Abstract
Evidence suggests that patients with irritable bowel syndrome (IBS) are hyper-responsive to environmental, physical, and visceral stimuli. IBS patients also frequently report poor sleep quality. This study compared serum cortisol and plasma catecholamine levels during sleep between women with IBS (n = 30) and healthy controls (n = 31), and among subgroups within the IBS sample based on predominant stool patterns, IBS-diarrhea (n = 14), IBS-constipation (n = 7), and IBS-alternators (n = 9). Cortisol was measured from serial blood samples drawn every 20 minutes, and catecholamines every hour, in a sleep laboratory from 8 PM until awakening. Because of the varied sleep schedules of the individual participants, each subject’s hormone series time base was referenced with respect to their onset of Stage-2 sleep. Overall, there were no significant differences in cortisol or catecholamine patterns between women with IBS and controls, nor were there any group by time interactions. However, women with constipation-predominant IBS demonstrated significantly increased norepinephrine, epinephrine, and cortisol levels throughout the sleep interval, and women with diarrhea-predominant IBS were significantly lower on norepinephrine and cortisol. These results suggest that differences in neuroendocrine levels during sleep among IBS predominant bowel pattern subgroups may be greater than differences between IBS women and controls. Neuroendocrine profiles during sleep may contribute to our understanding of symptom expression in IBS.
Keywords: cortisol, catecholamines, sleep, irritable bowel syndrome
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder. In the United States, approximately 5.4% of women in the population have been diagnosed with IBS.(1) Of those seeking health care services for a diagnosis of IBS the majority are women. The diagnosis of IBS is associated with significant health costs ranging from clinician visits and diagnostic studies to over-the-counter medications.(2-4) As a group, patients with IBS also report a lower health-related quality of life. (4, 5)
Laboratory studies have revealed that IBS patients are hypervigilant to external (stress)(6) and internal (barostat) stimuli.(7) Hypervigilance or hyperarousal can be defined as increased readiness or responsiveness to stress. Physiologically, hyperarousal may be associated with activation of neuroendocrine systems including the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal axis (HPA). Clinically, hyperarousal may be manifested in report of poor sleep as well as disruptions in sleep patterns.(8, 9)
Studies related to HPA axis activity in patients with IBS have yielded conflicting results. Several investigators measuring blood, urine, or saliva noted higher morning levels of cortisol in subgroups of patients with IBS relative to healthy controls.(10-13) Cortisol levels are also higher in anticipation of a stress (14) in heterogeneous groups of IBS patients as compared to those with only constipation and healthy controls. However, others have failed to find either IBS versus control or bowel pattern subgroup differences in basal cortisol levels (15, 16) as well as in response to dexamethasone suppression testing.(17) Discrepancies may be related to differences in measurement strategies, timing, patient characteristics such as bowel pattern predominance, and/or sleep quality.
Whether IBS bowel pattern subgroup differences exist in terms of neuroendocrine markers around the time of sleep onset and during sleep is the focus of this report. Therefore, the aims of these analyses are to 1) compare measures of neuroendocrine arousal (serum cortisol, plasma norepinephrine, and plasma epinephrine) immediately prior to and during sleep in IBS and control women, and 2) compare measures of neuroendocrine arousal among the bowel pattern subgroups IBS Constipation (IBS-C), IBS Diarrhea (IBS-D), and IBS Alternating (IBS-A). Based on the literature, we hypothesized that women with IBS would exhibit neuroendocrine arousal both pre- and during sleep as exhibited by higher levels of cortisol and catecholamines.
Materials and Methods
Sample
Women with IBS and healthy control women were recruited through community advertisements. Potential participants were initially screened for study eligibility. To be enrolled, the women in the IBS group had to have a medical diagnosis of IBS and currently be experiencing symptoms compatible with the Rome-II criteria for IBS.(18) Women in the control group were excluded if they had symptoms of a functional GI disorder. Women in either group were excluded if they had a: a) history of GI pathology, b) GI surgery or gynecological pathology, c) significant co-morbid condition, d) known cardiac dysrrhythmia, e) sleep disorder, f) currently taking medications that could interfere with sleep, cortisol, or catecholamines, (e.g., beta blockers, benzodiazepines, inhalant asthma medication, or antidepressants [e.g., SSRIs], or g) GI serotonergic agents. Women on hormonal contraceptives were excluded. Approval was obtained from the university’s human subjects’ institutional review board prior to recruitment. Eighty-eight women were initially enrolled; four IBS and three control women withdrew from the study before the sleep laboratory phase because of time commitments, persistently low hematocrit levels, or evidence during the diary phase (see procedures below) of two anovulatory cycles. Eight subjects did not complete the first two nights of the sleep protocol, and an additional twelve women did not complete the third (blood draw) night, resulting in a sample of 61 participants (30 IBS women and 31 control women) for the analyses presented in this report.
