Results from these studies reveal that some strains of Gram-positive bacteria exploit hypoxia-inducible factor-regulated platelet-activating factor receptor as a means for translocation through intestinal epithelial cells.
Abstract
Mucosal surfaces, such as the lung and intestine, are lined by a monolayer of epithelia that provides tissue barrier and transport function. It is recently appreciated that a common feature of inflammatory processes within the mucosa is hypoxia (so-called inflammatory hypoxia). Given the strong association between bacterial translocation and mucosal inflammatory disease, we hypothesized that intestinal epithelial hypoxia influences bacterial translocation. Initial studies revealed that exposure of cultured intestinal epithelia to hypoxia (pO2, 20 torr; 24–48 h) resulted in a increase of up to 40-fold in the translocation of some strains of Gram-positive bacteria, independently of epithelial barrier function. A screen of relevant pathway inhibitors identified a prominent role for the platelet-activating factor receptor (PAFr) in hypoxia-associated bacterial translocation, wherein pharmacologic antagonists of PAFr blocked bacterial translocation by as much as 80 ± 6%. Extensions of these studies revealed that hypoxia prominently induces PAFr through a hypoxia-inducible factor (HIF)-dependent mechanism. Indeed, HIF and PAFr loss of function studies (short hairpin RNA) revealed that apically expressed PAFr is central to the induction of translocation for the Gram-positive bacteria Enterococcus faecalis. Together, these findings reveal that some strains of Gram-positive bacteria exploit HIF-regulated PAFr as a means for translocation through intestinal epithelial cells.
INTRODUCTION
The gastrointestinal tract is supported by a rich and complex underlying vasculature. As a consequence, the intestinal epithelial cell layer is particularly susceptible to damage associated with diminished blood flow. The resulting hypoxia is a consequence of both decreased perfusion and increased metabolism within the mucosa (Karhausen et al., 2003). The metabolic shift may result in “cytopathic hypoxia,” a form of mitochondrial dysfunction leading to reduced intracellular oxygen and ATP availability (Fink, 1997; Fink, 2001). Although this damage poses a risk to the epithelial function of excluding harmful luminal entities (Keely et al., 2005, 2008), recent studies have shown that the intestinal epithelium is equipped with hypoxia-inducible adaptive mechanisms that sustain barrier function under these inflamed conditions (Karhausen et al., 2003, 2004, 2005). Indeed, compared with other surfaces, intestinal epithelial cells seem to be uniquely resistant to disruption by hypoxia (Furuta et al., 2001; Synnestvedt et al., 2002; Karhausen et al., 2004). Such observations may relate to the fact that intestinal epithelial cells are conditioned to a lower pO2 than other tissue sources (Taylor and Colgan, 2007).
Although there is strong correlation between barrier breakdown and bacterial translocation (Fink, 1991; Lenz et al., 2007; Zinkernagel et al., 2007), the molecular mechanisms of bacterial translocation from the lumen of the gastrointestinal (GI) tract to bloodstream are not well understood. Although there is an apparent increase in translocated bacteria with hypoxia, most evidence suggests that overall integrity if the intestinal epithelium remains intact even in relatively severe hypoxia (Furuta et al., 2001). This finding may indicate that bacterial translocation during intestinal inflammation is a consequence of increased transcellular bacterial movement, rather than a breakdown of epithelial integrity. As an example, Enterococcus faecalis has become increasingly important and is now the second most common hospital-acquired infection in the United States (Hageman et al., 2003; NNIS System, 2003), with increased prevalence of antibiotic resistant strains (Lam et al., 1995).
Based on these observations, we examined the passage of noninvasive, nonpathogenic enteric bacteria across intestinal epithelial cells subjected to hypoxia in an attempt to identify molecular mechanisms of such translocation. We hypothesized that noninvasive bacteria may exploit existing epithelial cell surface receptors to attach and invade the intestinal epithelium as a mechanism of invading the serosa. Using established in vitro models of infection, we identified platelet-activating receptor (PAFr) as an entry point into the cell by the Gram-positive enteric bacterium E. faecalis. We examined the induction and regulation of PAFr by HIF-1 and its regulation in intestinal inflammation in vivo. Our finding suggest that E. faecalis exploit existing epithelial pathways to overcome the intestinal barrier during inflammation and that competitive antagonism of PAFr may be a therapeutic strategy for patients at risk of sepsis.
MATERIALS AND METHODS
Cell Culture
Caco-2 intestinal epithelial cells were grown and maintained in T75 cell culture flasks (Corning Life Sciences, Lowell, MA) as described previously (Furuta et al., 2001). For translocation experiments, cells were grown on permeable supports (Corning Life Sciences) and maintained over 21 d to achieve fully differentiated, polarized monolayers as described previously (Keely et al., 2009).
Bacterial Cultures
Bacteria were either human isolates or ATCC strains (American Type Culture Collection, Manassas, VA). Primary bacterial cultures of E. faecalis were streaked on tryptone agar plates and incubated overnight at 37°C. All other strains were grown in LB agar plates. Colonies were removed from the plate and inoculated into 5 ml of the appropriate culture broth. This broth was incubated and shaken (250 rpm) overnight at 37°C, resulting in a bacterial population of ∼1 × 108 colony-forming units (CFU)/ml.
