Abstract
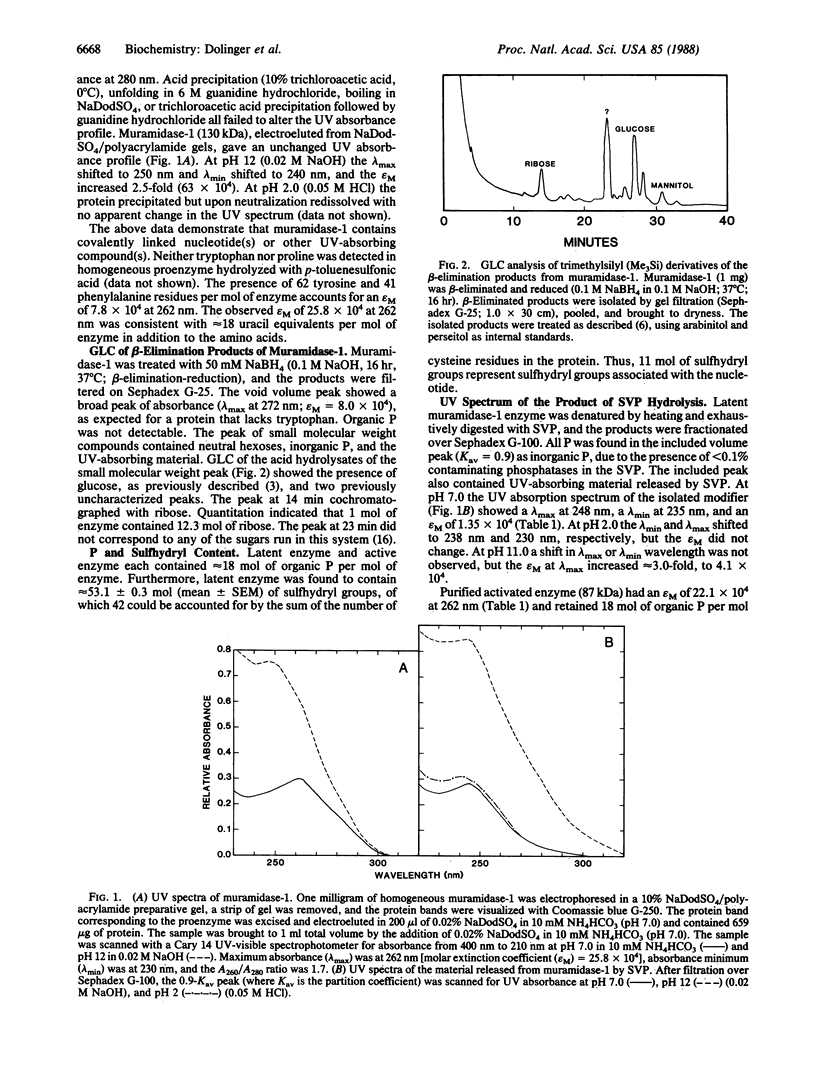
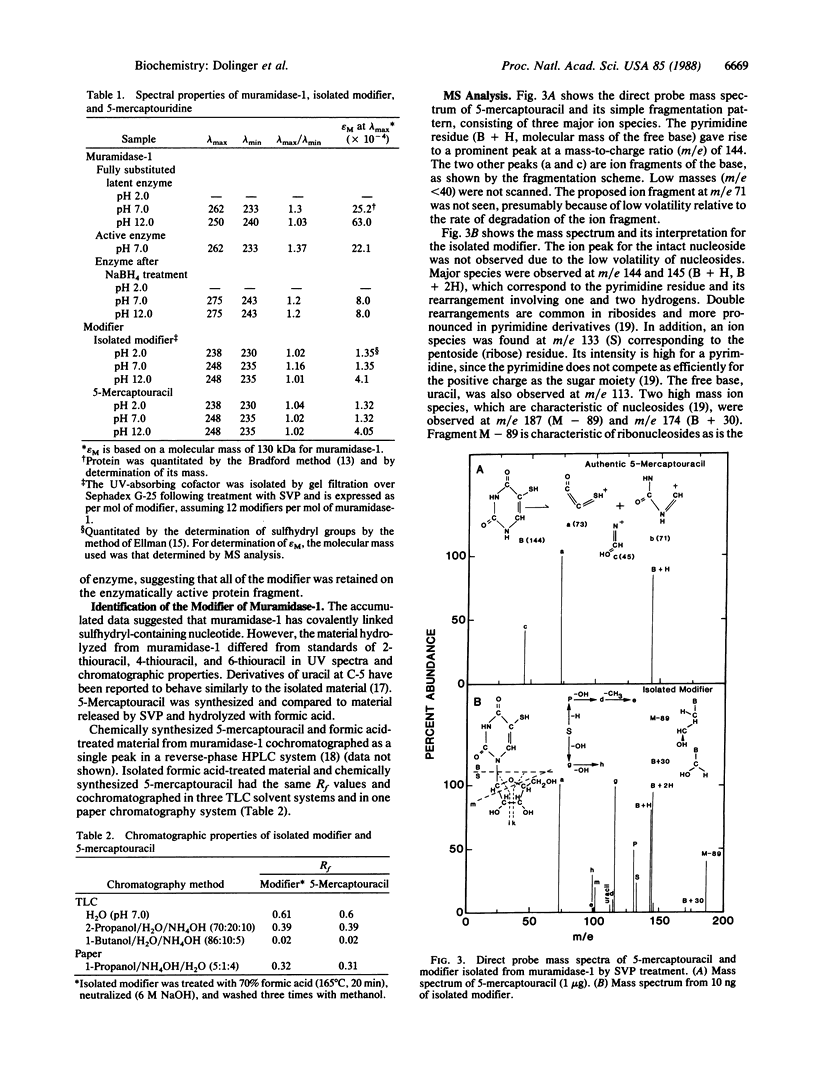
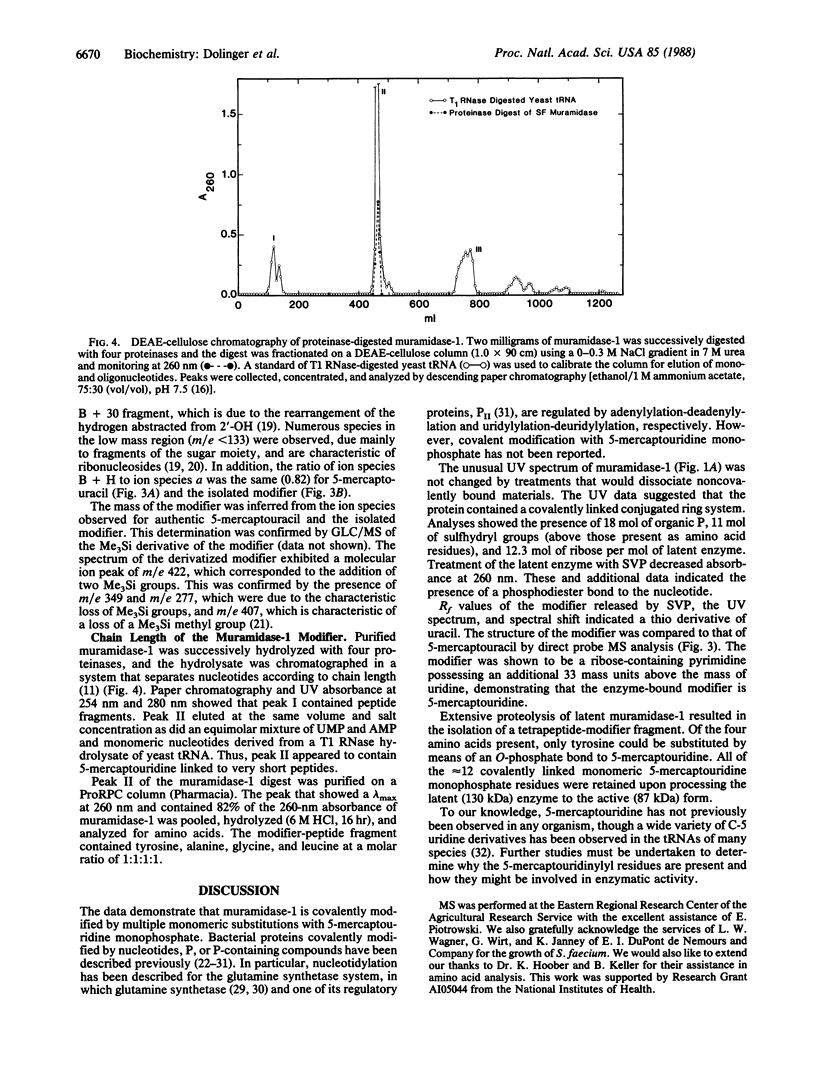
Purified beta-1,4-N-acetylmuramoylhydrolase (muramidase-1; EC 3.2.1.17) of Streptococcus faecium ATCC 9790 has been shown to be covalently substituted with approximately 12 mol equivalents of monomeric 5-mercaptouridine monophosphate. All 12 residues are present on the proteolytically processed 87-kDa active form of the enzyme. A peptide fragment containing 5-mercaptouridine, tyrosine, alanine, glycine, and leucine was isolated consistent with an O-phosphate linkage of the nucleotide to tyrosine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baczynskyj L., Biemann K., Hall R. H. Sulfur-containing nucleoside from yeast transfer ribonucleic acid: 2-thio-5(or 6)-uridine acetic acid methyl ester. Science. 1968 Mar 29;159(3822):1481–1483. doi: 10.1126/science.159.3822.1481. [DOI] [PubMed] [Google Scholar]
- Barrett J. F., Dolinger D. L., Schramm V. L., Shockman G. D. The mechanism of soluble peptidoglycan hydrolysis by an autolytic muramidase. A processive exodisaccharidase. J Biol Chem. 1984 Oct 10;259(19):11818–11827. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Segal A., Stadtman E. R. Modulation of glutamine synthetase adenylylation and deadenylylation is mediated by metabolic transformation of the P II -regulatory protein. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2949–2953. doi: 10.1073/pnas.68.12.