Abstract
Background & Aims
Somatic mutations provide uniquely specific markers for the early detection of neoplasia that can be detected in DNA purified from plasma or stool of patients with colorectal cancer. The primary purpose of the present investigation was to determine the parameters that were critical for detecting mutations using a quantitative assay. A secondary purpose was to compare the results of plasma and stool DNA testing using the same technology.
Methods
We examined DNA purified from the stool of 25 patients with colorectal cancers before surgery. In 16 of these cases, plasma samples also were available. Mutations in stool or plasma were assessed with an improved version of the BEAMing technology.
Results
Of the 25 stool DNA samples analyzed, 23 (92%) contained mutations that were present in the corresponding tumors from the same patients. In contrast, only 8 of the 16 (50%) plasma DNA samples analyzed had detectable levels of mutated DNA. We found that the DNA fragments containing mutations in both stool and plasma DNA typically were smaller than 150 bases in size. The sensitivity of the new method was superior to a widely used technique for detecting mutations, using single base extension and sequencing, when assessed on the same samples (92% vs 60%; P = .008, exact McNemar test).
Conclusions
When assessed with sufficiently sensitive methods, mutant DNA fragments are detectable in the stool of more than 90% of colorectal cancer patients. DNA purified from stool provides a better template for mutation testing than plasma.
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States. CRC generally can be cured by surgical excision if detected at any stage before distant metastasis to the liver and other organs. Unfortunately, about 35% of patients have such distant metastases, either occult or detectable, at the time of diagnosis, accounting for virtually all the deaths from the disease. The value of screening tests for colorectal neoplasia, particularly colonoscopy, has been highlighted in a variety of public awareness campaigns in the past several years. This likely has contributed to the decline in CRC-related deaths, but the large number of individuals still being diagnosed with surgically incurable cancers attests to the fact that current efforts in this regard are inadequate. In particular, there is an urgent need for noninvasive tests that can complement colonoscopy and be offered to patients who are hesitant to undergo this inconvenient and invasive procedure. This need has stimulated the development of new tests for early detection, including virtual colonoscopy, improved assays for the presence of blood in stool, immunohistologic tests for cancer cells or proteins in stool, and DNA-based tests for genetic or epigenetic alterations.1
Mutant DNA molecules offer unique advantages over cancer-associated biomarkers because they are so specific. Although mutations occur in individual normal cells at a low rate (∼10−9 to 10−10 mutations/bp/generation), such mutations represent such a tiny fraction of the total normal DNA that they are orders of magnitude below the detection limit of any test that has yet been described (including the one used in the current study). There is only one circumstance when a specific somatic mutation is present in an appreciable amount in any clinical sample: when it occurs in clonal fashion, that is, when the mutation is present in all cells of a specific population, thereby defining a neoplastic lesion.
Several studies have shown that mutant DNA can be detected in stool, urine, and blood of CRC patients.2 Moreover, technical factors that have limited the sensitivity of such assays gradually are being overcome. For example, improvements for stool-based testing include DNA stabilization after defecation,3 removal of polymerase chain reaction (PCR) inhibitors and bacterial DNA, cost-effective purification of sufficient amounts of human DNA for analysis,4 and the continuing delineation of mutant genes that can be assessed.5 Moreover, assays for detecting mutations have been developed that query each template molecule individually, dramatically increasing the signal-to-noise ratio. Such digital assays are particularly well suited for the analysis of DNA in clinical samples, such as stool or plasma, because the mutant DNA fragments in such samples are greatly outnumbered by normal DNA fragments.
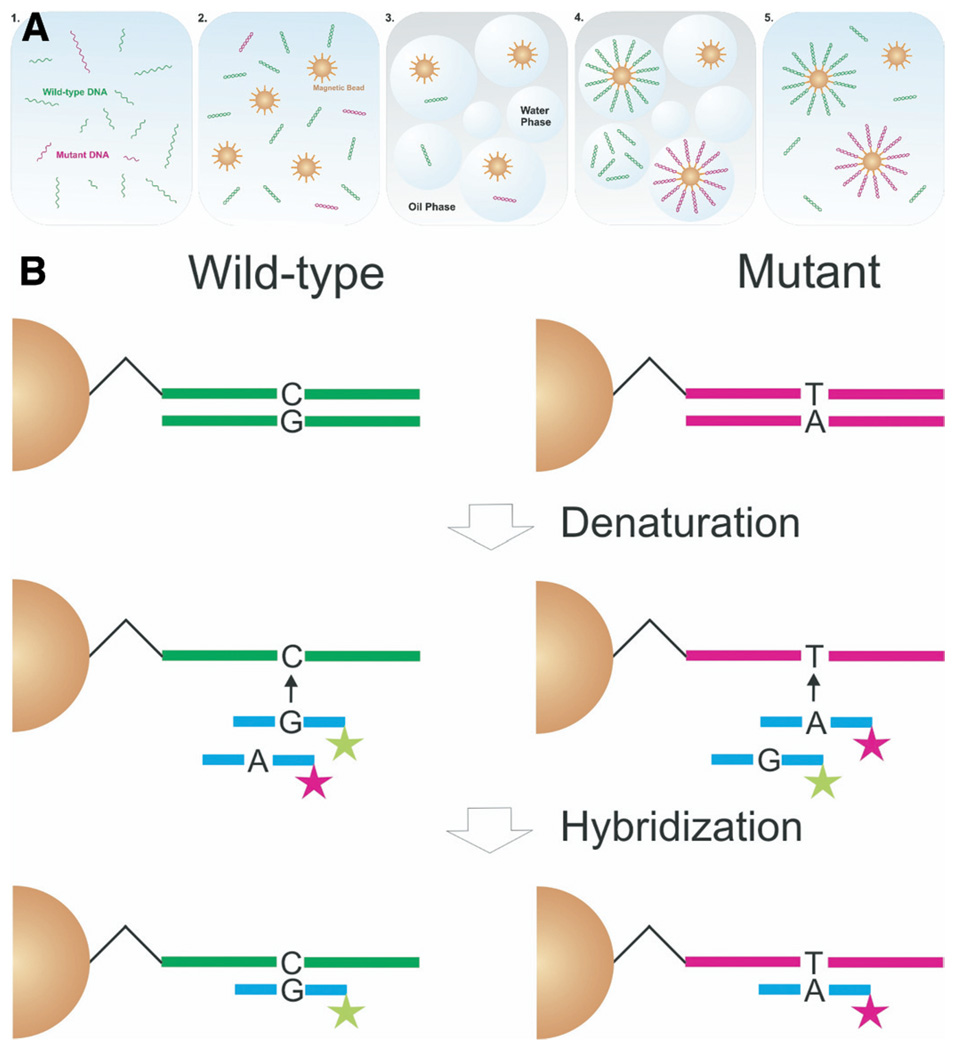
In the present study, we adopted a powerful digital assay called BEAMing to detect mutations in stool and plasma DNA from patients with CRCs (Figure 1). BEAMing was named after its components—beads, emulsions, amplification, and magnetics—and essentially converts single DNA template molecules to single beads containing tens of thousands of exact copies of the template.6 We used this method to determine how frequently mutations could be detected in the DNA from plasma or stool of the same patients as well as to investigate other parameters that could be useful in designing clinically applicable DNA-based tests in the future.
