Abstract
No broadly effective vaccines are available for prevention of group B meningococcal disease, which account for > 50% of all cases. The group B capsule is an autoantigen and is not a suitable vaccine target. Outer-membrane vesicle (OMV) vaccines appear to be safe and effective but serum bactericidal (SBA) responses of infants are specific for a porin protein (PorA), which is antigenically variable. To broaden protection, OMV vaccines have been prepared from more than 1 strain; from mutants with more than 1 PorA; or mutants with genetically detoxified endotoxin and overexpressed desirable antigens such as factor H-binding protein (fHbp). Also, recombinant protein vaccines such as fHbp, given alone or combined with other antigens, are in late-stage clinical development and may be effective against the majority of group B strains. Thus, the prospects have never been better for developing vaccines for prevention of meningococcal disease, including group B strains.
Keywords: Neisseria meningitidis; outer membrane vesicle, OMV; recombinant protein; factor H-binding protein, fHbp; PorA; GNA 2132; NadA
Introduction
Nearly half of all cases of meningococcal disease in the United States are caused by capsular group B strains for which there is no broadly effective vaccine [1]. In many European countries, the proportion is even higher (90%) [2, 3], in part, because of routine infant and/or toddler meningococcal group C polysaccharide-protein conjugate vaccination [4]. Group B strains cause a disproportionate number of cases in infants < 1 year, the age group with the highest incidence of disease [5–7]. These strains also cause prolonged epidemics, such as occurred in Cuba and Norway during the 1980s and, more recently, in New Zealand [8]. A quadrivalent group A, C, W-135, and Y polysaccharide-protein conjugate vaccine was introduced in the US and recommended for routine use beginning at age 11 years [9]. A more immunogenic quadrivalent conjugate vaccine [10, 11], and a Haemophilus influenezae type b-meningococcal group C and Y conjugate vaccine [12], both suitable for infants, are in late-stage clinical development. Control of meningococcal disease, however, will not be achieved until a broadly effective vaccine is available against group B strains, which is the subject of this review.
Protection Against Meningococcal Disease
Considerable evidence indicates that complement-mediated serum bactericidal antibody (SBA) confers protection against meningococcal disease (reviewed in [13, 14]). An SBA titer of 1:4 or greater when measured with human complement is generally accepted as a surrogate of protection [13]. Recent seroepidemiologic and experimental evidence also indicates that protection may be conferred by bactericidal activity present at serum dilutions < 1:4 and/or by opsonophagocytosis [15]. Nevertheless, because of high specificity, vaccine efficacy can be inferred from SBA titers ≥ 1:4, and the results can be used by national regulatory authorities for the licensure of new meningococcal vaccines.
Challenges for Group B Vaccine Development
When polysaccharides are conjugated to carrier proteins, the polysaccharide antigens become immunogenic in infants and prime for memory anticapsular antibody responses (reviewed in [16, 17]). The meningococcal group B polysaccharide, however, is a homolinear polymer of α(2→8) N-acetyl neuraminic acid (polysialic acid), and is an autoantigen [18]. The polysaccharide is expressed by a number of host tissues [19] and is a poor immunogen, even when conjugated to a protein carrier [20]. To increase immunogenicity, N-propionyl-derivatized group B polysaccharide-conjugate vaccines were prepared, which elicited SBA responses in mice [21] but not in humans [22]. Efforts to develop a group B vaccine, therefore, have focused on noncapsular antigens such as proteins or lipopolysaccharide (in meningococcus, referred to as lipooligosaccharide [LOS], because of the presence of repeating short saccharides instead of long-chain saccharides). The principal challenge has been to identify surface-exposed noncapsular antigens that are safe and antigenically conserved and that elicit broad SBA responses. Promising noncapsular group B vaccine approaches are discussed below and include outer-membrane vesicles (OMVs), recombinant proteins, and a combination of an OMV and recombinant proteins (Table 1).
Table 1.
Group B vaccines investigated in clinical trials
| Formulation | Vaccine | Clinical Status | Immunogenicity Results (humans) |
|---|---|---|---|
| Polysaccharide-protein conjugate | N-propionylated group B polysaccharide derivative [22] | Phase 1; completed | Did not elicit SBA |
| Detergent-treated OMVs | OMV from 1 strain* [28–33] | Phase 3; completed | Elicited SBA and opsonic activity |
| Mixture of OMV from 2 strains [40, 41] | Phase 1; completed | Elicited SBA | |
| Mixture of OMV from 2 mutants, each with 3 PorA proteins [45–47, 127, 128] | Phase 2; completed | Elicited SBA | |
| Mixture of OMV from 3 mutants, each with 3 PorA proteins [44] | Phase 1 | Not yet reported | |
| OMV from Neisseria lactamica [58] | Phase 1 | Elicited minimal SBA responses | |
| Native OMV (not treated with detergents) | Mutant with attenuated endotoxin, 2 PorA proteins, overexpressed fHbp, and other mutations [75] | Phase 1 | Not yet reported |
| Recombinant proteins | TbpB [92] | Phase 1; completed | SBA responses not reported |
| NspA [89] | Phase 1; completed | Did not elicit SBA | |
| fHbp (2 antigenic variants) [120–122] | Phase 2 | Elicited SBA | |
| 2 fusion proteins, GNA 2091-fHbp variant 1 and GNA2132-GNA1030, and Neisseria adhesin A (NadA) [119] (Figure 6) | Phase 1 | Elicited SBA and opsonic activity | |
| Recombinant proteins + detergent-treated OMV | 2 fusion proteins, GNA 2091-fHbp variant 1, and GNA2132-GNA1030, + NadA (Figure 6) + OMV [123, 124] | Phase 2/3 | Elicited SBA |
Abbreviations used: OMV, outer membrane vesicles; PorA, one of two porin proteins, designated A; fHbp, factor H binding protein; TbpB, transferring-binding protein B; NspA, Neisserial surface protein A; GNA, Genome-derived Neisserial Antigens; NadA, Neisserial adhesin A
Strategies for Group B Vaccine Development
Detergent-extracted OMV vaccines
OMVs can be separated from meningococcal bacteria (figure 1, panel A) [23], or isolated as membrane blebs, which are released into media during bacterial growth. The OMVs are treated with detergents to extract LOS and decrease endotoxin activity [24]. By sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the detergent-treated vesicles contain 4 or 5 major outer-membrane proteins (figure 1, panel B). Through more sensitive proteomic methods, the vesicles were shown to contain many other periplasmic and cytoplasmic proteins [25, 26]. The role of these proteins in safety or immunogenicity is unknown. Ample evidence indicates that OMV vaccines are safe [27] and effective in preventing group B meningococcal disease. OMV vaccines prepared from various wildtype strains have been tested in humans [28–33]. In 5 published studies, efficacy of 2 doses given to children 4 years or older, or to young adults, ranged from 57% to 83% against disease caused largely by homologous strains (reviewed in [15]). Recently, administration of 3 doses of an OMV vaccine to the New Zealand population 2 months to 20 years of age controlled a longstanding group B epidemic. Overall, vaccine efficacy was estimated at 73% [34]; and was 80% in the age group 6 months to 5 years [35].
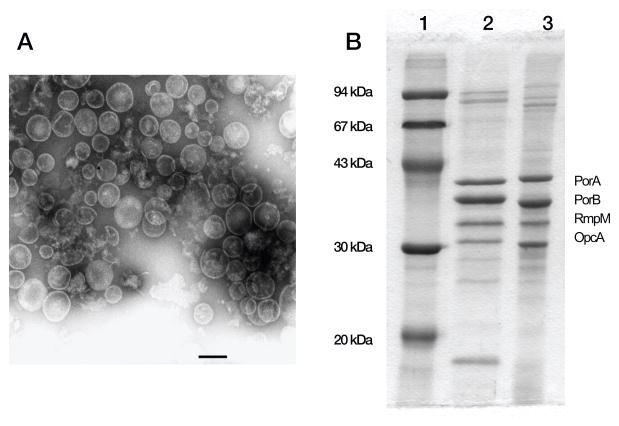
Figure 1.
Detergent-extracted OMV vaccines. Panel A. Electron micrograph of outer membrane vesicles of N. meningitides. The scale bar is 100 nm and the vesicle diameter is about 50–200 nm (80nm on average). Panel B. Major outer membrane proteins (PorA, PorB, reduction modifiable protein (RmpM) and opacity protein A (OpcA)) as visualized by Coommassie-stained SDS PAGE. Lane 1, molecular mass standards; lane 2, strain NZ98/254; lane 3, strain H44/76. After immunization, the SBA–responses of infants and children are directly predominantly against PorA. Adapted from published data [23]. Reprinted from Vaccine, vol 27, Supplement 2, 2009, Holst J, Martin D, Arnold R, et al. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis, with permission from Elsevier.