Infection Studies
Bacterial cultures in broth (1 × 108 CFU/ml) was centrifuged at 3000 × g for 10 min. The resulting pellet was resuspended in Hanks' balanced salt solution (supplemented with magnesium and calcium) buffer to give a concentration of 1 × 107 CFU/ml. Caco-2 cells were seeded on 0.33-cm2, 3-μm pore Transwell inserts and maintained for 21 d to allow polarization. Monolayers were incubated in either hypoxic (1% O2) or normoxic conditions at 37°C for 24 h before bacterial challenge. Monolayers were preincubated with either vehicle or PAFr antagonist CV-6209 (BIOMOL Research Laboratories, Plymouth Meeting, PA) on the apical side for 30 min. Monolayers were then apically challenged at a multiplicity of infection of 100:1 (3 × 107 bacteria to 3 × 105 epithelial cells) and incubated at 37°C. Samples of 100 μl in volume were taken from the basolateral chamber 15, 30, 60, and 120 min after challenge. Basolateral samples were diluted 1/100, and 50-μl samples were spot plated on tryptone agar plates. Plates were incubated overnight at 37°C, and plate counts were carried out to determine bacterial translocation.
For imaging, Caco-2 cells were seeded onto collagen-coated glass coverslips. E. faecalis was labeled with BacLight fluorescent bacterial stain (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions (Berney et al., 2007) before challenge (5–45 min). Cells were fixed in 4% formaldehyde, permeabilized with 0.01% Triton-X, blocked with 10% bovine serum albumin, and probed with anti-PAFr (Cayman Chemical, Ann Arbor, MI). Cells were counterstained with 4,6-diamidino-2-phenylindole (DAPI; Invitrogen) and imaged by fluorescent microscopy.
Stable Repression of HIF-1a and PAFr by Short Hairpin RNA (shRNA)
Lentiviral particles encoding a panel of shRNAs directed against either HIF-1α or HIF-2α (MISSION TRC shRNA; Sigma-Aldrich, St. Louis, MO) were used to transduce Caco-2 cells using standard protocols. Nontarget shRNA-encoding lentiviral particles were used to generate a control cell line. Stable integration was accomplished by prolonged exposure to puromycin (6 μg/ml). For repression of PAFr, hairpin primers with the sequence 5′-ACCTCACCACGGATACGGTCA-CTGAATCAAGAGTTCAGTGACCGTATCCGTGGTTT-3′ and 5′-CAAAAAACCACGGAT-ACGGTCACTGAACTCTTGATTCAGTGACCGTATCCGTGGTG-3′, corresponding to position 1088-1108 of the PAFr transcript, were annealed and ligated into the BbsI/BbsI-digested psiRNA-hH1neo G2 vector (InvivoGen, San Diego, CA). Caco-2 cells were transfected with plasmid by using FuGENE 6.0 according to the manufacturer's protocols (Roche Diagnostics, Indianapolis, IN). A control Caco-2 line was generated using psiRNA-hH1 neoSCR plasmid, encoding a scrambled shRNA with no known homology to any mouse or human transcript. Stable transfectants were selected with G418 (400 μg/ml). Repression was confirmed by real-time polymerase chain reaction (PCR) and Western blot analysis.
Transcriptional and Protein Expression Analysis
The transcriptional profile of Caco-2 and repression epithelial cells subjected to control (normoxia; pO2, 147 torr) or hypoxia (pO2, 20 torr for 6- or 18-h hypoxia) were assessed from total RNA by quantitative real-time PCR. (PAFr primer set: forward, 5′-AGAAGTTCCGCAAGCACCTC-3′ and reverse, 5′-GGATCTGGTTGAATGGCACA-3′) by using iQ SYBR mix (iCycler; Bio-Rad Laboratories, Hercules, CA), as described previously (Karhausen et al., 2004; Louis et al., 2008). Protein expression was ascertained by Western blot analysis. Protein isolated from normoxic and hypoxic monolayers was separated by SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, and probed with anti-PAFr (Cayman Chemical). β-Actin was used as a reference housekeeping protein. Blot analysis was quantified in terms of pixel number using ImageJ (National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/).
Chromatin Immunoprecipitation (ChIP) of Hif-1α
Chromatin immunoprecipitation was performed as described previously (Kong et al., 2004) by using anti-HIF-1α antibody (rabbit polyclonal; Novus Biologicals, Littleton, CO). HIF binding to PAFr promoter DNA was quantified by both standard and real-time PCR by using primers (forward, 5′-ctggcctcgggcgctgtcta-3′ and reverse, 5′-ccaagtcacccctgggaggaa-3′) designed to amplify a 153-base pair region of the PAFr promoter. Chromatin incubated with beads or beads plus rabbit immunoglobulin G (IgG) served as controls for nonspecific binding.
Dextran Sulfate Sodium (DSS) Colitis Model
DSS colitis was induced with a modification of the technique of Okayasu et al. (1990). Colitis was induced on day 0 by the addition of 3, 4.5, or 6% DSS (mol. wt. = 36,000–50,000; MP Biomedicals, Irvine, CA) solution in drinking water. Control animals received water alone. On day 6, animals were euthanized and the GI tract was excised. Colon length was recorded and epithelial cells were harvested from the colon by EDTA cell isolation (100 mM N-acetyl cysteine, 1 mM EDTA, and 1.5%, vol/vol HEPES in phosphate-buffered saline, vortexed at high speed for 15 min).