Figure 1.
BEAMing-based approach for detecting mutant DNA in stool samples from patients with CRC. (A) Stages in the process, starting with total fecal DNA. Step 1 represents the results of sequence-specific capture of mutant (red) and wild-type (green) single-stranded DNA molecules. After PCR-mediated amplification of gene fragments encompassing the queried mutation sites, the DNA is mixed with magnetic beads (spheres) that are bound to oligonucleotides (spikes on the spheres) complementary to sequences in the PCR products (step 2). In step 3, this mixture is segregated into billions of microcompartments in a water-in-oil emulsion. A small portion of these compartments contain a single bead and a single DNA template molecule, although the great majority of compartments contain neither (such as the blue bubble in the middle). When PCR is performed on these emulsions in step 4, individual DNA fragments are amplified within the microcompartments that contain them and become covalently bound to the surface of the bead. The resultant beads are coated with tens of thousands of copies of identical DNA fragments. In step 5, beads are recovered from the emulsion and the sequence of the bound DNA is deciphered by allele-specific hybridization, as depicted in B. (B) DNA amplified on magnetic microbeads by BEAMing initially is denatured to remove the noncovalently bound DNA strand. Differently labeled fluorescent probes are hybridized to the complementary target DNA covalently bound to the beads.11 Flow cytometry then is used to count beads individually, thereby determining the ratio of mutant to wild-type fragments originally present in the stool or plasma sample.
Materials and Methods
Study Design and Collection of Clinical Samples
For this study, specimens from subjects with CRC that had been acquired through a previous study were evaluated.7 Subjects were at average risk for CRC as determined by family history and had no personal history of any type of cancer. Patients with nonspecific abdominal symptoms or a history of basal cell or squamous cell carcinoma of the skin were not excluded. Stool and blood specimens were collected 6–12 days postcolonoscopy and before any bowel preparation for subsequent surgery. This study included 25 of the 40 previously identified cancer cases7 because 15 cases had inadequate amounts of residual material available. Patient characteristics are summarized in Table 1. Seven of the patients had stage I carcinomas, 7 had stage II, 8 had stage III, 2 had stage IV, and 1 was of unspecified stage. The blood samples were drawn in BD Vacutainer tubes with ethylenediaminetetraacetic acid (Becton Dickinson, Franklin Lakes, NJ) from 16 of the 25 patients. Plasma was prepared by centrifugation of blood at 1380g for 30 minutes. The supernatant was transferred to a fresh tube and recentrifuged. After centrifugation, the plasma was transferred to a Millipore Ultrafree-MC 0.45-µm filter device (Billerica, MA) to remove remaining cellular debris. The filter device was subjected to centrifugation at 1380g for 15 minutes. The cleared plasma was transferred to a new tube and stored at −20°C until processed.
Table 1.
Patient Characteristics
| Characteristic | Value (n = 25) |
|---|---|
| Age, y | |
| Mean | 67 |
| Median (range) | 66 (50–84) |
| Sex | |
| Female | 15 |
| Male | 10 |
| Stage | |
| I | 7 |
| II | 7 |
| III | 8 |
| IV | 2 |
| Unknown | 1 |
| Differentiation | |
| Well | 12 |
| Moderate | 9 |
| Poor | 3 |
| Unspecified | 1 |
| Number of mutations in tumor tissue | |
| 0 | 0 |
| 1 | 7 |
| 2 | 15 |
| 3 | 3 |
Identification of Mutations in Tumor Tissue
Tissues obtained at surgical resection were used for mutation analysis, as reported previously.4,5 Briefly, snap-frozen or paraffin-embedded microdissected tumor tissue was used for the isolation of tumor DNA using the QIAamp DNA mini kit (Qiagen, Valencia, CA). All DNA samples were analyzed for 22 common mutations in APC, TP53, and KRAS using a single base extension (SBE) assay and a sequencing approach for exons 9 and 20 of PIK3CA, exon 3 of CTNNB1, and exon 15 of APC. The sequencing was performed by using single-stranded DNA templates in 4 separate sequencing reactions, each containing a R110-labeled AcyloTerminator nucleotide (PerkinElmer, Waltham, MA) and a mixture of ThermoSequenase (GE, Piscataway, NJ) and AcycloPol (PerkinElmer). Combined, the 2 marker panels were able to identify at least 1 mutation in the 24 tumor samples available for this study (Table 2). The sensitivity of SBE and sequencing was 75% (18 of 24) and 79% (19 of 24), respectively (supplementary Table 1; see supplementary material online at www.gastrojournal.org).
Table 2.