One limitation of conventional detergent-treated OMV vaccines is that SBA responses of children are largely directed against surface-accessible loops on a porin protein, called PorA [36], which is antigenically variable [37]. The utility of OMV vaccines, therefore, is best suited for control of epidemics caused by a predominant strain [38, 39]. To broaden protection, OMV vaccines have been prepared from more than 1 strain [40, 41], or from mutant strains engineered to express more than 1 PorA molecule [42–47]. An OMV vaccine from 2 mutants (3 PorA variable region [VR) types per mutant) elicited PorA-specific SBA responses in human infants and primed for booster antibody responses [46, 48]. Certain PorA VR types, however, were poorly immunogenic, and point mutations in the PorA gene can result in small antigenic changes and resistance to SBA [49]. A vaccine consisting of a mixture of OMVs from 3 mutants, each expressing 3 PorA molecules (total of 9 PorA VR types), is in early-stage clinical development [44]. In the US, however, meningococcal disease is caused by strains with considerable PorA antigenic diversity (> 20 PorA VR types) [37, 50, 51]. Therefore, OMV vaccines that predominantly target PorA are unlikely to confer broad protection in infants and young children. These vaccines may be more useful in older age groups since SBA responses of OMV-immunized adults had broader SBA than those of immunized infants [36]. One possible reason is that most adults are naturally primed by exposure to Neisserial organisms and even small quantities of residual non-PorA antigens in the detergent-extracted OMV vaccines may be sufficient to boost memory SBA responses with broad activity. The quantity of these antigens, however, may be insufficient for immunogenicity in unprimed infants.
The incidence of meningococcal disease declines rapidly beginning in the second year of life coincident with the acquisition of colonization by N. lactamica, which is a common commensal of the nasopharynx of young children [52–54]. Exposure to crossreacting N. lactamica antigens and, later, to colonization by N. meningitidis, has been thought to contribute to naturally acquired meningococcal immunity although the specific antigenic targets and protective mechanisms are poorly understood [15]. To mimic naturally acquired immunity, and to circumvent possible immunodominance of PorA, which has been hypothesized to impair serum antibody responses to non-PorA antigens [55], an OMV vaccine was prepared from N. lactamica [52, 54, 56, 57], which does not express an ortholog of meningococcal PorA. In a phase 1 study, adults immunized with the N. lactamica OMV vaccine developed minimal SBA responses, even though nearly all of the subjects were naturally primed based on the presence of bactericidal activity in preimmunization sera [58]. The lack of SBA responses to the lactamica OMV vaccine is not surprising. Below age 10 years, when N. lactamica colonization is common, the prevalence of SBA is low [7, 16]. Also, although mice immunized with an OMV vaccine prepared from N. lactamica were protected against a lethal N. meningitides challenge [57], N. lactamica vaccines did not elicit SBA responses [57, 59]. The mechanism responsible for the mouse protection has not been defined.
Weynants et al. prepared detergent-treated OMV vaccines from mutant N. meningitidis strains in which the PorA gene had been inactivated to circumvent immunodominance [55]. The mutants also were engineered to overproduce several “minor” outer-membrane proteins that are normally expressed in low copy number (Transferrin-binding protein A, Neisserial surface protein A, and Outer Membrane Protein 85). The authors hypothesized that in the absence of PorA immunodominance, and with overexpression of these minor antigens, the breadth of the SBA responses to the mutant OMV vaccine would be increased. In immunized mice, only antibodies elicited by the OMV vaccine from the mutant in which all 3 minor antigens were overexpressed had SBA. The authors concluded that it was necessary to elicit antibodies directed against multiple “minor” antigens to achieve sufficient density of IgG on the surface of the bacteria to engage C1q and activate complement-mediated SBA. As described below, there may be exceptions to this model when the antibodies target a sparsely expressed antigen such as factor H-binding protein (fHbp), which regulates complement pathways [60].
Native (non detergent-treated) OMV vaccines
Detergent treatment, which is used to lower endotoxin activity of OMV vaccines, also extracts desirable antigens such as the lipoproteins, fHbp and Genome-derived Neisserial Antigen 2132 (GNA 2132), which are 2 recently discovered vaccine targets (see below) [61, 62]. To avoid the detergent step and preserve desirable detergent-soluble antigens, it may be possible to prepare native OMV vaccines from strains selected to have naturally low endotoxin activity [63], or to use genetic approaches to attenuate endotoxin activity [64–66].
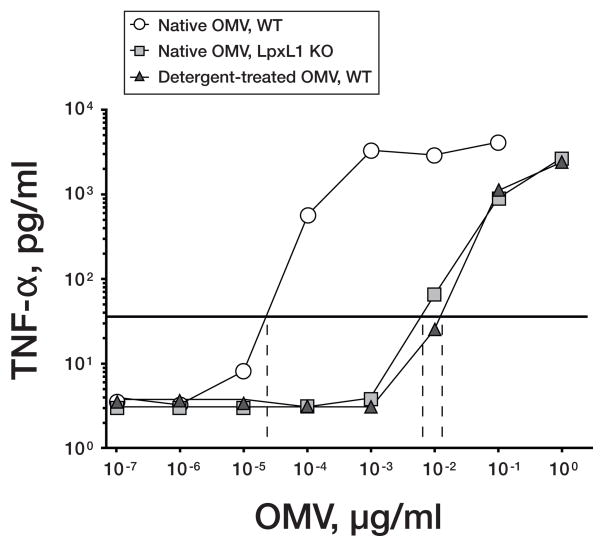
The lipid A portion of the LOS molecule is responsible for its endotoxin activity. One promising mutant with attenuated endotoxin activity contains a deletion in the LpxL1 gene (also referred to as the msbB gene) [67]. This mutation results in penta-acylated lipid A, which is poorly recognized by human toll-like receptor 4 (TLR4) [68], instead of the more toxic hexa-acylated lipid A, which is present in most wildtype strains [63, 69, 70]. When incubated with human PBMCs, a native OMV vaccine from a penta-acylated mutant had more than 100-fold less endotoxin activity based on lower stimulation of multiple proinflammatory cytokines than a control native OMV from the wildtype strain [65, 66]. The native mutant OMV vaccine showed similar stimulatory activity as control, detergent-treated wildtype OMV vaccines that had been administered safely to humans. Representative TNF-∝ responses to the different WT and mutant OMV vaccines are shown in figure 2.
Figure 2.
Release of TNF-α after incubation of human PBMCs for 4 hours with OMV vaccines. The OMV concentrations that resulted in a 10-fold increased release of TNF-α concentrations above background are shown on the X intercepts. White circles, native OMV from wildtype strain; gray squares, OMV vaccine prepared from LpxL1 KO mutants; black triangles, detergent-extracted OMV vaccines from corresponding wildtype strains. Adapted from published data [65] with permission from American Society for Microbiology.
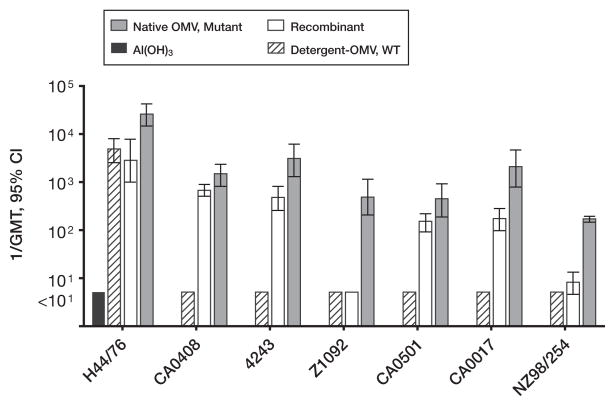
Many of the newly discovered vaccine targets identified by genome mining (an approach referred to as “reverse vaccinology” [71]), are naturally expressed in relatively low copy number by N. meningitidis strains (which is one reason why these antigens remained unrecognized before genome research). With a few exceptions, humans recovering from meningococcal disease showed low antibody responses to these antigens [72], and the antigens were poorly immunogenic in mice immunized with native OMV vaccines prepared from wildtype strains [66] [73]. To enhance immunogenicity, native (i.e. non–detergent-extracted) OMV vaccines have been prepared from mutants engineered to overexpress desirable antigens such as fHbp [65, 66, 74] (described in detail, below). In mice, a native OMV vaccine with genetically detoxified endotoxin and overexpressed fHbp elicited high titers of serum anti-fHbp antibodies with broad SBA (representative data, figure 3). By adsorption studies, the majority of the bactericidal antibodies were directed at fHbp [65, 66]. The native OMV vaccine, which expressed PorA, also elicited strain-specific bactericidal anti-PorA antibodies [65]. The safety and immunogenicity of a prototype native OMV vaccine from a mutant N. meningitidis strain with genetically attenuated endotoxin activity, overexpressed fHbp, more than one PorA VR type, and other mutations, is being investigated in a phase 1 clinical trial in adults [75].
