Abstract
Intraocular lens implantation after opacified natural lens removal is the primary treatment for cataracts in developed countries. Cataract surgery is generally considered safe, but entails significant risks in countries where sophisticated sterile operating theaters are not widely available. Post-operative infection (endophthalmitis) is a potential blinding complication. Infection often results from bacterial colonization of the new lens implant and subsequent antibiotic-tolerant biofilm formation. To combat this risk, we developed a polymeric hydrogel system that can deliver effective levels of antibiotic over an extended period of time within the globe of the eye. Norfloxacin™ antibiotic was loaded into cross-linked poly(2-hydroxyethyl methacrylate) (pHEMA) gels, which were subsequently surface-modified with octadecyl isocyanate to produce a hydrophobic rate-limiting barrier controlling norfloxacin release. Octadecyl surface modification was characterized using scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS). A 15-min modification leads to a uniform surface coating and near zero order release of norfloxacin from the matrix. Norfloxacin released from coated pHEMA kills Staphylococcus epidermidis in suspension and on a simulated medical implant surface. With these data, we demonstrate a new and effective system for sustained drug release from a hydrogel matrix with specific application for intraocular lens surgery.
Keywords: Drug delivery, Hydrogel, Intraocular lenses, Endophthalmitis, Infections
1. Introduction
A recent study estimates that there are more than 20.5 million cases of cataract in the United States alone, and the number is expected to rise to 30.1 million in the next decade [1]. Cataract removal is a common and safe procedure, usually performed in out-patient clinics, and it is often followed by the insertion of a synthetic artificial intraocular lens (IOL) to replace the extracted natural lens. These IOLs are generally very successful in restoring vision postoperatively. However, severe post-operative infection (endophthalmitis) after cataract surgery and IOL implantation is always a concern even with topical antibiotic coverage. Endophthalmitis rates in the developed nations are estimated between 0.07% and 0.12% associated with cataract removal and IOL insertion, and the rates are expected to be much higher in the developing world [2,3]. Endophthalmitis is most often caused by bacteria adhered to the surface of the IOL during insertion, which proliferate and form biofilms. Biofilms are associated with over 80% of microbial infections in the body. Biofilm-bound microbes exhibit lower susceptibility to antimicrobial treatment, and are often extremely difficult to remove once established on medical device surfaces [4,5]. Biofilm formation on IOL surfaces has been frequently observed and studied [3,6]. If endophthalmitis occurs, the infection is treated with injections of anti-infective medication directly into the eye because the penetration of topical agents is extremely limited. With the goal of reducing the incidence of post-operative intraocular infections, we have developed a polymeric system for sustained, rate-controlled intraocular delivery of an effective concentration of antibiotic.
Design of controlled-release drug delivery systems commonly employs polymers to effectively regulate the release of bioactive molecules with defined kinetics so as to maintain specific therapeutic concentrations. Polymers are ideal for drug delivery systems because of their bioinertness, processability and innate macro-molecular characteristics demonstrating phase transitions, swelling phenomenon, and changes in response to external stimuli (e.g., temperature, ultrasound, magnetic and electrical fields) that can be used to regulate drug release kinetics [7,8]. One class of polymers is hydrogels, which are cross-linked polymeric networks that reach an equilibrium degree of swelling in water enabling them to have: (1) a soft, rubbery consistency similar to living tissues that reduces mechanical friction; (2) good permeability in aqueous media that can allow small molecules to migrate within the polymer matrix efficiently; and (3) low interfacial tension that can reduce the tendency for protein adsorption [9]. Poly(2-hydroxyethyl methacrylate) (pHEMA) is a hydrogel polymer that is biostable, well tolerated in the body, and has an extensive history of use in ophthalmic applications such as contact lenses [10–12], and in drug delivery applications [13,14]. However, when used in controlled-release systems, it has been shown that an initial “burst release” occurs immediately after water or solvent hydration, making pHEMA gels potentially unsafe in drug delivery applications [15]. To counter this intrinsic burst release and achieve a controlled, sustained release, we modified the pHEMA surface by adding a dense, hydrophobic brush coating, which was achieved by reacting the hydroxyl groups of pHEMA with octadecyl isocyanate to form a surface-localized layer of methylene chains [16–18]. This surface modification creates a rate-limiting barrier to molecular transport into and out of the polymer matrix, modulating hydration and controlling drug delivery.
We specifically investigated here the release of the antibiotic norfloxacin™, a broad spectrum antibiotic belonging to the family of fluoroquinolones. Norfloxacin™ is active against both Gram positive and Gram negative microbes, including Staphylococcus epidermidis, Staphylococcus aureus, and Pseudomonas aeruginosa [19]. S. epidermidis is the organism responsible for most cases of endophthalmitis, followed by the other two species [20,21]. Choosing a broad spectrum antibiotic, which can be delivered continuously at effective concentrations, can provide a preventive treatment against most harmful species potentially colonizing the lens implant.