Mutations in Stool and Plasma DNA
| Tumor |
Stool DNA |
Plasma DNA |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Sex/age, y | Stage (TNM) | Histologya | Site | Size, mm | Gene | Mutation (codon) | Total DNA fragments per 362 mg stool | Mutant DNA, % | Score | Total DNA fragments per 2 mL plasma | Mutant DNA, % | Score |
| 1 | M/66 | I (T1N0M0) | Mod | R | 50 | APC | C2626T (876) | 50,600 | 4.0 | + | |||
| 2 | F/64 | I (T2N0M0) | Mod | Sig | 30 | APC | G3964T (1322) | 398,000 | 0.87 | + | 3676 | 0.000 | − |
| TP53 | G818A (273) | 302,000 | 0.20 | + | |||||||||
| 3 | F/70 | I (T2N0M0) | Mod | C | 45 | APC | 4237–4240delATGG (1413) | 1808 | 0.71 | + | 2397 | 1.88 | + |
| 4 | F/67 | I (T1N0Mx) | Well | Tr | 40 | APC | C4132T (1378) | 8460 | 0.39 | + | 9317 | 0.013 | + |
| 5 | M/69 | I (T2N0M0) | Well | Rs | 24 | APC | 4359delT (1453) | 1,030,000 | 0.000 | − | 3365 | 0.000 | − |
| KRAS | G35X (12)b | 1,030,000 | 0.000 | − | |||||||||
| 6 | M/84 | I (T2N0M0) | Mod | R | 25 | APC | C2626T (876) | 252,000 | 0.32 | + | |||
| APC | 4465delT (1489) | 252,000 | 0.78 | + | |||||||||
| TP53 | C742T (248) | 252,000 | 1.0 | + | |||||||||
| 7 | F/58 | I (T2N0Mx) | Well | Sig | 12 | APC | 4297delC (1433) | 13,840 | 1.0 | + | |||
| PIK3CA | C3075T (1025) | 8260 | 21 | + | |||||||||
| 8 | M/80 | II (T3N0Mx) | Well | Sig | 25 | APC | 4497delA (1499) | 7420 | 0.003 | − | |||
| KRAS | G38A (12) | 7420 | 0.21 | + | |||||||||
| 9 | M/70 | II (T3N0Mx) | Well | Sf | 50 | APC | C3980G (1327) | 59,600 | 15.0 | + | 10,652 | 0.002 | − |
| TP53 | G524A (175) | 59,600 | 0.3 | + | |||||||||
| 10 | F/58 | II (T3N0M0) | Well | Tr | 80 | APC | 4467 delA (1489) | 113,800 | 1.17 | + | 4530 | 0.002 | − |
| 11 | F/65 | II (T3N0M0) | Well | C | 50 | APC | 4661–4662insA (1554) | 106,600 | 1.09 | + | |||
| 12 | F/75 | II (T3N0M0) | Well | R | 25 | APC | G4135T (1379) | 540,000 | 0.37 | + | 4650 | 0.42 | + |
| 13 | M/80 | II (T3N0Mx) | Well | As | 65 | APC | C2626T (876) | 264,000 | 0.06 | + | |||
| APC | 4189–4190delGA (1397) | 264,000 | 0.04 | + | |||||||||
| 14 | M/66 | II (T3N0M0) | Mod | R | 45 | APC | C4348T (1450) | 15,740 | 0.2 | + | 3690 | 0.005 | − |
| PIK3CA | G1624A (542) | 5340 | 0.3 | + | |||||||||
| 15 | F/50 | III (T4N1M0) | Mod | R | 25 | APC | C4285T (1429) | 22,600 | 0.13 | + | 6422 | 0.062 | + |
| KRAS | G35A (12) | 22,600 | 0.2 | + | |||||||||
| 16 | M/64 | III (T3N1M0) | Poor | Sig | 30 | TP53 | G524A (175) | 6920 | 0.006 | − | 7047 | 0.033 | + |
| 17 | M/74 | III (T3N2M0) | Well | R | 30 | APC | 4126–4127insT (1376) | 7140 | 0.059 | + | 2679 | 0.17 | + |
| KRAS | G38A (12) | 7140 | 0.079 | + | |||||||||
| PIK3CA | G1624A (542) | 7140 | 0.050 | + | |||||||||
| 18 | M/57 | III (T3N1M0) | Mod/poor | Sig | 70 | APC | 3934delG (1312) | 18,700 | 0.28 | + | |||
| TP53 | G733A (245) | 17,460 | 1.3 | + | |||||||||
| 19 | F/65 | III (T3N2Mx) | Mod/poor | As | 50 | APC | C2626T (876) | 5920 | 0.18 | + | 11,716 | 0.002 | − |
| KRAS | G35C (12) | 3320 | 0.23 | + | |||||||||
| TP53 | C817T (273) | 3320 | 0.10 | + | |||||||||
| 20 | M/59 | III (T3N1Mx) | Well | Tr | 40 | APC | 4661–4662insA (1554) | 11,320 | 0.0062 | + | |||
| KRAS | G35A (12) | 11,320 | 0.3 | + | |||||||||
| 21 | M/73 | III (T2N1Mx) | Mod | Tr | 42 | APC | C4348T (1450) | 10,280 | 0.055 | + | 5043 | 0.007 | − |
| KRAS | G35A (12) | 7200 | 0.23 | + | |||||||||
| 22 | F/61 | III (T3N1M0) | Mod | R | NR | APC | 3980–3983delCAC (1327) | 62,800 | 7.60 | + | 4206 | 0.001 | − |
| KRAS | G35A (12) | 62,800 | 4.43 | + | |||||||||
| 23 | M/67 | IV (T3N2M1) | Mod | Sig | 60 | PIK3CA | C1636A (546) | 138,000 | 0.068 | + | 29,233 | 6.6 | + |
| 24 | M/65 | IV (T3N1M1) | Well | As | 30 | APC | G4189T (1397) | 254,000 | 0.62 | + | 4094 | 0.44 | + |
| PIK3CA | A3140G (1047) | 254,000 | 1.33 | + | |||||||||
| 25 | M/64 | NR | NR | R | 35 | APC | 3927–3931del AAAGA (1309) | 356,000 | 0.90 | + | |||
| TP53 | G524A (175) | 356,000 | 10.2 | + | |||||||||
As, ascending colon; C, cecum; NR, not received; R, rectum; Rs, rectosigmoid; Sf, splenic flexure; Sig, sigmoid colon; Tr, transverse colon.
Histology types were as follows: well, well-differentiated adenocarcinoma; mod, moderately differentiated adenocarcinoma; poor, poorly differentiated adenocarcinoma.
G35X means G35A, G35C, or G35T (specific base change not determined).
Isolation and Quantification of Stool DNA
Human DNA enriched for the target genes (APC, TP53, KRAS, and PIK3CA) was purified from total stool DNA using a Reversible Electrophoretic Capture Affinity Protocol,8 which is described in the supplementary Materials and Methods section (see supplementary material online at www.gastrojournal.org).
The copy number of gene fragments recovered from each stool sample was quantified using an iCycler IQ real-time PCR detection system (Bio-Rad, Hercules, CA). Duplicate reactions (50 µ,L) consisted of 5 µ,L of DNA, 10 × PCR buffer (Takara Bio, Madison, WI), 0.2 mmol/L deoxynucleoside triphosphates (Promega, Madison, WI), 0.5 µmol/L of sequence-specific primers (sequences available upon request), and 2.5 U LATaq DNA polymerase (Takara Bio). The PCR parameters were 95°C for 3.5 minutes for denaturation followed by 40 cycles of 95°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute.
