an initial investigation of flares’ potential relevance to locomotor behavior, we analyzed the temporal relationships between the two. We found a consistent order of events and a refractory character to flares’ occurrences.
Flares were refractory in that they arose more reliably at motor onset when there had been no prior recent movement. Thus, flares tended to accompany the initial but not the later movements within series of running bouts occurring in quick succession (Figure 6B). The percentage of cases in which flares occurred at motor onset rose from ~10% when the animal had moved in the previous 4 s (n = 27; 15 mice), to over ~86% if >32 s had elapsed since termination of the prior movement (n = 14; 15 mice) (Figure 6D). Only in 6.9 ± 5.9% (mean ± 68% confidence interval; n=87; 15 mice) of cases did flares of significant extent and sparkling rates (>100 Hz/mm2) occur without measurable rotation of the exercise ball, as assessed using the optical encoder.
Flares’ peak activity always occurred after movement of the exercise ball and never before (Figures 6B-D). Flares also had limited duration, regardless of how long the mouse’s locomotor episode lasted (Figure 6C). If >10 s had elapsed since termination of the prior locomotor episode, flares reached peak fluorescence 4.4 ± 2.5 s after re-initiation of movement (n = 93 events, 15 mice) (Figure 6C). Ca2+ increases during flares persisted for 9.7 ± 2.8 s (n = 93 events; 15 mice) (Figure 6C). Thus, if locomotion was brief (<4 s) flares outlasted the movement, but vice versa if motor activity persisted (>16 s).
Kinetic Relationships of Flares to Motor Behavior and Hemodynamics
Prior work in vitro and in anesthetized animals has shown the involvement of astrocytic Ca2+ signaling in hemodynamic modulation (Iadecola and Nedergaard, 2007; Nimmerjahn, 2009). This suggests a possible link between flares and blood flow regulation. Thus, we quantified the kinetic relationships between locomotion, hemodynamics, and flares to logically constrain the roles flares might play (Tables S3 and S4).
Time scales of cerebral hemodynamic responses have been well reported (Huettel and McCarthy, 2000; Martin et al., 2006; Martindale et al., 2005), but we verified the basic kinetics of responses that occur in our mouse locomotor assay by using laser Doppler measurements of blood perfusion levels in the vermis (Table S4), and we compared these kinetics to those of flares (Figure 7). Local blood perfusion levels rose (median amplitude ± s.d, 31 ± 11%; range, 16 - 68%; n = 134 events; 11 mice) over a very similar time course as Ca2+ flares (Figure 7C). However, perfusion levels did not follow similar time courses as flares throughout locomotion (Figure 7C and 7D). Instead, hemodynamic up-regulation persisted throughout motor behavior (median duration ± s.d, 13 ± 36 s; range, 1.2 – 226 s; n = 134 events; 11 mice) (Figure 7D), as documented for other types of movement (Logothetis and Wandell, 2004). This persistence of blood flow responses contrasts with flares’ limited duration (Figure 6B and C).
Figure 7. Temporal Relationships of Ca2+ Flares to Locomotor Evoked Blood Perfusion Increases.
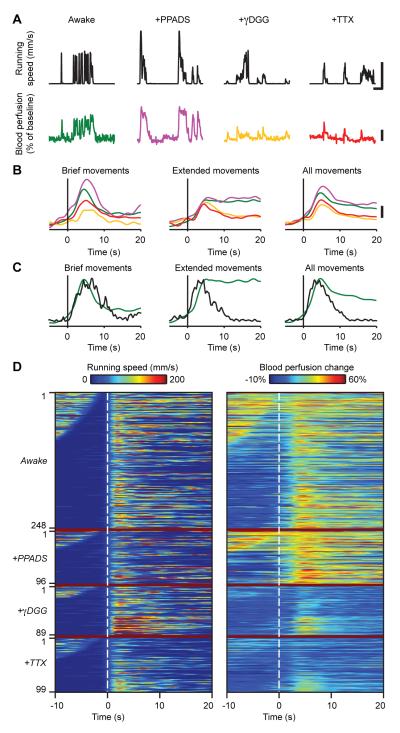
(A) Example traces showing blood perfusion increases (bottom) as a function of locomotor activity (top), under four conditions, awake-untreated (green), PPADS-(purple), γDGG- (orange) and TTX-treated (red). Data for each drug condition are from a different individual mouse. Upper scale bars, 50 s and 150 mm/s. Lower scale bar, 20 %.
(B) Population data showing overlay of average blood perfusion increases in untreated (green; n = 134; 11 mice), PPADS- (purple; n = 47; 3 mice), γDGG- (orange; n = 45; 4 mice), and TTX-treated mice (red; n = 35; 3 mice) triggered upon movement onset for brief (left), extended (center), and all movements (right). Traces from each trial were normalized by the peak running speed in that trial, prior to averaging. Note that TTX and γDGG reduce the amplitude of blood perfusion increases and reduce their persistence during extended running periods. Scale bar, 10 %.
(C) Overlay of average Ca2+ flare activation (black) and blood perfusion increase (green), triggered on movement onset (vertical lines), reveals similar kinetics during locomotor initiation in the untreated condition. Flares are not sustained during extended movements, but blood perfusion increases are. Flare traces are based on a set of 187 running bouts with peak running speeds >100 mm/s in 15 mice.