Procedures
Participants were enrolled at the initial visit, where they gave written consent, completed questionnaires, and were oriented to the sleep laboratory. At the start of the woman’s next menses, she completed a symptom/sleep diary each evening for one menstrual cycle, tested her urine for the luteinizing hormone (LH) surge (ClearPlan Easy, Unipath Research: Princeton, NJ, USA) and slept 3 consecutive nights in the laboratory 7 ± 2 days after a positive LH surge. On the third night, sequential blood samples were drawn every 20 minutes through a venous catheter between 2000 and 0700. At completion, the participating women were financially compensated.
Sleep Assessment Protocol
For this analysis, only sleep data collected on the third night were used. Prior to the sleep study, women were instructed to refrain from drinking caffeinated beverages, taking acetaminophen or aspirin within 6 hours of bedtime, drinking alcohol, or napping. Women came to the sleep laboratory at least 2 hours before their normal sleep time and had electrodes placed on their head, face, chest, and legs for a standard polysomnography (PSG) assessment.(19) Once the electrode placement was completed, subjects read or watched television in bed until their typical bedtime when the lights were turned out. During the first night, subjects were screened for two common disqualifying sleep disorders, apnea/hypopnea and periodic leg movements (PLMs). PSG recording included electroencephalography, electromyography, and electrooculography.(20)
Measures
Descriptive information collected from the participants included age in years, body mass index (BMI), ethnic affiliations, marital status, level of formal education, type of work, and job title. The Pittsburgh Sleep Quality Index (PSQI) was used to assess subjective sleep quality and sleep disturbances over the prior month.(21, 22) Detailed descriptions about the methods for measurement of objective sleep indices derived from PSG that were used in this study have been previously reported.(19, 20) In the present analyses, three standard sleep indices sensitive to disturbance in global sleep macro-architecture were used (Total Sleep Time, Sleep Efficiency Index, and Fragmentation Index). The time of first sustained passage into Stage 2 sleep was used as a reference point to synchronize the within-subject hormone time series.
A measure of retrospective psychological distress, the Symptom Checklist-90-R, includes 90 symptoms making up 9 subscales and a set of 7 additional items. The scales reported in the present study include: depression (SCL-DEP), anxiety (SCL-ANX), and global severity index (SCL-GSI).(23) In addition to the raw Likert item mean scaling, the SCL-90 subscales will be presented as standardized T-scores (based on a large (N=480) female non-patient normal control sample) and also with respect to criteria for “caseness” (individual subscale T-scores >= 63), both defined as described in the SCL-90R manual.(23) The 28-item Childhood Trauma Questionnaire (CTQ) was used to assess self-reported history of childhood physical, emotional, or sexual abuse.(24)
The blood samples for serum cortisol determination were collected in silicone coated vacutainer (Becton Dickinson and Company, Franklin Lakes, NJ, USA) tubes, allowed to coagulate and centrifuged. The serum layer was then removed and stored at −70 °C. Serum cortisol was assayed by chemiluminescence using the automated Immulite Analyzer (Siemens Healthcare Diagnostics, Deerfield, IL, USA). The results are reported as μg dL-1. The intra-assay variation was 5.4% and the inter assay variation was 9.87%. Blood samples for plasma catecholamine determination were collected with EDTA preserved vacutainer tubes and centrifuged at 1000 g for 10 minutes at 4 °C. The plasma layer was removed and stored at −70 °C. Plasma norepinephrine (NE) and epinephrine (E) were measured by 125I-Radio-immunoassay (Bi-CAT-RIA, ALPCO Diagnostics, Salem, NH, USA). The results are reported as pg mL-1. The intra-assay variation was 9.6% and the inter assay variation was 11.1%.
Preprocessing of the Physiological Data
All assays were conducted in duplicate and were manually screened for data quality. The blood draw collection logs were used to evaluate and to exclude unusual values based on evidence of procedural issues (e.g. difficult blood draw, blocked lines, recent subject movement or postural change including trips to restroom). Isolated missing or disqualified values interior to the within-subject hormone time series were replaced using running median smoothing and linear interpolation. In order to make the time course more comparable across subjects, the time base for each woman’s data sequence was synchronized to her first sustained passage into Stage 2 (or deeper) sleep. The within-subject hormone series were aggregated into hourly medians, based on time blocks representing whole hours before and after the onset of sleep. Because of variations in the length of participants’ sleep, for consistency of the data structure the subsequent statistical analyses were based on hourly median hormone profiles that uniformly extend out to 6 hours after the onset of sleep, although some subjects had as many as 9 hours of qualifying data.
Statistical Analyses
Demographic and personal characteristics were summarized as means and standard deviations for interval data, and percents for categorical data. Background group and subgroup differences were assessed with Student’s t-tests and oneway ANOVAs for the interval variables, and Fisher’s Exact Tests or Chi-Square independence tests for the categorical variables. A generalized repeated measures ANOVA, with a main group effect (G), a main time effect (T), and a group by time interaction (G×T) was used. To accommodate a small number of missing samples, analyses were executed using a linear mixed model repeated measures ANOVA (LMMRMA) with cubic representation of the within-subject time effect, and an AR(1) specification of the error correlation working matrix. The formal testing strategy used was for each of the three dependent variables (cortisol, NE, E) to be fitted to an omnibus four group (Controls, IBS-C, IBS-D, IBS-A) model. If the group-by-time interaction effect was not significant, those terms were dropped, and the main effects model was re-estimated. If the main group effect of the omnibus model was significant, then pairwise contrasts (e.g. IBS vs. Control, IBS-C vs. IBS-D, etc.) were computed. The reported p-values were not adjusted for multiple testing. Readers are advised to interpret the consistency of effects across the full pattern of results, but to treat the reported significance of individual subgroup comparisons cautiously.