Here, we demonstrate that antibiotic-loaded pHEMA constructs surface-modified with assembled n-alkyl chains can achieve sustained intraocular norfloxacin release sufficient to prevent bacterial infection and biofilm formation. X-ray photoelectron spectroscopy (XPS) was used to determine the surface chemical composition, while scanning electron microscopy (SEM) was performed to study surface morphology. Efficacy of the pHEMA constructs to prevent bacterial colonization and infection, both in suspension and on the polymer surfaces, was assessed.
2. Materials and methods
2.1. Materials
Ophthalmic grade HEMA (Cat. No. 04675) and tetraethylene glycol dimethacrylate (TEGDMA) (Cat. No. 02654) were obtained from Polysciences, Inc. (Warrington, PA). Ethylene glycol (Cat. No. 324558), ammonium persulfate (Cat. No. 161519), sodium metabisulfite (Cat. No. 248614), tetrahydrofuran (Cat. No. T5267), octadecyl isocyanate (Cat. No. O1807), dibutyl tin dilaurate (Cat. No. 291234), and norfloxacin (Cat. No. N9890) were obtained from Sigma-Aldrich (St. Louis, MO). Norfloxacin was protonated with hydrochloric acid to increase its solubility in water [22]. Briefly, a solution of norfloxacin in ethanol was acidified by adding hydrochloric acid drop wise until the pH reached 4. The acidified norfloxacin was filtered from the ethanol solution, and was rinsed with copious amount of water. After drying overnight in a fume hood, the acidified norfloxacinwas lyophilized and the resulting powder was ground into 90-μm particles and mixed with the HEMA monomer (see below). Norfloxacin-HCl will be referred to as norfloxacin for the remainder of this paper.
2.2. Preparation of pHEMA samples
PHEMA hydrogels were prepared as previously described [16,23]. Briefly, 5 mL of ophthalmic grade 2-HEMA monomer was mixed with 0.23 mL TEGDMA and added to a solvent mixture of water/ethylene glycol (1.0 mL/1.5 mL). 0.1 g of lyophilized norfloxacin was added to the monomer solution and vortexed prior to adding the catalyst and initiator, 0.5 mL of 15% sodium metabisulfite and 0.5 mL of 40% ammonium persulfate, respectively. The mixture was allowed to polymerize between two glass plates, separated by 0.015″ thick Teflon spacers, overnight at room temperature. Then the resulting polymer was removed from the glass plates, punched into 10-mm disks and allowed to soak in deionized water (dH2O) for 4 h, with frequent solvent changes to leach out unreacted monomers and initiators. The drug-loaded pHEMA disks were then vacuum-dried between two Teflon sheets and glass plates for at least 48 h.
2.3. Surface modification with octadecyl isocyanate
Dry pHEMA disks were transferred into an anhydrous nitrogen atmosphere (glove box). In a glass scintillation vial, 0.5 mL of octadecyl isocyanate (5% vol/vol) was added to 10 mL of anhydrous tetrahydrofuran (THF) with a stir bar and dry pHEMA disks. 20 μL of dibutyl tin dilaurate (0.3% vol/vol) was added as the catalyst. The vials were capped, removed from the glove box, and placed in an oil bath set at 50 °C. This surface modification involves attaching long methylene chains to the hydroxyl groups of pHEMA via the formation of a stable polyurethane bond. The samples were allowed to react for 15, 30, 45, and 60 min after which, they were retrieved, sonicated in a vial with fresh THF for 5 min, and blow dried with nitrogen.
2.4. Drug release characterization
To determine the release kinetics of the samples, the coated, norfloxacin-loaded pHEMA gels were placed in 2 mL dH2O. At specified time points (30 min, 1, 2, 3, 4, 8, 12, 24, 48, 72, 96, and 120 h), the samples were transferred to fresh dH2O. The norfloxacin concentration was determined by measuring the absorbance of the solution at λmax = 270 nm using a UV-VIS plate reader (SAFIRE II, Tecan Systems Inc, San Jose, CA). Using solutions of known concentrations, a calibration was performed to obtain the best-fit equation that will determine the concentration at any given absorbance. The absorbance was used to calculate the concentration, and knowing the exact volume, the mass of the drug in solution, mdrug, can be quantified. The release rates of norfloxacin from the polymer matrices were calculated using Eq. (1).
| (1) |
where, dmdrug is the change in mass of drug between time points, dt is the change in time, and A is the surface area of the samples, which is 1.57 cm2.