(D) Sets of paired traces of running speed (left) and corresponding blood perfusion increases (right) in untreated (n = 134; 11 mice), PPADS- (n = 47; 3 mice), γDGG- (n = 45; 4 mice), and TTX-treated mice (n = 35; 3 mice). Dashed lines indicate movement onset. For each condition, traces are ordered by the pause duration after the prior movement. TTX and γDGG particularly reduce the persistence of perfusion increases.
We also examined how the regulation of blood perfusion levels depended on neural activity and ATP-mediated signaling. Surface application to the vermis of either TTX or γDGG, but not PPADS, reduced the persistence of blood perfusion increases during extended periods of movement (Figure 7). Thus, the duration of blood flow responses was shortened by both TTX (6.1 ± 6.7 s, median ± s.d.; range, 1.4 – 27 s; n = 35 events; 3 mice; Wilcoxon rank sum test, p < 10−4) and γDGG (7.2 ± 6.2 s, median ± s.d.; range, 1.1 – 27 s; n = 45 events; 4 mice; Wilcoxon rank sum test, p < 10−5) but not by PPADS (16 ± 22 s, median ± s.d.; range, 1.8 – 87 s; n = 47 events; 3 mice; Wilcoxon rank sum test, p = 0.18). The peak amplitudes of blood perfusion increases were also reduced by both TTX (median amplitude, 21 ± 4.9%; range, 15 - 31%; n = 35 events; 3 mice; Wilcoxon rank sum test, p < 10−9) and γDGG (median amplitude, 23 ± 8.5%; range, 15 - 49%; n = 45 events; 4 mice; Wilcoxon rank sum test, p < 10−5) but not by PPADS (median amplitude, 42 ± 12%; range, 19 – 60%; n = 47 events; 3 mice; Wilcoxon rank sum test, p = 0.005). Taken together, the kinetic and pharmacological data (summarized in Table S4) suggest a potentially intricate relationship between flares, blood flow, and animal behavior (Discussion).
DISCUSSION
We conducted the initial study of Bergmann glial Ca2+-excitation in awake behaving animals and found three types of Ca2+-excitation: flares, bursts, and sparkles. Bursts and sparkles are ongoing in awake animals at rest, whereas flares are macroscopic events that initiate during locomotor behavior with similar rise times as blood perfusion levels. The modes of initiation and pharmacological data suggest sparkles and flares could share some common mechanisms distinct from those of bursts. Bursts require purinergic signaling but neither Na+ channel activation nor glutamatergic transmission. In contrast, sparkles and flares are affected by blockade of either Na+ channels or glutamatergic transmission, but not of purinergic signaling, and also by isoflurane anesthesia.
Isoflurane Anesthesia Disrupts Bergmann Glial Ca2+-excitation
Recent studies have explored Ca2+-excitation in astrocytes of anesthetized animals (reviewed in Nimmerjahn, 2009). Comparison of our recordings from awake mice to those from the same fields of view while the mice were under isoflurane anesthesia provides reason to be cautious in interpreting results on astrocytic dynamics from anesthetized subjects. Isoflurane exerts direct and indirect effects on astrocytes, influencing glutamate uptake and gap-junction-mediated signaling (Mantz et al., 1993; Miyazaki et al., 1997). We found isoflurane disrupts Bergmann glial Ca2+-excitation by eliminating flares and reducing sparkle rates, consistent with our other data suggesting sparkles and flares are each promoted by neural activity. Interestingly, isoflurane reduced the estimated rate of sparkles even more than TTX or γDGG (Table S1), indicating that isoflurane might diminish both the intrinsic and the neuronal activity-dependent sub-populations of sparkles. Hence, wakefulness and animal behavior seem to be key influences on the excitation of glial networks.
Ca2+ Excitation Differs in Bergmann Glia and Protoplasmic Astrocytes
Studies in anesthetized animals have found spontaneous and sensory-evoked Ca2+ transients in protoplasmic astrocytes of the neocortex. The spontaneous transients were temporally sparse and arose in both individual astrocytic processes and cell bodies (Hirase et al., 2004; Takata and Hirase, 2008), although Ca2+ activity was reported to be more frequent in astrocyte processes than in cell bodies (Wang et al., 2006). Sensory-evoked Ca2+ transients were also restricted to individual astrocytes (Schummers et al., 2008; Wang et al., 2006) and required glutamatergic transmission (Petzold et al., 2008; Wang et al., 2006). Spontaneous transients were largely unaffected by TTX and PPADS (Takata and Hirase, 2008). In contrast, we found Bergmann glia fibers show both highly localized and two forms of spreading Ca2+ transients. Spontaneously occurring bursts depended on purinergic signaling. Spontaneous Ca2+-excitation in Bergmann glial somata was rare, although sometimes Ca2+-excitation did spread among several Bergmann glial cell bodies during flares.
A recent study in the neocortical S1 hindlimb area of awake mice reported sub-populations of astrocytes that show Ca2+ activity correlated with running behavior (Dombeck et al., 2007). These Ca2+ increases lasted several seconds,and arose ~1-2 s after running onset. In comparison, locomotor-evoked cerebellar flares had no analogs in anesthetized mice, and displayed variable onset delays following running onset and relatively consistent durations. Whereas only a sub-population of astrocytes in the S1 hindlimb area showed strong correlations with running behavior (Dombeck et al., 2007), running-evoked flares reliably involved hundreds of Bergmann glial fibers (Figure 5), except during the refractory period immediately following a prior movement (Figure 6). No refractory period was reported for neocortical astrocytes.
