Abstract
Intestinal ischemia-reperfusion (IR)3 injury is initiated when natural IgM antibodies recognize neo-epitopes that are revealed on ischemic cells. The target molecules and mechanisms whereby these neo-epitopes become accessible to recognition are not well understood. Proposing that isolated intestinal epithelial cells (IEC) may carry IR-related neo-epitopes, we used in vitro IEC binding assays to screen hybridomas created from B cells of unmanipulated wild type C57BL/6 mice. We identified a novel IgM monoclonal antibody (mAb B4) that reacted with the surface of IEC by flow cytometric analysis and was alone capable of causing complement activation, neutrophil recruitment and intestinal injury in otherwise IR-resistant Rag1−/− mice. Monoclonal Ab B4 was found to specifically recognize mouse annexin IV. Pre-injection of recombinant annexin IV blocked IR injury in wild type C57BL/6 mice, demonstrating the requirement for recognition of this protein in order to develop IR injury in the context of a complex natural antibody repertoire. Humans were also found to exhibit IgM natural antibodies that recognize annexin IV. These data in toto identify annexin IV as a key ischemia-related target antigen that is recognized by natural Abs in a pathologic process required in vivo to develop intestinal IR injury.
Keywords: Natural antibodies, Annexin, Ischemia Reperfusion, Inflammation, Complement
Introduction
Ischemia-reperfusion (IR)-induced injury is a pathologic process that occurs when the normal blood flow to an organ or tissue is interrupted for a period of time that is sufficiently long to result in marked hypoxia and ischemia, following which the recirculation of blood is restored. A central concept in this process is that the introduction of oxygenated blood during the reperfusion phase results in the development of more severe target organ injury than that caused by the ischemia per se (1,2). The precise mechanism of injury during the reperfusion phase is a subject of intense investigation, as no specific therapy exists at the present time for the treatment of this pathologic process that underlies common clinical events such as myocardial infarction and stroke (3–6). IR-induced injury is especially prominent in the intestine and is frequently followed by multiple organ dysfunction and infection as secondary complications (7–10).
With regard to the pathogenesis of this condition, complement activation and neutrophil infiltration are two key events that are required for experimental intestinal IR injury induced by ligation and subsequent release of the mesenteric artery, as both neutrophil depletion (11–13) and complement blockade (14–16) protect mice from the development of local tissue damage. Initiation of complement activation by the classical and lectin pathways has been demonstrated after IR of the heart, intestine, and skeletal muscle (15,17–19), although tissue injury also apparently requires the engagement of the alternative pathway amplification loop (20). Initial evidence that natural antibodies (Abs) are centrally involved in IR-induced injury came from seminal findings that Rag1−/− mice are resistant to the induction of IR injury to the intestine as well as other vascularized organs (19,21,22). The same mice, when reconstituted with IgM purified from natural Ab in pooled sera from wild type mice, become fully susceptible to IR-induced injury (19,23,24). As a related finding, Cr2−/− mice that lack expression of the B lymphocyte complement receptors 1 (CR1) and CR2 are also resistant to IR-induced injury, despite exhibiting normal quantitative levels of polyclonal IgM. Infusion of natural IgM and IgG antibodies from Cr2+/+ but not Cr2−/− mice, or transfer of peritoneal B cells from Cr2+/+ to Cr2−/− mice, restores intestinal IR injury (23,25). These data have suggested that there are specific IRrelated neo-epitopes against which Cr2−/− mice do not develop normal quantitative or qualitative levels of natural Abs.
Natural Abs are immunoglobulins that are produced in the absence of deliberate immunization, and they are a major component of the repertoire of B1 cells, which produce IgM and in some cases IgG Abs (26,27). B1 cells in the adult mouse are found primarily in the peritoneum and pleura (28). Natural Abs are frequently found to be polyreactive at low affinity with multiple self antigens (29,30), and they are considered as an important part of the innate immune system defense against infection. For example, natural Abs have been found to be protective against challenge with bacterial as well as viral pathogens, and to play an important role in the clearance of endotoxin (31–33). In addition, natural Abs play important roles in the recognition of apoptotic cells, oxidized low-density lipoprotein, and nuclear and cytoplasmic components released from damaged cells (34–36).
The possibility that Abs recognizing specific antigens, or subsets of antigens, could be identified to play essential roles in IR-induced injury was suggested by Fleming et al (21) in experiments where IgG monoclonal antibodies (mAb) against negatively charged phospholipids and beta-2-glycoprotein 1, as well as polyclonal antisera with high titers against the same antigens, were able to reconstitute mesenteric IR-induced intestinal and lung injury in Cr2−/− mice. Unlike Cr2−/− mice, though, reconstitution of IR tissue damage in injury-resistant Rag1−/− mice required the infusion of both anti-beta-2-glycoprotein 1 and anti-phospholipid IgG mAbs, or polyclonal serum-derived Abs. Another Ab system important in IR injury has been shown to involve natural Abs recognizing an epitope on non-muscle myosin and glycogen phosphorylase (37,38). In contrast to studies by Fleming et al, an IgM mAb (designated CM22) recognizing these two antigens was found to be capable alone of inducing mesenteric and skeletal muscle IR injury in Rag1−/− mice, and a peptide mimic of the antigen also blocked in vivo the IR injury of intestine and skeletal muscle in wild type mice (22,37,38).
Annexin IV belongs to a family of proteins that are Ca2+- and phospholipid-binding proteins (39,40). The structure of annexins consists of a conserved Ca2+ and membrane binding core of four annexin repeats (eight for annexin VI) and variable N-terminal regions (41). Annexins are soluble cytosolic proteins, but despite the lack of obvious signal sequences and the apparent inability to enter the classical secretory pathway, annexins have been identified in extracellular fluids or associated with the external cell surface through poorly understood binding sites (40,42–44). Annexin IV is predominantly produced by epithelial cells and is also found at high levels in lung, intestine, pancreas, liver, and kidney. Depending on the cell type, annexin IV has been found either along the basolateral, basal or apical domains of the plasma membrane, and in some cell types it has been found to be present throughout the cytoplasm (45–47). With regard to its function, annexin IV has been shown to inhibit the epithelial calcium-activated chloride ion conductance (48), to play a role in the formation of pronephric tubules (49) and to regulate the passive membrane permeability to water and protons (50). Upregulation of annexin IV has been found in renal cell carcinoma (51,52). Finally, surface membrane expression of annexin IV has also been recognized as an early marker for apoptotic cell death (53,54).
Herein we report the identification of a novel pathogenic IgM mAb that is capable of inducing intestinal IR injury in Rag1−/− mice. The mAb was found to specifically recognize mouse annexin IV. Importantly, we also found that normal mouse sera contain natural Abs to annexin IV epitopes and that treatment of wild type C57BL/6 mice with recombinant annexin IV before the reperfusion phase prevents IR-induced intestinal injury. We propose that binding sites on annexin IV are essential neo-epitopes that are expressed on ischemic tissues and are targets for natural Ab binding during the reperfusion phase, with subsequent complement activation, neutrophil recruitment and tissue injury.
Materials and Methods
Mice
Adult male Rag-1−/− mice were obtained from Jackson Laboratory (Bar Harbor, ME) and maintained following shipment for at least a 7-day acclimation period in the Uniformed Services University for the Health Sciences animal facility. Adult male and female Cr2+/+ and Cr2−/− mice were maintained and bred as two sublines at UCDHSC as previously described (23). Animal studies at sites were approved by the local institutional review board. Human studies were approved by the Colorado Multi-Institutional Review Board.
Antibodies and reagents used for analysis
Biotinylated and FITC-conjugated goat anti-mouse IgG (Fcγ specific) or anti-mouse IgM (μ-chain specific) SA-FITC and SA-PE were obtained from Jackson ImmunoResearch Laboratories. Alkaline phosphatase-conjugated goat anti-mouse Ig Ab was obtained from Caltag Laboratories. Polyclonal rabbit anti-annexin IV Ab was obtained from ProteinTech Group, and anti-6xHis mAb was obtained from Novagen. Synthetic peptides were obtained from Synthetic Biomolecules.
Development and purification of IgM mAbs
Monoclonal Abs B4 and D5 were developed by the fusion of peritoneal, lymph node and spleen cells from unmanipulated wild-type C57BL/6 mice with the SP2/0-AG14 myeloma cell line by the standard protocol to establish hybridomas. Successful fusions were screened by Western blot analysis using IEC lysates and by flow cytometric analysis of isolated IEC. To purify mAbs, Ab from the exhausted supernatants of cultured B4 and D5 hybridomas was affinity purified on a column of agarose beads with goat anti-human IgM (Sigma-Aldrich). Bound Ab was eluted with a buffer containing 0.1 M glycine, pH 2.3, and collected into a buffer containing 1.5 M Tris, pH 8.8. Eluted mAb was dialyzed against PBS, pH 7.4, for 48 h and concentrated using centrifugal filtration on Centricon Plus-20 (Millipore). Ab concentration was determined by measuring the A280 of the sample, and purity was confirmed by analysis on a 10% SDS-PAGE gel.
IEC isolation and flow cytometric analysis
Isolation of IEC was performed using previously described methods (55,56). Briefly, after dissection of the mouse intestine into small pieces, the latter were incubated twice in HBSS with 1 mM DTT and 1 mM EDTA for 30 min at 37°C with shaking to detach IEC. By this method of isolation the cell mixture consists of 93–95% IEC, with less then 2% intra-epithelial lymphocyte (IEL) contamination 55. IEL can be readily gated out in time of flow cytometric analysis by size characteristics. Detached free cells and intact crypts were centrifuged and re-suspended in HBSS containing Ca++ and Mg++, or in 5% FBS DMEM culture media. Isolated IEC were washed in the staining buffer (2% FCS/0.01% NaN3/PBS), resuspended in the staining buffer containing hybridoma supernatant or pure mAb, and incubated for 30 min at room temperature (RT). After incubation, cells were washed in the staining buffer three times and then incubated with the secondary goat anti-mouse IgM (μ-chain specific) Abs (Jackson ImmunoResearch Laboratories) for 30 min at RT. Following incubation, cells were washed as above described and then resuspended in the staining buffer. Flow cytometry was performed using a BD Biosciences FACSCalibur (Oxford, U.K.).