Age and BMI were incorporated into all the ANOVA models as control covariates, in part because of modest empirical correlations with the neuroendocrine outcome variables. Mean nocturnal cortisol levels were significantly associated with age (p = 0.002). Epinephrine demonstrated a non-significant trend association with age (p = 0.061), but was significantly linked to BMI (p < 0.001). The impact on the ANOVA models of other potential factors (psychological distress, self-reported history of childhood abuse/neglect, abdominal pain severity, and subjective and objective measures of poor sleep) were studied in a sensitivity analysis by adding each covariable one at a time into the model. All three dependent variables (Cortisol, NE, E) were notably non-normal and skewed toward the right. Hence, logarithmic (base-10) transformations were applied. The means and 95% confidence intervals endpoints presented in the figures were computed using the log-transformed data values, then back-transformed to the original scale of measurement for ease of interpretation. Similarly the model results are reported as multiplicative effects. All statistical analyses were executed using SPSS version 15.0 (SPSS Inc, Chicago, Illinois, USA).
Results
Descriptive Characteristics
Table 1 summarizes the demographic and personal characteristics of the Control (n= 31) and IBS (n= 30) participants. There were no significant group differences in age, BMI, and other demographic characteristics. Not surprisingly, the IBS participants reported significantly more abdominal pain. Women in the IBS group reported more subjective sleep problems (p < 0.001) and psychological distress (p < 0.008) than the Control women. However, objective measures of sleep disturbance derived from Night-3 PSG were not significantly different (p > 0.35). Detailed information about the objective and subjective sleep characteristics on the first two nights of the protocol have been reported elsewhere.(19, 20) A history of childhood physical, emotional, or sexual abuse meeting threshold clinical criteria was reported by 50% of the IBS participants, compared with 19% of the Controls (p = 0.016). There were no statistically significant differences among the IBS subgroups in demographic characteristics, psychological distress, objective sleep measures, and report of child abuse. Overall the IBS-C group did tend to report higher levels of anxiety and depression as compared to the IBS-D group.
Table 1.
Demographic and Personal Characteristics of Control and and IBS Subgroups.
| Controls (n = 31) |
IBS (n=30) |
IBS-C (n = 7) |
IBS-D (n = 14) |
IBS-A (n = 9) |
|
|---|---|---|---|---|---|
|
Demographic:
|
|||||
| Age [years, Mean (SD)] | 32 (7) | 30 (8) | 31 (8) | 29 (7) | 32 (8) |
| BMI [Mean (SD)] | 24.1 (4.8) | 23.3 (4.2) | 21.8 (1.5) | 24.6 (5.3) | 22.5 (3.4) |
| Premenopausal [%] | 100 | 100 | 100 | 100 | 100 |
| Ethnicity [% Caucasian] | 55 | 70 | 57 | 78 | 88 |
| Married/Partnered [%] | 23 | 33 | 29 | 21 | 56 |
| College Degree [%] | 74 | 60 | 57 | 57 | 67 |
|
Subjective Sleep Disturbance:
|
|||||
| PSQI Global Score [Mean (SD)] | 3.9 (1.8) | 6.5 (3.6) | 7.0 (2.9) | 5.9 (3.2) | 7.0 (4.8) |
| Poor Sleep (% PSQI >= 5) | 41.9 | 70 | 85.7 | 57.1 | 77.8 |
| Very Poor Sleep (% PSQI >= 6) | 9.7 | 60 | 71.4 | 50.0 | 66.7 |
|