2.5. Surface characterization
X-ray photoelectron spectroscopy (XPS) is a technique used to quantitatively determine the atomic composition of a surface [24]. Survey and C1s high-resolution scans were performed for samples at depths between 50 and 80 Å using a Surface Science Instrument S-Probe with an Al K-alpha X-ray source. Two 800 μm spots were analyzed on each sample, and the spectra obtained were analyzed using Service Physics ESCA VB data reduction software to identify and calculate the area under each peak using a linear background function. The binding energies were normalized to the C1s binding energy (BE) of 285 eV. From the high-resolution carbon C1s scans, various carbon species were identified by resolving the carbon C1s peak into separate Gaussian peaks using a least-squares fitting routine in the SSI software. Each peak was referenced to the hydrocarbon peak at 285 eV to account for BE shifts. Peaks were fitted with a 100% Gaussian line function and a Shirley function was utilized for the background model.
Scanning electron microscope (SEM) images were also obtained of the surface features of the polymer matrices. The system used is an FEI Sirion SEM (Hillsboro, OR), which was combined with energy dispersive X-ray analysis (EDXA) to allow ultra-high-resolution digital imaging and X-ray mapping of the sample. Since the hydrogel samples were naturally non-conductive, they were sputter coated with a thin layer of gold in order to obtain better images. The entire surface of the sample was scanned, and representative images were saved as.jpg files.
2.6. Anti-bacterial assessment
To evaluate how these materials can prevent biofilm formation or bacterial proliferation, S. epidermidis (ATCC 35984) were incubated with simulated medical implants and concurrently exposed to norfloxacin-loaded pHEMA samples in media. S. epidermidis cultures were streaked on agar plates supplemented with 10 g/L tryptic soy broth (TSB) overnight at 37 °C until growing colonies reached desired sizes. A single, isolated colony was selected and placed in 25 mL of 10 g/L TSB medium; this flask was placed on a shaker in a 37 °C water bath for 24 h. 1 mL samples of freely-suspended S. epidermidis culture were then seeded into individual wells of a 48-well plate. To represent a medical implant, 6 mm diameter silicone disks were punched from a slab (0.762 mm thickness) and included in each well. These disks provided a target surface for the bacteria to attach. A norfloxacin-loaded pHEMA disk was also added to each well. At various time points after inoculation, i.e., 0, 2, 4, 8,12, and 24 h, the suspension was collected, and bacteria were stained with a LIVE/DEAD stain (BacLight L13152- Invitrogen) according to manufacturer’s protocol. Images were acquired by using an Axioskop2™ epifluorescent microscope (Carl Zeiss, Jena, Germany) equipped with an xenon bulb light source and two filter sets for FITC (ABS: 490–494 nm, emission: 517 nm) and Texas Red (ABS: 595 nm, emission 620 nm). Data analysis program, “Image J” version 1.38 × (Research Services Branch, National Institute of Mental Health, Bethesda, Maryland; (http://rsb.info.nih.gov/ij/)), was used to obtain cell counts. In addition, the silicone disks were also incubated with LIVE/DEAD stain, and bacteria on the surface were counted.
3. Results and discussion
The model for this drug delivery system was initially developed to provide on-demand, ultrasound-activated pulsatile delivery of ciprofloxacin, a member of the fluoroquinolone family of antibiotics, and insulin [17,18,25]. This previous system exhibited low background release, which was found to be adjustable by altering the surface modification reaction. This led to the development of the sustained release system described here suitable for prevention of infections, such as endophthalmitis after cataract surgery.