Isolation and Quantification of Plasma DNA
DNA was purified from 2 mL plasma using the QIAamp MinElute Virus Vacuum Kit (Qiagen) as recommended by the manufacturer. The DNA was eluted in EB buffer (Qiagen), and stored at −20°C. The amount of total DNA isolated from plasma was quantified using a modified version of a human LINE-1 quantitative real-time PCR assay, as described previously.9 Details are provided in the supplementary Materials and Methods section (see supplementary material online at www.gastrojournal.org).
Mutation Analysis by BEAMing
Plasma and stool DNA were analyzed for somatic mutations by BEAMing. In total, 18 amplification primer sets were designed for the analysis of 33 different mutations. For each stool sample, a total of 30,000 genome equivalents were analyzed. One genome equivalent was defined as 3.3 pg of genomic DNA and is equivalent to the DNA amount present in a haploid cell. A volume corresponding to the DNA purified from 2 mL of plasma was used for each BEAMing assay. The initial amplification was performed in multiples of 50-µL PCR reactions, each containing template DNA equivalent to 250 µL of plasma or 3750 genome equivalents of stool DNA. Each reaction consisted of 5X Phusion high-fidelity buffer, 1.5 U of Hotstart Phusion polymerase (both NEB, Ipswich, MA), 0.2 µmol/L of each primer, 0.25 mmol/L of each deoxynucleoside triphosphate, and 0.5 mmol/L MgCl2. Nested PCR reactions were performed for selected target regions; for the second amplification, 2 µL of the first PCR was added to a 20-µL PCR reaction of the same makeup as described earlier except that different primers were used. Primer sequences and cycling conditions are listed in supplementary Table 2 (see supplementary material online at www.gastrojournal.org). PCR products were pooled, diluted, and quantified using the PicoGreen double-stranded DNA assay (Invitrogen, Carlsbad, CA). The BEAming procedure has been described previously10,11 and modifications used in the current study are described in the supplementary Materials and Methods section and supplementary Table 2 (see supplementary material online at www.gastrojournal.org).
An LSR II flow cytometry system (BD Bioscience, Franklin Lakes, NJ) equipped with a high-throughput autosampler was used for the analysis of each bead population. On average, 5 × 106 beads were analyzed for each sample. The flow cytometric data were gated so that only single beads with extension products (as indicated by the control probe) were used for analysis. The mutation frequency was calculated as the number of gated beads attached to mutant sequences divided by the number of beads containing either mutant or wild-type sequences. For an assay to be scored as positive, it had to meet 2 criteria. First, the fraction of mutant beads had to be higher than the background emanating from polymerase errors arising during amplification. We used a Poisson distribution to estimate the expected variation in the background observed with DNA templates derived from normal lymphocyte DNA. A positive assay was scored as one in which the fraction was higher than 0.01%. The second criterion was that the calculated number of mutant sequences in the templates used for analysis had to be 1 or greater. For example, if in a sample only 1000 genomic equivalents were analyzed, yet the calculated fraction of beads bound to mutant sequences was 0.05% (1 in 2000), this sample was scored as negative because the number of mutant template molecules was only 0.5 (0.05% × 1000), which is less than 1.
Results
Detection of Somatic Mutations by BEAMing
We assessed the performance of BEAMing for the detection of 33 different base changes in either APC (20), KRAS (4), PIK3CA (4), or TP53 (5). The BEAMing procedure was performed and the resultant beads analyzed via hybridization to allele-specific probes, as previously described.11 Representative results are shown in Figure 1. The hybridization was performed with equimolar concentrations of fluorescently labeled oligonucleotides complementary to the immobilized wild-type or mutant DNA sequences. Optimal allele discrimination for all 33 base changes was reached by an initial denaturation step followed by a slow cooling process in a tetramethylammonium chloride-based buffer.11 All mutations we attempted to assess (transitions, transversions, insertions, or deletions ranging from 1 to 5 bp) were detected successfully, with high signal-to-noise ratios, using this single tetramethylammonium chloride-based hybridization procedure.
An example of allele-specific hybridization applied to beads generated by BEAMing is shown in Figure 2. Positive control DNA populations were prepared using long oligonucleotides representing the genomic sequences, with the mutations in the center. Negative controls were prepared from DNA isolated from lymphocytes of healthy donors. Because no polymerase is completely error-free, mutations introduced during the initial amplification step create a small number of beads with mutant DNA sequences even when no mutant DNA templates are present in the sample DNA.12 In the current study, we used Phusion DNA polymerase (NEB) because it has been shown to have the lowest error rate of any commercially available enzyme tested.12 This background was determined individually for each mutation analyzed in the current study. The median background of mutations stemming from polymerase errors in normal lymphocyte DNA was 0.0009% (range, 0.01%−0.00013%). Variations in background rates were observed between and within genes, presumably reflecting the nonrandom nature of polymerase errors. Accordingly, an assay for a given sample was scored as positive for mutation only if the mutant fraction was higher than the background by a conservative and statistically significant margin (see Materials and Methods section). In addition, samples were scored as positive only if the calculated number of beads bound to mutant sequences was higher than a threshold defined by the genomic equivalents used in the assay, as also explained in more detail in Wood et al11 (also see supplementary materials online at www.gastrojournal.org).