These differences in Ca2+-dynamics between star-shaped neocortical astrocytes and radially shaped Bergmann glia might be at least in part due to the distinct cellular organization and functional properties of these astrocytic cell types (Fiacco and McCarthy, 2006). Overall, our results highlight the need to compare astrocytic physiology across brain regions, between astrocytic sub-types, and under anesthesia to during animal behavior. Our results also show the utility of 3D imaging, which allowed us to reconstruct burst volumes and assess the axial extents of sparkles and flares. Comparisons of 3D data between different areas and layers will help reveal the diversities of roles astrocytes might play in brain dynamics.
Possible Causes of Sparkles
In vitro studies have shown Bergmann glia can respond to neuronal activity with intracellular Ca2+ transients and that Ca2+ signaling plays a key role in regulating structural and functional interactions between these cells and cerebellar neurons (Iino et al., 2001). It has been unclear if these responses evoked in vitro have analogs in vivo. We found Bergmann glia exhibit at least two types of spontaneous Ca2+ excitation. One of these types, sparkles, is highly localized and thus could be involved in synaptic function. We definitively identified sparkles within the palisade fibers, but our resolution in tissue bulk-loaded with OGB-1-AM does not permit us to judge whether the finer glial fibers closely adjacent to the palisades also undergo Ca2+ excitation during sparkles. Nor can we exclude a possible role for neuronal processes, particularly those that are closely entwined with the fine Bergmann glial processes. The sparkles detected within individual palisade fibers might be the result of intracellular Ca2+ waves that have initiated at microdomains in fine processes nearby synapses and propagated to reach the main Bergmann glial fibers (Grosche et al., 1999). The low rate of sparkles we report is comparable to rates of spontaneous Ca2+ activity seen in vivo in neocortical astrocytes (Hirase et al., 2004; Takata and Hirase, 2008). Due to our conservative detection threshold, our sparkle rate estimate should be understood as a lower bound.
Since sparkles persist at reduced rates under TTX, γDGG, and isoflurane, sparkles could partly be of intrinsic origin, as are spontaneous Ca2+ transients in neocortical astrocytes (Takata and Hirase, 2008). During locomotor activity, Ca2+ excitation due to flares arose across large numbers of palisade fibers, preventing us from making clear distinctions between isolated, individual sparkles and the widespread sparkle-like activity from the flares. Thus, it remains unclear whether there is a connection between sparkles and motor behavior.
Possible Causes of Bursts
Radially expanding bursts spread across the main glial fibers of the palisades as well as surrounding non-palisade regions in the cerebellar molecular layer where fine Bergmann glial fibers lie, as reported also in a recent study (Hoogland et al., 2009). Were a neuronal role to exist in bursts, it should be independent of Na+-channel mediated voltage depolarization and glutamatergic transmission, since bursts persist under TTX and γDGG.
Bursts propagated outwards from a central initiation site, which in some cases appeared to be an individual palisade fiber, with an approximate speed (~10 μm/s) comparable to reported speeds of intercellular Ca2+ waves seen in vitro in astrocytes of several types (Fiacco and McCarthy, 2006). Based on the size of bursts’ radial expansion, bursts typically cover fibers from ~10 but sometimes up to a few tens of Bergmann glia. Using 3D imaging we found bursts spread over an approximately ellipsoidal volume of ~86,000 μm3, yielding estimates that bursts traverse 55,000-70,000 parallel fiber-Purkinje cell synapses from ~3 adjoining Purkinje cells (Heinsen and Heinsen, 1983; Napper and Harvey, 1988). The 3D radially expanding waveform of bursts and the outward propagation of Ca2+ excitation suggest a diffusible messenger could mediate bursts. Bursts’ ellipsoidal volumes suggest the diffusion of any such messenger is anisotropic, which is plausible given the ordered, anisotropic architecture of cerebellar cortex. ATP is a likely candidate as a messenger, since blockade of P2 purinergic receptors eliminates bursts. However, since extracellular hydrolysis of ATP likely occurs within ~200 ms of release (Dunwiddie et al., 1997), ATP is probably not involved in bursts’ termination given that bursts last ~4-5 s.
The time course of bursts was well fit by a two-stage kinetic model and closely resembles that of large amplitude Ca2+ transients evoked in Bergmann glia upon parallel fiber stimulation in brain slices (Beierlein and Regehr, 2006; Piet and Jahr, 2007). These evoked transients also involved purinergic receptor activation and release of Ca2+ from intracellular stores. Given these similarities, we interpret the ongoing bursts we saw in vivo as the physiological analogs of the evoked transients recorded in vitro. Electrical stimulation of parallel fibers in vitro may be one means of eliciting sufficient ATP release to trigger bursts. However, based on the persistence of bursts in vivo in the presence of TTX and γDGG, parallel fiber activity may not be the normal physiological means of triggering the ATP release that leads to bursts.