RESULTS
Translocation of Bacteria through Hypoxic Monolayers
As an initial series of experiments, we examined a range of bacteria including noninvasive, nonpathogenic strains, for translocation across intestinal epithelial monolayers and whether this response was influenced by hypoxia. Two Gram-positive bacteria, E. faecalis and Streptococcus mitis, showed significantly increased translocation in response to hypoxia (Figure 1A). None of the Gram-negative bacteria species tested showed differences between normoxia and hypoxia (Figure 1A). As shown in Figure 1B, these studies revealed that in normoxia, E. faecalis translocated across confluent Caco2 monolayers at very low rates over a two hour period (corresponding to a rate of 4.0 ± 0.6 CFU/min). E. faecalis translocation changed quite significantly when epithelia were subjected to hypoxia (pO2, 20 torr; 48 h). Rates of E. faecalis translocation increased by 42 ± 6-fold after hypoxia (corresponding rate of 168.6 ± 7.2 CFU/min; p < 0.001). Fractional translocation comparing ratios of normoxia to hypoxia and individual time points (Figure 1C) revealed that the majority of this increase was evident at time points beyond 15 min. Importantly, this translocation occurred without significant changes in transepithelial resistance (TER; in the range of 400–500 Ω.cm2 at all times) or any increases in apparent permeability (Figure 1D; p > 0.05), wherein tumor necrosis factor (TNF)-α (10 ng/ml; 48 h), which is well established to increase epithelial permeability (Marano et al., 1998; Ma et al., 2005), served as a permeabilizing condition for these studies (p < 0.005). Such findings reveal that hypoxia increases bacterial translocation in a manner independent of paracellular permeability.
Figure 1.
Increased translocation of E. faecalis across hypoxic Caco-2 monolayers. (A) Influence of hypoxia on the translocation of a selection of Gram-negative and Gram-positive over 60 min. (B) Influence of hypoxia on the translocation of E. faecalis over time. (C) The rate of increased E. faecalis translocation with hypoxia. (D) Apparent permeability of Caco-2 monolayers after infection, TNF-α (10 ng/ml) is a positive control. (E) Translocation of E. faecalis across Caco-2 monolayers is temperature dependent. Results are derived from six or more experiments. Hx, hypoxia; Nx, normoxia, *p < 0.05, **p < 0.01, and ***p < 0.005 (Student's t test).
To further examine whether hypoxia-induced translocation was paracellular or transcellular, we studied the role of epithelial metabolism on bacterial translocation. To do this, we diminished epithelial metabolic rates by lowering incubation temperatures during E. faecalis translocation across epithelial monolayers, reasoning that transcellular processes, but not paracellular permeability, would not be influenced by decreased metabolic rates (Finlay et al., 1988). As shown in Figure 1E, bacterial translocation decreased with decreasing ambient temperature (p < 0.01 by analysis of variance [ANOVA]), thereby implicating the transcellular and not the paracellular pathway for bacterial translocation.
E. faecalis Translocation Is Inhibited by Antagonism of PAFr
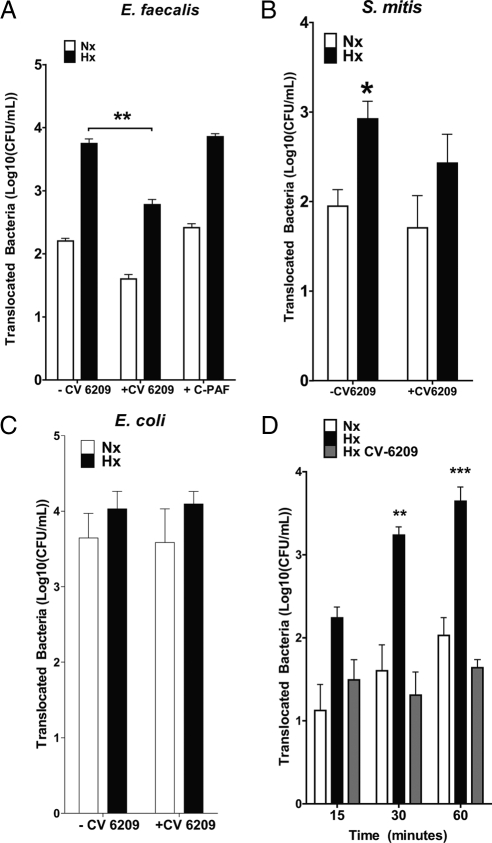
We next surmised that an apical surface receptor could contribute to the translocation of bacteria across intestinal epithelial cells. We approached the task of identifying candidate receptors by means of sterically hindering interaction of bacteria to putative binding sites with antibodies, antagonists or peptides known to interact with the surface protein. Within this screen, an antagonist for the Gram-positive bacterial pattern recognition-receptor PAFr (Fillon et al., 2006), CV-6209, significantly reduced the translocation of E. faecalis (Figure 2A, p < 0.01) and marginally reduced the translocation of S. mitis (Figure 2B) but not Escherichia coli (Figure 2C) across both hypoxic and normoxic Caco-2 monolayers. Such inhibition of E. faecalis translocation across post-hypoxic monolayers occurred in a time-dependent manner (Figure 2D; p < 0.005), thus implying that bacterial translocation of Gram-negative and Gram-positive species may be differentially regulated in hypoxia and that PAFr may serve as a pathway for the translocation of Gram-positive E. faecalis.