Western blot analysis
IEC were lysed on ice for 20 min in a buffer containing 0.5% Triton X-100, 0.5% Chaps, 20 mM Tris-HCl (pH 7.5), 1 mM EDTA, 10 μg/ml leupeptin and protease inhibitor cocktail (Roche Molecular Biochemicals). Lysates were cleared by centrifugation at 8000×g for 5 min. Following separation by 8% Tris-glycine SDS-PAGE, the proteins were transferred to a PVDF membrane. The membrane was blocked overnight with 5% non-fat milk dissolved in PBS. The membrane was washed in PBS and then probed with a primary Ab for 1–2 hours in 2% milk/PBS, washed and then incubated with horseradish-peroxidase-conjugated secondary Abs. A positive signal was visualized using the ECL system (Perkin Elmer)
Intestinal IR injury
The intestinal IR injury model was performed as previously described (23) by the surgical procedure of opening the abdominal cavity of an anesthetized mouse and occluding the superior mesenteric artery for 30 min, followed by removal of the clamp and 2 h reperfusion of the tissue. Ketamine/xylazine was used for anesthesia.
Histology and immunohistochemistry
Immediately after euthanasia, segments of small intestine specimens were fixed in 10% buffered formalin, embedded in paraffin, cut transversely in 5-μm sections and stained with Giemsa. Mucosal injury was graded on a six-tiered scale in a blinded manner, as described previously (23). Briefly, the average of villus damage in an ~2 cm intestinal section (50–100 villi) is determined after grading each villus in the section on a 0–6 scale. A score of 0 is assigned to a normal villus; a score of 1 to villi with tip distortion; a score of 2 is assigned when in addition goblet cells and Gugenheims’ spaces are missing; a score of 3 is assigned to villi with patchy disruption of the epithelial cells; a score of 4 is assigned to villi with exposed but intact lamina propria with epithelial cell sloughing; when the lamina propria is exuding a score of 5 is assigned; and lastly, a score of 6 is assigned to villi that display hemorrhage or to villi that are denuded. Additional tissue sections were fixed for 2 h in cold 4% paraformaldehyde in PBS before transfer to PBS for paraffin embedding and preparation of transverse sections. Following removal of paraffin from sections, nonspecific Ab binding sites were blocked by treatment with a blocking solution (DAKO) for 30 min. After washing in PBS, the tissues were incubated with goat anti-mouse C3 (Cappel) or appropriate control IgG (Jackson ImmunoResearch Laboratories) Ab overnight at 4°C. The primary antibody was detected with a biotinylated donkey anti-goat Ig (Jackson ImmunoResearch Laboratories), then SA-HPRO (Vector laboratories), and developed with Vector Nova Red Substrate (Vector Laboratories). For immunohistochemistry tissues were fixed at 4°C for 2 h in 4% paraformaldehyde in PBS. Nonspecific Ab binding sites were blocked by the incubation with a blocking solution PBS for 30 min. After washing in PBS, the tissues were incubated with isotype control Ab or FITC-conjugated anti-mouse C3, TRITC-conjugated anti-mouse Ig and DAPI to visualize the nucleus. After washing, the slides were mounted using ProLong Gold antifade reagent with DAPI and analyzed on an Olympus AX80 microscope.
Eicosanoid determination
The ex vivo generation of eicosanoids in small intestine tissue was determined using a previously described method (16,23). Briefly, sections of minced fresh mid-jejunum were washed and resuspended in 37°C oxygenated Tyrode’s buffer (Sigma, St. Louis, MO). After tissues were incubated for 20 min at 37°C, supernatants and tissue were collected and stored at −80°C until assayed. The concentrations of LTB4 and PGE2 were determined using an enzyme immunoassay (Cayman Chemical, Ann Arbor, MI). The tissue protein content was determined using the bicinchonic acid assay (Pierce, Rockford, IL) adapted for use with microtiter plates. Leukotriene B4 (LTB4) and prostaglandin E2 (PGE2) levels were expressed in pictograms per mg protein per 20 min.
Myeloperoxidase activity (MPO)
Supernatants generated for the eicosanoid assays were also used to determine peroxidase activity by measuring oxidation of 3,3′,5,5′-tetramethylbenzidine as described previously (16). Briefly, supernatants were incubated with equal volumes of 3,3′,5,5′-tetramethylbenzidine peroxidase substrate (Kirkegaard & Perry, Gaithersburg, MD) for 45 min. The reaction was stopped by the addition of 0.18 M sulfuric acid, and the OD450 was determined. The concentration of total peroxidase was determined using HRP (Sigma-Aldrich) as a standard and plotted as picograms of MPO activity per milligram of tissue.
Purification of IEC protein reactive with mAb B4
To purify protein reactive with mAb B4 for identification, a three-step purification procedure based on the method described by Vossennar and colleagues (57) was performed. In the first step, IEC lysates were resolved using preparative 8% SDS–PAGE. Two lanes from the gel were cut. The first lane was transferred to a membrane and probed with mAb B4, and the second lane was stained with Coomassie brilliant blue to localize precisely the position of the proteins. The rest of the gel was stained with a non-fixing gel stain GeBA SeeBand stain (Gentaur, Belgium) and protein-containing areas were excised and stored at 4°C. In the next step the gel strips were washed in a freshly prepared, warm (37°C) washing solution (2 mM Tris–HCl (pH 8.0), 8 M urea, and 1% NP-40). The washed gel strips were then loaded on an isoelectric focusing (IEF) gel (6 M urea, 1% NP-40, 15% (of total volume) acrylamide mix (39:1), 2% ampholytes (3–10), 0.47 μg/ml ammonium persulfate, 0.66 μl/ml TEMED). IEF was performed on a gel (15×15 cm) overnight at 200 V. The upper and lower buffers were 0.09 M NaOH and 0.85% phosphoric acid, respectively. Immediately after migration, the gel was fixed in the 20% trichloroacetic acid (TCA) for 20 min and then stained with Coomassie brilliant blue. Regions containing proteins in the IEF were excised and separated by 8% SDS-PAGE. Protein was localized by staining with GeBA SeeBand stain, and areas of interest were excised and used for identification of proteins using mass spectrometry (University of Chicago). A parallel SDS-PAGE was performed and, after transfer to PVDF membrane, Western blot analysis was performed to confirm the location of the antigen in the gel.
Protein identification by mass spectrometry
After separation the protein was digested in-gel with trypsin according to a modified protocol (University of Chicago). The aqueous peptide extract (10 μl) was analyzed using electrospray liquid chromatography MS (LC/MS/MS). An HPLC instrument (Agilent) was connected with an XCT ion trap mass spectrometer (Agilent). Sample was loaded automatically at 10 μl/min. Chromatography buffer solutions (buffer A, 2.5% methanol and 0.1% formic acid; buffer B, 99.9% acetonitrile and 0.1% formic acid) were used to make a 90-min gradient (8 min to 10% buffer B, 32 min from 10%–45%, hold 5 min, 5 min from 45%–90%, hold for 20 min, then 5 min to 0% B, hold for 15 min. A flow rate of 0.25 μl/min was used. The MASCOT program was used to search the mouse protein sequence database. Probability based mouse score (−10*log P) was used for protein identification, where P is the probability that the observed match is a random event. Individual ion scores higher than 40 indicate identity or extensive homology (p<0.05).
Annexin IV constructs
The original cDNA sequence of mouse annexin IV was taken from Invitrogen clone # 4947415 placed into the pET-32 Xa/LIC bacterial expression vector (Novagen). To accomplish this task, cDNAs encoding for annexin IV were amplified by PCR. The forward primer used was: 5′-GGT ATT GAG GGT CGC ATG GAA GCC AAA GGA GGA AC -3′. The reverse primer was: 5′-AGA GGA GAG TTA GAG CCT TAA TCA TCT CCT CCA CAG AGA ATG -3′. The ligation-independent cloning (LIC) was done in pETXa/LIC vector. The vector adds a Trx-Tag, His-Tag, and S-Tag to the N-terminus of the protein. These tags are cleavable with Factor Xa leaving a native version of the N-terminus. To express protein in mammalian cells the forward and reverse primers were 5′ CTG GTA CCA GCA TGG AAG CCA AAG GAG -3′ and 5′ TCT CGA GAA TCA TCT CCT CCA CAG AGA ATG-3′ respectively. The primers add restriction sites Acc65I [or Kpn I] to the start of annexin IV and Xho I restriction site to the end of annexin IV. The KpnI/XhoI fragment was amplified by PCR from genomic DNA using Pfu polymerase (Novagen). The KpnI/XhoI fragment was subsequently cloned into pSecTag2/Hygro B expression vector (Invitrogen) and expression of the protein was accomplished in the F-293 cell line (Invitrogen). The vector adds an Ig k-chain leader sequence to the N-terminus of the protein for secretion. After leader peptide is cleaved approximately 15 amino acids are left on the N-terminus of the protein. The vector adds also a myc epitope and a 6xHis tag to the C-terminus of the protein.
Expression and purification of recombinant annexin IV
The expression construct pETXa/LIC-A4 was transformed into E. coli Rosetta 2 (DE3) cells. Bacterial expression cultures were incubated at 37°C in LB medium containing ampicillin (50μg/mL) until an A600 of 0.6 was reached. Recombinant protein expression was induced by an addition of IPTG (Sanland-Chem) to a final concentration of 0.3 mM. Following 6 h incubation at 32°C, bacteria were harvested by centrifugation at 10,000×g for 10 min at 4°C. After harvesting the cells, they were resuspended in PBS with Complete, EDTA-Free Protease Inhibitor Cocktail Tablets (Roche Molecular Biochemicals). Bacteria were lysed by 4 freeze-thaw cycles. The lysate was then incubated with DNase and RNase for 30 min. Cell lysate was then centrifuged (10,000×g for 40 min) and cell pellet was resuspended in 6 M urea for 30 min. Centrifugaton by 10,000×g for 40 min was then used to remove undissolved debris. The pre-cleared supernatant was diluted (2:1) by PBS, its pH adjusted to 7.6 with NaOH and then applied to a TALON resin column (Clontech Laboratories) equilibrated with 4 M urea. Bound protein was refolded on the column using a discontinuous gradient from 4 M to 0.25 M urea, starting with the equilibration buffer and finishing with a buffer containing 10 mM imidazole in PBS pH 7.0. The refolded protein was eluted with a buffer containing a stepwise gradient of 19, 38, 75, and 150 mM imidazole. The presence of the protein and its purity was confirmed by SDS-PAGE and staining with Coomassie blue.
