During the development of neuronal polarity, the Kinesin-1 motor translocates preferentially to the axon. We show that Kinesin-1 selectivity does not depend on differences between axons and dendrites in microtubule stability or tubulin acetylation, but is likely specified by other tubulin posttranslational modifications.
Abstract
Polarized transport by microtubule-based motors is critical for neuronal development and function. Selective translocation of the Kinesin-1 motor domain is the earliest known marker of axonal identity, occurring before morphological differentiation. Thus, Kinesin-1–mediated transport may contribute to axonal specification. We tested whether posttranslational modifications of tubulin influence the ability of Kinesin-1 motors to distinguish microtubule tracks during neuronal development. We detected no difference in microtubule stability between axons and minor neurites in polarized stage 3 hippocampal neurons. In contrast, microtubule modifications were enriched in a subset of neurites in unpolarized stage 2 cells and the developing axon in polarized stage 3 cells. This enrichment correlated with the selective accumulation of constitutively active Kinesin-1 motors. Increasing tubulin acetylation, without altering the levels of other tubulin modifications, did not alter the selectivity of Kinesin-1 accumulation in polarized cells. However, globally enhancing tubulin acetylation, detyrosination, and polyglutamylation by Taxol treatment or inhibition of glycogen synthase kinase 3β decreased the selectivity of Kinesin-1 translocation and led to the formation of multiple axons. Although microtubule acetylation enhances the motility of Kinesin-1, the preferential translocation of Kinesin-1 on axonal microtubules in polarized neuronal cells is not determined by acetylation alone but is probably specified by a combination of tubulin modifications.
INTRODUCTION
Unidirectional signal transduction by neuronal cells is intimately linked to their highly polarized morphology. The biogenesis and maintenance of distinct axonal and dendritic compartments depends on the selective transport of specific vesicles and proteins along microtubules to these distinct cellular regions. Thus, one of the keys to understanding neuronal morphology and function involves discovering the molecular mechanisms responsible for the polarized transport of kinesin motors to axons or dendrites.
Recent work has shown that constitutively active (CA) forms of Kinesin-1 (also known as KIF5 or conventional kinesin) selectively accumulate within axonal but not dendritic growth cones of hippocampal neurons in culture (Nakata and Hirokawa, 2003). This ability to distinguish among different neurites begins before morphological polarization, as Kinesin-1 accumulates in only one or a small subset of neurites in unpolarized hippocampal neurons (Jacobson et al., 2006). The Kinesin-1 cargo protein c-Jun NH2-terminal kinase-interacting protein (JIP)1 follows a similar pattern as it also localizes to the axon in polarized cells and to a subset of neurites in nonpolarized cells (Verhey et al., 2001; Reed et al., 2006; Dajas-Bailador et al., 2008). Strikingly, Kinesin-1 accumulation in unpolarized stage 2 cells can be very dynamic, with the accumulation of active Kinesin-1 motors alternating between neurites within a matter of minutes (Jacobson et al., 2006). This indicates that the molecular signals that direct the transport of Kinesin-1 must also be dynamic.
What then are the molecular signals that drive the selective transport of Kinesin-1 motors to axons? As polarized accumulation is intrinsic to the Kinesin-1 motor domain, it seems likely that the motor–microtubule interaction is critical for specifying transport selectivity. In cultured neurons, axonal and dendritic microtubules differ in their populations of microtubule-associated proteins (MAPs), but it is not obvious how the differential localization of MAPs could influence the selectivity of kinesin transport. Axonal and dendritic microtubules also differ in their stability, as determined by resistance to depolymerization by nocodazole, and the local stabilization of axonal microtubules could influence kinesin translocation (Baas et al., 1991; Witte et al., 2008). The ability of Kinesin-1 motors to bind to, and in some cases move along, microtubules is enhanced by the presence of acetylation, detyrosination, or glutamylation modifications of tubulin (Larcher et al., 1996; Liao and Gundersen, 1998; Reed et al., 2006; Dompierre et al., 2007; Ikegami et al., 2007; Dunn et al., 2008; Cai et al., 2009; Konishi et al., 2009). Thus, we wondered whether axonal microtubules are preferentially subject to modifications of tubulin subunits and if so, whether selective posttranslational modifications (PTMs) of axonal microtubules are responsible for the preferential accumulation of truncated Kinesin-1 at the tip of the axon in polarized cells. We show that axons are enriched in tubulin PTMs and that manipulations that globally enhance tubulin PTMs alter the selectivity of Kinesin-1 translocation, allowing it to accumulate in all neurites. Increasing tubulin acetylation, without alterations in the levels of other tubulin PTMs, was not sufficient to alter the selectivity of Kinesin-1 accumulation.
MATERIALS AND METHODS
Plasmids and Antibodies
Constitutively active (CA) versions of the KHC subunit of Kinesin-1 [KHC(1-509) or KHC(1-560)] were generated from the rat KIF5C gene by using polymerase chain reaction (PCR) or convenient restriction sites and subcloned into plasmids for in-frame fusion to various fluorescent proteins and protein expression driven by the β-actin promoter. All constructs were verified by DNA sequencing. The following antibodies were used: total β-tubulin (E7; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), total α-tubulin (monoclonal DM1α [Sigma-Aldrich, St. Louis, MO]; polyclonal ab18251 or monoclonal ab7750 [Abcam, Cambridge, MA]), acetylated α-tubulin (monoclonal 6-11B-1 or a rabbit polyclonal antibody generated against an α-tubulin peptide that contains an acetylated lysine-40 residue [Sigma-Aldrich]), detyrosinated α-tubulin (AB3201 or a rabbit polyclonal antibody generated against an α-tubulin C-terminal peptide; Millipore Bioscience Research Reagents, Temecula, CA), polyglutamylated tubulin (GT335; a gift from C. Janke, CNRS, Montpellier, France), Tau-1 (MAB375; Millipore, Billerica, MA), MAP2 (AB5622; Millipore), and JIP1 polyclonal #152 (Meyer et al., 1999). A polyclonal anti-green fluorescent protein (GFP; Invitrogen, Carlsbad, CA) antibody was used in some immunofluorescence experiments to enhance the GFP signal of transfected CA-Kinesin-1.