3.1. Release from samples with lower density coatings
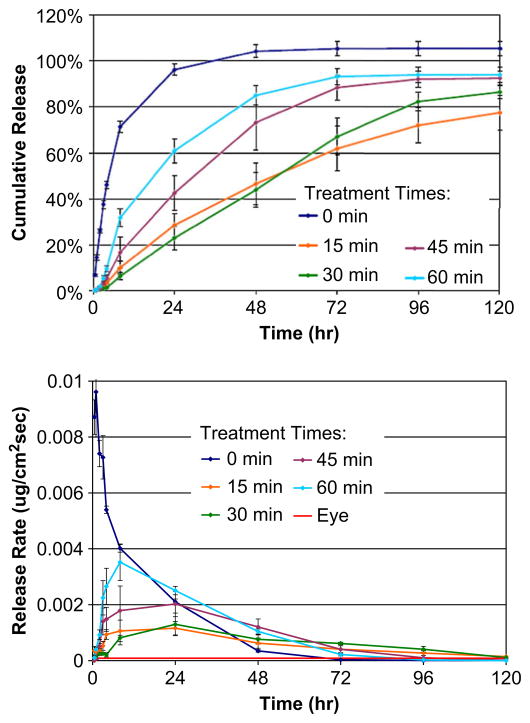
The release of norfloxacin from methylene-coated and uncoated pHEMA disks was characterized over a five-day period, and the cumulative release and release rates of norfloxacin from these samples are shown in Fig. 1a and b, respectively. As expected, pHEMA matrices without the isocyanate surface modification exhibit a burst release of norfloxacin, with the amount of nor-floxacin released decreasing rapidly with time. It was initially hypothesized that increasing the surface modification time would result in a denser packing of methylene chains, which would thus slow the release of norfloxacin. The methylene chain coating acts as a rate-limiting barrier to the drug, enabling better control of drug release and allowing a steady, sustained release [26]. Fig. 1a and b show the cumulative release and the release rates, respectively, of norfloxacin from 0 min, 15 min, 30 min, 45 min, and 60 min treated samples. In addition, the calculated release rate needed for achieving the minimum concentration of norfloxacin to inhibit bacterial proliferation in the eye, which is 8.48 × 10−5 μg/cm2s, is shown in Fig. 1b as a solid red line. It was observed that the samples coated for 15 min and 30 min slowed the release of norfloxacin as expected, while samples coated longer than that, i.e., 45 min and 60 min were not as effective, which is contradictory to our hypothesis about organized n-alkyl changes slowing release. Samples coated for longer times yielded faster release than the samples coated with 15 and 30 min of reaction, but still slower than the control samples, i.e., with no surface modification.
Fig. 1.
Release characteristics of norfloxacin from surface-modified pHEMA matrices. The cumulative release and release rates of various surface-modified pHEMA are plotted as a function of time, in A and B respectively. It was observed that release from 15 and 30 min surface-modified matrices are relatively slower and steadier in comparison to longer modified and unmodified matrices. The calculated minimum flux of norfloxacin from the device necessary to maintain the minimum inhibitory concentration against S. epidermidis is 8.48 × 10−5 ug/cm2 shown as a red line in B. Data is taken from two separate experiments, each with n = 3. Error bars are standard deviations.
The original publications on this alky isocyanate-coated pHEMA controlled-release system suggested that reactions time of 60 min might be essential to prevent rapid antibiotic diffusion from the gel matrix [16]. Improvements in this surface modification protocol, including the use of a dry box to prepare solutions for isocyanate reactions in an inert atmosphere and rigorously dried solvents, led to a more efficient reaction between the isocyanate and hydroxyl groups on the pHEMA [25]. This effectively decreased the time necessary to complete reaction of most of the hydroxyl groups in the surface zone of pHEMA. Thus, reactions longer than 30 min can lead to the disruption of the tightly packed methylene coating, possibly by adding so many n-alkyl chains that the pHEMA structure is sterically distorted (see the next section, below). Thus, it is believed that modifying the pHEMA surface with alkyl isocyanate groups between 15 and 30 min can achieve the most effective sustained release, maintaining the necessary flux to prevent bacterial infection as shown in Fig. 1b.
3.2. SEM analysis at longer reaction times
Although the increased reaction efficiency can explain why shorter surface modification times are effective in controlling norfloxacin from pHEMA matrices release in comparison to the longer times used in early studies, it does not clearly explain why release of drug is faster at longer coating reaction times. To fully understand this phenomenon, the surfaces of the polymer matrices were analyzed by SEM and XPS.
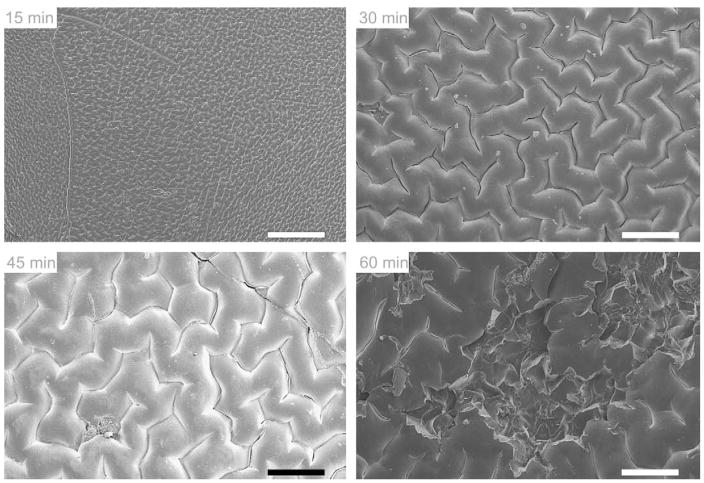
SEM images were taken at 100X magnification, and are shown in Fig. 2. After 15 min reaction with isocyanate, the surface of pHEMA displays a small, regular, corrugated pattern. After 30 min, the corrugated pattern is still observed, but the size of each unit in the pattern has increased. At 45 min, the pattern again appears slightly larger, but deep cracks are observed. Finally at 60 min, clearly visible crevices can be found in the coated pHEMA, and the surface appears damaged. Instead of forming a densely-packed monolayer of methylene chains, the rate-limiting barrier is breached and the gaps create spaces for norfloxacin to escape. With no more available surface hydroxyl groups to react with, the isocyanates in solution could have diffused deeper into the bulk of the polymer and attached themselves to the sub-surface hydroxyl groups causing deformities on the polymer surface. Also, they may react with the –NH portion of the carbamate bond forming allophanates. Surface mechanical disruption is a probable explanation as to why drug is released fastest after undergoing a 60 min modification reaction compared to surfaces modified for shorter times.