Although γDGG did not significantly alter the frequency of bursts, it did lengthen bursts’ duration by reducing the rate constant k2 in our kinetic model of two reaction steps. Thus, although glutamate transmission is not required for burst initiation, blockade of glutamate transmission does affect the termination of bursts. In vitro, blockade of AMPA glutamate receptors did not eliminate electrically evoked bursts, but did eradicate an initial component of the [Ca2+] increase in Bergmann glia that was small compared to the component sensitive to PPADS (Piet and Jahr, 2007). However, the available data do not permit us to make more precise conclusions about what glutamate’s specific role might be (Supplemental Discussion).
Evidence implicating a specific function for bursts remains lacking. In thalamus, ATP release from astrocytes during astrocytic Ca2+ waves leads to a partial suppression of glutamatergic neurotransmission that is mediated by the ATP hydrolysis product, adenosine (Bekar et al., 2008). By analogy, cerebellar Ca2+ bursts might also affect neurotransmission via adenosine signaling. However, in vitro studies have not found any influence of electrically evoked bursts upon parallel fiber to Purkinje cell synaptic transmission (Beierlein and Regehr, 2006). We saw considerable variability in burst rates between individual mice, and future work must identify the origins of this variability and the role of bursts in brain function. To date, we have not found any connection between bursts and locomotor activity.
Possible Causes of Flares
Ca2+ flares arose upon initiation of locomotor activity and often covered our entire field of view of 200-500 μm. At their initiation flares appear to be composed of multiple sparkles, combined with Ca2+ excitation in regions between the palisade fibers. Based on this appearance and the similar pharmacological sensitivities of flares and sparkles, it is tempting to speculate that sparkles could be seed or miniature flares. This speculation must be tempered on several grounds. Sparkles occur in animals at rest and persist at low rates under TTX or isoflurane, whereas the neural activity patterns needed to initiate flares in the areas we studied seem specific to motor behavior. Further, flares are more than just a composite of many sparkles, since flares activate both the main glial fibers in palisades and surrounding fine fibers. Our recordings do not have the resolution to eliminate the possibility that sparkles activate fine fibers closely adjacent to the palisades, but the data show conclusively the much tighter spatial association between sparkles and palisade fibers than for flares.
Regardless of whether sparkles are precisely limited to the palisades, it is reasonable to hypothesize that a smaller number of localized events, such as sparkles, might together initiate a composite macroscopic event, a flare. This hypothesis prompts many questions for future study, including how many palisade fibers must undergo Ca2+ activation to achieve a flare and under what conditions can this collective event occur? An interesting aspect of flares is their refractory period of ~30 s (Figure 6D). Given that Ca2+ excitation in astrocytes is thought to occur primarily through release of Ca2+ from intracellular stores (Fiacco and McCarthy, 2006), the refractory duration may correspond to the time needed to replenish these stores following Ca2+ depletion. Overall, network Ca2+ activation during flares contrasts with that of bursts, which appear to have a single initiation site, use different receptor pathways, and cover only tens of cells.
As with sparkles and bursts, although we can definitively state the involvement of Bergmann glial palisade fibers in flares, we cannot exclude a possible role for neurons in flares in regions outside the palisade fibers. On the contrary, in the scenario that flares mediate signals between neurons and blood vessels, neurons would clearly have a key role in flares’ initiation.
Possible Relationships of Flares to Motor Behavior and Hemodynamics
Given their size and behaviorally triggered character, flares seem well poised to influence neuronal or cerebral blood flow dynamics associated with motor behavior. Recent research has paid considerable attention to the potential role of astrocytes in functional hyperemia, the up-regulation of cerebral blood flow in response to heightened neural activity (Iadecola and Nedergaard, 2007). Like other cortical astrocytes Bergmann glia contact neuronal synapses and blood vessels. Hence, flares might help mediate locomotor driven blood flow increases. Our kinetic and pharmacological data are consistent with a possible role for Bergmann glial Ca2+ flares in blood flow responses, but do not conclusively prove a connection between the two phenomena. In the vermis, flares and blood perfusion responses arise over similar time courses following locomotor initiation and are both reduced by TTX and γDGG, but not PPADS. Notable differences are that flares have a refractory period and a limited duration, whereas blood perfusion responses are not refractory and persist throughout locomotor activity. TTX and γDGG, which block flares, preferentially reduce the persistence of blood flow responses. It is also well established cerebellar blood flow responses can be evoked in isoflurane anesthetized animals (Yang et al., 2000), whereas flares were absent under isoflurane anesthesia (Table S3, Figure S5).
These data are consistent with a number of plausible scenarios in which flares occur in direct response to the neural activity underlying locomotion and mediate changes in blood flow. Complications in considering these possibilities are that for technical reasons our imaging and laser Doppler studies used separate sets of animals, and that the ~1 mm depth of tissue sampled by our laser Doppler measurements was greater than the ~200 μm tissue depths sampled in our two-photon imaging studies. Thus, the failure of TTX and γDGG to abolish blood flow responses entirely could be due to incomplete effects of the drugs on the deeper tissues sampled in the blood perfusion studies. Prior laser Doppler studies in anesthetized animals have also found that functional hyperemia in the cerebellum is only partially reduced by surface application of TTX or glutamate antagonists (Iadecola et al., 1996; Yang and Iadecola, 1998).