Cell Culture and Drug Treatments
Primary hippocampal cultures were prepared from either E16 CD1 embryonic mice or E18 embryonic rats, essentially as described previously (Kaech and Banker, 2006). Neurons from mice were cultured in Neurobasal media with B27 supplement, and neurons from rats were cultured in minimal essential medium with N2 supplement. Experiments performed in mouse and rat neurons gave identical results. Transfection of DNA plasmids was done at the time of plating using a nucleofection protocol (Amaxa Biosystems, Gaithersburg, MD) or at 1–5 d after plating by using Lipofectamine 2000 (Invitrogen). Cells were treated for the indicated times and concentrations with Taxol (Sigma-Aldrich), the tubulin deacetylase inhibitors trichostatin A (TSA; Sigma-Aldrich) or a close structural analog of tubacin, MAZ1370, that is more potent in cell-based assays and referred to in the text as tubacin for simplicity (Reed et al., 2006), or the glycogen synthase kinase (GSK)3β inhibitor SB216763 (Sigma-Aldrich).
Microscopy and Image Processing
For live cell imaging, cells were maintained at 32–34°C. For immunofluorescence, cells were processed essentially as described previously (Verhey et al., 2001). Images were acquired on cooled 12-bit charge-coupled device cameras using high numerical aperture 40× or 60× objectives. The antibodies were used at subsaturation levels and showed no evidence of steric hindrance with each other (Supplemental Figure 1). Fluorochromes and filter sets were chosen to prevent fluorescence cross-talk between channels. Images were obtained under exposure conditions that did not result in saturated pixels and were quantified with ImageJ software (National Institutes of Health, Bethesda, MD) or MetaMorph (Molecular Devices, Sunnyvale, CA). Neurites were counted positive for JIP1 or CA-Kinesin-1 accumulation if the fluorescence intensity (due to immunostaining or fluorescent protein tags) in the growth cone was threefold higher than the fluorescence intensity of the neurite shaft.
To determine levels of microtubule posttranslational modifications, cells were double stained with antibodies specific for the modification and total tubulin. To determine the ratio of PTM versus total tubulin for individual neurites, we quantified the integrated fluorescence intensity of PTM staining or tubulin staining in the entirety of the axon, the minor neurites, and the whole cell after background subtraction. We then calculated the fraction of whole cell fluorescence originating from the axon and the minor neurites for each signal. The ratio of the fraction of PTM signal versus the fraction of tubulin signal is designated as the PTM/tubulin ratio.
Assay of Tubulin Stability
To assess the stability of axonal and dendritic microtubules, hippocampal neurons were electroporated with dendra2-α-tubulin at the time of plating. After 3 d in culture, neurons were imaged with a 60× Planapo (numerical aperture,1.4) objective on an LSM710 laser-scanning confocal microscope (Carl Zeiss, Thornwood, NY). The dendra2-α-tubulin signal was first visualized with 488 and 568 nm laser excitation, and then the dendra2 signal was photoconverted in specific regions of the cell using 405 nm laser illumination (1.2% of 30-mW, 6.3-μs per pixel dwell time; 10 iterations). The changes in fluorescence intensity in the photoconverted regions were monitored over time using 488 nm and 568 nm excitation. The decay of fluorescence in the red channel was analyzed using Prism 5 Software (nonlinear regression, two-phase decay; GraphPad Software, San Diego, CA) to estimate the half-times of the rapidly and slowly decaying components and the relative contribution of each.
RESULTS
Microtubule PTMs and Active Kinesin-1 Motors Are Both Enriched in the Developing Axon of Stage 3 Cells and the Same Subset of Neurites of Stage 2 Cells
Shortly after plating, dissociated hippocampal neurons put out several short, apparently identical processes, referred to as minor neurites (developmental stage 2; Dotti et al., 1988). Neurons become morphologically polarized when one neurite undergoes an extended period of growth and becomes the axon (developmental stage 3). The remaining neurites subsequently acquire the properties of dendrites (developmental stage 4) and develop an extensive arborization with numerous synapses (stage 5). We focused on cells at stages 2 and 3, before the appearance of a population of minus-end out microtubules in dendrites (Baas et al., 1989), when constitutively active Kinesin-1 can translocate only toward the neurite tips.
We first investigated whether axons are enriched in microtubule PTMs compared with minor neurites (immature dendrites) in polarized stage 3 hippocampal neurons. Immunofluorescence was performed with antibodies specific to α-tubulin and either acetylated α-tubulin, detyrosinated α-tubulin, or glutamylated α- and β-tubulin. To account for differences in tubulin levels in different neurites, we measured the ratio of modified tubulin to total tubulin in each neurite. The ratios of acetylation and detyrosination were significantly higher in the developing axon than in the minor neurites (Figure 1, A and B), whereas the ratio of glutamylated tubulin was not appreciably different between these subcellular regions (Figure 1, A and B). It is important to note, however, that glutamylation is a highly variable modification in that it occurs on both α- and β-tubulin subunits and involves the chain addition of one to six glutamate residues (Verhey and Gaertig, 2007; Hammond et al., 2008). Because the GT335 antibody recognizes mono- and polyglutamylation of both α- and β-tubulin (Wolff et al., 1992; van Dijk et al., 2007), we cannot rule out the possibility that specific glutamylation signals (α- vs. β-tubulin or chain length variations) could be enriched in axons or minor neurites.