Fig. 2.
Scanning electron micrographs of octadecyl isocyanate-modified pHEMA surfaces after 15, 30, 45, and 60 min. The 15-min reaction time shows a low-amplitude reticulated pattern, while longer times show increasing crevasse size and surface damage. Scale bars are 200 μm.
3.3. XPS evidence for surface modification
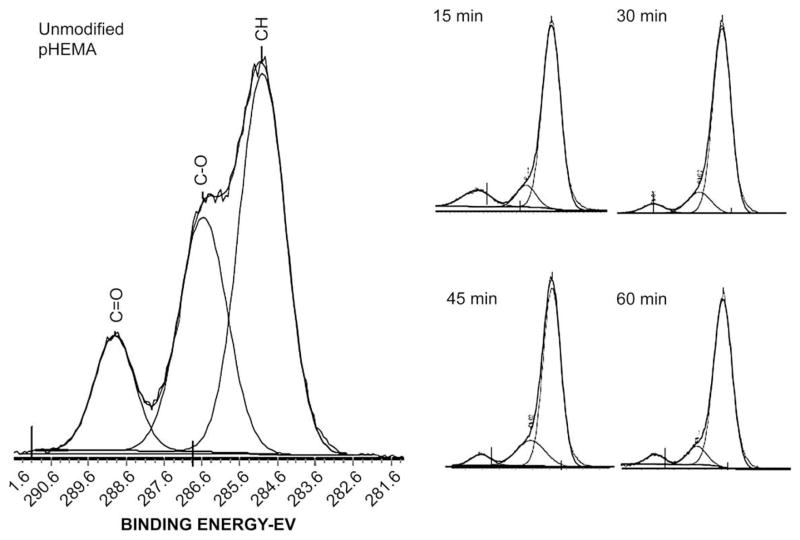
Examination of the chemical composition of the top 50–80Å of the surfaces by XPS after isocyanate modification verified that methylene chains were present on the pHEMA. Table 1 lists the theoretical stoichiometry and experimentally measured elemental composition values for methylene-coated and unmodified pHEMA. Analysis of the uncoated pHEMA showed 71.5% carbon (%C) and 26.7% oxygen (%O), compared to the theoretical composition of pHEMA of 66.7%C and 33.3%O. After a 15 min surface modification, the amount of carbon on the surface increases to 85.7%, while %O was reduced to 10.9%. Also, the surface is now found to contain nitrogen, 3.3% nitrogen (%N). These values are close to the theoretical values for a single HEMA polymer repeat unit reacted with one octadecyl isocyanate. Longer reaction times yield similar composition. Thus, consistent with the observations reported by Kwok et al., even short reaction times yield a multilayer surface coating of ordered methylene chains that is thicker than the approximate 100 Å sampling depth of the XPS. As the surface coating gets still thicker due to reaction of the isocyanate within the bulk of the gel, the outermost 100 Å is still the only region of the specimen sampled, so additional reaction cannot be measured by XPS. Furthermore, after 60 min modification, traces of fluorine were also detected, probably from norfloxacin drug particles exposed to the surface because of the damage induced by prolonged isocyanate reaction. High-resolution carbon 1s spectra were also obtained, and spectra verified previous observations of successful and efficient surface modification as shown in Fig. 3. Unmodified pHEMA has 50% C–C or C–H molecular environments, 33.3% C–O, and 16.7% C–O, which can be identified by their corresponding binding energies at 285 eV, 286.5 eV, and 288 eV, respectively. Even after 15 min of surface modification, the amount of C–C or C–H on the surface increased significantly, while the amount of other carbon species decreased. As with the survey scan, samples reacted with isocyanate at longer reaction times, did not yield significant increase in the surface hydrocarbon content. This change in the hydrocarbon composition before and after surface modification indicates, and verifies previous observations shown by survey and high-resolution carbon spectra, that the methylene coating is present in the surface zone of the pHEMA disks at all time points. Data obtained from XPS and the SEM both justify why 15 and 30 min surface modification times yield the optimal release of norfloxacin.