Objective Sleep: [Mean (SD)] |
|||||
| Total Sleep Time (minutes) | 361 (51) | 351 (67) | 340 (87) | 334 (54) | 379 (67) |
| Sleep Efficiency Index (%) | 79 (12) | 79 (13) | 78 (15) | 82 (14) | 80 (11) |
| Fragmentation Index (#/hour) | 8.1 (3.1) | 8.3 (3.0) | 7.8 (2.6) | 7.5 (3.1) | 9.7 (2.7) |
|
Psychological Distress:
|
|||||
| SCL-90R GSI [Mean(SD)] GSI T-Score [Mean (SD)] GSI T-Score > 63 [n (%)] |
0.31 (0.22) 50 (8) 1 (3) |
0.69 (0.73) 57 (12) 8 (27) |
0.93 (0.55) 62 (8) 4 (57) |
0.54 (0.77) 53 (13) 2 (14) |
0.72 (0.79) 58 (12) 2 (22) |
| SCL-90R ANX [Mean(SD)] ANX T-Score [Mean (SD)] ANX T-Score > 63 [n (%)] |
0.14 (0.39) 45 (11) 0 (0) |
0.65 (0.77) 54 (13) 8 (27) |
1.01 (0.72) 61 (10) 4 (57) |
0.51 (0.82) 50 (14) 2 (14) |
0.59 (0.71) 54 (13) 2 (22) |
| SCL-90R DEP [Mean(SD)] DEP T-Score [Mean (SD)] DEP T-Score > 63 [n (%)] |
0.49 (0.38) 52 (9) 2 (7) |
0.95 (0.97) 56 (14) 9 (30) |
1.04 (1.05) 63 (9) 3 (43) |
0.67 (0.87) 52 (15) 3 (21) |
0.96 (0.98) 57 (14) 3 (33) |
|
Childhood Abuse: [n (%)] |
|||||
| CTQ Physical Abuse | 2 (7) | 8 (27) | 2 (29) | 5 (36) | 1 (11) |
| CTQ Emotional Abuse | 3 (10) | 10 (33) | 4 (57) | 2 (14) | 4 (44) |
| CTQ Sexual Abuse | 2 (7) | 7 (23) | 2 (29) | 3 (21) | 2 (22) |
Notes: IBS = Irritable Bowel Syndrome, IBS-C = IBS constipation predominant. IBS-D = IBS diarrhea predominant. IBS-A = IBS alternator predominant, BMI = Body Mass Index, PSQI = Pittsburgh Sleep Quality Index, SCL-90R = Symptom Checklist 90 – Revised, SCL-90R GSI = SCL-90R Global Symptom Index, SCL-90R ANX = SCL-90R Anxiety subscale, SCL-90R DEP = SCL-90R Depression subscale, CTQ = Childhood Trauma Questionnaire.
Plasma Norepinephrine
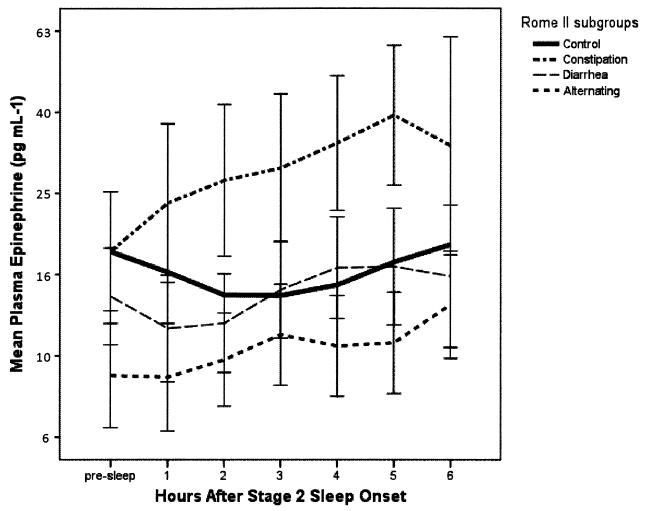
The geometric mean nocturnal plasma NE profiles for the four groups are plotted in Figure 1. Controlling for age and BMI in a four- group omnibus LMMRMA, the Group-by-Time interaction effect was not significant (p = 0.812). Both the omnibus group main effect and the time main effect were significant (p<0.001). Table 2 presents pair-wise comparisons of the groups. Because the outcome variable used in the analysis was the logarithm (base 10) transform of the measured NE values, the group comparisons are presented as multiplicative effects with respect to the scale of the original measurements. Consistent with Fig. 1, the IBS group as a whole was not significantly different in geometric mean value than the Control group (p = 0.896). The IBS-C subgroup maintained NE values that were 57% higher than the Control group women (p < 0.003), while the IBS-D subgroup was 37% lower than the Controls (p < 0.001). The largest contrast was between the IBS-C and the IBS- D subgroups (+149%).
Figure 1.
Plasma Norepinephrine During Sleep. The geometric mean profiles of plasma norepinephrine significantly vary as a function of hours since onset of polysomnographically confirmed sleep (p < 0.001). There is also a significant effect for group (p < 0.001). However the combined IBS sample was not significantly different than the control women. Vertical intervals describe geometric means +/− 1 SE. Error bars for the larger Control group are not shown. Note that the values on the ordinate are represented in linear measurement units, but are plotted on an axis with logarithmic spacing.
Table 2.
Main Effects Group Contrasts.