Figure 2.
PAFr antagonist CV-6209 (60 μM) prevents E. faecalis but not S. mitis or E. coli translocation across hypoxic Caco-2 monolayers. (A) Influence of CV-6209, a PAFr antagonist, and C-PAF, a PAFr agonist, on the translocation of E. faecalis across Caco-2 monolayers. (B) Influence of PAFr antagonism on the translocation of S. mitis across Caco-2 monolayers. (C) Influence of PAFr antagonism on the translocation of E. coli across Caco-2 monolayers. (D) Inhibition E. faecalis translocation by CV-6209 over time. Bacterial translocation over 60 min. Results are derived from six or more experiments. Hx, hypoxia; Nx, normoxia. **p < 0.01 and ***p < 0.005 (Student's t test).
The Gram-positive Pattern Recognition Receptor PAFr Is Hypoxia Inducible
Based on our finding that the PAFr antagonist CV-6209 selectively attenuates E. faecalis translocation, we next examined the expression pattern of PAFr in Caco-2 cells. As shown in Figure 3A, PAFr mRNA transcript was rapidly and prominently induced by hypoxia. Such induction was transient, wherein hypoxia induced PAFr at 2 and 4 h (p < 0.05) and measured levels were near baseline levels by 6 h. We next examined protein expression of PAFr in hypoxic Caco-2 cells. After 24 h in hypoxia, PAFr protein expression was increased (Figure 3B), and such induction in protein was shown to be significant by densitometry analysis (Figure 3C; p < 0.005). Analysis of apical and basolateral PAFr expression by differential biotinylation revealed that PAFr expression was predominantly apical in normoxia but was expressed on both apical and basolateral membranes during hypoxia (Figure 3D). These findings confirmed that the kinetics of bacterial translocation correlates with the increased expression of PAFr in hypoxia.
Figure 3.
Induction of PAFr in hypoxic Caco-2 monolayers and in DSS colitis. (A) Induction of PAFr mRNA transcript over time in Caco-2 monolayers incubated in hypoxia. (B) Expression of PAFr protein in Caco-2 monolayers after 24 h in hypoxia; 48 kDa represents apical cell surface PAFr (verified by biotinylation, densitometry analysis 3191 ± 248 pixels for apical PAFr vs. 319 ± 254 pixels for basolateral PAFr), whereas the 69-kDA ban represents cytoplasmic PAFr. (C) Densitometric analysis of PAFr protein expression by ImageJ pixel count. Results are derived from three experiments. (D) Apical (ap) and basolateral (bas) expression of PAFr in hypoxic and normoxic Caco-2 monolayers. (E) Induction of PAFr in DSS colitis. mRNA was isolated from epithelial cells from mice 6 d after induction of DSS colitis. (F) PAFr correlation with disease severity (weight loss). Results are derived from three experiments. For A, *p < 0.05 by one-way ANOVA. For B, p < 0.05 by linear regression analysis. Hx, hypoxia; Nx, normoxia. *p < 0.05 and ***p < 0.005 (Student's t test).
PAFr Is Induced in Epithelial Cells during Murine DSS Colitis
To place these findings in a physiological context, we investigated the role of PAFr in an in vivo setting. Here, we examined PAFr mRNA expression in epithelial cells isolated from the colon of mice after induction of DSS colitis, conditions known to elicit “inflammatory hypoxia” (Karhausen et al., 2005; Shah et al., 2008). In this setting, PAFr was shown to be induced in DSS colitic mice in a concentration-dependent manner (Figure 3E; p < 0.01 by ANOVA). Furthermore, PAFr induction correlated with disease severity as measured by correlation of PAFr mRNA and the magnitude of animal weight loss (Figure 3F; p < 0.05 by linear regression). These findings suggest that PAFr is induced in severe intestinal inflammation and may implicate that PAFr plays a role in sepsis as a result of inflammatory bowel disease.
shRNA-mediated Repression of PAFr Attenuates Translocation of E. faecalis
To further examine the direct role of PAFr in Gram-positive bacterial translocation, we generated a shRNA PAFr repression Caco-2 cell line. Screening of these cells by real-time PCR showed significant repression of PAFr at the transcript level (Figure 4A; p < 0.005) compared with nontarget control and wild-type cells. Furthermore, hypoxia-inducible protein expression of PAFr was abrogated in PAFr knockdown cell lines exposed to hypoxia (Figure 4B; p < 0.01 by densitometry). On confirmation of PAFr repression in the cell line, we performed bacterial translocation experiments on hypoxic and normoxic cells. This analysis revealed a significant reduction in the translocation of E. faecalis across hypoxic Caco-2 PAFr-shRNA monolayers (Figure 4C; p < 0.01). Furthermore, the PAF antagonist CV-6209 did not further reduce the translocation of E. faecalis, suggesting that the reduction in translocation was PAFr mediated. Notably, the translocation of E. coli was not influenced by the repression of PAFr (ΔCFU/ml = −9.11 × 102 ± 5.44 × 102 for Caco-2 PAFr-shRNA), suggesting at least some degree of specificity for Gram-positive bacteria.