Table 1.
Elemental composition (atomic %) of pHEMA before and after surface modification with octadecyl isocyanate as determined by XPS.
| Elemental composition (%) |
|||
|---|---|---|---|
| C | O | N | |
| PHEMA: no modification | |||
| Theory | 66.7 | 33.3 | 0 |
| 0 min | 71.5 | 26.7 | 0 |
| PHEMA: with surface modification | |||
| Theory | 83.3 | 13.3 | 3.3 |
| 15 min | 85.7 | 11.0 | 3.3 |
| 30 min | 89.8 | 7.5 | 2.7 |
| 45 min | 87.9 | 9.9 | 2.2 |
| 60 min | 85.1 | 12.5 | 2.2 |
Fig. 3.
XPS High-resolution carbon scans from unmodified and modified pHEMA surfaces. Modified pHEMA samples show a decrease in measured carbonyl and carboxyl bonds compared to unmodified pHEMA, confirming a surface composition of mostly long alkyl chains due to the reaction with octadecyl isocyanate, present at 285 eV.
3.4. Sustained antibiotic release against bacterial proliferation
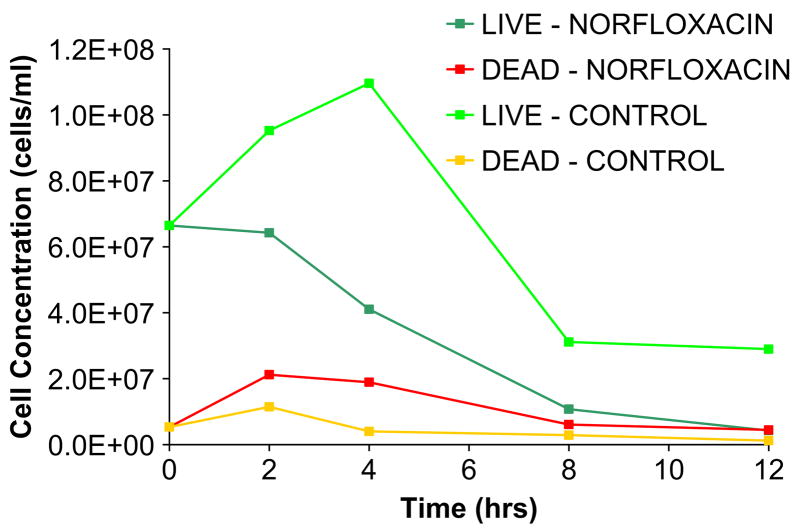
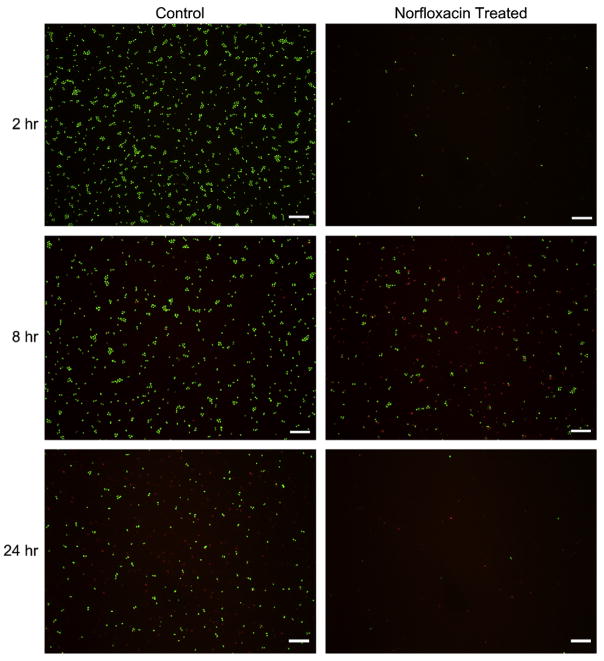
We confirmed that the norfloxacin released from the pHEMA gels retains its ability to kill bacteria. Norfloxacin-loaded pHEMA gels were placed in suspension with S. epidermidis, the most common species of bacteria associated with endophthalmitis infection, and also with a 6 mm diameter silicone rubber disk, representing a medical implant. Incubation with norfloxacin-loaded pHEMA disks show that there are fewer live cells and more dead cells in suspension within a 12 h period compared to the negative control, a pHEMA disk without norfloxacin (Fig. 4). On the silicone surface there are fewer adherent bacteria after 2 h, and after 8 h, although the cell number may be similar, more cells are dead in the norfloxacin-treated sample. At 24 h, there are almost no bacteria present on the silicone surface when norfloxacin is released from pHEMA (Fig. 5). This initial study verifies that nor-floxacin maintains its antibacterial activity towards bacteria in suspension for 24 h, thus reducing the number of viable cells that could possibly colonize the surface. It is important that Norfloxacin released from a pHEMA matrix is able to kill bacteria, both on the surface and in the solution-phase environments as shown in Fig. 5. Endophthalmitis-causing bacteria are initially introduced into the aqueous humor of the anterior chamber of the eye. Stopping initial infection is essential to the further prevention of bacterial colonization and biofilm formation on the intraocular lens. Once a biofilm is established, it is almost impossible to treat in the in vivo environment, so this strategy of destroying solution-phase bacteria and those initially attaching to the surface is justified.