| Multiplicative Effect |
Percent Difference |
95% CI (LCI, UCI |
p-value | |
|---|---|---|---|---|
|
Plasma Norepinephrine
|
||||
| IBS vs. Controls | 0.99 | −1% | (−19%, 20%) | 0.896 |
| IBS-C vs. Controls | 1.57 | 57% | (16%, 113%) | 0.003 |
| IBS-D vs. Controls | 0.63 | −37% | (−50%, −20%) | 0.001 |
| IBS-A vs. Controls | 1.04 | 4% | (−21%, 39%) | 0.766 |
| IBS-C vs. IBS-D | 2.49 | 149% | (77%, 250%) | 0.001 |
| IBS-C vs. IBS-A | 1.51 | 51% | (4%, 118%) | 0.031 |
| IBS-D vs. IBS-A | 0.61 | −39% | (−56%, −16%) | 0.003 |
|
Plasma Epinephrine
|
||||
| IBS vs. Controls | 0.89 | −11% | (−33%, 17%) | 0.383 |
| IBS-C vs. Controls | 2.03 | 103% | (31%, 213%) | 0.002 |
| IBS-D vs. Controls | 1.07 | 7% | (−24%, 50%) | 0.701 |
| IBS-A vs. Controls | 0.67 | −33% | (−56%, 1%) | 0.055 |
| IBS-C vs. IBS-D | 1.90 | 90% | (17%, 208%) | 0.009 |
| IBS-C vs. IBS-A | 3.04 | 204% | (77%, 419%) | 0.001 |
| IBS-D vs. IBS-A | 1.60 | 60% | (1%, 155%) | 0.047 |
|
Serum Cortisol
|
||||
| IBS vs. Controls | 0.99 | −1% | (−8%, 8%) | 0.948 |
| IBS-C vs. Controls | 1.16 | 16% | (2%, 32%) | 0.025 |
| IBS-D vs. Controls | 0.92 | −8% | (−17%, 2%) | 0.106 |
| IBS-A vs. Controls | 0.95 | −5% | (−16%, 6%) | 0.369 |
| IBS-C vs. IBS-D | 1.26 | 26% | (9%, 46%) | 0.002 |
| IBS-C vs. IBS-A | 1.22 | 22% | (5%, 43%) | 0.011 |
| IBS-D vs. IBS-A | 0.97 | −3% | (−15%, 11%) | 0.635 |
Notes: IBS = Irritable Bowel Syndrome, IBS-C = IBS Constipation, IBS-D = IBS Diarrhea, IBS-A = IBS Alternating. LCI = lower endpoint of the 95% confidence interval, UCI = upper endpoint of the 95% confidence interval. The multiplicative effect is a unitless ratio describing how many times larger one group is with respect than another. If the multiplicative effect is greater than 1.0, then the level for the first group is larger than the level for the second group. If the multiplicative effect is less than 1.0, the level of the first group is smaller than that of the second group.
Plasma Epinephrine
The geometric mean nocturnal plasma E profiles are plotted in Fig. 2. Group-by-Time interaction effects were not significant (p = 0.256), but the main effects for Group and Time were each strongly significant (P < 0.001). On average, the IBS group was 11% lower, but not significantly different, than the Control group (p = 0.383). The E values in the IBS-C subgroup were 102% higher than those of the Control group women (p = 0.002), while the IBS-A subgroup were 33% lower than the Controls (p = 0.055). As is apparent from Fig. 2, the largest contrast for the nocturnal E measures was between the IBS-C and the IBS-A subgroups (+203%, p <0.001).
Figure 2.
Plasma Epinephrine During Sleep. The geometric mean profiles of plasma epinephrine significantly vary as a function of hours since onset of sleep (p < 0.001), and by group (p < 0.001). The combined IBS sample was not significantly different than the control women. Vertical intervals describe geometric means +/− 1 SE. The values on the ordinate are represented in linear measurement units, but are plotted on an axis with logarithmic spacing.
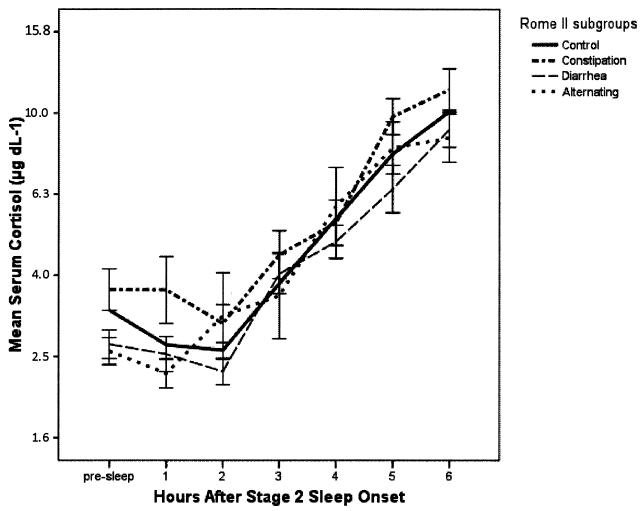
Serum Cortisol
The geometric mean serum cortisol profiles are plotted in Fig. 3. The omnibus main effects for group (p < 0.013) and the time (p < 0.001) were statistically significant, but the interaction effect for group and time was not significant (p = 0.796). The IBS group as a whole was not different with respect to the Control group (Table 2). However, the IBS-C subgroup maintained cortisol values that were 16% higher than the Control group women (p = 0.025), while the IBS-D subgroup tended to be 9% lower than the Controls (p < 0.106). The largest contrast effect size for serum cortisol was between the IBS-C and the IBS-D subgroups (+26%, p < 0.002).
Figure 3.
Serum Cortisol During Sleep. The geometric mean profiles of plasma epinephrine vary strongly as a function of hours since onset of sleep (p < 0.001), and slightly less strongly by group (p < 0.013). The combined IBS sample was not significantly different than the control women. Vertical intervals describe geometric means +/− 1 SE. The values on the ordinate are represented in linear measurement units, but are plotted on an axis with logarithmic spacing.