Figure 3.
Serum bactericidal activity elicited in mice by a native OMV vaccine prepared from a mutant with attenuated endotoxin activity and overexpressed fHbp. N. meningitidis strain designations are shown below the X axis (all expressed fHbp in the variant 1 group). Vaccine groups: recombinant (open bars), multicomponent vaccine containing 2 fusion proteins, GNA 2091-fHbp variant 1 and GNA 2132-GNA 1030, and NadA (See Figure 6); Detergent-OMV, WT (hatched bars), a clinical lot of detergent-treated OMV vaccine from Norway (strain H44/76); native OMV, mutant (gray bars), a native (not treated with detergent) OMV from a mutant strain of H44/76 with attenuated endotoxin (LpxL1KO) and overexpressed fHbp in the variant 1 group. Al(OH)3 (black bars), adjuvant alone (also used for the 3 vaccines). Copyright © 2008 by the Infectious Diseases Society of America. All rights reserved.
LOS has become another potential meningococcal vaccine target since anti-LOS antibodies were reported to have SBA and/or opsonic activity [76–78]. However, the lacto-N-neotetraose (Gal-GlcNAc-Gal-Glc tetrasaccharide) on meningococcal LOS is shared by antigens on human red blood cells, which raises safety concerns. Also, the typical concentrations of detergent used to extract LOS from OMV vaccines to decrease endotoxin activity decreased LOS immunogenicity [66, 67].
To develop an immunogenic LOS-enriched OMV vaccine, Weynants et al. prepared an LpxL1 KO mutant with attenuated endotoxin activity [67]. The mutant also was engineered to express a truncated LOS lacking the terminal galactose of lacto-N-neotetraose to eliminate antigenic crossreactivity with red cell antigens. PorA was deleted to avoid the hypothetical possibility of PorA immunodominance and suppression of anti-LOS antibody responses. In mice, the mutant OMV vaccine, which contained approximately 15% LOS after mild detergent treatment, elicited high serum anti-LOS antibody titers with broad SBA activity. The SBA responses, however, were measured with rabbit complement, which gives much higher titers than human complement [79, 80]. One reason is that rabbit complement factor H (fH) binds poorly to N. meningitidis [81]. In the absence of fH bound to the bacterial surface, complement activation is not down-regulated (Figure 4A and B). The result is that there are higher bactericidal titers with rabbit complement than with human complement since human fH is bound by the bacteria [81]. In other studies, mice immunized with native meningococcal OMV vaccines also developed high titers of anti-LOS antibodies by ELISA, but the antibodies appeared to be of low avidity [66] and had minimal SBA when assayed with human complement [65, 66, 73]. Thus, the vaccine potential of vaccines that target LOS antigens is unknown.
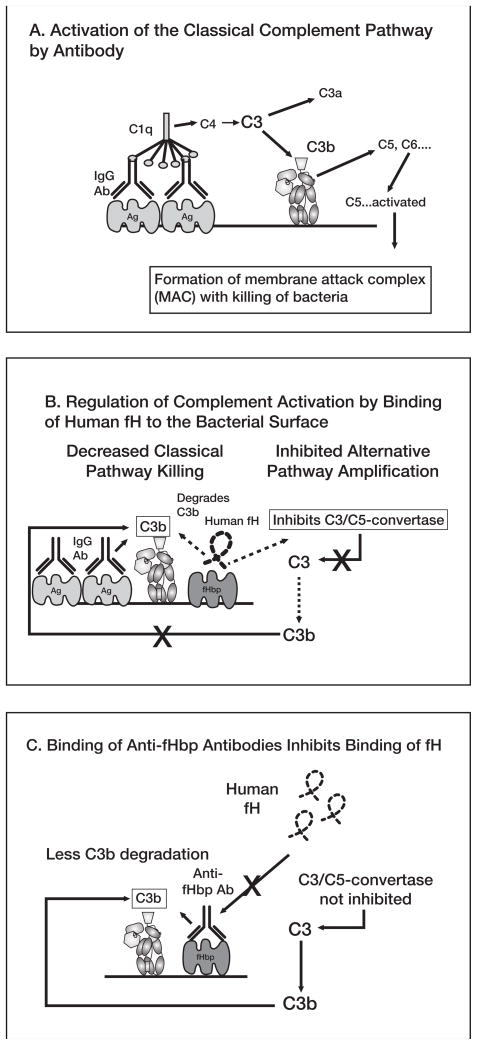
Figure 4.
A, Activation of classical complement pathway. Binding of 2 optimally spaced IgG molecules to the bacterial surface engages C1q and activates the classical complement pathway, which results in increased deposition of C3b. Bound C3b can serve as an opsonin and can also lead to bacteriolyis by cleavage of C5 and assembly of the C5b-9 membrane attack complex. Not shown are the components of the alternative pathway, which can be activated by the classical pathway and serve as an amplification loop. B, Regulation of complement activation by binding of human factor H (fH) to the bacterial surface. Human fH binds to surface-exposed fHbp. fH accelerates the decay of alternative pathway C3/C5 convertases, which downregulates the positive feedback amplification loop of the alternative pathway. Binding of fH also leads to degradation of C3b by factor I (not shown), which decreases classical pathway activation and amplification by the alternative pathway. C, Binding of antibodies to fHbp activates classical complement pathway bacteriolysis and also inhibits binding of fH to the bacterial surface. With decreased amounts of fH bound to the bacterial surface, there is less downregulation of complement activation and the organism becomes more susceptible to complement-mediated bacteriolysis.
Recombinant protein vaccines
New vaccine discovery approaches including genome mining [62, 71, 82–84], proteomics [85, 86], and immunological approaches [87] have identified a large number of novel vaccine targets for prevention of group B meningococcal disease. These include Neisserial surface protein A (NspA) [87–89], Transferrin-binding proteins (Tbp) [90–92], Opacity proteins (Opc) [93–95], Genome-derived Neisserial Antigen (GNA) 2132 [96–99], fHbp (previously referred to as GNA 1870 or LP2086 [61, 100]), FetA [101], Neisserial adhesin A (NadA, also referred to as GNA 1994) [102–105], and others [62, 106]. To date, the vaccine potential of nearly all of these candidates has been limited by either antigenic variability (e.g., FetA and Opc), lack of the gene in strains from some hypervirulent lineages (e.g. NadA), phase-variability (Opc), or low constitutive expression of the antigen by some strains (fHbp, GNA 2132, and NspA). There also may be poor expression of important conformational epitopes by the recombinant protein (NspA) [89, 107]. Thus, a vaccine containing only 1 recombinant antigen is unlikely to be sufficient for broad protective meningococcal immunity.
One of the most promising of the new protein antigens is fHbp, which is a surface-exposed lipoprotein present in all N. meningitidis strains [61, 100, 108]. An important function of fHbp is to bind the human complement protein fH [60]. Binding of fH to the bacterial surface accelerates decay of C3/C5 convertase, which decreases alternative pathway activation (Figure 4B), and contributes to the ability of the organism to avoid complement-mediated killing by non-immune human serum or blood [60, 109–111]. In the presence of anti-meningococcal antibodies, binding of fH to the bacteria also decreases complement-mediated SBA titers [81] by enhancing factor I-mediated degradation of C3b, which decreases classical complement pathway activation, and by decreasing alternative complement pathway amplification (Figure 4B). Additional indirect evidence that fH contributes to the pathogenesis of meningococcal disease comes from a report that persons homozygous for a single nucleotide polymorphism in the promoter region, C496T, have increased serum fH protein levels, and also have an increased risk of developing meningococcal disease [112]. With increased fH concentrations, the bacteria became more resistant to SBA.
As described above, binding of fH to N. meningitidis was reported to be specific for human fH [81]. This human specificity adds another mechanism to explain why N. meningitidis is strictly a human pathogen. A crystal structure of a portion of fH in complex with fHbp has been reported [113], which provided a structural basis for specificity of binding human fH. As a vaccine antigen, fHbp is unique in that antibodies to fHbp both activate classical complement pathway bacteriolysis directly and block binding of fH to the bacterial surface [60] [114]. Inhibition of fH binding would be expected to enhance susceptibility of the organism to classical and alternative pathway bacteriolysis (Figure 4C).