Figure 1.
Presence of higher levels of tubulin PTMs correlates with the polarized localization of CA-Kinesin-1 motors. (A) Immunofluorescence of polarized stage 3 hippocampal neurons stained with antibodies to total α-tubulin and either acetylated (top), detyrosinated (middle), or polyglutamylated (bottom) tubulin subunits. (B) Quantification of the average ratio of PTM tubulin to total tubulin in axons or minor neurites (paired t test: acetylation, p < 0.001; detyrosination, p = 0.004; polyglutamylation, p = 0.11; 40 cells each). (C) Per-cell comparison of the ratios of PTM tubulin to total tubulin in the axon and minor neurites. For each cell, the PTM-to-total ratio in the minor neurites was set to 1. The relative level of PTM-to-total ratio in the axon of the same cell was then plotted and connected by a colored line. (D) Immunofluorescence of unpolarized stage 2 hippocampal neurons expressing CA-Kinesin-1-GFP stained with antibodies to total α-tubulin and acetylated α-tubulin. (E) Quantification of the average ratio of PTM tubulin to total tubulin in unpolarized stage 2 neurites that have accumulated CA-Kinesin-1 motors (with CA-Kinesin-1) or not (without CA-Kinesin-1) (t test: acetylation, p < 0.001, n = 28; detyrosination, p = 0.70, n = 28; polyglutamylation, p = 0.55, n = 20). Bars, 20 μm.
When examined on a cell-by-cell basis, axonal microtubules were preferentially acetylated in 39 of 40 cells, preferentially detyrosinated in 30 of 40 cells, and preferentially glutamylated in only 19 of 40 cells (Figure 1C). Truncated CA Kinesin-1 motors selectively accumulate in the axon in ∼ 90% of transfected cells (Jacobson et al., 2006). Thus, the enrichment of certain microtubule PTMs in axons correlates with the selective accumulation of CA-Kinesin-1 motors to this subcellular destination. This indicates that microtubule PTMs could provide a biochemical cue that directs the polarized transport of Kinesin-1 to axons.
We then tested whether microtubule PTMs are enriched in a subset of neurites in unpolarized stage 2 neurons and if so, whether Kinesin-1 preferentially traffics to these neurites. Previous results indicate that the localization of expressed truncated kinesins is a useful measure of where these motors translocate in neurons (Nakata and Hirokawa, 2003; Jacobson et al., 2006). The CA-Kinesin-1 motors [KIF5C(1-560) or KIF5C(1-509)] that were used contain the motor and stalk domains required for microtubule-based motility of a dimeric motor, but lack the C-terminal tail domains that contribute to autoinhibition and cargo binding. When tagged with fluorescent proteins and expressed in neuronal cells, such truncated motors accumulate at the tips of some neurites, providing a direct readout of Kinesin-1 activity regulated primarily by the microtubule/motor interface (Nakata and Hirokawa, 2003; Jacobson et al., 2006). Unpolarized stage 2 hippocampal neurons expressing CA-Kinesin-1-GFP motors were fixed and stained with antibodies against total α-tubulin and specific tubulin PTMs. We found that the ratio of acetylated tubulin to total tubulin was higher in one or two neurites than the others and that CA-Kinesin-1 motors accumulated more frequently in these neurites with higher levels of acetylation (Figure 1, D and E). In contrast, detyrosination and glutamylation were not significantly enriched in one neurite over the others (Figure 1E; data not shown) and were not significantly different between neurites that contained or lacked CA-Kinesin-1 motors (Figure 1E). Based on its enrichment in the axon of stage 3 cells and the subset of stage 2 neurites preferred by Kinesin-1, microtubule acetylation is a likely candidate to direct the polarized transport of Kinesin-1.
The Enrichment of Tubulin PTMs Is Not a Result of Increased Microtubule Stability in the Axon
PTMs occur preferentially on stable (i.e., long-lived) microtubules. In mature sympathetic neurons, axonal microtubules are preferentially resistant to nocodazole-induced depolymerization (Baas et al., 1991), a measure thought to correlate with microtubule stability. Similar results were obtained in stage 3 hippocampal neurons (Witte et al., 2008). Thus, we wondered whether the differential distribution of tubulin PTMs in axons versus dendrites could be explained by a difference in the stability of microtubules in these domains.
To analyze microtubule stability in cultured hippocampal neurons, we used an α-tubulin construct tagged with dendra2, a photoswitchable variant of GFP whose fluorescence emission changes from green to red after illumination with 405 nm light (Gurskaya et al., 2006). In polarized stage 3 hippocampal neurons, expressed dendra2-α-tubulin exhibited a diffuse distribution and a filamentous pattern (Figure 2, A and B), indicating that it had been incorporated into the microtubule network. Before photoconversion, dendra2-α-tubulin fluorescence was detectable only in the green channel (Figure 2, A and B). After photoconversion of a small segment in the proximal region of the axon or minor neurite, a red dendra2-α-tubulin signal was detected only in the illuminated region. In both axons and minor neurites, the red dendra2-α-tubulin fluorescence decayed initially rapidly and then more slowly so that after one hour, a significant fraction of the red fluorescence remained in both axons and minor neurites (Figure 2, A and C), suggesting that each contains a significant population of stable microtubules. As a test of the sensitivity of this method, we also examined the decline in red dendra2-α-tubulin fluorescence in growth cones that contain predominantly dynamic, and hence unstable, microtubules. When dendra2-α-tubulin was photoconverted in the growth cone region, the red fluorescence signal disappeared within minutes (Figure 2, B and C), confirming that microtubules in the growth cone turn over more rapidly than those in neurite shafts.