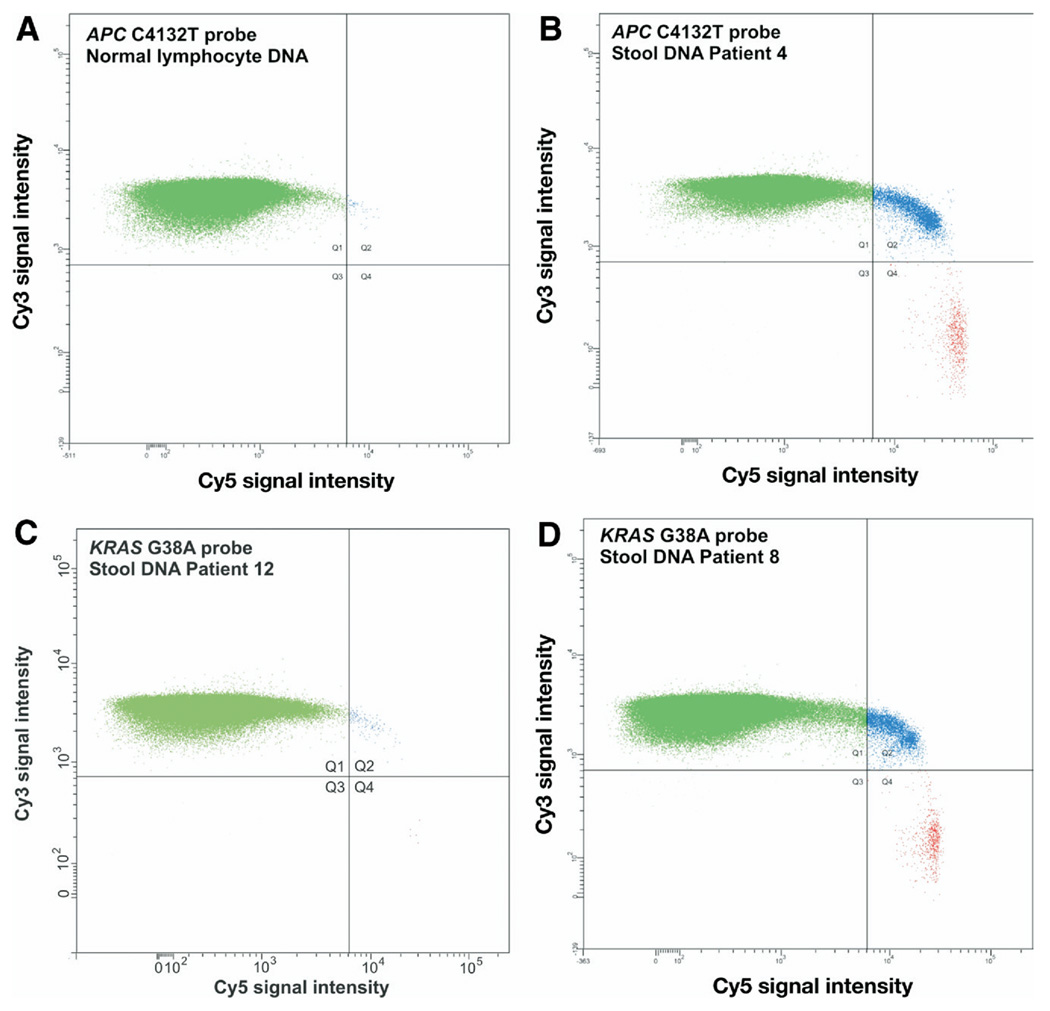
Figure 2.
Scatter plot of beads analyzed by flow cytometry. BEAMing assay for APC C4132T mutation using (A) normal lymphocyte DNA or (B) stool DNA from patient 4. For lymphocyte DNA the total number of beads analyzed (all quadrants) was 253,723, with no bead containing mutant DNA (red, quadrant 4). The total number of beads analyzed for patient 4 was 192,513, of which 747 were mutant. (C) BEAMing assay for KRAS G38A using stool DNA from patient 12, whose tumor did not contain this mutation. Five mutant beads were present among 305,449 analyzed beads, which were introduced by the DNA polymerase used for the initial amplification and scored as negative (see the Materials and Methods section). (D) Assay of stool DNA from patient 7 whose tumor did contain a KRAS G38A. A total of 333,630 beads were analyzed, of which 685 beads were mutant.
Quantity and Quality of the DNA Purified From Stool
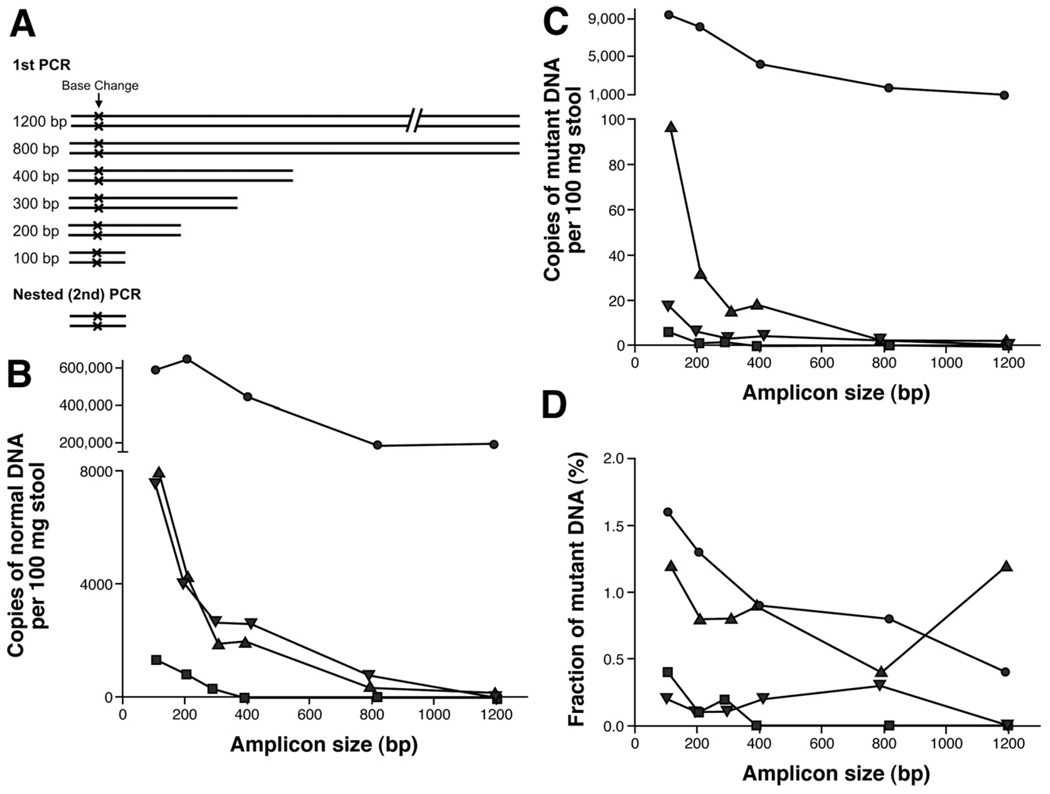
Because BEAMing cannot only be used to detect mutant DNA templates but also to precisely quantify their abundance, it could be used to determine both the quantity and quality of cell-free mutant and normal DNA present in the stool of CRC patients. We therefore began the current study by analyzing the sizes of the mutant DNA fragments present in the stool of CRC patients. For this purpose, 6 PCR primer sets were designed for the amplification of DNA fragments that encompassed different APC mutations found in 4 patients with localized CRCs. Two of the patients harbored stage I cancer and 2 patients harbored stage II cancer (supplementary Table 3 see supplementary material online at www.gastrojournal.org). The amplicon sizes obtained with these primers varied between 104 bp and 1197 bp, with the mutations located in the middle of each amplicon (Figure 3A). The DNA purified from an equal mass (181 mg) of stool was used in each assay. The number of mutant or normal template molecules was calculated by multiplying the respective fraction of beads bound to mutant or normal DNA sequences by the DNA concentrations measured by quantitative real-time PCR (see Materials and Methods section). In all 4 patients, the number of amplifiable normal DNA fragments decreased with increasing amplicon size. In patient 2, this decrease was only 3-fold, whereas the decrease was more severe–up to 1000-fold–in the other 3 patients (Figure 3B). The mutant DNA fragments decreased with size in a similar, but not identical, fashion (Figure 3C). As a result, the fraction of mutant DNA fragments was highest in the smallest amplicons (Figure 3D); in patients 4 and 14 we could not detect any mutant DNA fragments when the largest amplicon size (1200 bp) was used. These findings were important because they suggested that the sensitivity of tests for mutations in fecal DNA can be optimized by using small amplicons. Based on this result, all the BEAMing assays used in the subsequent phases of this study were performed with approximately 100-bp amplicons whenever possible, and never longer than 126 bp (supplementary Table 1; see supplementary materials online at www.gastrojournal.org).