Annexin IV ELISA
Immulon 1B plates (Dynatech) were coated with 5 μg/ml of annexin IV purified protein in PBS. Wells were blocked with 1% BSA in PBS. Serial dilution of serum samples were made in blocking buffer and samples were applied to wells. After incubation and washing, bound Abs were detected using AP-conjugated anti-mouse IgG (Fcγ specific) or anti-mouse IgM (μ-chain specific) Abs, followed by p-nitrophenyl phosphate (Sigma-Aldrich) at 1 mg/ml. Plates were read at 405 nm.
Results
Generation of monoclonal antibodies
As a strategy to identify mAbs that would recognize neo-epitopes on ischemic tissues, we hypothesized that intact intestinal epithelial cells (IEC), when isolated as a single cell suspension, might expose on the surface the same neo-epitopes that are targets on the ischemic cells for pathogenic IgM Abs in vivo during intestinal IR. Consistent with this, in pilot experiments we found that whole serum from Cr2+/+ mice exhibited higher binding of immunoglobulins to IEC than Cr2−/− mice (data not shown). Based on this hypothesis, we used freshly isolated IEC to screen hybridomas obtained by fusion with the SP2/0-AG14 myeloma cell line of B cells from wild type unmanipulated C57BL/6 mice derived from peritoneum, lymph nodes and spleen. Wells were chosen for further subcloning based on the positive surface staining of IEC by flow cytometry or reactivity by Western blot analysis on tissue lysates. Cells were then serially re-cloned in order to obtain monoclonal cell lines stably producing a single mAb.
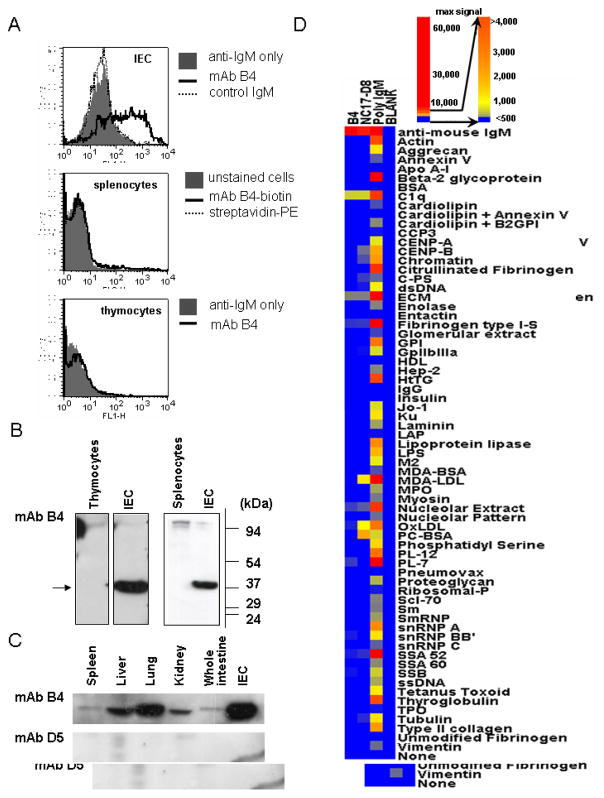
One such product of this strategy, designated mAb B4, was obtained from a fusion with spleen cells and is an IgM kappa isotype Ab. As shown using flow cytometric analysis, mAb B4 binds a surface epitope on IEC but not on freshly isolated splenocytes or thymocytes (Fig. 1A). By Western blot analysis, mAb B4 recognizes a protein with a molecular weight of 37 kDa in IEC lysates but not lysates from freshly isolated splenocytes or thymocytes (Fig. 1B). When other tissue lysates were probed by Western blot with mAb B4, the epitope was found to be widely distributed, with the highest relative expression in lung and isolated IEC (Fig. 1C). As an isotype control we used mAb D5 directed to an epitope on mouse cytokeratin 19 (our unpublished data). The results shown in Fig. 1C demonstrate that when the whole spleen was taken to make lysates, a weak band corresponding to the B4 antigen is seen, and a band of the same size was seen in lysates from whole thymus (data not shown). These data suggest that the mAb B4 epitope is expressed in non-lymphoid stromal cells, perhaps of epithelial origin, in these tissues.
Figure 1.
Characterization of mAb B4 epitope expression. A, Representative flow cytometric histograms show binding of mAb B4 to a single cell suspension of IEC (top), splenocytes (middle) and thymocytes (bottom). Cells were incubated with mAb B4, and bound Ab was detected by using anti-mouse IgM (μ-chain specific) for IEC and thymocytes. In the case of splenocytes, mAb B4 labeled with biotin was used. B and C, Monoclonal Ab B4 epitope expression was determined by Western blot analysis of isolated from thymus, spleen or intestine cell in (B) or lysates were prepared from whole organs in (C). Data are representative of two independent experiments. D, Binding of mAb B4 to proteins typically found as targets of polyreactive natural Abs was studied by micro-array analysis. The positive control for mAb B4 binding are C1q and anti-mouse IgM antibody, positive controls for the micro-array are mAb NC-17D8 and polyclonal IgM, and the negative control is designated blank.
To determine whether mAb B4 is typical of polyreactive natural Abs or has a more restricted reactivity, micro-array analysis was performed using a series of antigens typically found to be targets of this class of Abs (29,30). The accession number for the data is GSE14862; the data can be accessed on the website http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14862. Positive controls for the assay were mAb NC17-D8 that recognizes phosphatidyl choline (58,59) and polyclonal mouse IgM. As shown in Fig. 1D, mAb B4 demonstrates only minimal reactivity with a subset of antigens that is far below that demonstrated by mAb NC17-D8 or polyclonal IgM. Monoclonal Ab B4 also does not recognize negatively charged phospholipids when using ELISA analysis (see Fig. 5 below). Thus, mAb B4 does not appear to be typical of the polyreactive subset of natural Abs.
Figure 5.
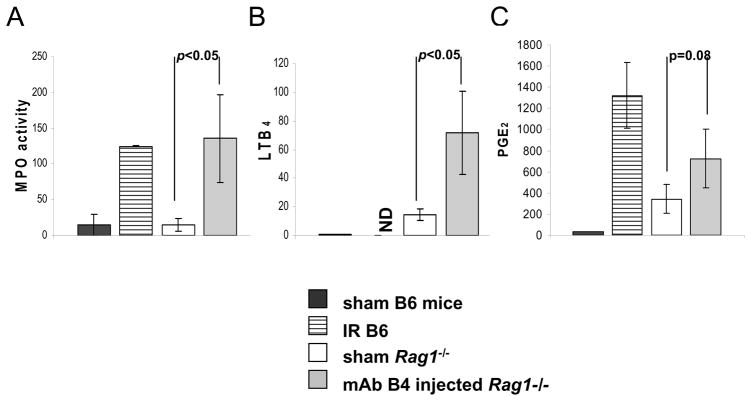
Association of neutrophil infiltration and eicosanoid generation with IR in Rag1−/− mice treated with mAb B4. Intestinal tissue from sham operated wildtype C57Bl/6 mice (black bar), wildtype mice undergoing IR (horizontally stripped bar), or sham operated Rag1−/− mice (unfilled bar), and Rag1−/− mice pre-injected with mAb B4 and undergoing IR (gray bar) were collected. The concentrations of MPO (left), LTB4 (middle) and PGE2 (right) were determined ex vivo using enzyme immunoassays. The data are presented as picogram per milligram of tissue for LTB4 and PGE2, and as MPO activity. Each bar is the average ± SEM with four to six animals per group. Statistical significance was determined using an unpaired 2-tailed Student’s t test. ND: data not obtained.
Monoclonal Ab B4 restores IR injury in Rag1−/− mice
To determine whether mAb B4 exhibited the desired characteristic of induction of intestinal IR injury, we used Rag1−/− mice that are normally protected from IR injury (19). Purified mAb B4 or control IgM mAb D5 was injected i.v. 60 min prior to the reperfusion phase, and the intestines from mice undergoing IR were examined for injury.
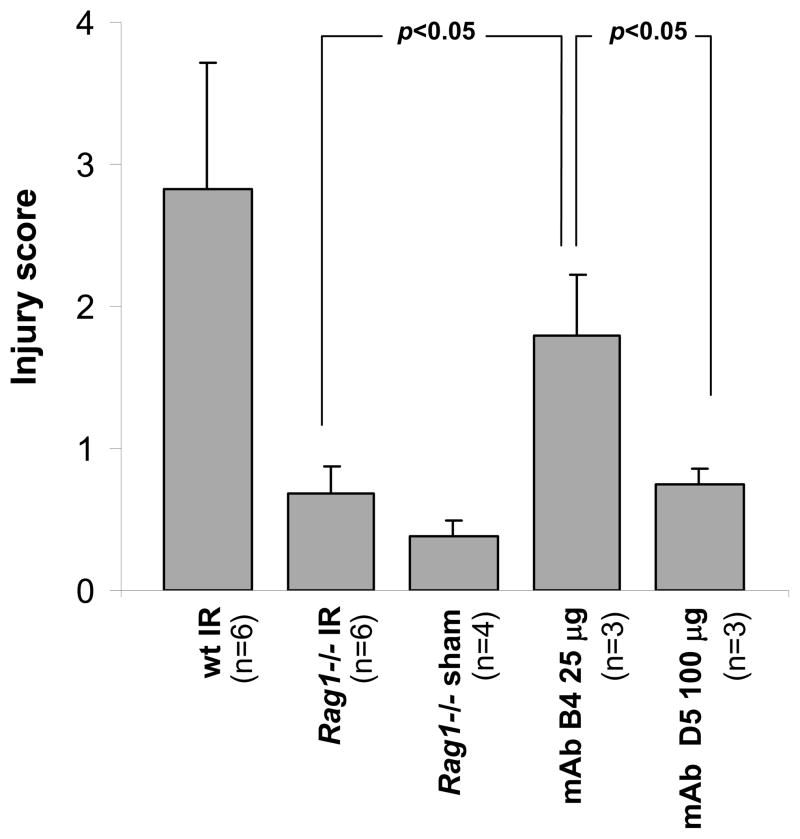
Analysis of intestinal injury revealed that the sham treated Rag1−/− mice as well as Rag1−/− mice that underwent the IR protocol did not show significant damage as compared to wild type C57BL/6 mice that demonstrated an injury score of 2.82 ± 0.9 (Fig. 2). In contrast, Rag1−/− mice injected with 25 μg of mAb B4 antibody demonstrated an injury score of 1.8 ± 0.42. In contrast to the effects of mAb B4, the isotype control mAb D5, generated during the same screening protocol, did not cause IR injury in Rag1−/− mice even at a dose of 100 μg per mouse (score 0.75 ± 0.09). In other experiments, treatment of mice with doses of mAb B4 ranging from 9 to 81 μg also led to significantly increased IR injury (data not shown), and thus there is not a narrow dose-response interval for its biologic effect.