Figure 2.
Rate of tubulin turnover is similar in axons and dendrites. (A) To estimate the temporal stability of axonal and dendritic microtubules in polarized stage 3 neurons, dendra2-α-tubulin was subjected to photoconversion in a segment of the axon (box 1) and one minor neurite (box 2). Before photoconversion, no signal was detected in the red channel. Photoconversion (at t = 0) produced a bright signal in the red channel that declined over time, but significant fluorescence persisted in both the axon and minor neurite more than 1 h later. (B) Dendra2-α-tubulin photoconversion was carried out in a segment of the axon (box) and the growth cone of a minor neurite (circle). The red signal in the growth cone declined within minutes, whereas the signal in the axon persisted much longer. (C) Decay of fluorescence of photoconverted dendra2-α-tubulin in growth cones (n = 6) and in the shafts of axons (n = 20) and minor neurites (n = 14). The fitted curves show a biphasic decline in fluorescence in axons and dendrites, with the following values (means and 95% confidence intervals): fraction of tubulin in the rapidly decaying component: axon, 50.9% (43.6–58.3%); dendrite, 45.5% (35.1–55.8%); half-life of the rapidly decaying component: axon, 1.4 min (1.1–2.0 min); dendrite, 1.4 min (1.4–3.5 min); half-life of the slowly decaying component: axon, 61.0 min (43.6–103.0 min); dendrite: 41.6 (35.1–55.9). Fluorescence decay in the growth cones could be fitted using a one-phase decay curve with a mean half-life of 0.86 min (0.76–0.99 min).
The decay in dendra2-α-tubulin fluorescence in the axons and minor neurites could be mathematically fit by a two-component model. The rapid component decayed with a half-time of ∼1.5 min, whereas the slow component decayed with a half-time of ∼1 h. The rapidly decaying component probably represents the diffusion of soluble tubulin and the turnover of dynamic microtubules, including the loss of subunits due to depolymerization. The slowly decaying component probably represents tubulin subunits that have been incorporated into “stable” microtubules. The rate of decline of both the fast and slow phases was similar in axons and minor neurites (Figure 2C). Although the half-time of stable microtubules was slightly longer in axons (64 vs. 42 min, respectively), this difference was not statistically significant. Furthermore, the distribution of dendra2-α-tubulin between the two phases is similar in axons and minor neurites (Figure 2C). In contrast to these results, the dendra2-α-tubulin fluorescence decay in growth cones could be fit with a one-phase decay model, which yielded an average half life of 0.9 min, indicating that there were no slow-decaying components in this region. These results demonstrate that the increased levels of tubulin PTMs observed in the axon of stage 3 neurons cannot be accounted for by a higher proportion of stable microtubules in this domain. Instead, the activities of the enzymes responsible for tubulin modification must differ between the axon and minor neurites.
Increased Acetylation Is Not Sufficient to Alter the Selective Localization of Kinesin-1 Motors and Cargoes
Acetylated tubulin is present in higher levels in the developing axon in polarized stage 3 hippocampal neurons and in a subset of the neurites in unpolarized stage 2 neurons (Figure 1). Microtubule acetylation can influence Kinesin-1 trafficking events in unpolarized (stage 2) neurons in that hyperacetylation causes the Kinesin-1 cargo JIP1 to accumulate in nearly all neurite tips rather than in only a subset of neurite tips as seen in control cells (Reed et al., 2006). We thus tested whether microtubule acetylation is sufficient to direct Kinesin-1 to axons as opposed to minor neurites in polarized (stage 3) neurons.
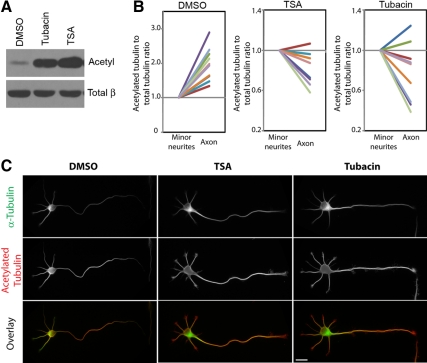
Microtubule acetylation is a reversible modification that involves the addition of an acetyl group to lysine 40 of α-tubulin. In fibroblasts, microtubule acetylation can be greatly enhanced by TSA and tubacin, inhibitors of a known α-tubulin deacetylase histone deacetylase (HDAC)6 (Hubbert et al., 2002; Matsuyama et al., 2002; Haggarty et al., 2003). We verified that TSA and tubacin treatments also cause a global increase in microtubule acetylation in polarized stage 3 neuronal cells by Western blotting and immunofluorescence. Treatment with TSA or tubacin caused a significant increase in the overall levels of microtubule acetylation compared with control dimethyl sulfoxide (DMSO) treatment (Figure 3A). The increase in acetylated tubulin levels occurred in both the axon and the minor neurites (Figure 3C) and resulted in nearly equivalent levels of acetylation between these two compartments (Figure 3B). Thus, HDAC6 inhibition efficiently abolished the selective enrichment of acetylated microtubules in the developing axon. These results also suggest that tubulin acetylation occurs at about the same rate in minor neurites and axons but that a higher deacetylase activity in the minor neurites reduces the level of acetylated tubulin in this compartment.
Figure 3.