Figure 3.
Quality and quantity of normal and mutant DNA isolated from stool of patients with CRC. (A) Experimental design. Stool DNA was amplified with differently sized primer pairs that encompass a patient-specific DNA mutation. Real-time PCR was used to determine the total number of stool DNA fragments obtained for each amplicon size. These amplified fragments subsequently were analyzed by BEAMing to determine the number of (B) normal and (C) mutant DNA fragments as well as the (D) fraction of mutant to normal molecules present in the feces (-●-, patient 2; -■-, patient 4; - ▲ -, patient 7; - ▼ -, patient 14).
Mutation Detection in Stool DNA by BEAMing
The clinicopathologic characteristics of the 25 patients included in this study are summarized in Table 1. Tumors ranged in size from 12–80 mm, with a mean size of 41 mm (median, 40 mm). Fourteen (56%) patients were early stage (stage I or II), 10 (40%) were late stage (stage III or IV), and 1 patient was of unknown stage. As outlined earlier, of the 24 patients for whom tumor tissue had been available, all had at least 1 mutation in the primary tumor (Materials and Methods section and supplementary Table 5; see supplementary material online at www.gastrojournal.org). For patient 25, for whom no tissue was available, 2 mutations were identified in stool DNA by the SBE assay (supplementary Table 5; see supplementary material online at www.gastrojournal.org).
Forty-five BEAMing assays were performed to assess the 33 different mutations in these samples (13 patients had at least 1 mutation found in another patient; Table 2). Of the 25 patients, 23 (92%; 95% confidence interval, 74%–99%) had detectable levels of mutant DNA in their stool samples. Mutations were detected as readily in patients with early stage colorectal cancers (stages I and II) as in patients with late-stage cancers (stages III and IV) (supplementary Table 4 see supplementary material online at www.gastrojournal.org). Interestingly, in 1 of the 2 patients in whom mutant DNA fragments could not be identified in the stool, the amount of normal DNA was very high (patient 5; see Discussion section).
The median fraction of mutant DNA present in stool samples was 0.32%, but varied widely (range, 0.0062%– 21.1%; Table 2). In most cases wherein 2 mutations could be assessed in the same stool sample, the fraction of mutant DNA molecules was similar. However, in 4 cases (patients 7, 8, 20, and 25) there was more than a 5-fold difference in the fraction of mutant DNA fragments from one gene compared with those in another gene. Possible reasons for this variability are outlined in the Discussion section.
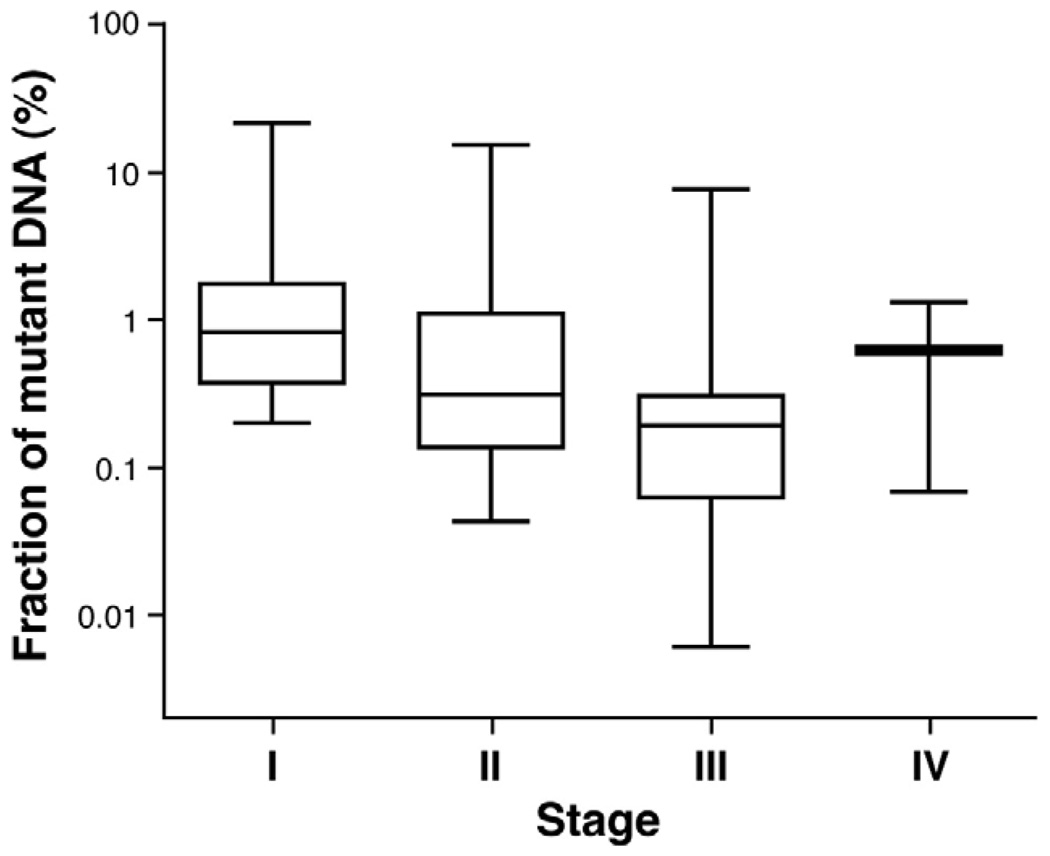
Another important observation was that the median fraction of mutant DNA fragments in stool samples did not vary significantly across the stage of the patient’s tumor: 0.83%, 0.31%, 0.20%, and 0.62% for stages I, II, III, and IV, respectively (Figure 4).