Figure 4.
shRNA-mediated repression of PAFr in Caco-2 monolayers reduces translocation of E. faecalis. (A) PAFr is not induced by hypoxia in PAFr knockdown Caco-2 monolayers incubated in hypoxia. (B) Loss of protein induction by PAFr knockdown Caco-2 monolayers after 24 h in hypoxia. (C) Translocation of E. faecalis across hypoxic and normoxic PAFr knockdown Caco-2 monolayers. CV-6209 (60 μM) does not reduced translocation across PAFr knockdown Caco-2 monolayers. Bacterial translocation was over 120 min. Results are derived from six experiments. Hx, hypoxia; Nx, normoxia. **p < 0.01 and ***p < 0.005 (Student's t test).
In an attempt to define the association of PAFr expression and bacterial translocation, we examined the association of E. faecalis and Caco-2 monolayers by confocal microscopy. As shown in Figure 5, A–C, a marked induction of PAFr expression in Caco-2 monolayers exposed to hypoxia (Figure 5B) over normoxic cells (Figure 5A). However, hypoxic PAFr shRNA knockdown cells (Figure 5C) showed little expression of PAFr and a reduced capacity for bacterial uptake compared with hypoxic scrambled monolayers. The x-z axis imaging of monolayers by confocal showed adhesion and internalization of E. faecalis, confirming transcellular entry of the bacteria (Figure 5D). Together, these findings demonstrate further the central role of PAFr in the translocation of Gram-positive bacteria across hypoxic intestinal epithelial cells.
Figure 5.
E. faecalis adherence-to and invasion-of scr-shRNA and PAFr-shRNA Caco-2 monolayers. (A) Fluorescent staining of PAFr and E. faecalis on Caco-2 scr-shRNA monolayers. (B) Fluorescent staining of PAFr and E. faecalis on hypoxic Caco-2 scr-shRNA monolayers. (C) Fluorescent staining of PAFr and E. faecalis on hypoxic Caco-2 PAFr-shRNA monolayers. (D) Internalization of E. faecalis by hypoxic hypoxic Caco-2 PAFr-shRNA monolayers over time. Magnification at 40× for top-down fluorescent imaging and at 63× for confocal X-Z imaging, Blue, DAPI nuclear stain (Invitrogen); red, anti-PAFr antibody (Cayman Chemical); and green, BacLight labeled E. faecalis (Invitrogen). Results are derived from three experiments and images are representative.
shRNA-mediated Repression of HIF-1 Reduces PAFr Expression and E. faecalis Translocation
We next investigated the molecular mechanisms of PAFr induction by hypoxia. As a global regulator of hypoxia, HIF functions as a central regulator of hypoxia-mediated gene expression. To address the role of HIF-1 in PAFr induction, and consequently E. faecalis translocation, we screened five separate lentiviral shRNAs directed against HIF-1α in Caco-2 epithelial cells. As shown in Figure 6A, Western blot analysis of individual lentivirus-transduced lines revealed significant reduction in HIF-1α in all lines under baseline conditions but only shRNAs 3, 4, and 5 in hypoxia. There was no significant reduction in HIF-2α levels in any of these cell lines (Figure 6A). Based on these results, we proceeded with our analysis using the shRNA4 cell line.
Figure 6.
Knockdown of HIF-1 in Caco-2 monolayers prevents PAFr induction in hypoxia and the translocation of E. faecalis. (A) Expression of both HIF-1α and HIF-2α isoforms in intestinal epithelial cells. Western blot analysis of HIF knockdown Caco-2 cells cultured for 16 h under normoxic conditions (Nx) or in hypoxia (Hx) with the prolyl hydroxylase domain inhibitor dimethyloxallyl glycine (DMOG; 0.5 mg/ml). Nuclear lysates were immunoprobed with antibodies against HIF-1α and HIF-2α. TATA-binding protein levels were monitored as a reference loading control. Highest level of repression was observed in the stable cell line harboring shRNA 4 (70% repression); this cell line was used in all further experiments. sh, short hairpin RNA. (B) Loss of PAFr is not induced by hypoxia in HIF-1 knockdown Caco-2 monolayers incubated in hypoxia. (C) PAFr protein induction is reduced in HIF-1 knockdown Caco-2 monolayers after 24 h in hypoxia. (D) Translocation of E. faecalis across hypoxic and normoxic HIF-1 knockdown Caco-2 monolayers. (E) ChIP analysis to examine HIF-1 binding to the PAFr promoter in hypoxic Caco-2 cells. Reaction controls included immunoprecipitations using nonspecific IgG and beads only as well as by PCR performed using total Caco-2 DNA (Input). Results are derived from three to six experiments. Hx, hypoxia; Nx, normoxia. *p < 0.05, **p < 0.01, and ***p < 0.005 (Student's t test).
We next examined PAFr transcript levels in the shRNA HIF-1α knockdown line. As shown in Figure 6B, this analysis revealed the specific loss of hypoxia mediated PAFr induction in response to hypoxia (p < 0.005). Further examination of PAFr protein expression revealed a nearly complete loss in cells lacking HIF-1α compared with scrambled controls (Figure 6C). On verification of loss of PAFr induction in these cells, we performed bacterial translocation assays and revealed a significant reduction in the translocation of E. faecalis across Caco-2 cells lacking HIF-1α when exposed to hypoxia (Figure 6D).