Figure 2.
Restoration of IR injury in Rag1−/− mice by mAb B4. Sixty min prior to the induction of ischemia by mesenteric artery occlusion, 25 μg of purified mAb B4, or 100 μg of control IgM mAb D5, was injected i.v. Following completion of the IR protocol, mice were sacrificed and Giemsa-stained intestinal sections from each treatment group were scored for mucosal injury (0–6) as described in Materials and Methods. Monoclonal Ab B4 injected into Rag1−/− mice induced substantial injury in the mice undergoing IR (mAb B4 IR) in contrast to sham operated mice (Rag1−/− sham) or Rag1−/− mice undergoing IR (Rag1−/− IR). MAb D5 treated Rag1−/− mice (mAb D5 IR) did not demonstrate substantial injury. As a positive control for the level of intestinal injury, wildtype C57Bl/6 mice undergoing IR were included (wt IR). All measurements were obtained at x200 magnification. The figure is a representative of two independent experiments. Each bar is the average ± SEM with 3–6 animals per group. Statistical analysis was performed by Student’s t test.
Monoclonal Ab B4 recognizes annexin IV
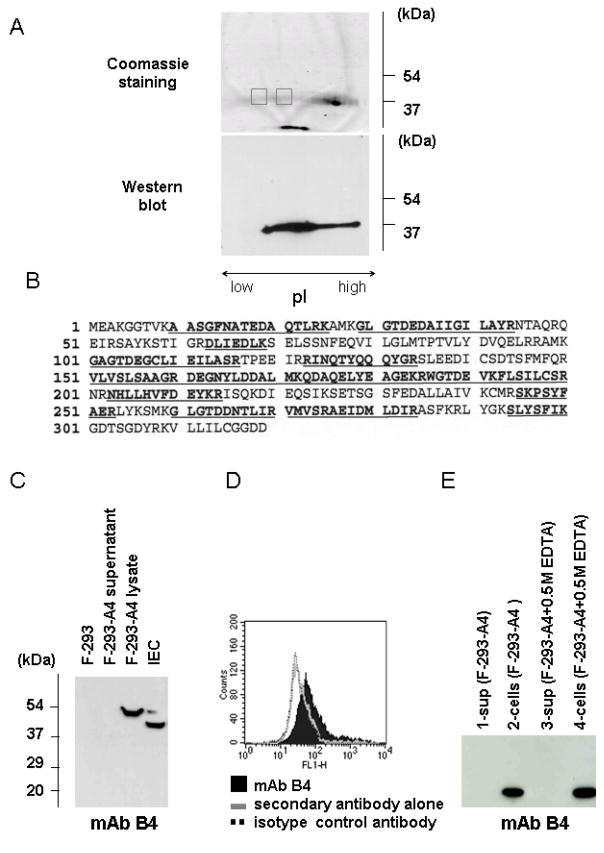
The finding that mAb B4 was able to mediate intestinal IR injury in Rag1−/− mice led us to perform further studies and identify the protein specifically recognized by this mAb on IEC. We used a multi-step process described in Materials and Methods which culminated in a two-dimensional gel separation of the proteins according to their molecular weight and charge, following which the protein reactive with mAb B4 was characterized by proteolysis and mass spectroscopic (MS) analysis (Fig. 3). Of note, the isoelectric focusing gel, wherein the separation of proteins occurs based on charge, revealed that the protein of interest formed a long smear, thus making it difficult to localize it in a single spot (Fig. 3A). To overcome the problem we cut two separate areas from the gel and analyzed the proteins in each of these areas, considering that the same protein would be found in each section. MS analysis of fragmented protein in the two areas identified the same protein recognized by mAb B4 as mouse annexin IV (Fig. 3B). Consistent with this result, using commercial polyclonal antibodies to annexin IV we were able to determine that anti-annexin IV antibodies recognize the same extended band on the isoelectric focusing gel to which mAb B4 binds (data not shown).
Figure 3.
Identification of the antigen recognized by mAb B4. A, Lysates prepared from isolated IEC were separated using a sequential three gel separation protocol as described in Materials and Methods. The presence of the B4 antigen was confirmed by Western blot analysis (right). Two gel samples (gray color boxes) were analyzed by MS. B, The MASCOT search results for the protein isolated from first sample was identified as mouse annexin IV based on the highest score number of 785; 78 matched peptides covering 53% of the annexin IV sequence were identified (underlying bold letters). C, Lysates of untransformed F-293 cells (lane 1) and F-293 cells transformed with the pSecTag2/Hygro B expression vector carrying annexin IV insert (F-293-A4) (lane 3) together with culture supernatants from transformed cells (lane 2) were probed by Western blot analysis with mAb B4. D, Flow cytometric analysis of F-293 cells expressing recombinant annexin IV. F-293-A4 cells were probed in flow cytometry with mAb B4. E, Supernatant (lane1) and lysate (lane 2) from F-293-A4 cells expressing recombinant annexin IV that had been incubated in PBS alone, or supernatant (lane 3) and lysate (lane 4) from the same cells incubated with 0.5 M EDTA, were probed by Western blot analysis with mAb B4. Recombinant annexin IV was not released from F-293-A4 cells in the absence of free Ca2+.
Monoclonal Ab B4 recognizes recombinant annexin IV expressed in mammalian cells
To confirm that murine annexin IV is the protein recognized by mAb B4, we expressed recombinant annexin IV in mammalian and bacterial cell systems. When recombinant annexin IV was expressed in the human F-293 cell line using the pSecTag2/Hygro B expression vector, Western blot analysis showed that the recombinant annexin IV protein expressed was recognized by mAb B4 (Fig. 3C). The presence of annexin IV on the Western blot was confirmed by probing the membrane with polyclonal anti- annexin IV and anti-6xHis antibodies (data not shown). We also performed flow cytometric analysis of transfected F293 cells to show that mAb B4 recognizes cells expressing recombinant murine annexin IV (Fig. 3D).
When transfected F-293-A4 cells were probed with mAb B4 using flow cytometric analysis, the protein was found to be localized on the surface of the cells (Fig. 3D). These results are consistent with the known localization of annexin IV whereby the protein is found to be closely associated with the plasma membrane. Previous results have suggested that annexin proteins may bind to the plasma membrane through a Ca2+-dependent mechanism (41,60). However, when the recombinant annexin IV-expressing F-293-A4 cells were incubated in a buffer containing 0.5 M EDTA, immunoreactive protein was not released from the cell surface (Fig. 3E). These data correlate with the observation that IEC isolated in EDTA-containing buffer display the mAb B4 epitope on the surface of IEC as detected by flow cytometric analysis. In sum, several lines of evidence using mammalian recombinant protein expression methods confirm that mAb B4 specifically recognizes mouse annexin IV.
Monoclonal Ab B4 does not recognize antigens previously shown to be targets for pathogenic antibodies in intestinal IR injury
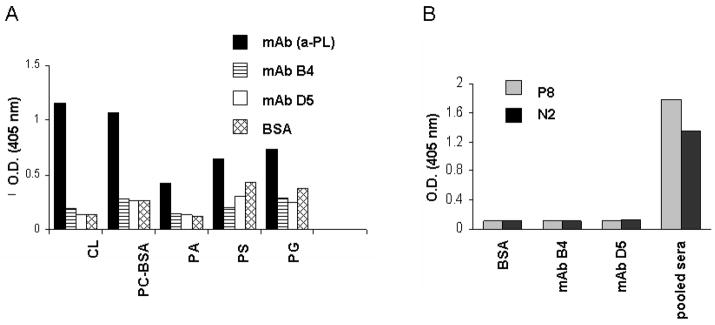
Two classes of antigens have previously been suggested as candidates for IR-related neo-epitopes and as targets for pathogenic natural antibodies. Although mAb B4 was found to recognize annexin IV, we wanted to specifically determine if it would also cross-react with these other antigens. First, since it had been shown previously that Cr2−/− and Rag1−/− mice reconstituted with Abs recognizing negatively charged phospholipids demonstrated IR injury (21), we used an anti-phospholipid Ab ELISA to determine if mAb B4 would recognize this class of antigens. We were unable to detect any binding of mAb B4 to the same phospholipids recognized in previous studies (Fig. 4A). Second, as reported by the Carroll group, the CM22 IgM mAb (37,38) that is capable of inducing IR injury in Rag1−/− mice recognizes a synthetic peptide (designated N2) derived from one of its antigens, non-muscle myosin, or a mimicking peptide (designated P8) that was identified from a phage display library. When the same synthetic peptides were probed in ELISA for binding by mAb B4, no detectable signal was apparent, in contrast to pooled sera from wild type C57BL/6 mice that demonstrated robust binding to both peptides (Fig. 4B). Thus, mAb B4 does not appear to cross react with previously described antigens or epitopes important in this process.
Figure 4.
Monoclonal Ab B4 does not cross-react with previously published antigens recognized by pathogenic natural Abs in intestinal IR injury. A, Monoclonal Ab B4 was tested in an anti-phospholipid Ab ELISA for the binding to cardiolipin (CL), phosphorylcholine-BSA (PC-BSA), phosphatidic acid (PA), phosphatidylserine (PS), and phosphatidylglycerol (PG). A broadly reactive IgM anti-phospholipid mAb (our unpublished data) was used as a positive control. Bound Abs were detected by AP-conjugated goat anti-mouse IgM. Data are shown as OD405nm. B, Bar graph shows the lack of reactivity of mAb B4 in ELISA with synthetic peptides reported to be targets for the pathogenic CM22 IgM mAb. Synthetic peptides coated to an ELISA plate were incubated with either mAb B4 or control mAb D5. Bound antibodies were detected by AP-conjugated goat anti-mouse IgM Ab. Pooled sera from C57BL/6 mice at a dilution of 1/50 were used as a positive control. Data are shown as OD405nm and are representative of two independent experiments.