Figure 4.
Mutations in fecal DNA and TNM stage. The horizontal bar shows the median fraction of mutant DNA. The whiskers represent the minimum and maximum values that were found for each indicated stage.
Finally, it was of interest to compare the results of BEAMing assays in these stool samples with those obtained previously using a modified sequencing approach5 and SBE7 (supplementary Table 5; see supplementary material online at www.gastrojournal.org). Of the 25 patients assessed in the current study, these assays combined were able to detect at least one mutation in only 15 patients (60% of the 25 analyzed) whereas BEAMing detected 23 (92% of the same 25 patients). This difference was statistically significant (Table 2; P = .008, exact McNemar test). The SBE assay alone, which comprises the component of a commercially available DNA test that assesses 22 specific mutations in APC, TP53, and KRAS, performed about as well as the sequencing-based assay (60% [12 of 20] vs 56% [10 of 18]). Our data also revealed a potential basis for the lower sensitivity of the SBE and sequencing tests compared with BEAMing. Those mutations that were not detected with these tests constituted 0.11% ± 3.0% of the analyzed fragments. In contrast, those mutations that were detectable with SBE or sequencing were 9 times more abundant (median, 1.0% ± 5.0%).
Mutation Detection in Stool and Plasma DNA by BEAMing
Sixteen pairs of matched samples of blood and plasma were available for analysis. For each sample, one of the mutations found in the patients’ tumor was selected for analysis. As noted in Table 2, 14 of these 16 (87.5%) patients’ stool samples contained mutations at detectable levels. Mutant DNA fragments were found in a smaller proportion of the plasma samples (8 of 16 [50%]; P value for difference between the number of patients positive in the plasma and stool assays was .07 by the exact McNemar test). There was only 1 patient who was negative for both tests (patient 5) and 1 patient with a negative stool test but a positive plasma test (patient 16). In patients who scored positive, the median fraction of mutant DNA was similar in stool (0.37%) and plasma (0.42%).
Discussion
Though many previous studies have reported the presence of mutations in fecal DNA; this study analyzed them in a highly sensitive and quantitative manner. Other publications have reported the identification of genetic alterations in plasma or serum; we compared the results obtained with circulating DNA with those obtained with fecal DNA using identical techniques. The comparisons and quantifications reported here are important for guiding the development of sensitive and specific noninvasive screening tests for colorectal tumors in the future.
The quantitative analysis of fecal DNA highlighted several issues that are important for further research in this area. First, the highest sensitivities were realized when the amplicons were small, optimally less than 100 bp (Figure 3). This is undoubtedly owing to the DNA degradation that occurs either in cancer cells undergoing apoptosis or necrosis in situ or after they are released into the fecal stream. A similar size dependence for DNA mutation detection has been described in plasma.13 Note that this observation is not contradictory to studies showing that an increase in DNA integrity can be used as a marker for CRC.14 Mutant DNA present in the stool of cancer patients represents only a minor fraction (median, 0.32%; mean, 1.89%) of the total DNA and therefore has little influence on the measurement of the integrity of the total (mutant plus normal) DNA. The observed increase of DNA integrity in cancer patients is most likely caused by the release of larger DNA fragments from normal cells within the tumor environment into the fecal stream. Indeed, recent results have shown that cancers are infiltrated routinely with particular types of inflammatory cells that could contribute relatively large DNA fragments of normal sequence.15
Second, the results make it clear that a minimum number of DNA template molecules must be obtained to realize the sensitivity afforded by BEAMing. The sensitivity of BEAMing for any of the analyzed mutations is such that at least one mutant template can be detected among 10,000 normal templates (0.01%). For some mutations, the sensitivity is as high as one mutant template among 800,000 normal templates (0.0013%). The sensitivity is limited only by the error rate of the polymerase used in the initial amplification.12 The use of this high technical sensitivity in practice, however, requires an adequate number of DNA templates. For example, if only 2000 template molecules are used per assay, then the maximum sensitivity that can be achieved is 0.05% rather than 0.01%. Obtaining this number of templates is not problematic with stool samples, but often is problematic for plasma. In the current study, 2 mL of plasma contained a median of 4590 genome equivalents of DNA. This may be why the plasma-based assay was less sensitive (60%) than the stool-based assay (88%) in the same patients. To routinely obtain 30,000 genome equivalents from plasma (the number used for the stool-based tests), 50 mL of blood would be necessary. Although this may be feasible in future prospective studies, it is unlikely to be available in retrospective studies such as ours.
Although stool provides a nearly limitless supply of DNA, there are other technical issues that affect the assay results. For example, stool contains a variety of PCR inhibitors and a large excess of bacterial DNA, necessitating sequence-specific capture of human genomic DNA. Cost-effective methods for such capture have been developed and were used in the current study. However, they have not yet been optimized for the isolation of small DNA fragments that contain the mutations of interest. As shown in Figure 3, the sizes of normal and mutant DNA fragments corresponding to specific genetic regions is not necessarily the same. The fraction of mutant fragments as a function of size is likely to vary with the particular mutation in a patient-specific manner because it depends both on the source of the normal DNA as well as the extent of degradation of the tumor DNA fragments. This issue could have affected our results in 2 ways. First, it could be responsible for the wide variations among the fractions of mutant fragments observed within 2 different genes in 2 patients (eg, patient 7 in Table 2). Second, it could explain why we were unable to detect mutations in some patients. For example, 1 of these 2 patients (patient 5) had a very large number of normal fragments in his stool, more than 2-fold that of any other patient. Optimization of the capture probes could in the future increase sensitivity over and above the 92% obtained in the current study.
The new results also inform discussion of the relative advantages and disadvantages of stool vs plasma analysis for early detection. As noted earlier, it is easier to obtain sufficient amounts of DNA from stool than from plasma. However, plasma is more convenient to collect from a practical standpoint because it can be obtained during routine office visits, and it is easier to purify DNA from plasma than from stool. The sensitivity of detecting mutations in plasma from CRC patients (50%) is less than that in stool, but this perhaps could be increased by using more plasma in each assay. Perhaps the greatest advantage of stool vs plasma, however, is in the relative fractions of mutations observed in the feces of patients with different stage tumors. As shown in Figure 4 and Table 4, the fraction of mutations in stool of early stage patients was as high as that in late-stage patients. In contrast, our previous studies have shown that the fraction of mutations in the plasma of early stage patients is considerably lower than that of late-stage patients (not apparent in the current study because of the small numbers of patients with positive plasma samples).13 Furthermore, the situation is likely to be even more pronounced in patients with large adenomas because mutant DNA is much more difficult to detect in the plasma than in the stool of patients with these benign, but clinically significant, neoplasms.