Exploratory evaluation of the impact of other potentially relevant covariables
The sensitivity of the group and subgroup effects described above to other potentially mediating or confounding factors (abdominal pain severity, psychological distress, self-reported physical, emotional, or sexual abuse history, and subjective and objective measures of sleep) was assessed by adding each of these variables, one at a time, as covariates in the previously described analysis models. None of these variables altered the direction, strength, or significance of the results presented in Table 1. However, several of these covariates were significant contributors to the overall models. The SCL-90 GSI was a significant covariate for plasma NE (p = 0.018) and E (p = 0.017), but presented a non-significant weak trend for serum cortisol (p = 0.082). SCL- 90 Depression and Anxiety subscales were not significant covariates in any of the models (p > 0.20). The PSQI Global score was a significant covariate for cortisol (p = 0.013), but not for NE and E. Abdominal pain severity, objective sleep measures (Total Sleep Time, Sleep Efficiency, Sleep Fragmentation) and the variables encoding childhood physical, emotional, or sexual abuse were non-significant covariates. Thus while several global indices of psychological distress and subjective sleep difficulties contributed significantly to the total explained variance as covariates in analysis models predicting NE, E, and cortisol, the inclusion of these variables did not alter the patterns of the main effects for group and time.
Discussion
In this study, group mean profiles of serum cortisol, plasma NE, and plasma E collected during sleep were not different between the overall IBS sample and the control group, either in level or pattern over time since onset of sleep. We confirmed the common finding that subjective sleep problems, psychological distress, and childhood abuse are reported at significantly higher levels in women with IBS than control women. A simple hypothesis that women with IBS uniformly sustain a state of biobehavioral hyperarousal through the sleep period, with HPA and ANS neuroendocrine involvement, is not well-supported in this sample.
Chang and colleagues in a carefully controlled study of 24 hour measures of cortisol and ACTH found that plasma cortisol levels were marginally higher between 0200 and 0600 in women IBS patients.(25) However, plasma ACTH levels were markedly lower during the day and early morning hours, suggesting HPA dysfunction particularly upon awakening during the day in women with IBS. An earlier study from our group also found higher levels of urine cortisol in a morning first voided sample in a mixed group of IBS patients (26). In both of these studies, the IBS samples were composed of IBS-A, IBS-C and IBS-D women and bowel subgroup differences were not explored.
When IBS subgroups based on predominant bowel pattern were considered, a different story emerged. Women with constipation-predominant IBS had elevated NE, E, and cortisol levels throughout the sleep period, while women with diarrhea-predominant IBS were lower on NE and cortisol. The observed neuroendocrine profile differences among subgroups based on predominant bowel pattern were not diminished by controlling for history of childhood abuse, abdominal pain severity, or objective measures of sleep disturbance.
In this sample, a specific subgroup (IBS-C) of the women presented a nocturnal neuroendocrine profile that would be consistent with a hyperarousal hypothesis affecting both HPA axis and ANS functioning. But based on these findings, another subgroup (IBS-D) might equally be described as hypo-aroused during sleep. Such subgroup differences may not be unique to sleep. Another group using a daytime laboratory meal stimulus found higher levels of salivary cortisol pre-meal in IBS-C (n=12) as compared to controls (n=20). In addition, they noted bowel pattern subgroup differences in response to the meal itself with the IBS-D group (n=12) showing increased salivary cortisol levels and vagal withdrawal.
Similarly our findings of higher night time levels of NE and E in the IBS-C group are not inconsistent with the study of Mazur and colleagues who measured a single daytime adrenaline and noradrenaline level and HRV indices in a laboratory setting.(27) In addition to finding a higher level serum catcholamines (adrenaline and noradrenaline) in IBS-C patients (n=23) relative to healthy controls (n=30), they also found that the elevated levels of catecholamines were significantly correlated with simultaneously measured low vagal tone (reduced HRV). These findings suggest that pattern of neuroendocrine arousal in patients with IBS-C is not unique to sleep.
Findings that subgroups of IBS patients might have different physiological characteristics are not unprecedented. Prior studies have shown ANS differences by bowel pattern predominance subgroups. In an earlier study, employing 24 hour heart rate variability (HRV) measurement, we found lower levels of indicators of parasympathetic activity in IBS-C women, and higher levels in IBS-D women, especially in the IBS patients reporting severe or very severe pain.(28, 29)
Based on nights one and two sleep data for the same set of subjects described in the current report, we previously reported lower HRV parasympathetic activity in IBS-C as compared to IBS-D. (20) Early REM and NREM sleep periods were the time of greatest group differences. Together these findings of enhanced adrenal secretion of cortisol and higher levels of NE and E (on Night-3) and reduced vagal activity (on Night-1 and Night-2) suggest a consistent pattern of hyperarousal in IBS-C. However, the relatively small sample sizes and the fact that indicators of psychological distress were somewhat higher in the IBS-C group means the results should be interpreted with caution. Even though controlling for psychological distress did not substantially change the results, it is possible that psychological distress does play a role in the relationship between predominant bowel pattern and arousal.