Treatment with deacetylase inhibitors results in increased microtubule acetylation in both the axon and minor neurites. (A) Polarized stage 3 cortical cells were treated for 3 h with DMSO (control), 125 nM TSA, or 10 μM tubacin and then lysed and analyzed by Western blotting using antibodies to acetylated α-tubulin or total α-tubulin. (B and C) Polarized stage 3 hippocampal neurons were treated with DMSO, 100 nM TSA, or 2 μM tubacin for 6 h and then fixed and stained with antibodies to acetylated and total tubulin. (B) Per-cell comparison of the ratio of acetylated to total α-tubulin in the axon or minor neurites. For each cell, the acetylated-to-total ratio in the minor neurite was set to 1. The relative level of acetylated-to-total ratio in the axon of the same cell was then plotted and connected by a colored line. n = 10 cells each. (C) Representative images of control (left) and treated (middle and right) cells.
We next tested whether hyperacetylation of microtubules throughout both axons and minor neurites was sufficient to misdirect CA-Kinesin-1 into minor neurites. Polarized stage 3 hippocampal neurons were treated for 3–4 h with DMSO, TSA, or tubacin. The cells were then transfected with CA-Kinesin-1-mCherry along with yellow fluorescent protein (YFP) as a soluble marker of the transfected cells and allowed to express the exogenous proteins under additional drug treatment for 4–5 h. Treatment with deacetylase inhibitors did not cause CA-Kinesin-1-mCherry motors to accumulate in the minor neurites. Rather, CA-Kinesin-1-mCherry motors localized specifically to the developing axon in both control and treated cells (Figure 4, A and B). Similar results were obtained in mature (stage 5) neurons where treatment with deacetylase inhibitors did not alter the selective accumulation of CA-Kinesin-1 motors in the axon (Figure 4B).
Figure 4.
Increased microtubule acetylation does not alter the polarized sorting of CA-Kinesin-1 motors or JIP1 cargoes in polarized stage 3 neurons. (A) stage 3 hippocampal neurons were treated for 2–4 h with DMSO, 125 nM TSA, or 10 μM tubacin. The cells were then transfected with CA-Kinesin-1-mCherry and soluble YFP, allowed to express the exogenous proteins under additional treatment for 4–5 h, and then fixed and imaged. (B) Quantification of the data in stage 3 (A) and stage 5 (not shown) neurons. The data are expressed as the percentage of stage 3 minor neurites or stage 5 dendrites that have accumulated CA-Kinesin-1-mCherry motors. Error bars, SEM. (C) stage 3 hippocampal neurons were treated for 3 h with DMSO, 125 nM TSA, or 10 μM tubacin. The cells were fixed and stained with antibodies to endogenous JIP1 and acetylated α-tubulin. Arrows, tips of minor neurites. Arrowheads, tips of axons. (D) Quantification of the data in C. The data are expressed as the percentage of stage 3 minor neurites and axons that have accumulated JIP1 at their tips. Error bars, SEM. Bars, 20 μm. t test: p > 0.05 for all conditions in B and D compared with the DMSO-treated control.
We then tested whether hyperacetylation of microtubules could misdirect the localization of the Kinesin-1 cargo protein JIP1 from the developing axon in polarized stage 3 cells. In cells treated with DMSO, TSA, or tubacin for 3 h and then fixed and stained with antibodies to JIP1 and acetylated α-tubulin, JIP1 was still delivered exclusively to axons (Figure 4, C and D). Together, the results on CA-Kinesin-1 motor and JIP1 cargo localization suggest that α-tubulin acetylation is not sufficient to provide the biochemical cue that drives the selective axonal translocation of Kinesin-1 motors in polarized neurons.
Taxol Treatment Results in Increases in Microtubule Posttranslational Modifications and Misdirection of Kinesin-1 Trafficking
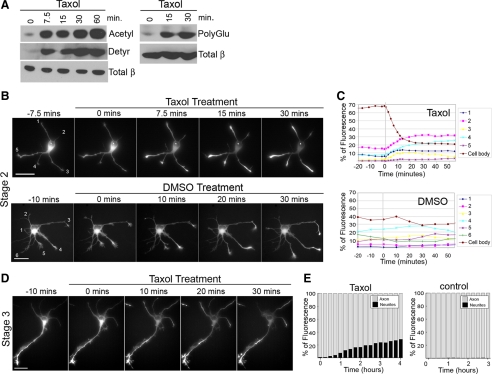
What biochemical cues other than microtubule acetylation could account for the preferential accumulation of the Kinesin-1 motor domain in axons? One clue comes from a study of mature neurons (stage 5), which showed that treatment with low doses of Taxol resulted in accumulation of CA-Kinesin-1 motors in both axons and dendrites (Nakata and Hirokawa, 2003). We thus asked whether the loss of selective Kinesin-1 accumulation after Taxol treatment could be due to changes in multiple microtubule PTMs. To explore this possibility, we tested whether Taxol treatment alters the levels of microtubule PTMs in neuronal cells. At submicromolar concentrations, Taxol suppresses microtubule dynamics without an increase in polymer mass (Jordan and Wilson, 1998). Consistent with this, low dose Taxol treatment of polarized stage 3 neurons caused a significant increase in the fraction of stable microtubules in both axons and minor neurites (Supplemental Figure 3). Importantly, low-dose Taxol treatment of polarized stage 3 neurons also resulted in a significant increase in overall levels of tubulin acetylation, detyrosination, and glutamylation (Figure 5A).
Figure 5.