IR injury restored by mAb B4 in Rag1−/− mice is accompanied by neutrophil infiltration and the local generation of pro-inflammatory eicosanoids
To confirm that the intestinal IR injury in Rag1−/− mice reconstituted with mAb B4 demonstrated similar characteristics to wild type mice, we evaluated complement C3 deposition (see next Fig. 6) as well as the infiltration of neutrophils and generation of eicosanoids (Fig. 5), all of which have been found in wild type mice undergoing IR injury (16). To measure the level of neutrophil invasion into injured tissue, we performed biochemical analyses of MPO activity (Fig. 5). Rag1−/− mice reconstituted with mAb B4 demonstrated elevated MPO activity indistinguishable from wild type mice undergoing IR injury. Previous studies have shown that the neutrophil chemoattractant leukotriene B4 (LTB4) is rapidly produced in response to intestinal I/R injury (14,16), and consistent with the increases in neutrophil invasion, the level of LTB4 in Rag1−/− mice reconstituted with mAb B4 was greatly increased as compared to LTB4 generation in sham-operated animals (Fig. 5). Finally, the pro-inflammatory eicosanoid PGE2 also was elevated in samples from mice injected with mAb B4, but with marginal significance. In toto, mAb B4 appears to induce a pro-inflammatory response in Rag1−/− mice that is similar in nature to those in wild type mice.
Figure 6.
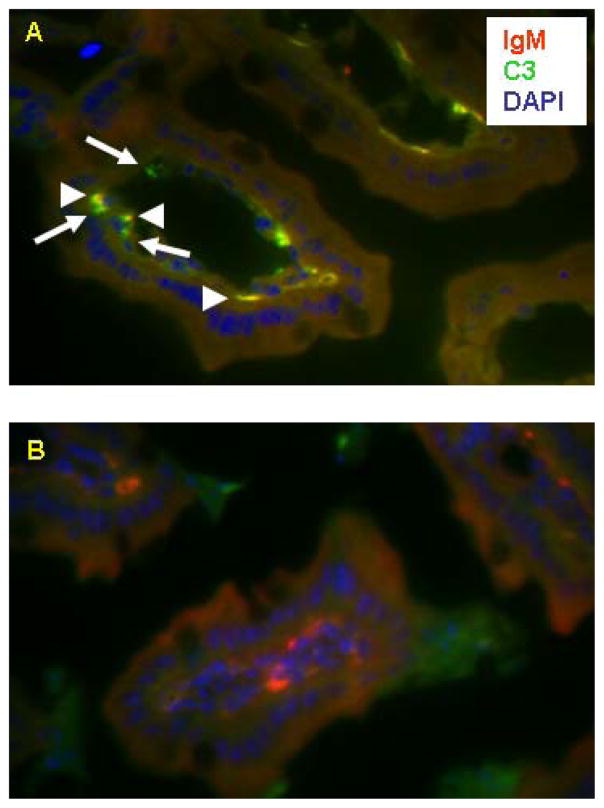
Monoclonal Ab B4, when injected into Rag1−/− mice and inducing IR injury, clusters in the microvillus beneath the enterocyte layer. Three color immunofluorescence staining for C3 (green), mouse Ig (red), and DAPI (blue) was performed. Clusters of C3 deposition co-localized with mouse Ig (arrow head) and C3 deposition alone (arrow) were seen in intestine from Rag1−/− mice reconstituted by mAb B4 before IR (upper picture). Slides made from the same intestine were probed with anti-mouse Ig and isotype control antibody for C3 antibody (lower picture). Note absence of the yellow color clusters therein.
In situ analysis of mAb B4 localization and complement C3 deposition
It has been previously shown that complement C3 is deposited during intestinal IR injury (19,23). The deposition of C3 in relation to mAb B4 in the intestines of mAb B4 treated Rag1−/− mice subjected to IR was determined by multi-color immunofluorescence microscopy (Fig. 6). As shown, when Rag1−/− mice were reconstituted with mAb B4 and IR injury was induced, mAb B4 was detected in the intestinal microvillus under the enterocyte layer, possibly being attached to the basal membrane of these cells, and co-localizing with C3.
Recombinant annexin IV blocks intestinal IR injury in wild type mice
Although our data using mAb B4 in Rag1−/− mice revealed that this Ab was sufficient to mediate intestinal IR injury, it was uncertain as to whether this reactivity was necessary in context of the entire natural Ab repertoire in wild type mice to develop injury. In order to address this question, we utilized recombinant annexin IV as an “inhibitor” of the annexin IV reactive natural Ab repertoire. We used annexin IV made in bacteria because of the low yields and inability to purify large amounts of recombinant protein from F-293 cell membranes. Bacterial annexin IV was produced as described in Materials and Methods and was >95% pure by SDS-PAGE (data not shown). As a control for the experiments, we also produced and purified to a similar level a soluble fragment of cytokeratin 19, to which the control mAb D5 reacted (data not shown).
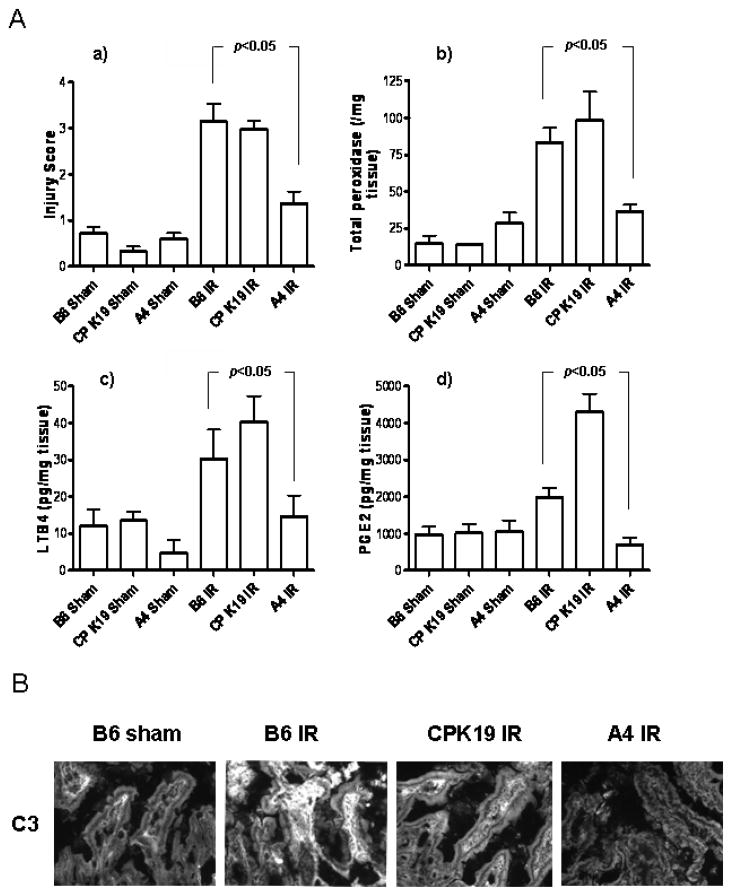
Following i.v. injection of wild type mice 5 minutes prior to intestinal reperfusion phase with 50 μg/mouse of purified recombinant annexin IV or cytokeratin 19 as a relevant control, it was observed that the injection of annexin IV significantly reduced intestinal injury to the level of the sham operated animals (Fig. 7A(a)). Reduction of IR injury in the intestine of mice injected with annexin IV was confirmed using additional approaches. First, the tissue MPO content (Fig. 7A(b)), which correlates with neutrophil activation and invasion, was also specifically reduced when compared to controls. Absence of inflammation in intestine of mice injected by annexin IV before reperfusion phase was also confirmed by the finding of specifically reduced levels of LTB4 and PGE2 (Fig. 7A(c and d)). Finally, immunofluorescence analysis revealed a greatly diminished C3 deposition in annexin IV treated mice as compared to controls (Fig. 7B). These data demonstrate that natural Ab reactivity with annexin IV is required for the full elaboration of intestinal IR injury.
Figure 7.
A, Intestinal IR injury is ameliorated in wildtype C57Bl/6 mice receiving recombinant annexin IV prior to the ischemic phase. Reduction of the of IR induced injury to the level of sham operated animals (B6 sham) was observed in wild type mice when they were injected with 50 μg/mouse of annexin IV 5 min prior to the reperfusion phase (A4 IR). Injury in mice pre-injected with control CPK19 protein (CP K19 IR) was comparable with the injury in C57Bl/6 mice undergoing IR (B6 IR) (a). Small intestine tissue samples were processed as in Fig. 6 for myeloperoxidase activity (b) and the eicosanoids LTB4 (c) and PGE2 (d). Each bar is the average ± SEM with 3–6 - animals per group. Statistical significance was determined using one way ANOVA. B, Small intestine of annexin IV pre-injected mice (far right), similarly to sham operated wild type mice (far left) does not show C3 deposition in IR induced injury, in contrast to wild type mice (second from left) and mice pre-injected with the CPK19 control protein undergoing IR (second from right).