The primary limitation affecting the study is the sample size. The study was statistically powered to be able to detect a moderate effect size difference between the combined IBS group and the control group, if one existed. But the most interesting findings were in contrasts among the smaller subgroups defined by IBS bowel pattern-predominance. Another concern is the self selection of subjects for participation in the study. Those IBS patients who were willing to undergo three nights in a sleep lab with an IV line during the third night may be a select subset of the population of IBS patients. Subjects were recruited with community advertisements and were screened for conditions and medications that might influence sleep and/or ANS measures. Thus, the included subjects may not be typical of the IBS women who present clinically, especially to consultants in tertiary GI clinics.
Conclusions
While there were no distinguishing differences in the nocturnal profiles for plasma NE, E, and serum cortisol between the IBS women as a whole and the control women, there were differences among the IBS subgroups defined by bowel pattern predominance. IBS-C women had higher average nocturnal levels of all three neuroendocrine measures. IBS-D women had lower levels of nocturnal NE and cortisol. These results suggest that the hypothesis of a physiological state of hyperarousal of the ANS and HPA axis could be true among IBS-C but not IBS-D. While consistent with other published reports, further research would be useful to confirm these findings.
Acknowledgements and Disclosures
This study was supported by NINR, NIH NR01094 and P30 NR04001.
We wish to thank Anne Poppe, Dr. Beth Hacker, Joyce Tsuji, James Rothermel, Ernest Tolentino, Wimon Deechakawan, and especially, the women who gave so generously of their time to participate in this study.
Abbreviations
- IBS
Irritable Bowel Syndrome
- GI
Gastrointestinal
- IBS-D
Diarrhea-predominant IBS
- IBS-C
Constipation-predominant IBS
- IBS-A
Alternating predominant stool pattern in IBS
- NE
Plasma Norepinephrine
- E
Plasma Epinephrine
- PSQI
Pittsburgh Sleep Quality index
- PSG
Polysomnography
- SCL-GSI
Symptoms Checklist 90-Revised Global Severity Index
- SCL-ANX
Symptoms Checklist 90-Revised Anxiety Subscale
- SCL-DEP
Symptoms Checklist 90-Revised Depression Subscale
- CTQ
Childhood Trauma Questionnaire
- ANOVA
Analysis of Variance
- LMMRMA
Linear Mixed Model Repeated Measures ANOVA
- BMI
Body Mass Index, (weight(kg) height (m)−2)
- ANS
Autonomic Nervous System
- HPA
Hypothalamic-Pituitary-Adrenal
Footnotes
Competing interests: the authors have no competing interests.
References
- 1.Chey WD, Olden K, Carter E, Boyle J, Drossman D, Chang L. Utility of the Rome I and Rome II criteria for irritable bowel syndrome in U.S. women. American Journal of Gastroenterology. 2002;97:2803–2811. doi: 10.1111/j.1572-0241.2002.07026.x. [DOI] [PubMed] [Google Scholar]
- 2.van Tilburg MA, Palsson OS, Levy RL, et al. Complementary and alternative medicine use and cost in functional bowel disorders: a six month prospective study in a large HMO. BMC Complement Altern Med. 2008;8:46. doi: 10.1186/1472-6882-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyrop KA, Palsson OS, Levy RL, et al. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment Pharmacol Ther. 2007;26:237–248. doi: 10.1111/j.1365-2036.2007.03370.x. [DOI] [PubMed] [Google Scholar]
- 4.Brun-Strang C, Dapoigny M, Lafuma A, Wainsten JP, Fagnani F. Irritable bowel syndrome in France: quality of life, medical management, and costs: the Encoli study. Eur J Gastroenterol Hepatol. 2007;19:1097–1103. doi: 10.1097/MEG.0b013e3282f1621b. [DOI] [PubMed] [Google Scholar]
- 5.Rey E, Garcia-Alonso MO, Moreno-Ortega M, Alvarez-Sanchez A, Diaz-Rubio M. Determinants of quality of life in irritable bowel syndrome. J Clin Gastroenterol. 2008;42:1003–1009. doi: 10.1097/MCG.0b013e31815af9f1. [DOI] [PubMed] [Google Scholar]
- 6.Naliboff BD, Waters AM, Labus JS, et al. Increased acoustic startle responses in IBS patients during abdominal and nonabdominal threat. Psychosom Med. 2008;70:920–927. doi: 10.1097/PSY.0b013e318186d858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang L, Mayer EA, Labus JS, et al. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;291:R277–284. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- 8.Bastien CH, St-Jean G, Morin CM, Turcotte I, Carrier J. Chronic psychophysiological insomnia: hyperarousal and/or inhibition deficits? An ERPs investigation. Sleep. 2008;31:887–898. doi: 10.1093/sleep/31.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. Chronic Insomnia and Stress System. Sleep Med Clin. 2007;2:279–291. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patacchioli FR, Angelucci L, Dellerba G, Monnazzi P, Leri O. Actual stress, psychopathology and salivary cortisol levels in the irritable bowel syndrome (IBS) J Endocrinol Invest. 2001;24:173–177. doi: 10.1007/BF03343838. [DOI] [PubMed] [Google Scholar]