Although our study represents a step towards clinical implementation of a new, more sensitive and quantitative assay than currently available commercially, several additional steps will be necessary to realize this goal. In addition to clinical studies using large numbers of patients with varying stages of colorectal tumors and equally large numbers of controls, there are still technical issues to be overcome. In particular, cost-effective methods for querying a panel of genetic markers with BEAMing must be developed. In this regard, it is notable that mutations in all 25 patients in the current study were revealed by the study of a relatively small number of common mutations. We envision that nearly 86% of patients with either CRC or large adenomas would harbor at least one of the 100 most common mutations. Implementation of such an assay would include parallel capture of approximately 10 exons and the subsequent multiplex PCR amplification of these DNA fragments. The newly described hybridization-based approach for mutation detection also has an advantage in that it can be easily automated. Next-generation sequencing has the potential to further simplify the approach; the beads obtained by BEAMing can be analyzed by sequencing rather than by flow cytometry.16 In addition, the mutation marker panel could be reduced in size by including epigenetic markers.17 Indeed, the lessons learned from the current study could be applied to optimize quantitative assays for methylation-based BEAMing or for any other tests for tumor-specific DNA variations that are developed in the future.
Supplementary Material
Acknowledgments
This work was supported by the Virginia and D.K. Ludwig Fund for Cancer Research, The Miracle Foundation, The National Colorectal Cancer Research Alliance, and National Institutes of Health grants CA43460, CA57345, CA62924, and CA129825. Under agreements between the Johns Hopkins University and Exact Sciences, Inc, Genzyme Molecular Oncology, Inc, and Beckman Instruments, Inc, Kenneth W. Kinzler and Bert Vogelstein are entitled to a share of the royalties received by the University on sales of products related to certain genes described in this article. The University and these authors also own stock in Exact Sciences, Inc, and Genzyme, Inc, which is subject to certain restrictions under University policy. The terms of these arrangements are being managed by the University in accordance with its conflict of interest policies. K.H.D. and K.J.M. are employees and shareholders of Exact Sciences Corp.
Abbreviations used in this paper
- CRC
colorectal cancer
- PCR
polymerase chain reaction
- SBE
single base extension
Footnotes
Supplementary Data
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi:10. 1053/j.gastro.2008.05.039.
References
- 1.Ouyang DL, Chen JJ, Getzenberg RH, et al. Noninvasive testing for colorectal cancer: a review. Am J Gastroenterol. 2005;100:1393–1403. doi: 10.1111/j.1572-0241.2005.41427.x. [DOI] [PubMed] [Google Scholar]
- 2.Osborn NK, Ahlquist DA. Stool screening for colorectal cancer: molecular approaches. Gastroenterology. 2005;128:192–206. doi: 10.1053/j.gastro.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 3.Olson J, Whitney DH, Durkee K, et al. DNA stabilization is critical for maximizing performance of fecal DNA-based colorectal cancer tests. Diagn Mol Pathol. 2005;14:183–191. doi: 10.1097/01.pas.0000176768.18423.7e. [DOI] [PubMed] [Google Scholar]
- 4.Whitney D, Skoletsky J, Moore K, et al. Enhanced retrieval of DNA from human fecal samples results in improved performance of colorectal cancer screening test. J Mol Diagn. 2004;6:386–395. doi: 10.1016/S1525-1578(10)60536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kann L, Han J, Ahlquist D, et al. Improved marker combination for detection of de novo genetic variation and aberrant DNA in colorectal neoplasia. Clin Chem. 2006;52:2299–2302. doi: 10.1373/clinchem.2007.070896. [DOI] [PubMed] [Google Scholar]
- 6.Dressman D, Yan H, Traverso G, et al. Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proc Natl Acad Sci U S A. 2003;100:8817–8822. doi: 10.1073/pnas.1133470100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itzkowitz SH, Jandorf L, Brand R, et al. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol. 2007;5:111–117. doi: 10.1016/j.cgh.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Kent Moore J, Smith JA, Whitney DH, et al. An electrophoretic capture method for efficient recovery of rare sequences from heterogeneous DNA. Biotechniques. 2008;44:363–374. doi: 10.2144/000112702. [DOI] [PubMed] [Google Scholar]
- 9.Rago C, Huso DL, Diehl F, et al. Serial assessment of human tumor burdens in mice by the analysis of circulating DNA. Cancer Res. 2007;67:9364–9370. doi: 10.1158/0008-5472.CAN-07-0605. [DOI] [PubMed] [Google Scholar]
- 10.Diehl F, Li M, He Y, et al. BEAMing: single-molecule PCR on microparticles in water-in-oil emulsions. Nat Methods. 2006;3:551–559. doi: 10.1038/nmeth898. [DOI] [PubMed] [Google Scholar]
- 11.Wood WI, Gitschier J, Lasky LA, et al. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985;82:1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Diehl F, Dressman D, et al. BEAMing up for detection and quantification of rare sequence variants. Nat Methods. 2006;3:95–97. doi: 10.1038/nmeth850. [DOI] [PubMed] [Google Scholar]
- 13.Diehl F, Li M, Dressman D, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci U S A. 2005;102:16368–16373. doi: 10.1073/pnas.0507904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boynton KA, Summerhayes IC, Ahlquist DA, et al. DNA integrity as a potential marker for stool-based detection of colorectal cancer. Clin Chem. 2003;49:1058–1065. doi: 10.1373/49.7.1058. [DOI] [PubMed] [Google Scholar]
- 15.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 16.Shendure J, Porreca GJ, Reppas NB, et al. Accurate multiplex polony sequencing of an evolved bacterial genome. Science. 2005;309:1728–1732. doi: 10.1126/science.1117389. [DOI] [PubMed] [Google Scholar]
- 17.Chen WD, Han ZJ, Skoletsky J, et al. Detection in fecal DNA of colon cancer-specific methylation of the nonexpressed vimentin gene. J Natl Cancer Inst. 2005;97:1124–1132. doi: 10.1093/jnci/dji204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.