One scenario consistent with our data is that flares help mediate the persistence, but not the initial rise, of blood flow responses. Elimination of flares by TTX or γDGG would thus lead to the preferential decline in persistent blood perfusion responses that we observed (Figure 7). However, an additional signaling mechanism would be needed to explain how blood flow responses are maintained over the duration of longer movements and then terminated. This other signal could either maintain persistent blood flow responses over the duration of motor activity, or alternatively this other signal could mediate the termination of blood flow responses. Combinations of these alternatives are also possible.
In another plausible scenario, the similarity in the rise times of flares and blood flow up-regulation is due to a direct role for flares in the initiation of blood flow responses. In this case, persistent blood flow responses would be initiated and maintained by other signals, such as direct signals sent from neurons to blood vessels. In anesthetized animals it has been shown that the activity of cerebellar molecular layer interneurons triggers blood flow responses via release of nitric oxide (NO) (Yang et al., 1999; Yang et al., 2000). Since flares are not of sufficient duration to have a role in the maintenance of blood flow responses during extended periods of neural activity (Figure 5), NO signals from interneurons might have this maintenance role.
In contrast to the two scenarios just considered in which flares influence blood flow, another conceivable possibility is that increases in blood flow, rather than increases in neural activity, trigger flares. In this case, flares might provide retrograde signaling from blood vessels to neuropil (Moore and Cao, 2008), although there is presently less data to support such a possibility. It is also conceivable flares are part of a dynamical interplay between neurons and astrocytes that mediates animal behavior. For example, flares might cause Bergmann glia to release neuro-active molecules, in turn affecting the neural activity underlying locomotion. Other possibilities involve combinations of such scenarios. To dissect these issues, microscopy in behaving mice must be combined with cell-type specific manipulations, such as by genetic or optogenetic means.
EXPERIMENTAL PROCEDURES
Animals and Surgery
All procedures were approved by the Stanford APLAC. We used male C57BL/6 mice (6-10 weeks old). Surgery was done under isoflurane (1.5–2.5%, mixed with 1-2 L/min O2)..
1–2 weeks prior to imaging the skull was exposed and cleaned above neocortex and cerebellum. A custom metal plate allowing cranial access and repeated head-fixation was fixed to the skull with dental acrylic (Coltene/Whaledent, H00335).
On the imaging day, a craniotomy (0.5–3.5 mm diameter) was opened (−6.5 mm posterior to bregma; 0.5 mm lateral). Exposed tissue was irrigated with warm artificial cerebral spinal fluid (ACSF; 125 mM NaCl, 5 mM KCl, 10 mM D-Glucose, 10 mM HEPES, 2 mM CaCl2, 2 mM MgSO4; pH to 7.4 with NaOH). To dampen heartbeat- and breathing-induced brain motion, the craniotomy was filled with agarose (1.5%; Type III-A, high EEO; Sigma) in ACSF and covered by a coverslip fixed to the head plate, creating an optical window. The dura was left intact for Ca2+-indicator injections and control experiments regarding mechanical stress, but generally removed just prior to agarose application to allow pharmacological interventions and electrical recordings. In some cases teflon-coated silver wires (A-M Systems, 785500 and 787000) were implanted for electro-corticogram (ECoG) recordings.
Indicator Labeling
Targeted bulk-loading of cerebellar tissue with the Ca2+-indicator Oregon green 488 1,2-bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetate-1 acetoxymethyl (OGB-1-AM; Molecular Probes) was done as described (Stosiek et al., 2003). Concentrations of OGB-1-AM and DMSO in our pipette solution were 500 μM and 2-5%, respectively. To counterstain Bergmann glia, the red fluorescent astrocyte marker sulforhodamine 101 (SR101; 200-333 μM in ACSF; Invitrogen) was added to the injection solution or applied topically (1-5 min) in cases in which the dura was removed (Nimmerjahn et al., 2004).
Imaging
After surgical implantation of the head-plate and a recovery of 1-7 days, mice were habituated in 3-10 sessions (usually 1 session/day) to accept increasingly longer periods (15-135 min sessions; 2.5-9.25 h total habituation time) of head restraint while on a 11.9-cm-diameter exercise ball (Habitrail, 62065) covered with copper mesh. The ball rotated on one axis, allowing the animal to stride forward or backward at liberty.
We used a custom upright two-photon microscope equipped with an ultrafast Ti:sapphire laser (Tsunami, Spectra-Physics), a nanofocusing system (P-725-PIFOC, Physik Instrumente), two detection channels, and a 20× water-immersion objective (Olympus, 0.95 NA, XLUMPlanFl). For simultaneous excitation of OGB-1-AM and SR101 we ordinarily used 800-820 nm. To minimize aftereffects of anesthesia, mice recovered from isoflurane for >60 min prior to recordings. To minimize the possibility of photo-induced alterations in physiology, the duration and average power of continuous illumination typically were <7 min and <35 mW at a given brain location.
Images were typically 256 × 256 pixels (10.2–11.1 Hz frame rate) or 128 × 128 pixels (20.4 Hz), but sometimes 512 × 512 pixels (2 Hz frame rate) to obtain higher resolution. In 3D recordings, image stacks contained 5-20 optical sections acquired at 5, 10, or 20 μm axial spacing (1-4 Hz stack acquisition rate).
Behavioral Analysis
Rotations of the exercise ball were monitored using an optical encoder (US Digital, E7PD-720-118). In addition, mouse behavior was recorded in Mini-DV format using a digital Camcorder (Sony DCR-VX2000).