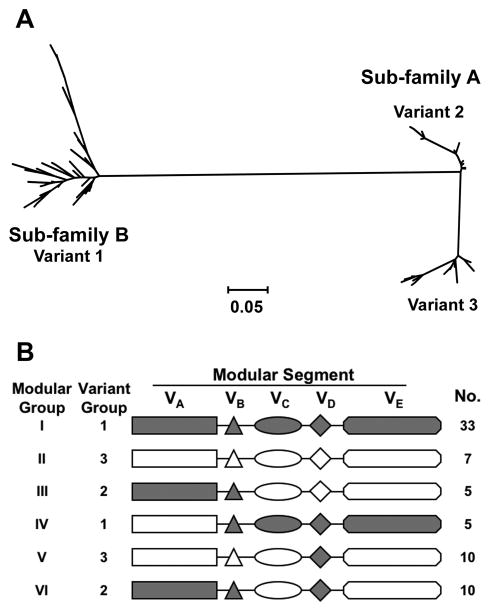
Meningococcal fHbp can be subclassified into 3 antigenic variant groups based on antigenic crossreactivity and sequence similarity of the entire protein [61] (figure 5A). In general, antibodies prepared against fHbp in the variant 1 group (also referred to as subfamily B by Fletcher et al. [100]) were bactericidal against strains expressing fHbp from the variant 1 group but not against strains expressing fHbp in the variant 2 or 3 groups (together, referred to as subfamily A), and vice versa [61, 100, 103]. There also are subvariants of fHbp within each of the variant groups (proteins that differ by < 10% of amino acids from those of the canonical protein of the respective antigenic variant group) [103, 115]. Recently, the molecular architecture of fHbp has been shown to be “modular” (each fHbp variant contains different combinations of five variable segments, each of which is derived from one of two genetic lineages [116], Figure 5B). The breadth of protection conferred by anti-fHbp antibodies against strains expressing subvariants of fHbp, or fHbps from different “modular” groups, remains to be determined.
Figure 5.
A. Phylogram of 70 unique fHbp amino acid sequences showing division of the proteins into 2 subfamilies, designated as A and B by Fletcher et al. [100]. Subfamily B contains the proteins in the variant 1 group described by Masignani et a. [61]. Subfamily A is subdivided into 2 branches, designated by Masignani et al. as variants 2 and 3, respectively. Each branch represents a distinctive protein sequence. The scale bar represents 5 amino acid differences per 100 amino acids. B. Modular structure. The architecture of fHbp consists of different combinations of five variable segments, designated VA to VD [116]. Each segment is derived from one of two genetic lineages, designated alpha (gray segments) or beta (white segments). All of the distinctive fHbp protein amino acid sequences referred to in Panel A could be assigned to one of six “modular groups”, designated I–VI. Panel B is reprinted from Microbiology 2009;155:2873–83. Copyright ©2009 by the Society for General Microbiology.
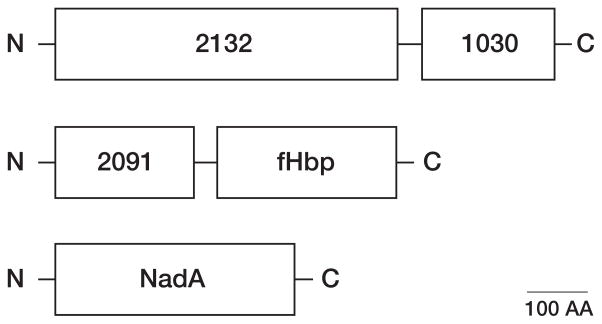
Factor H-binding proteins are part of 2 promising meningococcal vaccines being investigated in humans. One vaccine, referred to as LP2086, contains 2 recombinant lipidated proteins from the fHbp subfamilies A and B. The second vaccine contains fHbp in the variant 1 group (subfamily B) as part of a multicomponent vaccine [96] (figure 6). Two of the components are fusion proteins (GNA 2091 fused with fHbp and GNA 2132 fused with GNA 1030), and the third component is recombinant NadA. Of these 5 antigens, fHbp, GNA 2132, and NadA were reported to be responsible for most of the SBA responses in mice [96].
Figure 6.
Schematic showing three recombinant proteins (five antigens) contained in a multicomponent meningococcal vaccine [96, 119]. Two of the components are fusion proteins (GNA 2132 fused with GNA 1030, and GNA 2091 fused with fHbp. The third component is recombinant NadA. N- and C- refer to the amino- and carboxy terminal portions, respectively, of the proteins. The scale bar represents 100 amino acids (AA). Of the five antigens, fHbp, GNA 2132 and NadA, shown in gray, were reported to be responsible for most of the SBA responses in mice [96]. In the vaccine formulation being investigated in phase 3 clinical trials, the three recombinant proteins are combined with a detergent-extracted OMV vaccine from group B strain NZ98/254.
GNA 2132 antigen is a surface-accessible lipoprotein of unknown function. The gene was detected in all N. meningitidis strains tested to date, and also was present in strains of N. lactamica and N. gonorrhoeae [62]. Based on sequence alignments, portions of GNA 2132 were highly conserved. In mice, recombinant GNA 2132 elicited SBA responses, although only a subset of strains was susceptible with human complement [99]. Human adults immunized with an OMV vaccine combined with recombinant GNA 2132 had higher SBA responses measured with human complement than those who were given a control OMV vaccine without the recombinant protein [97].
NadA is an adhesin/invasin that binds to epithelial cells in vitro [102]. In mice, recombinant NadA elicited SBA responses [105]. The antigen is conserved (> 96% amino acid identity) among group B strains but the gene is absent from strains from certain genetic lineages, which are responsible for approximately 40% of group B disease [103, 105, 117, 118].
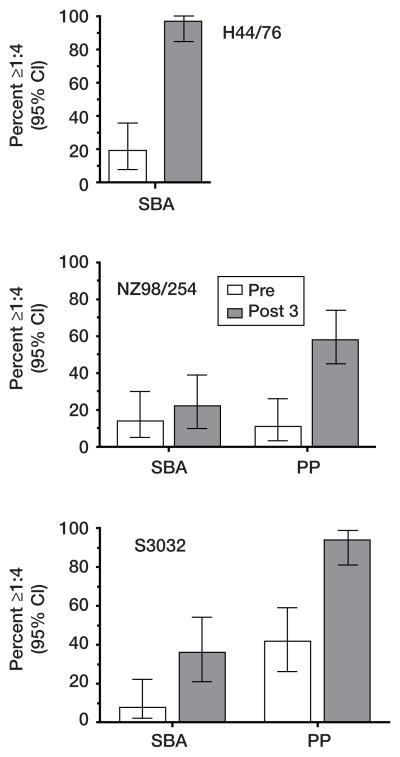
In a phase 1 study [119], adults were immunized with the multicomponent recombinant protein vaccine described in figure 6. Their sera were assayed for bactericidal activity against three representative test strains, H44/76, NZ98254 and S3032, each from different genetic clonal complexes: Two of the strains were responsible for large group B epidemics in Norway (H44/76) and New Zealand (NZ98254), and S3032 was from a patient in the United States. High SBA responses were observed against strain H44/76, which expressed a homologous fHbp to the recombinant protein antigen in the vaccine (Serum GMT < 1:4 before immunization, which increased to 1:64 in sera obtained 4 weeks after a third dose of vaccine); 97% of subjects developed protective titers of ≥ 1:4 when measured with human complement (figure 7). Against the 2 other test strains, which were selected because neither had the gene for NadA and both expressed fHbp antigens mismatched for the vaccine antigen, SBA responses were much lower (figure 7). The majority of the postimmunization sera, however, conferred passive protection against these stains in an ex vivo human bacteremia model (figure 7) [119]. The greater sensitivity of the passive protection ex vivo bacteremia assay may reflect higher complement concentrations in the whole human blood assay (90%) than in the SBA assay (20%–25%), and/or the presence of phagocytic cells in the blood assay.
Figure 7.
Summary of the proportion of immunized subjects with titers ≥ 1:4 in SBA (3 strains), or passive protection (PP) in a human blood bacteremia model with group B strains NZ98/254 and S3032 (strain H44/76 was not tested). The bars represent the proportion of pre- (open bars) or postimmunization sera (closed bars) that were positive when tested at a 1:4 dilution in each assay and the respective 95% confidence intervals. Postimmunization passive protective activity was 2.6-to 2.8-fold more frequent then an SBA titer ≥ 1:4. Adapted with permission. Copyright 2009© American Society for Microbiology, Clin Vaccine Immunol, 16:785–91, 2009.