Natural Abs to annexin IV are diminished in Cr2−/− mice
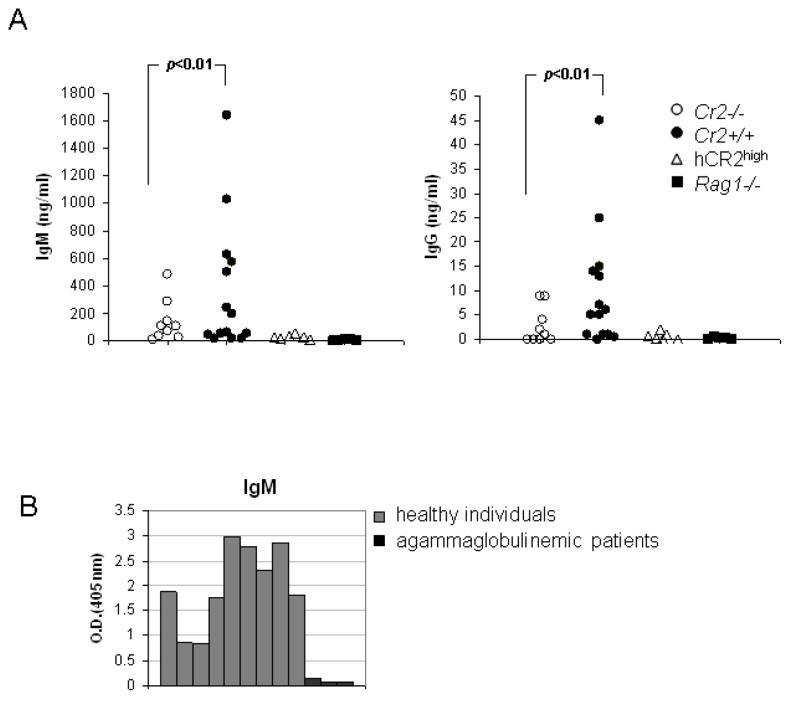
Because of the protection from the development of intestinal IR injury in Cr2−/− mice, we sought to determine whether differential reactivity to this particular antigen might underlie the difference in IR susceptibility. To address this question, we used an annexin IV ELISA assay to directly compare the level of anti-annexin IV Ab in serum samples from Cr2+/+ and Cr2−/− mice. As a negative control sera from Rag1−/− mice was used (Fig. 8A). Although we were not able to see complete abrogation of anti-annexin IV aAb in Cr2−/− mice, it is likely that the diminished natural Ab reactivity found is one reason that Cr2−/− mice are protected from IR injury.
Figure 8.
Presence of natural antibodies to annexin IV. A, Cr2+/+ in contrast to Cr2−/− mice demonstrate higher levels of IgM natural Ab to annexin IV. Serum samples from three cohorts of mice, Cr2−/− (n=9), Cr2+/+ mice (n=14) and negative control Rag1−/− (n=5) mice were evaluated for the presence of natural IgM (left) and IgG (right) Abs to bacterial annexin IV. The figures are representative of two independent experiments. Statistical significance was determined using a Wilcoxson test. B, 9 serum samples from healthy humans and three from agammaglobulinemic patients were tested in the anti-annexin IV ELISA. Substantial levels of anti-annexin IV IgM antibody IgM is present in the sera of normal humans.
Human annexin IV is a similar protein to mouse annexin IV and exhibits a 92% protein identity. We reasoned that it was relevant to determine whether similar natural Ab reactivity to annexin IV is present in humans. Indeed, when compared to sera from patients with agammaglobulinemia, there is IgM reactivity to annexin IV demonstrable in human serum (Fig. 8B). These data suggest that a similar process of neo-epitope recognition could be present in humans with IR injury.
Discussion
At the initiation of these studies, we hypothesized that neo-epitopes necessary for the development of IR injury could be identified by creating mAbs reactive with IEC, a major cellular target of reperfusion injury. Using this approach we successfully identified a pathogenic IgM mAb, designated B4, that could alone mediate the induction of IR injury in Rag1−/− mice. In this experimental setting mAb B4 could lead to the fixation of complement C3 as well as the infiltration of neutrophils and elaboration of pro-inflammatory leukotrienes. Using several approaches, annexin IV was demonstrated to be the specific target antigen of mAb B4, and no evidence of cross reactivity of mAb B4 with two previously suggested natural antibody targets, phospholipids and non-muscle myosin, was found. Most importantly, the essential role for pathogenic natural Abs reactivity with annexin IV was confirmed by the specific inhibition of IR injury in wild type mice using systemically administered recombinant annexin IV. Pretreatment of mice with annexin IV blocked tissue injury as measured by histologic criteria as well as greatly diminished the deposition of C3 and the elaboration of additional pro-inflammatory mediators in the intestine. Sera from Cr2−/− mice, which are relatively protected from intestinal IR injury, showed diminished reactivity with annexin IV. Finally, sera from normal human subjects also demonstrated IgM Ab reactivity with annexin IV, suggesting that a similar recognition process may play a key role in IR injury in man. In sum, annexin IV and natural Ab reactivity to this membrane-associated protein play an essential role in this medically important pathologic process.
One major question is how access to annexin IV by natural Abs is regulated. It is likely, based on what is known regarding the normal distribution of annexin IV in vivo, that IEC have an intracellular or internal membrane bilayer-associated pool of protein that transfers to the extracellular membrane upon initial cellular injury. Whether endothelial cells also elaborate this protein during IR injury is not known but such a situation might also contribute to the initial recognition of annexin IV by natural Abs. There was no evidence of reactivity of mAb B4 with endothelial surfaces as shown in Fig. 6; however, recognition of endothelial cells may be below the level of detection of that immunofluorescence technique. Natural Abs then likely bind to several epitopes on annexin IV, activate complement and through this mechanism initiate the inflammatory cascade.
Annexin IV is a relatively widely distributed protein and a member of a family of largely membrane associated proteins. Several annexin family members are expressed on the cell surface, for example annexin II which serves as an angiostatin receptor (61). Annexin VI and annexin II are found in the extracellular space and bind fetuin-A 43. The precise mechanisms by which annexins are transported to the surface of the cells are not certain. The N-terminal domain of annexins is responsible for binding to other proteins (62–64) and it is thus possible that the extracellular annexin IV we have detected is attached to the IEC membrane by being bound to another intrinsic membrane protein. Annexin IV has also been found in lipid rafts (65). The mechanism of raft inclusion is unknown; however, based on analogy to other lipid raft associated proteins this may be through attachment to a GPI-anchor or through acylation with palmitate or myristate (66). A new ligand for annexin IV in the pancreas, GPI-anchored sialoprotein GP-2, was described recently (67). Our finding that annexin IV is detected on the IEC surface even if the purification protocol for these cells contains EDTA, as well as the finding that recombinant annexin IV despite of presence of a leader peptide was not released from F-293 cells but rather remained attached to the surface in a Ca2+ independent process, strongly suggests that it is not using the well-described Ca2+ dependent phospholipid binding mechanism (40). The possibility of such Ca2+ independent binding by annexins was recently shown by others while demonstrating that annexin IV could bind PS and PA containing liposomes at low pH in Ca2+ independent manner (68), and that hypoxia sufficient to induce an intracellular pH change can promote certain annexins to translocate to the surface of the plasma membrane (69).
The histological analyses clearly showed that when mAb B4 was injected into Rag1−/− mice it was bound beneath the basal membrane of enterocytes in the villi. Monoclonal Ab B4 co-localizes with C3 deposition in intestine of mice undergoing IR injury, strongly suggesting that the binding of the mAb to the antigen results in complement activation. It is likely that annexin IV translocates to the cell surface when cells undergo hypoxia, early apoptosis and perhaps necrosis. This translocation could also be facilitated by post-translational modifications of annexin IV (53).
There is also a possibility that the chronic injury of epithelial cells may increase autoantibody production to annexin IV, as it has been demonstrated that patients with chronic alcoholism exhibit elevated levels of IgG autoantibodies to annexin IV (70,71). This effect may increase the level of self-reactivity and promote the greater development of IR injury in the setting of similar levels of initial ischemia.
Natural Abs to IR related neo-epitopes are a component of the class of self-protein-directed Abs circulating in the body, the majority of which are products of B-1 B cells. The development of B-1 B cells secreting self reactive Abs is believed to be antigen dependent (72). In this regard, it is notable that Cr2−/− mice, despite exhibiting normal quantitative levels of IgG and IgM isotype antibodies 24, clearly show differences in natural Ab reactivity to annexin IV. It is likely that the absence of mouse CR2 is the major cause of that difference (23,25,73). In addition, while it has been shown that there is a decrease in autoantibody production in experimental immunization-induced models of self-reactivity in the absence of CR2, for example collagen-induced arthritis (74), this is the first report to our knowledge of a marked decrease in natural Ab reactivity to specific self antigens in Cr2−/− mice in the absence of directed immunization.
Of note, CD5+ B-1 cells do express mouse CR2; however, they do not amplify Ca2+ influx in response to BCR/C3dg co-engagement (75) and also demonstrate a lower level of CR2 expression (76,77). These data have been interpreted to indicate that, since these cells are already pre-activated by antigen binding to the BCR, the cells are anergic due to chronic low grade chronic stimulation in vivo. The lower levels of mouse CR2 may be due to receptor shedding or internalization after being engaged with its ligand (76,77). If natural Ab reactivity to annexin IV is derived primarily from B-1 cells, and CR2 is required on B-1 cells for this process, this suggests that in vivo responses through CR2 are substantially different than those found in vitro using co-ligation as an analytic tool. Of course, since coligation of BCR with CR2 lowers the activation signal in B-2 cells several thousand-fold (78), it is also possible that B-2 cells play the major role in driving natural Ab self reactivity to annexin IV. Finally, since the absence of mouse CR2 not only changes the B-2 cell activation threshold to immune complexes (IC) but also greatly alters follicular dendritic cell (FDC)/IC networks (79) and diminishes Ab responses to T-independent antigens (73), additional mechanisms may play important roles in the lack of development of natural Abs to annexin IV in the absence of this receptor.
Finally, it is worth considering how natural Ab reactivity to annexin IV relates to non-muscle myosin and phosopholipids, both of which have been previously suggested as targets for natural Abs in IR-induced injury and, at least with regard to non-muscle myosin, as necessary for the development of tissue injury in vivo. There are several reasons that might explain these findings. One possibility is that serial recognition of several epitopes that are displayed in a stereotypic fashion during the development of cell apoptosis/necrosis is required to initiate complement-dependent injury. In that setting, interruption of reactivity of natural Abs to any one of the antigens could ameliorate the process. A second possibility is that epitopes are expressed at the same time but on unique cell populations, for example non-muscle myosin on endothelial cells and annexin IV on IECs, and recognition of each cell type is necessary for full injury development. A third possibility is that all of these proteins come together in protein/phospholipid complexes that are exposed during IR, and binding of natural Abs to several of the epitopes is necessary to activate complement and induce injury. What argues against these hypotheses is that individual IgM mAbs can transfer injury in Rag1−/− mice. However, it is possible that a high dose of individual injected IgM mAbs can overcome restriction points that would be present in wild type mice with lower levels of natural Abs. Certainly going forward it will be necessary to better understand the physicochemical and quantitative relationships between the various neo-epitopes that are important in injury as well as the natural Abs that recognize them.