We extended these findings to determine whether HIF-1α binds to the PAFr gene promoter in response to hypoxia. Sequence analysis of the proximal human PAFr promoter (from chromosome 16, NM_000952) revealed a potential binding site for HIF at positions −133 bp relative to the transcription start site). As shown in Figure 5E, ChIP analysis of nuclei derived from Caco-2 cells revealed a prominent band of 153 base pairs from hypoxic samples. No bands were evident in control IgG immunoprecipitates, and input samples (preimmunoprecipitation) revealing the predicted 153-base pair band under these conditions. Such results indicate that hypoxia induces HIF-1α binding to the distal 153-base pair region of the PAFr promoter. Together, these results provide strong evidence for a functional hypoxia-inducible activity, mediated by HIF-1α, in the distal 5′-region the PAFr promoter.
DISCUSSION
In this study, we aimed to elucidate whether conditions found in the inflamed mucosa might influence the translocation of noninvasive bacteria. These studies are founded on the observation that commensal bacteria and/or bacterial products seem to penetrate the mucosa and do so under conditions where the epithelium remains intact. Such translocation contributes to the activation of host immune defense mechanisms, subsequent autointoxication and tissue destruction (Meakins and Marshall, 1986). Here, we propose a mechanism by which noninvasive Gram-positive E. faecalis override host phosphorylcholine binding receptors (PAFr) during instances of acute inflammation and concomitant tissue hypoxia.
Our initial studies revealed a profound increase in noninvasive bacterial translocation across posthypoxic intestinal epithelia. As has been demonstrated in the past (Furuta et al., 2001), intestinal epithelia seem to be uniquely tolerant to hypoxia with regard to barrier integrity, and our findings here were no exception. A broader examination revealed that although Gram-negative E. coli translocation was also increased, the magnitude was far greater for Gram-positive E. faecalis. This is interesting given the current state of nonsocomial infections. E. faecalis, for example, has traditionally been regarded as an opportunistic pathogen but has become increasingly important as an antibiotic-resistant strain of bacteria associated with hospital-acquired infections in the United States (Hageman et al., 2003; NNIS System, 2003).
A search for potential membrane components which might contribute to increased bacterial translocation identified PAFr, an apically expressed G protein-coupled cell surface receptor (Claud et al., 2002). Indeed, the finding that the PAFr antagonist CV-6209 reduced the capacity of E. faecalis to translocate across hypoxic Caco-2 monolayers was particularly interesting. In addition to its role in signaling via PAF, PAFr functions as an innate immune recognition receptor phosphorylcholine moiety of Gram-positive lipoteichoic acid (LTA) (Cundell et al., 1995; Fillon et al., 2006; Barbier et al., 2008), possibly mirroring the role of Toll receptors in Gram-negative lipopolysaccharide recognition (Lemjabbar and Basbaum, 2002). Notable were differences in hypoxia-inducible translocation between various Gram-positive bacterial strains and species. Although we do not know the exact nature of these differences, it is well established LTA structures vary significantly between various Gram-positive bacterial species and even within bacterial strains (Draing et al., 2006). It is possible, for example, that these differences in LTA structure could contribute to variability in bacterial uptake across the plasma membrane. Moreover, several studies suggest that PAFr may also mediate the recruitment of neutrophils and macrophages to the sites of inflammation and infection (Wallace, 1988; Hirayama et al., 2003; Han et al., 2006). Antagonistic blocking of the PAFr receptor has been shown to reduce the translocation of heat killed bacteria across Caco-2 monolayers and an M-cell coculture model (Tirer et al., 2006). Repression of the PAFr gene in Caco-2 monolayers similarly reduced E. faecalis translocation across hypoxic monolayers.
Several levels of analysis in Caco-2 epithelial cells revealed a prominent, transcription-dependent induction of PAFr by hypoxia. These observations lead us to define the contribution of HIF to such induction. Studies using HIF-1α loss of function (shRNA) revealed a prominent role for HIF-1α in both PAFr induction and in bacterial translocation. Additional studies using ChIP analysis defined a binding site for HIF in the proximal PAFr promoter. PAFr has been shown to be a pathway for nasopharyngeal passage of pneumococci (Cundell et al., 1995) and has been involved the transcytosis of pneumococci through brain microvascular endothelial cells (Ring et al., 1998). More recently PAFr has been implicated in inflammatory response during pneumococcal pneumonia and colitis (Hirayama et al., 2003; Rijneveld et al., 2004). In this study, we show that PAFr facilitates Gram-positive E. faecalis translocation across epithelial barriers. Notable are the observations that epithelial HIF is protective during mucosal inflammation, particularly in murine models of colitis. Studies using conditional loss of intestinal epithelial HIF-1α (Karhausen et al., 2004) and more recent pharmacologic studies strongly implicate an important adaptive role for HIF in mucosal inflammation. In the current studies, PAFr mRNA was increased in DSS colitic tissue and the degree of PAFr induction was positively correlated with the degree of disease (weight loss measurements).