Taxol treatment increases all microtubule PTMs and disrupts the polarized localization of CA-Kinesin-1 motors and JIP-1 cargoes in polarized stage 3 and unpolarized stage 2 neurons. (A–E) Effects of Taxol in polarized stage 3 cells. (A) stage 3 cortical neurons were treated for 3 h with DMSO, 10 or 100 nM Taxol, and then lysates were analyzed by Western blotting with antibodies to acetylated α-tubulin, detyrosinated α-tubulin, polyglutamylated α- and β-tubulin, or total β-tubulin. Polyglutamylated samples are from nonadjacent lanes of the same gel. (B) Stage 3 hippocampal neurons were treated for 2–4 h with DMSO, 10 or 100 nM Taxol. The cells were then transfected with CA-Kinesin-1-mCherry and soluble YFP, allowed to express the exogenous proteins under additional treatment for 4–5 h, and then fixed and imaged. (C) Quantification of the data in B. The data are expressed as the percentage of cells that have accumulated CA-Kinesin-1 mCherry motors in minor neurites. (D) Stage 3 hippocampal neurons were treated for 3 h with DMSO, 10 or 100 nM Taxol, and then fixed and stained with antibodies to JIP1 and acetylated α-tubulin. Arrows, tips of minor neurites. Arrowheads, tips of axons. (E) Quantification of the data in D. The data are expressed as the percentage of stage 3 minor neurites or axons that have accumulated JIP1 protein. (F–H) Effects of Taxol in unpolarized stage 2 cells. (F) Stage 2 hippocampal neurons expressing CA-Kinesin-1-YFP motors since the time of plating were untreated or treated with 10 nM Taxol for 20 min and then imaged. Bar, 10 μm. (G) Stage 2 hippocampal neurons were treated for 3 h with DMSO, 10 or 100 nM Taxol, and then fixed and stained with antibodies to JIP1 and acetylated α-tubulin. (H) Quantification of the data in F and G. The data are expressed as the percentage of stage 2 neurites that have accumulated CA-Kinesin-1 motors or JIP1 cargoes. Bars, 20 μm. Error bars, SEM. t test: *p < 0.01 compared with control DMSO-treated cells.
We verified that Taxol treatment results in misrouting of Kinesin-1 to dendrites in mature stage 5 neurons as described by Nakata and Hirokawa (2003); Supplemental Figure 2). Analysis of Kinesin-1 translocation at this stage of development is complicated by the presence of minus-end out microtubules in the dendrites. Thus, we examined the effects of Taxol treatment on Kinesin-1 accumulation at earlier stages of development, when the microtubules in all neurites are oriented plus end-out (Baas et al., 1989). Treatment of polarized stage 3 neurons with Taxol markedly reduced the selectivity of Kinesin-1 accumulation (Figure 5B). Specifically, Taxol treatment resulted in the accumulation of CA-Kinesin-1-mCherry in the minor neurites in >70% of the cells as compared with only 13% of DMSO-treated cells (Figure 5, B and C). Similar effects were found on the localization of the endogenous JIP1 cargo protein; Taxol treatment caused JIP1 to accumulate in the minor neurites in 57% (100 nM Taxol) or 70% (10 nM Taxol) of cells compared with only 16% of DMSO treated cells (Figure 5, D and E). In unpolarized stage 2 cells, Taxol treatment also caused a mislocalization of both active Kinesin-1 motors and JIP1 cargoes to a majority rather than a subset of neurite tips (Figure 5, F–H). Together these results demonstrate that Taxol-induced changes in microtubule stability regulate the selective accumulation of Kinesin-1 and its cargoes in both unpolarized and polarized neurons. This change in Kinesin-1 sorting correlates with increased levels of multiple tubulin PTMs.
We next determined whether the Taxol-induced changes in microtubule PTMs and Kinesin-1 accumulation occur on the same time scale. Western blot analysis of unpolarized stage 2 neurons treated with 100 nM Taxol for 0–60 min revealed that the increase in microtubule PTMs (specifically acetylated, detyrosinated, and polyglutamylated tubulin) occurs rapidly, within 7.5 min of Taxol treatment (Figure 6A). To investigate the reaction time of CA-Kinesin-1 to Taxol treatment, we used time-lapse microscopy to observe unpolarized (stage 2) or polarized (stage 3) neuronal cells that had been transfected with CA-Kinesin-1-monomeric citrine (mCit) plasmid at the time of plating. In unpolarized stage 2 cells where Kinesin-1 was evenly distributed throughout the cell before Taxol treatment, the motor began concentrating in nearly all neurite tips within minutes after Taxol treatment but not after DMSO treatment (Figure 6, B and C), and accumulation was nearly complete by 10–15 min after treatment. Thus, in the same time scale, Taxol-induced changes in microtubule structure, stability, and PTMs resulted in loss of the preferential accumulation of CA-Kinesin-1. Taxol treatment also limited the dynamic nature of CA-Kinesin-1 accumulation in different neurite tips over time. With Taxol treatment, CA-Kinesin-1 remained in neurite tips for the duration of the recording (Figure 6, B and C) and no longer underwent transient accumulations, as reported for untreated cells (Jacobson et al., 2006).
Figure 6.
Taxol-induced alterations in CA-Kinesin-1 trafficking and tubulin PTMs occur on the same time scale. (A) A mix of stage 2/3 hippocampal neurons were treated with 100 nM Taxol for the indicated times. Cell lysates were analyzed by western blotting with antibodies to acetylated α-tubulin, detyrosinated α-tubulin, and total β-tubulin (left) or polyglutamylated α-/β-tubulin and total β-tubulin (right). (B and C) Time course of CA-Kinesin-1 accumulation in stage 2 cells. (B) Unpolarized, stage 2 hippocampal neurons expressing CA-Kinesin-1-mCit motors since the time of plating were analyzed by time-lapse microscopy before and after 0–30 min of treatment with 100 nM Taxol (top) or DMSO vehicle (bottom). (C) Quantification of the mCit fluorescence in each neurite over time for the cells in (B). The data are expressed as the percentage of total mCit fluorescence in each neurite and the cell body. (D and E) Time course of CA-Kinesin-1 accumulation in polarized, stage 3 cells. (D) Polarized, stage 3 cells expressing CA-Kinesin-1-mCit were analyzed as described in B. (E) Quantification of the localization of CA-Kinesin-1-YFP over time in a stage 3 control cell and a cell treated with 10 nM Taxol. The data are expressed as the percentage of YFP fluorescence in the axon and minor neurites at times after addition of Taxol. Bars, 20 μm.