Fig. 4.
S. epidermidis were incubated with a pHEMA disk containing norfloxacin. A blank pHEMA disk was used as a positive control. It was observed that there are less viable cells, and more dead cells in the norfloxacin-treated samples compared to controls.
Fig. 5.
S. epidermidis present on a 6 mm silicone rubber disk after incubation with a norfloxacin-releasing pHEMA disk or with a disk containing no norfloxacin. Released norfloxacin reduces initial bacterial adhesion (2 h), kills more bacteria (8 h), and shows few cells still adherent after 24 h. Scale bars = 10 μm.
We also confirmed that the antibiotic concentration achieved and maintained by this controlled-release system is theoretically sufficient to kill S. epidermidis in the dynamic environment of the anterior chamber of the eye. The flow rate in the anterior chamber of the eye is 1.5–2.5 μL/min and 4 μg/mL is the minimum inhibitory concentration needed to kill S. epidermidis [19,27]. Therefore, the flux of Norfloxacin from our device must be maintained above 8.48 × 10−5 μg/cm2 s (Fig. 1 – Release Rate, red line) in order to be effective. This calculated flux is necessary to prevent endophthalmitis infection and biofilm formation following cataract surgery and insertion of an intraocular lens.
4. Conclusion
In this report, we have demonstrated that steady, sustained antibiotic release from a hydrogel polymer matrix can be achieved by modifying the surface with a hydrophobic, n-alkyl coating. Specifically, octadecyl isocyanate reacted efficiently and sufficiently with surface hydroxyl groups of pHEMA in 15 min producing a controlled-release barrier preventing the expected burst release associated with matrix drug delivery. The surfaces were characterized by SEM and XPS, helping to explain release characteristics and verifying the modification by octadecyl isocyanate. Lastly, norfloxacin released from pHEMA retained its anti-bacterial activity to eliminate bacteria in suspension, and reduced the number of live, surface-adherent bacterial that would otherwise lead to biofilm formation. We believe that our data demonstrate the potential of this sustained antibiotic delivery system to effectively prevent infection on medical implants such as the intraocular lens. We also propose the adaptation of this technology for infection prevention with other medical devices, as well as the use of this system for the delivery of other small molecule drugs and small proteins.
Acknowledgments
This work was funded, in part, from a Translational Partnership Award from the Wallace H. Coulter Foundation to the University of Washington Department of Bioengineering, and a grant from the National Science Foundation (#EEC-0121881) and NIBIB EB007575-01.
Appendix
Figures with essential colour discrimination. Figs. 1, 4 and 5 in this article may be difficult to interpret in black and white. The full colour images can be found in the on-line version, at doi:10.1016/j.biomaterials.2009.06.047.
References
- 1.Parsons C, Jones DS, Gorman SP. The intraocular lens: challenges in the prevention and therapy of infectious endophthalmitis and posterior capsular opacification. Expert Rev Med Devices. 2005;2(2):161–73. doi: 10.1586/17434440.2.2.161. [DOI] [PubMed] [Google Scholar]
- 2.Kresloff MS, Castellarin AA, Zarbin MA. Endophthalmitis. Surv Ophthalmol. 1998;43(3):193–224. doi: 10.1016/s0039-6257(98)00036-8. [DOI] [PubMed] [Google Scholar]
- 3.Aaberg TM, Jr, Flynn HW, Jr, Schiffman J, Newton J. Nosocomial acute-onset postoperative endophthalmitis survey. A 10-year review of incidence and outcomes. Ophthalmology. 1998;105(6):1004–10. doi: 10.1016/S0161-6420(98)96000-6. [DOI] [PubMed] [Google Scholar]