In the multicomponent vaccine formulation that has advanced to phase 3 testing, the 3 recombinant proteins (5 antigens) were combined with a detergent-treated OMV vaccine that had been used to control a group B epidemic in New Zealand [34]. As of Fall 2009, there are no published reports of the results of clinical trials with this vaccine or a bivalent fHbp vaccine. Early clinical data from testing these vaccines have been reported at conferences [120–124]. Both vaccines were well tolerated and elicited SBA responses in children and adults. Although the data were promising, detailed information is needed for critical assessment of the safety and efficacy of these vaccines.
Challenges in Assessing Vaccine Efficacy
Because of the low incidence of meningococcal group B disease, it is not feasible to perform prospective, randomized, controlled clinical trials to assess the efficacy of new group B vaccines. For vaccine licensure, the breadth of protection will be estimated from immunogenicity studies conducted in different age groups and in different geographic locales, and by testing SBA against genetically diverse strains. This approach presents several challenges. First, the quantity of sera available from the infant trials will be limited, which precludes performing assays against a large number of genetically diverse test strains. Given variability in antigen sequence and expression, it will be necessary to select a representative strain panel that insures that the resulting SBA data can be extrapolated to estimate protection against disease-causing strains in the population. Second, as discussed above, by relying only on SBA titers ≥ 1:4 as a measure of protection, the immunogenicity data likely will underestimate protection induced by vaccination [15, 97, 119]. A more sensitive assay is needed. Determining whether protection is conferred by antibodies that only have opsonic activity, or have SBA at titers < 1:4, would permit use of expanded serologic assays and advance vaccine development, as well as help formulate optimal public health strategies for introduction of the new vaccines. Third, up to a third of the effect of group C conjugate vaccines on control of meningococcal disease in a population has been attributed to decreased colonization and transmission of group C organisms (herd immunity) [125]. No information is available on whether protein-based meningococcal vaccines affect transmission of the organism, and no clinical information is available on protection against non-group B strains. The protein antigens used for group B vaccines are shared across strains with other capsular groups [126], and mice immunized with OMV vaccines from group B mutants developed broad SBA responses against epidemic group A, W-135, and X isolates from Africa [126]. Referring to the new protein vaccines as “group B” vaccines is, therefore, a misnomer since the vaccines likely also will protect against capsular group A, C, W-135, and Y strains, which will be an added bonus to vaccination.
Acknowledgments
Financial support. This research was supported by Public Health Service grant R01 AI046464 from the National Institute of Allergy and Infectious Diseases, NIH. The work at Children’s Hospital Oakland Research Institute was performed in a facility funded by Research Facilities Improvement Program grant number C06 RR-16226 from the National Center for Research Resources, NIH.
Footnotes
Conflict of interest. The author is principal investigator of laboratory research conducted on behalf of Children’s Hospital Oakland Research Institute that is funded by grants from Novartis Vaccines and Diagnostics, and Sanofi Pasteur. He also holds a paid consultancy from Novartis and is an inventor on patents or patent applications in the area of meningococcal B vaccines.
References
- 1.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27:B51–B63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 2.Trotter CL, Chandra M, Cano R, et al. A surveillance network for meningococcal disease in Europe. FEMS Microbiol Rev. 2007;31:27–36. doi: 10.1111/j.1574-6976.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 3.Gray SJ, Trotter CL, Ramsay ME, et al. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol. 2006;55:887–96. doi: 10.1099/jmm.0.46288-0. [DOI] [PubMed] [Google Scholar]
- 4.Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–7. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- 5.Shepard CW, Rosenstein NE, Fischer M. Neonatal meningococcal disease in the United States, 1990 to 1999. Pediatr Infect Dis J. 2003;22:418–22. doi: 10.1097/01.inf.0000066876.77453.04. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344:1378–88. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 7.Trotter C, Findlow J, Balmer P, et al. Seroprevalence of bactericidal and anti-outer membrane vesicle antibodies to Neisseria meningitidis group B in England. Clin Vaccine Immunol. 2007;14:863–8. doi: 10.1128/CVI.00102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker MG, Martin DR, Kieft CE, Lennon D. A 10-year serogroup B meningococcal disease epidemic in New Zealand: descriptive epidemiology, 1991–2000. J Paediatr Child Health. 2001;37:S13–9. doi: 10.1046/j.1440-1754.2001.00722.x. [DOI] [PubMed] [Google Scholar]
- 9.Pace D, Pollard AJ, Messonier NE. Quadrivalent meningococcal conjugate vaccines. Vaccine. 2009;27:B30–B41. doi: 10.1016/j.vaccine.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Perrett KP, Snape MD, Ford KJ, et al. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J. 2009;28:186–93. doi: 10.1097/INF.0b013e31818e037d. [DOI] [PubMed] [Google Scholar]
- 11.Snape MD, Perrett KP, Ford KJ, et al. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. Jama. 2008;299:173–84. doi: 10.1001/jama.2007.29-c. [DOI] [PubMed] [Google Scholar]
- 12.Nolan T, Lambert S, Roberton D, et al. A novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus-toxoid conjugate vaccine is immunogenic and induces immune memory when co-administered with DTPa-HBV-IPV and conjugate pneumococcal vaccines in infants. Vaccine. 2007;25:8487–99. doi: 10.1016/j.vaccine.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Borrow R, Carlone GM, Rosenstein N, et al. Neisseria meningitidis group B correlates of protection and assay standardization--international meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine. 2006;24:5093–107. doi: 10.1016/j.vaccine.2006.03.091. [DOI] [PubMed] [Google Scholar]
- 14.van Alphen L, van den Dobbelsteen G. Meningococcal B vaccine development and evaluation of efficacy. Hum Vaccin. 2008;4:158–61. doi: 10.4161/hv.4.2.4871. [DOI] [PubMed] [Google Scholar]
- 15.Granoff DM. Relative importance of complement-mediated bactericidal and opsonic activity for protection against meningococcal disease. Vaccine. 2009;27 (Suppl 2):B117–25. doi: 10.1016/j.vaccine.2009.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granoff DM, Harrison L, Borrow R. Meningoccal Vaccines. In: Plotkin SA, Offit P, Orenstein WA, editors. Vaccines. 5. Philadelphia: Saunders Elsevier; 2008. pp. 399–434. [Google Scholar]
- 17.Granoff DM, Pollard AJ. Reconsideration of the use of meningococcal polysaccharide vaccine. Pediatr Infect Dis J. 2007;26:716–22. doi: 10.1097/INF.0b013e3180cc2c25. [DOI] [PubMed] [Google Scholar]
- 18.Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;2:355–7. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 19.Finne J, Bitter-Suermann D, Goridis C, Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J Immunol. 1987;138:4402–7. [PubMed] [Google Scholar]
- 20.Jennings HJ, Lugowski C. Immunochemistry of groups A, B, and C meningococcal polysaccharide- tetanus toxoid conjugates. J Immunol. 1981;127:1011–8. [PubMed] [Google Scholar]
- 21.Jennings HJ, Gamian A, Ashton FE. N-propionylated group B meningococcal polysaccharide mimics a unique epitope on group B Neisseria meningitidis. J Exp Med. 1987;165:1207–11. doi: 10.1084/jem.165.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruge J, Bouveret-Le Cam N, Danve B, Rougon G, Schulz D. Clinical evaluation of a group B meningococcal N-propionylated polysaccharide conjugate vaccine in adult, male volunteers. Vaccine. 2004;22:1087–96. doi: 10.1016/j.vaccine.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Holst J, Martin D, Arnold R, et al. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009;27 (Suppl 2):B3–12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 24.Fredriksen JH, Rosenqvist E, Wedege E, et al. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1991;14:67–79. discussion 79–80. [PubMed] [Google Scholar]