In summary we propose that annexin IV is a major IR related neo-epitope that is recognized by pathogenic natural Abs. Further explorations of the mechanisms of natural Ab induced injury injury, a determination of how and where annexin IV epitopes are displayed, and the development of a better understanding of how this component of the natural Ab repertoire is positively selected by the presence of CR2 are particularly relevant areas for future investigation.
Footnotes
Abbreviations used in this paper: IR, ischemia-reperfusion; MS, mass spectrometry; IEC, intestinal epithelial cells; MPO, myeloperoxidase; LTB4, leukotriene B4; PGE2, prostaglandin E2.
This work was funded by U.S. Army Medical Research and Materiel Command Award W81XWH-06-1-0520 and MRMC W81XWH-07-1-0286; Alliance for Lupus Research; and National Institutes of Health R01 AI31105, A161691, AI46637, DK41873 and DK55357.
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org
References
- 1.Ozmen S, Ayhan S, Demir Y, Siemionow M, Atabay K. Impact of gradual blood flow increase on ischaemia-reperfusion injury in the rat cremaster microcirculation model. J Plast Reconstr Aesthet Surg. 2007;61:939–948. doi: 10.1016/j.bjps.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman BJ, Granger DN. Mechanisms of reperfusion injury. Am J Med Sci. 1994;307:284–292. doi: 10.1097/00000441-199404000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Mack WJ, Sughrue ME, Ducruet AF, Mocco J, Sosunov SA, Hassid BG, Silverberg JZ, Ten VS, Pinsky DJ, Connolly ES., Jr Temporal pattern of C1q deposition after transient focal cerebral ischemia. J Neurosci Res. 2006;83:883–889. doi: 10.1002/jnr.20775. [DOI] [PubMed] [Google Scholar]
- 4.Mocco J, Wilson DA, Komotar RJ, Sughrue ME, Coates K, Sacco RL, Elkind MS, Connolly ES., Jr Alterations in plasma complement levels after human ischemic stroke. Neurosurgery. 2006;59:28–33. doi: 10.1227/01.NEU.0000219221.14280.65. [DOI] [PubMed] [Google Scholar]
- 5.Vinten-Johansen J, Zhao ZQ, Jiang R, Zatta AJ. Myocardial protection in reperfusion with postconditioning. Expert Rev Cardiovasc Ther. 2005;3:1035–1045. doi: 10.1586/14779072.3.6.1035. [DOI] [PubMed] [Google Scholar]
- 6.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Macfie J, O’boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223–228. doi: 10.1136/gut.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukatsu K, Sakamoto S, Hara E, Ueno C, Maeshima Y, Matsumoto I, Mochizuki H, Hiraide H. Gut ischemia-reperfusion affects gut mucosal immunity: a possible mechanism for infectious complications after severe surgical insults. Crit Care Med. 2006;34:182–187. doi: 10.1097/01.ccm.0000196207.86570.16. [DOI] [PubMed] [Google Scholar]
- 9.Deitch EA. Role of the gut lymphatic system in multiple organ failure. Curr Opin Crit Care. 2001;7:92–98. doi: 10.1097/00075198-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Burns BJ, Brandt LJ. Intestinal ischemia. Gastroenterol Clin North Am. 2003;32:1127–1143. doi: 10.1016/s0889-8553(03)00093-1. [DOI] [PubMed] [Google Scholar]
- 11.Simpson R, Alon R, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Neutrophil and nonneutrophil-mediated injury in intestinal ischemia-reperfusion. Ann Surg. 1994;218:444–453. doi: 10.1097/00000658-199310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 13.Crawford MH, Grover FL, Kolb WP, McMahan CA, O’Rourke RA, McManus LM, Pinckard RN. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circulation. 1988;78:1449–1458. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- 14.Eror AT, Stojadinovic A, Starnes BW, Makrides SC, Tsokos GC, Shea-Donohue T. Anti-inflammatory effects of soluble complement receptor type 1 promote rapid recovery of ischemia/reperfusion injury in rat small intestine. Clin Immunol. 1999;90:266–275. doi: 10.1006/clim.1998.4635. [DOI] [PubMed] [Google Scholar]
- 15.Pemberton M, Anderson G, Vetvicka V, Justus DE, Ross GD. Microvascular effects of complement blockade with soluble recombinant CR1 on ischemia/reperfusion injury of skeletal muscle. J Immunol. 1993;150:5104–5113. [PubMed] [Google Scholar]
- 16.Rehrig S, Fleming SD, Anderson J, Guthridge JM, Rakstang JK, McQueen C, Holers VM, Tsokos GC, Shea-Donohue T. Complement inhibitory Crry-Ig attenuates intestinal damage after the onset of mesenteric ischemia/reperfusion injury in mice. J Immunol. 2001;167:5921–5927. doi: 10.4049/jimmunol.167.10.5921. [DOI] [PubMed] [Google Scholar]
- 17.Chan RK, Ibrahim SI, Takahashi K, Kwon E, McCormack M, Ezekowitz A, Carroll MC, Moore FD, Jr, Austen WG., Jr The differing roles of the classical and mannose-binding lectin complement pathways in the events following skeletal muscle ischemia-reperfusion. J Immunol. 2006;177:8080–8085. doi: 10.4049/jimmunol.177.11.8080. [DOI] [PubMed] [Google Scholar]
- 18.Jordan JE, Montalto MC, Stahl GL. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation. 2001;104:1413–1418. doi: 10.1161/hc3601.095578. [DOI] [PubMed] [Google Scholar]
- 19.Williams JP, Pechet TT, Weiser MR, Reid R, Kobzik L, Moore FD, Jr, Carroll MC, Hechtman HB. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol. 1999;86:938–942. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- 20.Stahl GL, Xu Y, Hao L, Miller M, Buras JA, Fung M, Zhao H. Role for the alternative complement pathway in ischemia/reperfusion injury. Am J Pathol. 2003;162:449–455. doi: 10.1016/S0002-9440(10)63839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming SD, Egan RP, Chai C, Girardi G, Holers VM, Salmon J, Monestier M, Tsokos GC. Anti-phospholipid antibodies restore mesenteric ischemia/reperfusion-induced injury in complement receptor 2/complement receptor 1-deficient mice. J Immunol. 2004;173:7055–7061. doi: 10.4049/jimmunol.173.11.7055. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Michael LH, Grosjean SA, Kelly RA, Carroll MC, Entman ML. The role of natural IgM in myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2006;41:62–67. doi: 10.1016/j.yjmcc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Fleming SD, Shea-Donohue T, Guthridge JM, Kulik L, Waldschmidt TJ, Gipson MG, Tsokos GC, Holers VM. Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J Immunol. 2002;169:2126–2133. doi: 10.4049/jimmunol.169.4.2126. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Austen WG, Jr, Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Jr, Carroll MC. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Pro Natl Acad Sci. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid RR, Woodstock S, Shimabukuro-Vornhagen A, Austen WG, Jr, Kobzik L, Zhang M, Hechtman HB, Moore FD, Jr, Carroll MC. Functional activity of natural antibody is altered in Cr2-deficient mice. J Immunol. 2002;169:5433–5440. doi: 10.4049/jimmunol.169.10.5433. [DOI] [PubMed] [Google Scholar]
- 26.Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749–2754. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 27.Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006;18:547–555. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Kawahara T, Ohdan H, Zhao G, Yang YG, Sykes M. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J Immunol. 2003;171:5406–5414. doi: 10.4049/jimmunol.171.10.5406. [DOI] [PubMed] [Google Scholar]
- 29.Herzenberg LA. B1 cells: the lineage question revisited. Immunol Rev. 2000;175:9–22. [PubMed] [Google Scholar]
- 30.Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Cur Opin Immunol. 2001;13:195–201. doi: 10.1016/s0952-7915(00)00204-1. [DOI] [PubMed] [Google Scholar]
- 31.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg L, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 33.Buza J, Sileghem M, Gwakisa P, Naessens J. CD5+ B lymphocytes are the main source of antibodies reactive with non-parasite antigens in Trypanosoma congolense-infected cattle. Immunology. 1997;92:226–233. doi: 10.1046/j.1365-2567.1997.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26:385–404. doi: 10.1007/s00281-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 35.Shaw PX, Horkko S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silverman GJ, Shaw PX, Luo L, Dwyer D, Chang M, Horkko S, Palinski W, Stall A, Witztum JL. Neo-self antigens and the expansion of B-1 cells: lessons from atherosclerosis-prone mice. Curr Top Microbiol Immunol. 2000;252:189–200. doi: 10.1007/978-3-642-57284-5_20. [DOI] [PubMed] [Google Scholar]
- 37.Chan RK, Verna N, Afnan J, Zhang M, Ibrahim S, Carroll MC, Moore FD., Jr Attenuation of skeletal muscle reperfusion injury with intravenous 12 amino acid peptides that bind to pathogenic IgM. Surgery. 2005;139:236–243. doi: 10.1016/j.surg.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoko M, Chan R, Friend D, Mahmood U, Weissleder R, Moore FD, Jr, Carroll MC. Identification of the target self antigens in reperfusion injury. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan RO, Martin-Almedina S, Iglesias JM, Gonzalez-Florez MI, Fernandez MP. Evolutionary perspective on annexin calcium-binding domains. Biochim Biophys Acta. 2004;1742:133–140. doi: 10.1016/j.bbamcr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Rescher U, Gerke V. Annexins--unique membrane binding proteins with diverse functions. J Cell Sci. 2004;117:2631–2639. doi: 10.1242/jcs.01245. [DOI] [PubMed] [Google Scholar]
- 41.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 42.Kim J, Hajjar KA. Annexin II: a plasminogen-plasminogen activator co-receptor. Front Biosci. 2002;7:d341–d348. doi: 10.2741/kim. [DOI] [PubMed] [Google Scholar]