- 4.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–45. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 5.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science (New York, NY) 1999;284(5418):1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez WG, Galea S, Miller J, Ahern J, Chiang W, Kennedy EL, et al. Health status among emergency department patients approximately one year after consecutive disasters in New York City. Acad Emerg Med. 2005;12(10):958–64. doi: 10.1197/j.aem.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Kost J, Langer R. Responsive polymeric delivery systems. Adv Drug Deliv Rev. 2001;46(1–3):125–48. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 8.Sershen S, West J. Implantable, polymeric systems for modulated drug delivery. Adv Drug Deliv Rev. 2002;54(9):1225–35. doi: 10.1016/s0169-409x(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 9.Ratner BD, Hoffman AS. Synthetic hydrogels for biomedical applications. In: Andrade JD, editor. Hydrogels for medical and related applications. Washington D.C: 1975. pp. 1–36. [Google Scholar]
- 10.Wichterle O, Lim D. Hydrophilic gels for biological use. Nature. 1960;185:117–8. [Google Scholar]
- 11.Peppas NA, Yang WHM. Properties-based optimization of the structure of polymers for contact lens applications. Contact Intraocul Lens Med J. 1981;7:300–21. [PubMed] [Google Scholar]
- 12.Tighe BJ. The design of polymers for contact lens applications. Br Polym J. 1976;8:71–90. [Google Scholar]
- 13.Peppas NA. Hydrogels and drug delivery. Crit Opin Colloid Interface Sci. 1997;2:531–7. [Google Scholar]
- 14.Peppas NA Elsevier. Encyclopedia of materials: science and technology. Amsterdam: 2001. Gels for drug delivery; pp. 3492–5. [Google Scholar]
- 15.Tsou TL, Tang ST, Huang YC, Wu JR, Young JJ, Wang HJ. Poly(2-hydroxyethyl methacrylate) wound dressing containing ciprofloxacin and its drug release studies. J Mater Sci. 2005;16(2):95–100. doi: 10.1007/s10856-005-5954-2. [DOI] [PubMed] [Google Scholar]
- 16.Kwok CS, Mourad PD, Crum LA, Ratner BD. Surface modification of polymers with self-assembled molecular structures: multitechnique surface characterization. Biomacromolecules. 2000;1(1):139–48. doi: 10.1021/bm000292w. [DOI] [PubMed] [Google Scholar]
- 17.Kwok CS, Mourad PD, Crum LA, Ratner BD. Self-assembled molecular structures as ultrasonically-responsive barrier membranes for pulsatile drug delivery. J Biomed Mater Res. 2001;57(2):151–64. doi: 10.1002/1097-4636(200111)57:2<151::aid-jbm1154>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Norris P, Noble M, Francolini I, Vinogradov AM, Stewart PS, Ratner BD, et al. Ultrasonically controlled release of ciprofloxacin from self-assembled coatings on poly(2-hydroxyethyl methacrylate) hydrogels for Pseudomonas aeruginosa biofilm prevention. Antimicrob Agents Chemother. 2005;49(10):4272–9. doi: 10.1128/AAC.49.10.4272-4279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merck & Co. I Noroxin. [cited www.merck.com/product/usa/pi_circulars/n/noroxin/noroxin_pi.pdf].
- 20.Ren Z, Wang ZQ, Li R, Luo SY, Deng SJ, Sun XG. Etiological analysis on bacterial endophthalmitis. Zhonghua Yan Ke Za Zhi. 2007;43(12):1106–9. [PubMed] [Google Scholar]
- 21.Benz MS, Scott IU, Flynn HW, Jr, Unonius N, Miller D. Endophthalmitis isolates and antibiotic sensitivities: a 6-year review of culture-proven cases. Am J Ophthalmol. 2004;137(1):38–42. doi: 10.1016/s0002-9394(03)00896-1. [DOI] [PubMed] [Google Scholar]
- 22.Procedimiento para la obtencion del clorhidrato del acido del 1-etil-6-fluoro-1,4-dihidro-4-oxo-7-(1-piperazinil)-3-quinolinico. Spain: 1985. [Google Scholar]
- 23.Ratner BD, Miller IF. Transport through cross-linked poly(2-hydroxyethyl methacrylate) hydrogel membranes. J Biomed Mater Res. 1973;7:353–67. doi: 10.1002/jbm.820070407. [DOI] [PubMed] [Google Scholar]
- 24.Ratner BD, Castner D. Electron spectroscopy for chemical analysis. In: Vickerman JC, editor. Surface analysis: the principal techniques. New York: John Wiley & Sons, Ltd; 1997. pp. 43–98. [Google Scholar]
- 25.Noble ML, Mourad PD, Ratner BD. Controlled release of antibiotics from pHEMA-based matrices using ultrasound. in preparation. [Google Scholar]
- 26.Heller J, Hoffman AS. Drug delivery systems. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials science. San Diego: Elsevier; 2004. pp. 628–9. [Google Scholar]
- 27.McLaren JW, Trocme SD, Relf S, Brubaker RF. Rate of flow of aqueous humor determined from measurements of aqueous flare. Invest Ophthalmol Vis Sci. 1990;31(2):339–46. [PubMed] [Google Scholar]