- 25.Vipond C, Suker J, Jones C, Tang C, Feavers IM, Wheeler JX. Proteomic analysis of a meningococcal outer membrane vesicle vaccine prepared from the group B strain NZ98/254. Proteomics. 2006;6:3400–13. doi: 10.1002/pmic.200500821. [DOI] [PubMed] [Google Scholar]
- 26.Williams JN, Skipp PJ, Humphries HE, Christodoulides M, O’Connor CD, Heckels JE. Proteomic analysis of outer membranes and vesicles from wild-type serogroup B Neisseria meningitidis and a lipopolysaccharide-deficient mutant. Infect Immun. 2007;75:1364–72. doi: 10.1128/IAI.01424-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nokleby H, Aavitsland P, O’Hallahan J, Feiring B, Tilman S, Oster P. Safety review: two outer membrane vesicle (OMV) vaccines against systemic Neisseria meningitidis serogroup B disease. Vaccine. 2007;25:3080–4. doi: 10.1016/j.vaccine.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Bjune G, Gronnesby JK, Hoiby EA, Closs O, Nokleby H. Results of an efficacy trial with an outer membrane vesicle vaccine against systemic serogroup B meningococcal disease in Norway. NIPH Ann. 1991;14:125–30. discussion 130–2. [PubMed] [Google Scholar]
- 29.Hoiby EA, Bjune G, Froholm LO, et al. The Norwegian meningococcal serogroup B outer membrane vesicle vaccine protection trials: case tracing, meningococcal antigen detection and serological diagnosis. NIPH Ann. 1991;14:107–21. discussion 121–3. [PubMed] [Google Scholar]
- 30.de Moraes JC, Perkins BA, Camargo MC, et al. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet. 1992;340:1074–8. doi: 10.1016/0140-6736(92)93086-3. [DOI] [PubMed] [Google Scholar]
- 31.Noronha CP, Struchiner CJ, Halloran ME. Assessment of the direct effectiveness of BC meningococcal vaccine in Rio de Janeiro, Brazil: a case-control study. Int J Epidemiol. 1995;24:1050–7. doi: 10.1093/ije/24.5.1050. [DOI] [PubMed] [Google Scholar]
- 32.O’Hallahan J, Lennon D, Oster P, et al. From secondary prevention to primary prevention: a unique strategy that gives hope to a country ravaged by meningococcal disease. Vaccine. 2005;23:2197–201. doi: 10.1016/j.vaccine.2005.01.061. [DOI] [PubMed] [Google Scholar]
- 33.Oster P, Lennon D, O’Hallahan J, Mulholland K, Reid S, Martin D. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine. 2005;23:2191–6. doi: 10.1016/j.vaccine.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 34.Kelly C, Arnold R, Galloway Y, O’Hallahan J. A prospective study of the effectiveness of the New Zealand meningococcal B vaccine. Am J Epidemiol. 2007;166:817–23. doi: 10.1093/aje/kwm147. [DOI] [PubMed] [Google Scholar]
- 35.Galloway Y, Stehr-Green P, McNicholas A, O’Hallahan J. Use of an observational cohort study to estimate the effectiveness of the New Zealand group B meningococcal vaccine in children aged under 5 years. Int J Epidemiol. 2009;38:413–8. doi: 10.1093/ije/dyn228. [DOI] [PubMed] [Google Scholar]
- 36.Tappero JW, Lagos R, Ballesteros AM, et al. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. Jama. 1999;281:1520–7. doi: 10.1001/jama.281.16.1520. [DOI] [PubMed] [Google Scholar]
- 37.Tondella ML, Popovic T, Rosenstein NE, et al. Distribution of Neisseria meningitidis serogroup B serosubtypes and serotypes circulating in the United States. The Active Bacterial Core Surveillance Team. J Clin Microbiol. 2000;38:3323–8. doi: 10.1128/jcm.38.9.3323-3328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holst J. Strategies for development of universal vaccines against meningococcal serogroup B disease: the most promising options and the challenges evaluating them. Hum Vaccin. 2007;3:290–4. doi: 10.4161/hv.4513. [DOI] [PubMed] [Google Scholar]
- 39.Holst J, Feiring B, Naess LM, et al. The concept of “tailor-made”, protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine. 2005;23:2202–5. doi: 10.1016/j.vaccine.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 40.Sandbu S, Feiring B, Oster P, et al. Immunogenicity and safety of a combination of two serogroup B meningococcal outer membrane vesicle vaccines. Clin Vaccine Immunol. 2007;14:1062–9. doi: 10.1128/CVI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boutriau D, Poolman J, Borrow R, et al. Immunogenicity and safety of three doses of a bivalent (B:4:p1.19,15 and B:4:p1.7-2,4) meningococcal outer membrane vesicle vaccine in healthy adolescents. Clin Vaccine Immunol. 2007;14:65–73. doi: 10.1128/CVI.00230-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claassen I, Meylis J, van der Ley P, et al. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine. 1996;14:1001–8. doi: 10.1016/0264-410x(96)00020-5. [DOI] [PubMed] [Google Scholar]
- 43.van der Ley P, van der Biezen J, Poolman JT. Construction of Neisseria meningitidis strains carrying multiple chromosomal copies of the porA gene for use in the production of a multivalent outer membrane vesicle vaccine. Vaccine. 1995;13:401–7. doi: 10.1016/0264-410x(95)98264-b. [DOI] [PubMed] [Google Scholar]
- 44.Rots NY, Kleijne DE. Safety of a nonavalent meningococcal serogroup B vaccine in healthy adult volunteers in a randomised, controlled, single blind study. 16th International Pathogenic Neisseria Conference; Rotterdam, The Netherlands. 2008. [Google Scholar]
- 45.de Kleijn ED, de Groot R, Labadie J, et al. Immunogenicity and safety of a hexavalent meningococcal outer-membrane- vesicle vaccine in children of 2–3 and 7–8 years of age. Vaccine. 2000;18:1456–66. doi: 10.1016/s0264-410x(99)00423-5. [DOI] [PubMed] [Google Scholar]
- 46.Cartwright K, Morris R, Rumke H, et al. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine. 1999;17:2612–9. doi: 10.1016/s0264-410x(99)00044-4. [DOI] [PubMed] [Google Scholar]
- 47.Peeters CC, Rumke HC, Sundermann LC, et al. Phase I clinical trial with a hexavalent PorA containing meningococcal outer membrane vesicle vaccine. Vaccine. 1996;14:1009–15. doi: 10.1016/0264-410x(96)00001-1. [DOI] [PubMed] [Google Scholar]
- 48.Longworth E, Borrow R, Goldblatt D, et al. Avidity maturation following vaccination with a meningococcal recombinant hexavalent PorA OMV vaccine in UK infants. Vaccine. 2002;20:2592–6. doi: 10.1016/s0264-410x(02)00151-2. [DOI] [PubMed] [Google Scholar]
- 49.Martin SL, Borrow R, van der Ley P, Dawson M, Fox AJ, Cartwright KA. Effect of sequence variation in meningococcal PorA outer membrane protein on the effectiveness of a hexavalent PorA outer membrane vesicle vaccine. Vaccine. 2000;18:2476–81. doi: 10.1016/s0264-410x(00)00047-5. [DOI] [PubMed] [Google Scholar]
- 50.Russell JE, Jolley KA, Feavers IM, Maiden MC, Suker J. PorA variable regions of Neisseria meningitidis. Emerg Infect Dis. 2004;10:674–8. doi: 10.3201/eid1004.030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sacchi CT, Whitney AM, Popovic T, et al. Diversity and prevalence of PorA types in Neisseria meningitidis serogroup B in the United States, 1992–1998. J Infect Dis. 2000;182:1169–76. doi: 10.1086/315833. [DOI] [PubMed] [Google Scholar]
- 52.Bennett JS, Griffiths DT, McCarthy ND, et al. Genetic diversity and carriage dynamics of Neisseria lactamica in infants. Infect Immun. 2005;73:2424–32. doi: 10.1128/IAI.73.4.2424-2432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trotter CL, Gay NJ, Edmunds WJ. The natural history of meningococcal carriage and disease. Epidemiol Infect. 2006;134:556–66. doi: 10.1017/S0950268805005339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gorringe AR. Can Neisseria lactamica antigens provide an effective vaccine to prevent meningococcal disease? Expert Rev Vaccines. 2005;4:373–9. doi: 10.1586/14760584.4.3.373. [DOI] [PubMed] [Google Scholar]
- 55.Weynants VE, Feron CM, Goraj KK, et al. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect Immun. 2007;75:5434–42. doi: 10.1128/IAI.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Finney M, Vaughan T, Taylor S, et al. Characterization of the key antigenic components and pre-clinical immune responses to a meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Hum Vaccin. 2008;4:23–30. doi: 10.4161/hv.4.1.4806. [DOI] [PubMed] [Google Scholar]