In both unpolarized stage 2 cells (data not shown) and polarized stage 3 cells (Figure 6, D and E) where CA-Kinesin-1 was already concentrated in one neurite tip before Taxol treatment, a longer time frame (e.g., hours) was required for redistribution of the motor. In polarized stage 3 cells, most CA-Kinesin-1 motors were accumulated in the axon before Taxol treatment, but they began to accumulate in minor neurites after Taxol treatment, with gradual increases occurring over the 4-h imaging period (Figure 6, D and E). Presumably, accumulated motors are more likely to reuse the Taxol-stabilized microtubule tracks in the axon rather than diffuse back to the cell body and choose microtubules leading to another neurite. Taxol treatment resulted in localization of CA-Kinesin-1 to the tips of 95% of axons and 66% of minor neurites compared with 98% of axons and 3% of minor neurites in control cells. Taxol treatment did not alter the polarized distribution of the MAPs Tau and MAP2 (axonal and dendritic markers, respectively; Supplemental Figure 4), demonstrating that the influence of Taxol on CA-Kinesin-1 motors is not due to Taxol-induced alterations in the association of MAPs with microtubules (Black, 1987; Samsonov et al., 2004; Kim et al., 2006). We conclude that Taxol induces rapid increases in microtubule PTMs and results in mistargeting of CA-Kinesin-1 motors to minor neurites.
Inhibition of GSK3β Activity Also Results in Increased Microtubule Posttranslational Modifications and Misdirection of Kinesin-1
Taxol treatment of cultured neurons was recently reported to result in the formation of multiple axons at the expense of dendrites (Witte et al., 2008). Our demonstration that Taxol treatment results in alterations in the selective axonal targeting of CA-Kinesin-1 motors and JIP cargoes is consistent with this effect. Another treatment that has been reported to disturb axon/dendrite formation in cultured neurons is the inhibition of GSK3β. Global inhibition of GSK3β results in the formation of multiple axons and/or increased axonal branching (Jiang et al., 2005; Yoshimura et al., 2005; Gartner et al., 2006; Kim et al., 2006). We wondered whether the formation of multiple axons upon GSK3β inhibition could be linked, like Taxol treatment, to enhanced microtubule PTMs and a change in the selectivity of Kinesin-1.
We first compared the ability of Taxol, GSK3β inhibitors, and deacetylase inhibitors to induce the formation of supernumerary axons. Primary hippocampal neurons were incubated in low levels of Taxol, SB216763 (GSK3β inhibitor), or tubacin (HDAC6 inhibitor) for 6 d in vitro, when both axons and dendrites are actively growing, and then we fixed and stained them with antibodies to Tau and MAP2. Both Taxol treatment and GSK3β inhibition led to the formation of multiple axons whereas tubacin treatment had no effect (Supplemental Figure 5). Taxol treatment resulted in multiple axon formation in a large percentage of cells (85.9 ± 1.6%) compared with control (DMSO-treated) cells (13.1 ± 1.4%). SB216763 treatment also led to a significant, yet smaller, increase in the percentage of cells with multiple axons (36.7 ± 2.5%) consistent with previous reports (Jiang et al., 2005; Yoshimura et al., 2005; Gartner et al., 2006).
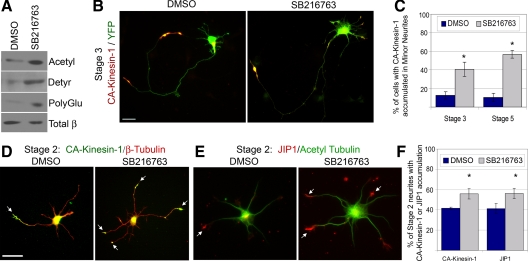
We then tested whether inhibition of GSK3β results in an alteration of microtubule PTMs and a corresponding misdirection of CA-Kinesin-1 motors and JIP1 cargoes. Polarized (stage 3) neurons were treated with SB216763 and the levels of specific PTMs were analyzed by Western blotting of the cell lysates. SB216763 treatment resulted in increased levels of microtubule acetylation, detyrosination, and polyglutamylation (Figure 7A). Inhibition of GSK3β activity by SB216763 treatment also resulted in a misdirection of CA-Kinesin-1 motors into minor neurites rather than only to axons, as seen in control cells (Figure 7, B and C). Similar results were obtained in mature (stage 5) neurons where SB216763 treatment caused a significant increase in the amount of CA-Kinesin-1 motors that accumulated at the tips of dendrites (Figure 7C). Thus, GSK3β inhibition in polarized cells caused the formation of supernumerary axons as well as increased levels of microtubule PTMs and mistargeting of CA-Kinesin-1 motors. Interestingly, inhibition of GSK3β was not as effective in altering the selective targeting of CA-Kinesin-1 motors and JIP1 cargoes in unpolarized (stage 2) cells. In this case, SB216763 treatment caused a small, but still significant, misdirection of CA-Kinesin-1 motors (Figure 7, D and F) and JIP1 cargoes (Figure 7, E and F) to more neurites than in control DMSO-treated cells.
Figure 7.