- 43.Kundranda MN, Ray S, Saria M, Friedman D, Matrisian LM, Lukyanov P, Ochieng J. Annexins expressed on the cell surface serve as receptors for adhesion to immobilized fetuin-A. Biochim Biophys Acta. 2004;1693:111–123. doi: 10.1016/j.bbamcr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Perretti M, Gavins FN. Annexin 1: an endogenous anti-inflammatory protein. News Physiol Sci. 2003;18:60–64. doi: 10.1152/nips.01424.2002. [DOI] [PubMed] [Google Scholar]
- 45.Massey-Harroche D, Mayran N, Maroux S. Polarized localizations of annexins I, II, VI and XIII in epithelial cells of intestinal, hepatic and pancreatic tissues. J Cell Sci. 1998;111(Pt 20):3007–3015. doi: 10.1242/jcs.111.20.3007. [DOI] [PubMed] [Google Scholar]
- 46.Massey D, Traverso V, Rigal A, Maroux S. Cellular and subcellular localization of annexin IV in rabbit intestinal epithelium, pancreas and liver. Biol Cell. 1991;73:151–156. doi: 10.1016/0248-4900(91)90097-7. [DOI] [PubMed] [Google Scholar]
- 47.Mayran N, Traverso V, Maroux S, Massey-Harroche D. Cellular and subcellular localizations of annexins I, IV, and VI in lung epithelia. Am J Physiol. 1996;270:L863–L871. doi: 10.1152/ajplung.1996.270.5.L863. [DOI] [PubMed] [Google Scholar]
- 48.Piljic A, Schultz C. Annexin A4 self-association modulates general membrane protein mobility in living cells. Mol Biol Cell. 2006;17:3318–3328. doi: 10.1091/mbc.E06-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seville RA, Nijjar S, Barnett MW, Masse K, Jones EA. Annexin IV (Xanx-4) has a functional role in the formation of pronephric tubules. Development. 2002;129:1693–1704. doi: 10.1242/dev.129.7.1693. [DOI] [PubMed] [Google Scholar]
- 50.Hill WG, Kaetzel MA, Kishore BK, Dedman JR, Zeidel ML. Annexin A4 reduces water and proton permeability of model membranes but does not alter aquaporin 2-mediated water transport in isolated endosomes. J Gen Physiol. 2003;121:413–425. doi: 10.1085/jgp.200308803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi T, Dong F, Liou LS, Duan ZH, Novick AC, Didonato JA. Differential protein profiling in renal-cell carcinoma. Mol Carcinog. 2004;40:47–61. doi: 10.1002/mc.20015. [DOI] [PubMed] [Google Scholar]
- 52.Zimmermann U, Balabanov S, Giebel J, Teller S, Junker H, Schmoll D, Protzel C, Scharf C, Kleist B, Walther R. Increased expression and altered location of annexin IV in renal clear cell carcinoma: a possible role in tumour dissemination. Cancer Lett. 2004;209:111–118. doi: 10.1016/j.canlet.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Herzog A, Kuntz S, Daniel H, Wenzel U. Identification of biomarkers for the initiation of apoptosis in human preneoplastic colonocytes by proteome analysis. Int J Cancer. 2004;109:220–229. doi: 10.1002/ijc.11692. [DOI] [PubMed] [Google Scholar]
- 54.Sohma H, Ohkawa H, Hashimoto E, Sakai R, Saito T. Ethanol-induced augmentation of annexin IV expression in rat C6 glioma and human A549 adenocarcinoma cells. Alcohol Clin Exp Res. 2002;26:44S–48S. doi: 10.1097/01.ALC.0000026975.39372.A5. [DOI] [PubMed] [Google Scholar]
- 55.Grossmann J, Maxson JM, Whitacre CM, Orosz DE, Berger NA, Fiocchi C, Levine AD. New isolation technique to study apoptosis in human intestinal epithelial cells. Am J Pathol. 1998;153:53–62. doi: 10.1016/S0002-9440(10)65545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grossmann J, Walther K, Artinger M, Kiessling S, Scholmerich J. Apoptotic signaling during initiation of detachment-induced apoptosis (“anoikis”) of primary human intestinal epithelial cells. Cell Growth Differ. 2001;12:147–155. [PubMed] [Google Scholar]
- 57.Vossenaar ER, Despres N, Lapointe E, van der HA, Lora M, Senshu T, van Venrooij WJ, Menard HA. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004;6:R142–R150. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mercolino TJ, Arnold LW, Hawkins LA, Haughton G. Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl choline. Relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J Exp Med. 1988;168:687–698. doi: 10.1084/jem.168.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mercolino TJ, Locke AL, Afshari A, Sasser D, Travis WW, Arnold LW, Haughton G. Restricted immunoglobulin variable region gene usage by normal Ly-1 (CD5+) B cells that recognize phosphatidyl choline. J Exp Med. 1989;169:1869–1877. doi: 10.1084/jem.169.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Junker M, Creutz CE. Ca(2+)-dependent binding of endonexin (annexin IV) to membranes: analysis of the effects of membrane lipid composition and development of a predictive model for the binding interaction. Biochemistry. 1994;33:8930–8940. doi: 10.1021/bi00196a010. [DOI] [PubMed] [Google Scholar]
- 61.Syed SP, Martin AM, Haupt HM, renas-Elliot CP, Brooks JJ. Angiostatin receptor annexin II in vascular tumors including angiosarcoma. Hum Pathol. 2007;38:508–513. doi: 10.1016/j.humpath.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 62.Cheng CW, Rifai A, Ka SM, Shui HA, Lin YF, Lee WH, Chen A. Calcium-binding proteins annexin A2 and S100A6 are sensors of tubular injury and recovery in acute renal failure. Kidney Int. 2005;68:2694–2703. doi: 10.1111/j.1523-1755.2005.00740.x. [DOI] [PubMed] [Google Scholar]
- 63.Hayes MJ, Rescher U, Gerke V, Moss SE. Annexin-actin interactions. Traffic. 2004;5:571–576. doi: 10.1111/j.1600-0854.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 64.Willshaw A, Grant K, Yan J, Rockliffe N, Ambavarapu S, Burdyga G, Varro A, Fukuoka S, Gawler D. Identification of a novel protein complex containing annexin A4, rabphilin and synaptotagmin. FEBS Lett. 2004;559:13–21. doi: 10.1016/S0014-5793(03)01513-8. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen HT, Amine AB, Lafitte D, Waheed AA, Nicoletti C, Villard C, Letisse M, Deyris V, Roziere M, Tchiakpe L, Danielle CD, Comeau L, Hiol A. Proteomic characterization of lipid rafts markers from the rat intestinal brush border. Biochem Biophys Res Commun. 2006;342:236–244. doi: 10.1016/j.bbrc.2006.01.141. [DOI] [PubMed] [Google Scholar]
- 66.Brown D. Structure and function of membrane rafts. Int J Med Microbiol. 2002;291:433–437. doi: 10.1078/1438-4221-00150. [DOI] [PubMed] [Google Scholar]
- 67.Tsujii-Hayashi Y, Kitahara M, Yamagaki T, Kojima-Aikawa K, Matsumoto I. A potential endogenous ligand of annexin IV in the exocrine pancreas. Carbohydrate structure of GP-2, a glycosylphosphatidylinositol-anchored glycoprotein of zymogen granule membranes. J Biol Chem. 2002;277:47493–47499. doi: 10.1074/jbc.M206572200. [DOI] [PubMed] [Google Scholar]
- 68.Zschornig O, Opitz F, Muller M. Annexin A4 binding to anionic phospholipid vesicles modulated by pH and calcium. Eur Biophys J. 2007;36:415–424. doi: 10.1007/s00249-007-0147-1. [DOI] [PubMed] [Google Scholar]
- 69.Monastyrskaya K, Tschumi F, Babiychuk EB, Stroka D, Draeger A. Annexins sense changes in intracellular pH during hypoxia. Biochem J. 2008;409:65–75. doi: 10.1042/BJ20071116. [DOI] [PubMed] [Google Scholar]
- 70.Sohma H, Ohkawa H, Hashimoto E, Toki S, Ozawa H, Kuroki Y, Saito T. Alteration of annexin IV expression in alcoholics. Alcohol Clin Exp Res. 2001;25:55S–58S. doi: 10.1097/00000374-200106001-00013. [DOI] [PubMed] [Google Scholar]
- 71.Sohma H, Ohkawa H, Sakai R, Hashimoto E, Ukai W, Saito T. Augmentation of ethanol-induced cell damage and activation of nuclear factor-kappa B by annexin IV in cultured cells. Alcohol Clin Exp Res. 2003;27:64S–67S. doi: 10.1097/01.ALC.0000078615.47704.73. [DOI] [PubMed] [Google Scholar]
- 72.Hayakawa K, Asano M, Shinton SA, Gui M, Allman DM, Steward CL, Silver J, Hardy RR. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 73.Holers VM, Kulik L. Complement receptor 2, natural antibodies and innate immunity: Inter-relationships in B cell selection and activation. Mol Immunol. 2007;44:64–72. doi: 10.1016/j.molimm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Kuhn KA, Cozine CL, Tomooka B, Robinson WH, Holers VM. Complement receptor CR2/CR1 deficiency protects mice from collagen-induced arthritis and associates with reduced autoantibodies to type II collagen and citrullinated antigens. Mol Immunol. 2008;45:2808–2819. doi: 10.1016/j.molimm.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 75.Lyubchenko T, dal Porto J, Cambier JC, Holers VM. Co-ligation of the B cell receptor with complement receptor type 2 (CR2/CD21) using its natural ligand C3dg: activation without engagement of an inhibitory signaling pathway. J Immunol. 2005;174:3264–3272. doi: 10.4049/jimmunol.174.6.3264. [DOI] [PubMed] [Google Scholar]
- 76.Tessier J, Cuvillier A, Glaudet F, Khamlichi AA. Internalization and molecular interactions of human CD21 receptor. Mol Immunol. 2007;44:2415–2425. doi: 10.1016/j.molimm.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 77.Masilamani M, Kassahn D, Mikkat S, Glocker MO, Illges H. B cell activation leads to shedding of complement receptor type II (CR2/CD21) Eur J Immunol. 2003;33:2391–2397. doi: 10.1002/eji.200323843. [DOI] [PubMed] [Google Scholar]
- 78.Fearon DT, Carter RH. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Ann Rev Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 79.Fang Y, Xu C, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a normal antigen-specific IgG response. J Immunol. 1998;160:5273–5279. [PubMed] [Google Scholar]