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarquets G., Cortay J. C., Cozzone A. J. Two-dimensional analysis of proteins phosphorylated in E. coli cells. FEBS Lett. 1984 Aug 6;173(2):337–341. doi: 10.1016/0014-5793(84)80801-7. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Enami M., Ishihama A. Protein phosphorylation in Escherichia coli and purification of a protein kinase. J Biol Chem. 1984 Jan 10;259(1):526–533. [PubMed] [Google Scholar]
- Ferro-Luzzi Ames G., Nikaido K. Phosphate-containing proteins of Salmonella typhimurium and Escherichia coli. Analysis by a new two-dimensional gel system. Eur J Biochem. 1981 Apr;115(3):525–531. [PubMed] [Google Scholar]
- Holbrook I. B., Leaver A. G. A procedure to increase the sensitivity of staining by Coomassie brilliant blue G250-perchloric acid solution. Anal Biochem. 1976 Oct;75(2):634–636. doi: 10.1016/0003-2697(76)90118-4. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Shockman G. D. Purification and some properties of the endogenous, autolytic N-acetylmuramoylhydrolase of Streptococcus faecium, a bacterial glycoenzyme. J Biol Chem. 1983 Aug 10;258(15):9514–9521. [PubMed] [Google Scholar]
- Kersten H. On the biological significance of modified nucleosides in tRNA. Prog Nucleic Acid Res Mol Biol. 1984;31:59–114. doi: 10.1016/s0079-6603(08)60375-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCloskey J. A., Lawson A. M., Tsuboyama K., Krueger P. M., Stillwell R. N. Mass spectrometry of nucleic acid components. Trimethylsilyl derivatives of nucleotides, nucleosides, and bases. J Am Chem Soc. 1968 Jul 17;90(15):4182–4184. doi: 10.1021/ja01017a062. [DOI] [PubMed] [Google Scholar]