It remains to be determined whether HIF-mediated, PAFr-dependent bacterial translocation represents a physiological clearance mechanism or rather serves as a pathophysiologic mechanism whereby bacteria exploit PAFr as a route of entry. An example of the former are provided by the observation that Pseudomonas uses apically expressed cystic fibrosis transmembrane regulator (CFTR) in the lung as a route of entry and as a mechanism of bacterial clearance (Pier et al., 1997). Clinically relevant mutants of CFTRs that do not bind Pseudomonas fail to clear bacteria and result in overgrowth pneumonia in some patients with cystic fibrosis. Thus, it is possible that the PAFr functions as a membrane surface sensor, which when bound (e.g., by Gram-positive LTA), results in internalization, translocation, and clearance. Alternatively, PAFr may represent a previously unappreciated pathway for bacterial uptake and subsequent sepsis. Necrotizing enterocolitis (NEC), for example, is a mucosal disease of unknown etiology that has been strongly associated with prematurity, enteral feeding and hypoxia (Caplan et al., 2005). Important for the current studies, a primary proinflammatory pathway linked to NEC is PAF, and several studies in murine models have suggested that PAFr antagonism may be beneficial to the development of NEC (Caplan et al., 1997). Additional studies and appropriate models are necessary to define whether PAFr-mediated bacterial translocation is protective or pathogenic in mucosal disease.
ACKNOWLEDGMENTS
We acknowledge the outstanding technical assistance by Melanie Scully and Brittelle Bowers throughout the course of this study. This work was supported by National Institutes of Health grants HL-60569, DE-016191, and DK-50189 and by a Research Fellowship Award from the Crohn's and Colitis Foundation of America.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-07-0573) on December 23, 2009.
REFERENCES
- Barbier M., Oliver A., Rao J., Hanna S. L., Goldberg J. B., Alberti S. Novel phosphorylcholine-containing protein of Pseudomonas aeruginosa chronic infection isolates interacts with airway epithelial cells. J. Infect. Dis. 2008;197:465–473. doi: 10.1086/525048. [DOI] [PubMed] [Google Scholar]
- Berney M., Hammes F., Bosshard F., Weilenmann H. U., Egli T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight Kit in combination with flow cytometry. Appl. Environ. Microbiol. 2007;73:3283–3290. doi: 10.1128/AEM.02750-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan M. S., Hedlund E., Adler L., Lickerman M., Hsueh W. The platelet-activating factor receptor antagonist WEB 2170 prevents neonatal necrotizing enterocolitis in rats. J. Pediatr. Gastroenterol. Nutr. 1997;24:296–301. doi: 10.1097/00005176-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Caplan M. S., Simon D., Jilling T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin. Pediatr. Surg. 2005;14:145–151. doi: 10.1053/j.sempedsurg.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Claud E. C., Li D., Xiao Y., Caplan M. S., Jilling T. Platelet-activating factor regulates chloride transport in colonic epithelial cell monolayers. Pediatr. Res. 2002;52:155–162. doi: 10.1203/00006450-200208000-00005. [DOI] [PubMed] [Google Scholar]
- Cundell D. R., Gerard N. P., Gerard C., Idanpaan-Heikkila I., Tuomanen E. I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- Draing C., Pfitzenmaier M., Zummo S., Mancuso G., Geyer A., Hartung T., von Aulock S. Comparison of lipoteichoic acid from different serotypes of Streptococcus pneumoniae. J. Biol. Chem. 2006;281:33849–33859. doi: 10.1074/jbc.M602676200. [DOI] [PubMed] [Google Scholar]
- Fillon S., et al. Platelet-activating factor receptor and innate immunity: uptake of gram-positive bacterial cell wall into host cells and cell-specific pathophysiology. J. Immunol. 2006;177:6182–6191. doi: 10.4049/jimmunol.177.9.6182. [DOI] [PubMed] [Google Scholar]
- Fink M. Cytopathic hypoxia in sepsis. Acta Anaesthesiol. Scand. Suppl. 1997;110:87–95. doi: 10.1111/j.1399-6576.1997.tb05514.x. [DOI] [PubMed] [Google Scholar]
- Fink M. P. Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Crit. Care Med. 1991;19:627–641. doi: 10.1097/00003246-199105000-00009. [DOI] [PubMed] [Google Scholar]
- Fink M. P. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit. Care Clin. 2001;17:219–237. doi: 10.1016/s0749-0704(05)70161-5. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Gumbiner B., Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J. Cell Biol. 1988;107:221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta G. T., Turner J. R., Taylor C. T., Hershberg R. M., Comerford K., Narravula S., Podolsky D. K., Colgan S. P. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman J. C., Fridkin S. K., Mohammed J. M., Steward C. D., Gaynes R. P., Tenover F. C. Antimicrobial proficiency testing of National Nosocomial Infections Surveillance System hospital laboratories. Infect. Control Hosp. Epidemiol. 2003;24:356–361. doi: 10.1086/502214. [DOI] [PubMed] [Google Scholar]
- Han S. H., Kim J. H., Seo H. S., Martin M. H., Chung G. H., Michalek S. M., Nahm M. H. Lipoteichoic acid-induced nitric oxide production depends on the activation of platelet-activating factor receptor and Jak2. J. Immunol. 2006;176:573–579. doi: 10.4049/jimmunol.176.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama K., Yokoi Y., Sakaguchi T., Nakamura T., Kashiwabara H., Sunayama K., Nakamura S. Platelet-activating factor, a critical mediator in the pathogenesis of dextran sulfate sodium-induced colitis in rats. Dis. Colon Rectum. 2003;46:100–110. doi: 10.1007/s10350-004-6503-7. [DOI] [PubMed] [Google Scholar]