- 11.Katayama M, Nomura K, Ujihara M, Obara T, Demura H. Age-dependent decline in cortisol levels and clinical manifestations in patients with ACTH-independent Cushing’s syndrome. Clin Endocrinol (Oxf) 1998;49:311–316. doi: 10.1046/j.1365-2265.1998.00551.x. [DOI] [PubMed] [Google Scholar]
- 12.Heitkemper M, Jarrett M, Levy R, et al. Self-management for women with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2004;2:585–596. doi: 10.1016/s1542-3565(04)00242-3. [DOI] [PubMed] [Google Scholar]
- 13.Elsenbruch S, Orr WC. Diarrhea- and constipation-predominant IBS patients differ in postprandial autonomic and cortisol responses. Am J Gastroenterol. 2001;96:460–466. doi: 10.1111/j.1572-0241.2001.03526.x. [DOI] [PubMed] [Google Scholar]
- 14.Walter SA, Aardal-Eriksson E, Thorell LH, Bodemar G, Hallbook O. Pre-experimental stress in patients with irritable bowel syndrome: high cortisol values already before symptom provocation with rectal distensions. Neurogastroenterol Motil. 2006;18:1069–1077. doi: 10.1111/j.1365-2982.2006.00833.x. [DOI] [PubMed] [Google Scholar]
- 15.Bohmelt AH, Nater UM, Franke S, Hellhammer DH, Ehlert U. Basal and stimulated hypothalamic-pituitary-adrenal axis activity in patients with functional gastrointestinal disorders and healthy controls. Psychosom Med. 2005;67:288–294. doi: 10.1097/01.psy.0000157064.72831.ba. [DOI] [PubMed] [Google Scholar]
- 16.Munakata J, Mayer E, Chang L, et al. Autonomic and neuroendocrine responses to recto-sigmoid stimulation. Gastroenterology. 1998;114:A808. [Google Scholar]
- 17.Karling P, Norrback KF, Adolfsson R, Danielsson A. Gastrointestinal symptoms are associated with hypothalamic-pituitary-adrenal axis suppression in healthy individuals. Scand J Gastroenterol. 2007;42:1294–1301. doi: 10.1080/00365520701395945. [DOI] [PubMed] [Google Scholar]
- 18.Drossman D, Corazziari E, Talley N, Thompson W, Whitehead W. Rome II: The functional gastrointestinal disorders. 2nd edn Degnon Associates; McLean: 2000. [Google Scholar]
- 19.Heitkemper M, Jarrett M, Burr R, et al. Subjective and objective sleep indices in women with irritable bowel syndrome. Neurogastroenterol Motil. 2005;17:523–530. doi: 10.1111/j.1365-2982.2005.00700.x. [DOI] [PubMed] [Google Scholar]
- 20.Jarrett ME, Burr RL, Cain KC, Rothermel JD, Landis CA, Heitkemper MM. Autonomic nervous system function during sleep among women with irritable bowel syndrome. Dig Dis Sci. 2008;53:694–703. doi: 10.1007/s10620-007-9943-9. [DOI] [PubMed] [Google Scholar]
- 21.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 22.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 23.Derogatis L. SCL-90R: Administration, Scoring and Procedures Manual. NCS Pearson, Inc; Minneapolis: 1994. [Google Scholar]
- 24.Bernstein D, Fink L. Childhood Trauma Questionnaire. The Psychological Corporation; San Antonio: 1998. [Google Scholar]
- 25.Chang L, Sundaresh S, Elliott J, et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2008 doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heitkemper M, Jarrett M, Cain K, et al. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. Am J Gastroenterol. 1996;91:906–913. [PubMed] [Google Scholar]
- 27.Mazur M, Furgala A, Jablonski K, et al. Dysfunction of the autonomic nervous system activity is responsible for gastric myoelectric disturbances in the irritable bowel syndrome patients. J Physiol Pharmacol. 2007;58(Suppl 3):131–139. [PubMed] [Google Scholar]
- 28.Heitkemper M, Jarrett M, Cain KC, et al. Autonomic nervous system function in women with irritable bowel syndrome. Dig Dis Sci. 2001;46:1276–1284. doi: 10.1023/a:1010671514618. [DOI] [PubMed] [Google Scholar]
- 29.Cain KC, Jarrett ME, Burr RL, Hertig VL, Heitkemper MM. Heart rate variability is related to pain severity and predominant bowel pattern in women with irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:110–118. doi: 10.1111/j.1365-2982.2006.00877.x. [DOI] [PubMed] [Google Scholar]
- 30.Elsenbruch S, Thompson JJ, Hamish MJ, Exton MS, Orr WC. Behavioral and physiological sleep characteristics in women with irritable bowel syndrome. Am J Gastroenterol. 2002;97:2306–2314. doi: 10.1111/j.1572-0241.2002.05984.x. [DOI] [PubMed] [Google Scholar]