- 57.Oliver KJ, Reddin KM, Bracegirdle P, et al. Neisseria lactamica protects against experimental meningococcal infection. Infect Immun. 2002;70:3621–6. doi: 10.1128/IAI.70.7.3621-3626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorringe AR, Taylor S, Brookes C, et al. Phase I safety and immunogenicity study of a candidate meningococcal disease vaccine based on Neisseria lastamica outer membrane vesicles. Clin Vaccne Immunol. doi: 10.1128/CVI.00118-09 ed, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Dwyer CA, Reddin K, Martin D, et al. Expression of heterologous antigens in commensal Neisseria spp: preservation of conformational epitopes with vaccine potential. Infect Immun. 2004;72:6511–8. doi: 10.1128/IAI.72.11.6511-6518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madico G, Welsch JA, Lewis LA, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177:501–10. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masignani V, Comanducci M, Giuliani MM, et al. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med. 2003;197:789–99. doi: 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pizza M, Scarlato V, Masignani V, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–20. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 63.Fransen F, Heckenberg SG, Hamstra HJ, et al. Naturally occurring lipid A mutants in Neisseria meningitidis from patients with invasive meningococcal disease are associated with reduced coagulopathy. PLoS Pathog. 2009;5:e1000396. doi: 10.1371/journal.ppat.1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fisseha M, Chen P, Brandt B, Kijek T, Moran E, Zollinger W. Characterization of Native Outer Membrane Vesicles from lpxL Mutant Strains of Neisseria meningitidis for Use in Parenteral Vaccination. Infect Immun. 2005;73:4070–80. doi: 10.1128/IAI.73.7.4070-4080.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koeberling O, Giuntini S, Seubert A, Granoff DM. Meningococcal outer membrane vesicle vaccines derived from mutant strains engineered to express factor H binding proteins from antigenic variant groups 1 and 2. Clin Vaccine Immunol. 2009;16:156–62. doi: 10.1128/CVI.00403-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koeberling O, Seubert A, Granoff DM. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J Infect Dis. 2008;198:262–70. doi: 10.1086/589308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weynants V, Denoel P, Devos N, et al. Genetically modified L3,7 and L2 lipooligosaccharides from Neisseria meningitidis serogroup B confer a broad cross-bactericidal response. Infect Immun. 2009;77:2084–93. doi: 10.1128/IAI.01108-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steeghs L, Keestra AM, van Mourik A, et al. Differential activation of human and mouse Toll-like receptor 4 by the adjuvant candidate LpxL1 of Neisseria meningitidis. Infect Immun. 2008;76:3801–7. doi: 10.1128/IAI.00005-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steeghs L, Tommassen J, Leusen JH, van de Winkel JG, van der Ley P. Teasing apart structural determinants of ‘toxicity’ and ‘adjuvanticity’: implications for meningococcal vaccine development. J Endotoxin Res. 2004;10:113–9. doi: 10.1179/096805104225004059. [DOI] [PubMed] [Google Scholar]
- 70.van der Ley P, Steeghs L, Hamstra HJ, ten Hove J, Zomer B, van Alphen L. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect Immun. 2001;69:5981–90. doi: 10.1128/IAI.69.10.5981-5990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rappuoli R, Covacci A. Reverse vaccinology and genomics. Science. 2003;302:602. doi: 10.1126/science.1092329. [DOI] [PubMed] [Google Scholar]
- 72.Litt DJ, Savino S, Beddek A, et al. Putative vaccine antigens from Neisseria meningitidis recognized by serum antibodies of young children convalescing after meningococcal disease. J Infect Dis. 2004;190:1488–97. doi: 10.1086/424464. [DOI] [PubMed] [Google Scholar]
- 73.Moe GR, Zuno-Mitchell P, Hammond SN, Granoff DM. Sequential immunization with vesicles prepared from heterologous Neisseria meningitidis strains elicits broadly protective serum antibodies to group B strains. Infect Immun. 2002;70:6021–31. doi: 10.1128/IAI.70.11.6021-6031.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hou VC, Koeberling O, Welsch JA, Granoff DM. Protective antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed genome-derived neisserial antigen 1870. J Infect Dis. 2005;192:580–90. doi: 10.1086/432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zollinger WD, Donets M, Brandt BL, et al. Multivalent group B meningococcal vaccine based on native outer membrane vesicles has potential for providing safe, broadly protective immunity. 16th International Pathogenic Neisseria Conference; Rotterdam, The Netherlands. 2008. [Google Scholar]
- 76.Cox AD, Zou W, Gidney MA, et al. Candidacy of LPS-based glycoconjugates to prevent invasive meningococcal disease: developmental chemistry and investigation of immunological responses following immunization of mice and rabbits. Vaccine. 2005;23:5045–54. doi: 10.1016/j.vaccine.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 77.Plested JS, Harris SL, Wright JC, et al. Highly conserved Neisseria meningitidis inner-core lipopolysaccharide epitope confers protection against experimental meningococcal bacteremia. J Infect Dis. 2003;187:1223–34. doi: 10.1086/368360. [DOI] [PubMed] [Google Scholar]
- 78.Estabrook MM, Jarvis GA, McLeod Griffiss J. Affinity-purified human immunoglobulin G that binds a lacto-N-neotetraose-dependent lipooligosaccharide structure is bactericidal for serogroup B Neisseria meningitidis. Infect Immun. 2007;75:1025–33. doi: 10.1128/IAI.00882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zollinger WD, Mandrell RE. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983;40:257–64. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santos GF, Deck RR, Donnelly J, Blackwelder W, Granoff DM. Importance of complement source in measuring meningococcal bactericidal titers. Clin Diagn Lab Immunol. 2001;8:616–23. doi: 10.1128/CDLI.8.3.616-623.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun. 2009;77:764–9. doi: 10.1128/IAI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Groot AS, Rappuoli R. Genome-derived vaccines. Expert Rev Vaccines. 2004;3:59–76. doi: 10.1586/14760584.3.1.59. [DOI] [PubMed] [Google Scholar]
- 83.Capecchi B, Serruto D, Adu-Bobie J, Rappuoli R, Pizza M. The genome revolution in vaccine research. Curr Issues Mol Biol. 2004;6:17–27. [PubMed] [Google Scholar]
- 84.Grifantini R, Sebastian S, Frigimelica E, et al. Identification of iron-activated and -repressed Fur-dependent genes by transcriptome analysis of Neisseria meningitidis group B. Proc Natl Acad Sci U S A. 2003;100:9542–7. doi: 10.1073/pnas.1033001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bernardini G, Braconi D, Lusini P, Santucci A. Postgenomics of Neisseria meningitidis: an update. Expert Rev Proteomics. 2009;6:135–43. doi: 10.1586/epr.09.3. [DOI] [PubMed] [Google Scholar]
- 86.Bernardini G, Braconi D, Martelli P, Santucci A. Postgenomics of Neisseria meningitidis for vaccines development. Expert Rev Proteomics. 2007;4:667–77. doi: 10.1586/14789450.4.5.667. [DOI] [PubMed] [Google Scholar]
- 87.Martin D, Cadieux N, Hamel J, Brodeur BR. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J Exp Med. 1997;185:1173–83. doi: 10.1084/jem.185.7.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moe GR, Tan S, Granoff DM. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect Immun. 1999;67:5664–75. doi: 10.1128/iai.67.11.5664-5675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Halperin SA, Langley JM, Smith B, et al. Phase 1 first-in-human studies of the reactogenicity and immunogenicity of a recombinant meningococcal NspA vaccine in healthy adults. Vaccine. 2007;25:450–7. doi: 10.1016/j.vaccine.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 90.West D, Reddin K, Matheson M, et al. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect Immun. 2001;69:1561–7. doi: 10.1128/IAI.69.3.1561-1567.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rokbi B, Mignon M, Maitre-Wilmotte G, et al. Evaluation of recombinant transferrin-binding protein B variants from Neisseria meningitidis for their ability to induce cross-reactive and bactericidal antibodies against a genetically diverse collection of serogroup B strains. Infect Immun. 1997;65:55–63. doi: 10.1128/iai.65.1.55-63.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Danve B, Lissolo L, Guinet F, et al. Safety and immunogenicity of a Neisseria meningitidis group B transferrin binding protein vaccine in adults. Eleventh international pathogenic Neisserial conference; Paris: EDK; 1998. [Google Scholar]