Electrophysiology
For ECoG recording the stripped tip of a 140-μm-diameter Teflon-coated silver wire (A-M Systems, 785500) was placed on the pial surface in one corner of the craniotomy. A 330-μm-diameter reference electrode (A-M Systems, 787000) was placed over neocortex near bregma through a small hole in the parietal bone. ECoG signals were acquired using a differential AC amplifier (A-M Systems, 1700; gain, 1,000-10,000; Notch filtered).
Pharmacology
TTX (25-125 μM), γDGG (50 mM) and PPADS (1-2 mM) were dissolved in ACSF and applied in aqueous solution onto the optical window in the cranium. Drug action was generally verified by observing changes in ECoG recordings. For each drug treatment, we always performed within animal comparisons of Ca2+ transients occurring at the same field of view before and during the treatment. Since wash out of drugs can be imperfect, we used a separate set of animals for each drug tested.
Laser Doppler Studies
We tracked blood perfusion levels using an instrument (PF-5010-LDPM, Perimed) with a 1-mm diameter fiber-optic probe (PR 407-1) that we affixed either to the cranium or an optical window using a miniature probe holder (PH 07-6). A smoothing filter with a 0.03 s time-constant was applied to all perfusion traces.
General Methods of Image Analysis
As a first analysis step we corrected any lateral motion artifacts in the imaging data. We then corrected for photobleaching and for spectral overlap in the emission spectra of the two dyes. Regions of interest (ROIs) containing glial fiber palisades were identified in SR101 images by applying a spatial high-pass filter and then finding contiguous regions above a threshold intensity value. To obtain Ca2+ signals we examined the glial palisade ROIs within the OGB-1-AM images and extracted ΔF(t)/F traces for each ROI.
Statistical Analysis
Data analysis algorithms were written in MATLAB (Mathworks). All values reported in the text are mean ± standard deviations, except those noted as median ± standard deviations and the errors in event percentages and rates, which are 68% confidence intervals.
Supplementary Material
Movie S1. Ca2+ Bursts Occur Spontaneously in Alert Resting Animals and Appear as a Radial Wave Expanding in Three Dimensions
A video clip taken from an 8 min recording acquired by three-dimensional (3D) two-photon microscopy in an awake, head-restrained mouse standing on the exercise ball, following injection of the cell membrane-permeant Ca2+-sensitive indicator OGB-1-AM into the cerebellar vermis. The video shows two examples of radially spreading bursts recorded in the cerebellar molecular layer across multiple optical planes at different depths below the pial surface. The planes shown range from 25-100 μm in depth spaced at 5 μm increments and were consecutively sampled at a rate of 49 ms per plane. The entire 3D stack was sampled at 1.1 Hz. Image data are displayed in a mosaic arrangement, with raw (left) and normalized (right) fluorescence signals. Normalized changes in fluorescence, ΔF(t)/F, were computed for each pixel as the difference between the instantaneous fluorescence, F(t), and the time-averaged fluorescence, F, divided by F. Elapsed time in seconds is shown in the upper right corner. Width of each individual image is 220 μm. Scale bar: 25 μm.
Movie S2. Ca2+ Flares are Triggered by Locomotion and Occur Across Areas Hundreds of Microns in Extent
This exemplary video taken from a 9.8 min recording in an awake head-restrained mouse shows Ca2+-activation in numerous Bergmann glial fibers across a 232 μm field of view before, during and after voluntary locomotor activity on an exercise ball. The time in seconds relative to movement onset is indicated in the upper right corner. Instantaneous running speed on the ball (in mm/s) as measured by the optical encoder is shown in the lower right corner. Fluorescence data were recorded in the cerebellar vermis by dual-color, two-photon microscopy following injection of the green fluorescent, cell membrane-permeant Ca2+-sensitive indicator OGB-1-AM (left) and topical application of the red fluorescent astrocyte marker SR101 (center). The right panel shows an overlay of the SR101 signals and normalized changes (ΔF(t)/F) in the OGB-1-AM signals, with the OGB-1-AM ΔF(t)/F signals represented in blue. Both primary glial fibers in the palisades highlighted by SR101 and the surrounding tissue areas participate in the Ca2+ flare. Virtually no motion artifacts are apparent, even when the mouse is running. The image acquisition rate was 20.35 frames per second, but to aid visualization of Ca2+-activation each frame in the movie shows an average of 10 consecutive frames. Scale bar, 25 μm.
ACKNOWLEDGEMENTS
We thank B.A. Barres, B. Stevens, and members of the Barres lab for helpful conversations and S100β-EGFP mice, D. Profitt and Stanford’s Varian Machine Shop for expert machining, A. Lui for programming assistance and help with habituating animals, and Q. T. Nguyen and D. Kleinfeld for providing imaging software and electronics. Our work was supported by grants to MJS from NIDCD, the NIH NDC for Optical Control of Biological Function, the Stanford Bio-X program, and the Klingenstein, Sloan, and Packard Foundations, postdoctoral fellowships to AN from the Alexander von Humboldt-Foundation and the International Human Frontier Science Program Organization, and an NSF Graduate Fellowship to EAM.