- Ohashi Z., Maeda M., McCloskey J. A., Nishimura S. 3-(3-Amino-3-carboxypropyl)uridine: a novel modified nucleoside isolated from Escherichia coli phenylalanine transfer ribonucleic acid. Biochemistry. 1974 Jun 4;13(12):2620–2625. doi: 10.1021/bi00709a023. [DOI] [PubMed] [Google Scholar]
- Parkin D. W., Leung H. B., Schramm V. L. Synthesis of nucleotides with specific radiolabels in ribose. Primary 14C and secondary 3H kinetic isotope effects on acid-catalyzed glycosidic bond hydrolysis of AMP, dAMP, and inosine. J Biol Chem. 1984 Aug 10;259(15):9411–9417. [PubMed] [Google Scholar]
- Shapiro B. M., Kingdon H. S., Stadtman E. R. Regulation of glutamine synthetase. VII. Adenylyl glutamine synthetase: a new form of the enzyme with altered regulatory and kinetic properties. Proc Natl Acad Sci U S A. 1967 Aug;58(2):642–649. doi: 10.1073/pnas.58.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Cheney M. C. Autolytic enzyme system of Streptococcus faecalis. V. Nature of the autolysin-cell wall complex and its relationship to properties of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Jun;98(3):1199–1207. doi: 10.1128/jb.98.3.1199-1207.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Koshland D. E., Jr Evidence for protein kinase activities in the prokaryote Salmonella typhimurium. J Biol Chem. 1978 Nov 10;253(21):7605–7608. [PubMed] [Google Scholar]