- 93.Perez RE, Lasa AM, Rodriguez RS, Menendez EC, Suarez JG, Balaguer HD. Scale-up of recombinant Opc protein production in Escherichia coli for a meningococcal vaccine. J Biotechnol. 2006;127:109–14. doi: 10.1016/j.jbiotec.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 94.Jolley KA, Appleby L, Wright JC, Christodoulides M, Heckels JE. Immunization with recombinant Opc outer membrane protein from Neisseria meningitidis: influence of sequence variation and levels of expression on the bactericidal immune response against meningococci. Infect Immun. 2001;69:3809–16. doi: 10.1128/IAI.69.6.3809-3816.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Callaghan MJ, Buckee CO, Jolley KA, Kriz P, Maiden MC, Gupta S. The effect of immune selection on the structure of the meningococcal opa protein repertoire. PLoS Pathog. 2008;4:e1000020. doi: 10.1371/journal.ppat.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giuliani MM, Adu-Bobie J, Comanducci M, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A. 2006;103:10834–9. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Plested JS, Granoff DM. Vaccine-induced opsonophagocytic immunity to Neisseria meningitidis group B. Clin Vaccine Immunol. 2008;15:799–804. doi: 10.1128/CVI.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jacobsson S, Thulin S, Molling P, et al. Sequence constancies and variations in genes encoding three new meningococcal vaccine candidate antigens. Vaccine. 2006;24:2161–8. doi: 10.1016/j.vaccine.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 99.Welsch JA, Moe GR, Rossi R, Adu-Bobie J, Rappuoli R, Granoff DM. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J Infect Dis. 2003;188:1730–40. doi: 10.1086/379375. [DOI] [PubMed] [Google Scholar]
- 100.Fletcher LD, Bernfield L, Barniak V, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun. 2004;72:2088–100. doi: 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thompson EA, Feavers IM, Maiden MC. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology. 2003;149:1849–58. doi: 10.1099/mic.0.26131-0. [DOI] [PubMed] [Google Scholar]
- 102.Capecchi B, Adu-Bobie J, Di Marcello F, et al. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol Microbiol. 2005;55:687–98. doi: 10.1111/j.1365-2958.2004.04423.x. [DOI] [PubMed] [Google Scholar]
- 103.Beernink PT, Welsch JA, Harrison LH, Leipus A, Kaplan SL, Granoff DM. Prevalence of factor H-binding protein variants and NadA among meningococcal group B isolates from the United States: implications for the development of a multicomponent group B vaccine. J Infect Dis. 2007;195:1472–9. doi: 10.1086/514821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Comanducci M, Bambini S, Caugant DA, et al. NadA diversity and carriage in Neisseria meningitidis. Infect Immun. 2004;72:4217–23. doi: 10.1128/IAI.72.7.4217-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Comanducci M, Bambini S, Brunelli B, et al. NadA, a novel vaccine candidate of Neisseria meningitidis. J Exp Med. 2002;195:1445–54. doi: 10.1084/jem.20020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grifantini R, Bartolini E, Muzzi A, et al. Previously unrecognized vaccine candidates against group B meningococcus identified by DNA microarrays. Nat Biotechnol. 2002;20:914–21. doi: 10.1038/nbt728. [DOI] [PubMed] [Google Scholar]
- 107.Hou VC, Moe GR, Raad Z, Wuorimaa T, Granoff DM. Conformational epitopes recognized by protective anti-neisserial surface protein a antibodies. Infect Immun. 2003;71:6844–9. doi: 10.1128/IAI.71.12.6844-6849.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murphy E, Andrew L, Lee KL, et al. Sequence Diversity of the Factor H Binding Protein Vaccine Candidate in Epidemiologically Relevant Strains of Serogroup B Neisseria meningitidis. J Infect Dis. 2009 doi: 10.1086/600141. [DOI] [PubMed] [Google Scholar]
- 109.Schneider MC, Exley RM, Chan H, et al. Functional significance of factor H binding to Neisseria meningitidis. J Immunol. 2006;176:7566–75. doi: 10.4049/jimmunol.176.12.7566. [DOI] [PubMed] [Google Scholar]
- 110.Welsch JA, Ram S, Koeberling O, Granoff DM. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J Infect Dis. 2008;197:1053–61. doi: 10.1086/528994. [DOI] [PubMed] [Google Scholar]
- 111.Seib KL, Serruto D, Oriente F, et al. Factor H-binding protein is important for meningococcal survival in human whole blood and serum and in the presence of the antimicrobial peptide LL-37. Infect Immun. 2009;77:292–9. doi: 10.1128/IAI.01071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haralambous E, Dolly SO, Hibberd ML, et al. Factor H, a regulator of complement activity, is a major determinant of meningococcal disease susceptibility in UK Caucasian patients. Scand J Infect Dis. 2006;38:764–71. doi: 10.1080/00365540600643203. [DOI] [PubMed] [Google Scholar]
- 113.Schneider MC, Prosser BE, Caesar JJ, et al. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature. 2009;458:890–3. doi: 10.1038/nature07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beernink PT, Welsch JA, Bar-Lev M, Koeberling O, Comanducci M, Granoff DM. Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate, factor H-binding protein. Infect Immun. 2008;76:4232–4240. doi: 10.1128/IAI.00367-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beernink PT, Granoff DM. Bactericidal antibody responses induced by meningococcal recombinant chimeric factor H-binding protein vaccines. Infect Immun. 2008;76:2568–75. doi: 10.1128/IAI.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beernink PT, Granoff DM. The modular architecture of meningococcal factor H-binding protein. Microbiology. 2009;155:2873–83. doi: 10.1099/mic.0.029876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jacobsson S, Hedberg ST, Molling P, et al. Prevalence and sequence variations of the genes encoding the five antigens included in the novel 5CVMB vaccine covering group B meningococcal disease. Vaccine. 2009;27:1579–84. doi: 10.1016/j.vaccine.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 118.Bambini S, Muzzi A, Olcen P, Rappuoli R, Pizza M, Comanducci M. Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus. Vaccine. 2009;27:2794–803. doi: 10.1016/j.vaccine.2009.02.098. [DOI] [PubMed] [Google Scholar]
- 119.Plested JS, Welsch JA, Granoff DM. Ex vivo model of meningococcal bacteremia using human blood for measuring vaccine-induced serum passive protective activity. Clin Vaccine Immunol. 2009;16:785–91. doi: 10.1128/CVI.00007-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Richmond P, Marshall H, Nissen MD, et al. In: van Alphen L, van Ley P, van den Dobbelsteen G, editors. A randomized, observer-blinded, active control, phase 1 trial of meningococcal serogroup B rLP2086 vaccine in healthy children and adolescents aged 8 to 14 years; 16th International Pathogenic Neisseria Conference; Rotterdam, The Netherlands. 2008. [Google Scholar]
- 121.Marshall H, Nissen MD, Richmond P, et al. In: van Alphen L, van Ley P, van den Dobbelsteen G, editors. A randomized, placebo-controlled, double-blind, phase 1 trial of ascending doses of meningococcal group B rLP2086 vaccine in healthy adults; 16th International Pathogenic Neisseria Conference; Rotterdam, The Netherlands. 2008. [Google Scholar]
- 122.Jansen KU, McNeil LK, Dragalin V, et al. In: van Alphen L, van Ley P, van den Dobbelsteen G, editors. Bivalent recombinant LP2086 vaccine to provide broad protection against Neisseria meningitidis B disease: Immunological correlates of protection and how to assess coverage against invasive MnB strains; 16th International Pathogenic Neisseria Conference; Rotterdam, The Netherlands. 2008. abstract 064. [Google Scholar]
- 123.Rappuoli R. In: van Alphen L, van Ley P, van den Dobbelsteen G, editors. The application of reverse vaccinology, Novartis MenB vaccine developed by design; 16th International Pathogenic Neisseria Conference; Rotterdam, The Netherlands. 2008. [Google Scholar]
- 124.Snape MD, Dawson T, Morant A, et al. In: Alphen Lv, Dobbelsteen Gvd, Ley Pvd., editors. Immunogenicity and reactogenicity of a novel serogroup B Neisseria meningitidis vaccine administered from 6 months of age; 16th International Pathogenic Neisseria Conference; Rotterdam, The Netherlands. 2008. [Google Scholar]
- 125.Maiden MC, Ibarz-Pavon AB, Urwin R, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–43. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beernink PT, Caugant DA, Welsch JA, Koeberling O, Granoff DM. Meningococcal factor H-binding protein variants expressed by epidemic capsular group A, W-135, and X strains from Africa. J Infect Dis. 2009;199:1360–8. doi: 10.1086/597806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Kleijn ED, de Groot R, Lafeber AB, et al. Prevention of meningococcal serogroup B infections in children: a protein-based vaccine induces immunologic memory. J Infect Dis. 2001;184:98–102. doi: 10.1086/320993. [DOI] [PubMed] [Google Scholar]
- 128.van der Voort ER, van der Ley P, van der Biezen J, et al. Specificity of human bactericidal antibodies against PorA P1.7,16 induced with a hexavalent meningococcal outer membrane vesicle vaccine. Infect Immun. 1996;64:2745–51. doi: 10.1128/iai.64.7.2745-2751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]