Footnotes
See Supplemental Data for more details.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersson G, Armstrong DM. Complex Spikes in Purkinje-Cells in the Lateral Vermis (B-Zone) of the Cat Cerebellum during Locomotion. Journal of Physiology-London. 1987;385:107–134. doi: 10.1113/jphysiol.1987.sp016487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nature Reviews Neuroscience. 2005;6:297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Regehr WG. Brief bursts of parallel fiber activity trigger calcium signals in Bergmann glia. J Neurosci. 2006;26:6958–6967. doi: 10.1523/JNEUROSCI.0613-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, Lovatt D, Williams E, Takano T, Schnermann J, et al. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med. 2008;14:75–80. doi: 10.1038/nm1693. [DOI] [PubMed] [Google Scholar]
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular Signaling in Glial-Cells - Calcium Waves and Oscillations in Response to Mechanical Stimulation and Glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- Chen G, Hanson CL, Dunbar RL, Ebner TJ. Novel form of spreading acidification and depression in the cerebellar cortex demonstrated by neutral red optical imaging. J. Neurophysiol. 1999;81:1992–1998. doi: 10.1152/jn.1999.81.4.1992. [DOI] [PubMed] [Google Scholar]
- De Blas AL. Monoclonal-Antibodies to Specific Astroglial and Neuronal Antigens Reveal the Cytoarchitecture of the Bergmann Glia Fibers in the Cerebellum. J Neurosci. 1984;4:265–273. doi: 10.1523/JNEUROSCI.04-01-00265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. Astrocyte calcium elevations: Properties, propagation, and effects on brain signaling. Glia. 2006;54:676–690. doi: 10.1002/glia.20396. [DOI] [PubMed] [Google Scholar]
- Gobel W, Kampa BM, Helmchen F. Imaging cellular network dynamics in three dimensions using fast 3D laser scanning. Nat Methods. 2007;4:73–79. doi: 10.1038/nmeth989. [DOI] [PubMed] [Google Scholar]
- Greenberg DS, Houweling AR, Kerr JND. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat. Neurosci. 2008;11:749–751. doi: 10.1038/nn.2140. [DOI] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat. Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Heinsen H, Heinsen YL. Quantitative Studies on Regional Differences in Purkinje-Cell Dendritic Spines and Parallel Fiber Synaptic Density. Anatomy and Embryology. 1983;168:361–370. doi: 10.1007/BF00304274. [DOI] [PubMed] [Google Scholar]
- Hirase H, Qian LF, Bartho P, Buzsaki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS. Biol. 2004;2:494–499. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogland TM, Kuhn B, Gobel W, Huang W, Nakai J, Helmchen F, Flint J, Wang SS. Radially expanding transglial calcium waves in the intact cerebellum. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3496–3501. doi: 10.1073/pnas.0809269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, McCarthy G. Evidence for a refractory period in the hemodynamic response to visual stimuli as measured by MRI. Neuroimage. 2000;11:547–553. doi: 10.1006/nimg.2000.0553. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Li J, Xu S, Yang G. Neural mechanisms of blood flow regulation during synaptic activity in cerebellar cortex. J. Neurophysiol. 1996;75:940–950. doi: 10.1152/jn.1996.75.2.940. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, Ozawa S. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292:926–929. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- Jorntell H, Ekerot C, Garwicz M, Luo XL. Functional organization of climbing fibre projection to the cerebellar anterior lobe of the rat. J Physiol. 2000;522(Pt 2):297–309. doi: 10.1111/j.1469-7793.2000.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekkoek SKE, Den Ouden WL, Perry G, Highstein SM, De Zeeuw CI. Monitoring kinetic and frequency-domain properties of eyelid responses in mice with magnetic distance measurement technique. J. Neurophysiol. 2002;88:2124–2133. doi: 10.1152/jn.2002.88.4.2124. [DOI] [PubMed] [Google Scholar]
- Leicht R, Schmidt RF. Somatotopic Studies on Vermal Cortex of Cerebellar Anterior Lobe or Unanesthetized Cats. Experimental Brain Research. 1977;27:479–490. doi: 10.1007/BF00239037. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annual Review of Physiology. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Mantz J, Cordier J, Giaume C. Effects of General-Anesthetics on Intercellular Communications Mediated by Gap-Junctions between Astrocytes in Primary Culture. Anesthesiology. 1993;78:892–901. doi: 10.1097/00000542-199305000-00014. [DOI] [PubMed] [Google Scholar]
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage. 2006;32:33–48. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Martindale J, Berwick J, Martin C, Kong YZ, Zheng Y, Mayhew JEW. Long duration stimuli and nonlinearities in the neural-haemodynamic coupling. Journal of Cerebral Blood Flow and Metabolism. 2005;25:651–661. doi: 10.1038/sj.jcbfm.9600060. [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Nakamura Y, Arai T, Kataoka K. Increase of glutamate uptake in astrocytes - A possible mechanism of action of volatile anesthetics. Anesthesiology. 1997;86:1359–1366. doi: 10.1097/00000542-199706000-00018. [DOI] [PubMed] [Google Scholar]
- Moore CI, Cao R. The hemo-neural hypothesis: On the role of blood flow in information processing. J. Neurophysiol. 2008;99:2035–2047. doi: 10.1152/jn.01366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napper RMA, Harvey RJ. Number of Parallel Fiber Synapses on an Individual Purkinje-Cell in the Cerebellum of the Rat. Journal of Comparative Neurology. 1988;274:168–177. doi: 10.1002/cne.902740204. [DOI] [PubMed] [Google Scholar]
- Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J. Neurophysiol. 2002;87:528–537. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A. Astrocytes going live: advances and challenges. Journal of Physiology-London. 2009 doi: 10.1113/jphysiol.2008.167171. doi: 10.1113/jphysiol.2008.167171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Kerr JND, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods. 2004;1:31–37. doi: 10.1038/nmeth706. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910. doi: 10.1016/j.neuron.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piet R, Jahr CE. Glutamatergic and purinergic receptor-mediated calcium transients in Bergmann glial cells. J Neurosci. 2007;27:4027–4035. doi: 10.1523/JNEUROSCI.0462-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, Siegel A, Rickmann M, Wolff JR, Noone D, Robinson SR. Distribution of Bergmann Glial Somata and Processes - Implications for Function. J. Brain Res.-J. Hirnforsch. 1995;36:509–517. [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci. 2006;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Yu HB, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Shibuki K, Gomi H, Chen L, Bao SW, Kim JSK, Wakatsuki H, Fujisaki T, Fujimoto J, Katoh A, Ikeda T, et al. Deficient cerebellar long-term depression, impaired eyeblink conditioning, and normal motor coordination in GFAP mutant mice. Neuron. 1996;16:587–599. doi: 10.1016/s0896-6273(00)80078-1. [DOI] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Hirase H. Cortical layer 1 and layer 2/3 astrocytes exhibit distinct calcium dynamics in vivo. PLoS ONE. 2008;3:e2525. doi: 10.1371/journal.pone.0002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XH, Lou NH, Xu QW, Tian GF, Peng WG, Han XN, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- Yang G, Chen G, Ebner TJ, Iadecola C. Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 1999;277:R1760–R1770. doi: 10.1152/ajpregu.1999.277.6.R1760. [DOI] [PubMed] [Google Scholar]
- Yang G, Huard JMT, Beitz AJ, Ross ME, Iadecola C. Stellate neurons mediate functional hyperemia in the cerebellar molecular layer. J Neurosci. 2000;20:6968–6973. doi: 10.1523/JNEUROSCI.20-18-06968.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Iadecola C. Stimulation of the cerebellar climbing fibers increases local blood flow by activation of glutamate receptors via nitric oxide and cGMP. Stroke. 1998;29:324–324. doi: 10.1161/01.str.29.2.499. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lubischer JL, Kang H, Tian L, Mikesh M, Marks A, Scofield VL, Maika S, Newman C, Krieg P, Thompson WJ. Fluorescent proteins expressed in mouse transgenic lines mark subsets of glia, neurons, macrophages, and dendritic cells for vital examination. J Neurosci. 2004;24:10999–11009. doi: 10.1523/JNEUROSCI.3934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Ca2+ Bursts Occur Spontaneously in Alert Resting Animals and Appear as a Radial Wave Expanding in Three Dimensions
A video clip taken from an 8 min recording acquired by three-dimensional (3D) two-photon microscopy in an awake, head-restrained mouse standing on the exercise ball, following injection of the cell membrane-permeant Ca2+-sensitive indicator OGB-1-AM into the cerebellar vermis. The video shows two examples of radially spreading bursts recorded in the cerebellar molecular layer across multiple optical planes at different depths below the pial surface. The planes shown range from 25-100 μm in depth spaced at 5 μm increments and were consecutively sampled at a rate of 49 ms per plane. The entire 3D stack was sampled at 1.1 Hz. Image data are displayed in a mosaic arrangement, with raw (left) and normalized (right) fluorescence signals. Normalized changes in fluorescence, ΔF(t)/F, were computed for each pixel as the difference between the instantaneous fluorescence, F(t), and the time-averaged fluorescence, F, divided by F. Elapsed time in seconds is shown in the upper right corner. Width of each individual image is 220 μm. Scale bar: 25 μm.
Movie S2. Ca2+ Flares are Triggered by Locomotion and Occur Across Areas Hundreds of Microns in Extent
This exemplary video taken from a 9.8 min recording in an awake head-restrained mouse shows Ca2+-activation in numerous Bergmann glial fibers across a 232 μm field of view before, during and after voluntary locomotor activity on an exercise ball. The time in seconds relative to movement onset is indicated in the upper right corner. Instantaneous running speed on the ball (in mm/s) as measured by the optical encoder is shown in the lower right corner. Fluorescence data were recorded in the cerebellar vermis by dual-color, two-photon microscopy following injection of the green fluorescent, cell membrane-permeant Ca2+-sensitive indicator OGB-1-AM (left) and topical application of the red fluorescent astrocyte marker SR101 (center). The right panel shows an overlay of the SR101 signals and normalized changes (ΔF(t)/F) in the OGB-1-AM signals, with the OGB-1-AM ΔF(t)/F signals represented in blue. Both primary glial fibers in the palisades highlighted by SR101 and the surrounding tissue areas participate in the Ca2+ flare. Virtually no motion artifacts are apparent, even when the mouse is running. The image acquisition rate was 20.35 frames per second, but to aid visualization of Ca2+-activation each frame in the movie shows an average of 10 consecutive frames. Scale bar, 25 μm.