- Karhausen J., Furuta G. T., Tomaszewski J. E., Johnson R. S., Colgan S. P., Haase V. H. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhausen J., Haase V. H., Colgan S. P. Inflammatory hypoxia: role of hypoxia-inducible factor. Cell Cycle. 2005;4:256–258. [PubMed] [Google Scholar]
- Karhausen J., Ibla J. C., Colgan S. P. Implications of hypoxia on mucosal barrier function. Cell. Mol. Biol. 2003;49:77–87. [PubMed] [Google Scholar]
- Keely S., Rawlinson L. A., Haddleton D. M., Brayden D. J. A tertiary amino-containing polymethacrylate polymer protects mucus-covered intestinal epithelial monolayers against pathogenic challenge. Pharm. Res. 2008;25:1193–1201. doi: 10.1007/s11095-007-9501-3. [DOI] [PubMed] [Google Scholar]
- Keely S., Rullay A., Wilson C., Carmichael A., Carrington S., Corfield A., Haddleton D. M., Brayden D. J. In vitro and ex vivo intestinal tissue models to measure mucoadhesion of poly (methacrylate) and N-trimethylated chitosan polymers. Pharm. Res. 2005;22:38–49. doi: 10.1007/s11095-004-9007-1. [DOI] [PubMed] [Google Scholar]
- Keely S., Ryan S. M., Haddleton D. M., Limer A., Mantovani G., Murphy E. P., Colgan S. P., Brayden D. J. Dexamethasone-pDMAEMA polymeric conjugates reduce inflammatory biomarkers in human intestinal epithelial monolayers. J. Control Release. 2009;135:35–43. doi: 10.1016/j.jconrel.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong T., Eltzschig H. K., Karhausen J., Colgan S. P., Shelley C. S. Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of beta2 integrin gene expression. Proc. Natl. Acad. Sci. USA. 2004;101:10440–10445. doi: 10.1073/pnas.0401339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S., Singer C., Tucci V., Morthland V. H., Pfaller M. A., Isenberg H. D. The challenge of vancomycin-resistant enterococci: a clinical and epidemiologic study. Am. J. Infect. Control. 1995;23:170–180. doi: 10.1016/0196-6553(95)90038-1. [DOI] [PubMed] [Google Scholar]
- Lemjabbar H., Basbaum C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 2002;8:41–46. doi: 10.1038/nm0102-41. [DOI] [PubMed] [Google Scholar]
- Lenz A., Franklin G. A., Cheadle W. G. Systemic inflammation after trauma. Injury. 2007;38:1336–1345. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Louis N. A., Robinson A. M., MacManus C. F., Karhausen J., Scully M., Colgan S. P. Control of IFN-alphaA by CD 73, implications for mucosal inflammation. J. Immunol. 2008;180:4246–4255. doi: 10.4049/jimmunol.180.6.4246. [DOI] [PubMed] [Google Scholar]
- Ma T. Y., Boivin M. A., Ye D., Pedram A., Said H. M. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G422–G430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- Marano C. W., Lewis S. A., Garulacan L. A., Soler A. P., Mullin J. M. Tumor necrosis factor-alpha increases sodium and chloride conductance across the tight junction of CACO-2 BBE, a human intestinal epithelial cell line. J. Membr. Biol. 1998;161:263–274. doi: 10.1007/s002329900333. [DOI] [PubMed] [Google Scholar]
- Meakins J. L., Marshall J. C. The gastrointestinal tract: the “motor” of multiple organ failure. Arch. Surg. 1986;121:197. [Google Scholar]
- NNIS System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2003, issued August 2003. Am. J. Infect. Control. 2003;31:481–498. doi: 10.1016/j.ajic.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Grout M., Zaidi T. S. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc. Natl. Acad. Sci. USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijneveld A. W., Weijer S., Florquin S., Speelman P., Shimizu T., Ishii S., van der Poll T. Improved host defense against pneumococcal pneumonia in platelet-activating factor receptor-deficient mice. J. Infect. Dis. 2004;189:711–716. doi: 10.1086/381392. [DOI] [PubMed] [Google Scholar]
- Ring A., Weiser J. N., Tuomanen E. I. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J. Clin. Invest. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah Y. M., Ito S., Morimura K., Chen C., Yim S. H., Haase V. H., Gonzalez F. J. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134:2036–2048. 2048, e2031–e2033. doi: 10.1053/j.gastro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synnestvedt K., Furuta G. T., Comerford K. M., Louis N., Karhausen J., Eltzschig H. K., Hansen K. R., Thompson L. F., Colgan S. P. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. T., Colgan S. P. Hypoxia and gastrointestinal disease. J. Mol. Med. 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- Tyrer P., Foxwell A. R., Cripps A. W., Apicella M. A., Kyd J. M. Microbial pattern recognition receptors mediate M-cell uptake of a gram-negative bacterium. Infect. Immun. 2006;74:625–631. doi: 10.1128/IAI.74.1.625-631.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L. Release of platelet-activating factor (PAF) and accelerated healing induced by a PAF antagonist in an animal model of chronic colitis. Can. J. Physiol. Pharmacol. 1988;66:422–425. doi: 10.1139/y88-071. [DOI] [PubMed] [Google Scholar]
- Zinkernagel A. S., Johnson R. S., Nizet V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J. Mol. Med. 2007;85:1339–1346. doi: 10.1007/s00109-007-0282-2. [DOI] [PubMed] [Google Scholar]