Inhibition of GSK3β results in increased levels of tubulin PTMs and disrupts the polarized sorting of CA-Kinesin-1 and JIP1 in polarized stage 3 and unpolarized stage 2 cells. (A–C) Effects of GSK3β inhibition in polarized stage 3 cells. (A) Stage 3 cortical neurons were treated for 8 h with 10 μM SB216763. Cell lysates were analyzed by Western blotting with antibodies to acetylated α-tubulin, detyrosinated α-tubulin, polyglutamylated tubulin, or total β-tubulin. (B) Stage 3 hippocampal neurons were treated for 2–4 h with DMSO or 5–10 μM SB216763. The cells were then transfected with CA-Kinesin-mCherry and soluble YFP, allowed to express the exogenous proteins under additional treatment for 4–5 h, and then fixed and imaged. (C) Quantification of the percentage of stage 3 minor neurites or stage 5 dendrites that have accumulated CA-Kinesin-mCherry after treatment described in B. (D–F) Effects of GSK3β inhibition in unpolarized stage 2 cells. (D) Stage 2 hippocampal neurons expressing CA-Kinesin-1-mCit since the time of plating were treated for 8 h with DMSO or 10 μM SB216763 and then fixed and stained for total β-tubulin. (E) Stage 2 hippocampal neurons were treated for 8 h with DMSO or 10 μM SB216763 and then fixed and stained with antibodies to JIP1 and acetylated α-tubulin. (F) Quantification of the percentage of stage 2 neurites with CA-Kinesin-1 (D) or JIP1 (E) accumulated at the tips after treatment with DMSO or SB216763. Bars, 20 μm. Error bars, SEM. t test: *p < 0.01 compared with control DMSO-treated cells.
Under some circumstances, GSK3β activity contributes to the regulation of microtubule stability in cultured cells (Owen and Gordon-Weeks, 2003; Eng et al., 2006). In polarized stage 3 hippocampal neurons, SB216763 treatment caused a small increase in the fraction of stable microtubules and in the calculated half-life of stable microtubules in the shaft regions of both axons and minor neurites, as measured by the fluorescence decay of photoconverted dendra2-α-tubulin (Supplemental Figure 3), although the differences between treated and control cells were not statistically significant. We conclude that inhibition of GSK3β promotes an “axonal signal” that may be comprised, at least in part, of microtubule PTMs that can influence Kinesin-1 translocation in primary hippocampal neurons.
DISCUSSION
Role of Microtubule PTMs in Directing Kinesin-1 Translocation
Based on analysis of the accumulation of constitutively active kinesin motors in cultured neurons, Kinesin-1 is thought to translocate selectively into the axon (Nakata and Hirokawa, 2003). This selective translocation is the earliest known marker of axonal identity, occurring before morphological differentiation (Jacobson et al., 2006). Because polarized translocation is intrinsic to the Kinesin-1 motor domain, we have investigated whether microtubule-based molecular signals drive the selective localization of Kinesin-1 motors to axons in polarized hippocampal neurons.
We show that two microtubule modifications, acetylation and detyrosination, are enriched in axons compared with minor neurites in polarized (stage 3) cells. The axonal enrichment of detyrosinated tubulin has also been shown recently by Konishi and Setou (2009). In contrast, we did not detect differences in microtubule stability between axons and minor neurites at this stage of development. Although there may be subtle differences in microtubule stability that cannot be detected by current methods, our results indicate that the enrichment of microtubule PTMs in axons is probably due to differences in the localization or local regulation of the enzymes that regulate tubulin PTMs rather than to differences in microtubule stability.
We show that selective translocation of Kinesin-1 in polarized neurons can be abolished by low-dose Taxol treatment and inhibition of GSK3β. Both treatments increased the levels of microtubule PTMs, diminished the selective localization of CA-Kinesin-1 motors to axons, and resulted in the formation of multiple axons. Taxol treatment and GSK3β inhibition are unlikely to reduce Kinesin-1 axonal selectivity by altering the MAP distribution between compartments or by simply enhancing microtubule stability in both axons and minor neurites. Thus, microtubule PTMs may provide the biochemical cue that directs the preferential translocation of Kinesin-1 on axonal microtubules.
Microtubule acetylation can influence the binding and motility of Kinesin-1 in vitro (Reed et al., 2006; Konishi and Setou, 2009). Microtubule acetylation also influences, at least in part, the selective localization of Kinesin-1 to a subset of microtubules in fibroblasts and the selective accumulation of Kinesin-1 in a subset of neurites in unpolarized stage 2 neurons (Reed et al., 2006; Cai et al., 2009). We show here that the acetylation of lysine 40 of α-tubulin is not sufficient to direct the selective translocation of Kinesin-1 motors to axons in polarized stage 3 neurons. Konishi and Setou present evidence that the selective axonal translocation of Kinesin-1 is governed by detyrosination of α-tubulin in hippocampal neurons (Konishi and Setou, 2009). Our observations on the effects of Taxol and GSK3β inhibitors are also consistent with a role for detyrosinated tubulin in polarized neurons, but we note that a significant percentage of stage 3 cells lack axonal enrichment of detyrosinated microtubules (Figure 1), whereas Kinesin-1 is nearly always targeted to axons. Together, our observations are consistent with the idea that kinesin translocation is influenced by a tubulin code that involves multiple PTMs (Verhey and Gaertig, 2007). Defining the mechanistic basis by which specific PTMs influence Kinesin-1 is an important future goal.
Microtubule PTMs, Signaling Pathways, and the Specification of Axonal Identity
A major unresolved question in neuronal development is how the axon is specified from the morphologically equivalent neurites in stage 2 cells. Witte et al. (2008) showed that microtubules at the axon tip are preferentially resistant to nocodazole-induced depolymerization when compared with microtubules at the tips of minor neurites. These authors also showed that treatment with Taxol induces the formation of multiple axons and that photoactivation of caged Taxol in a single minor neurite of unpolarized cells enhances the probability that this neurite will become the axon. These observations led to the hypothesis that microtubule stabilization in one minor neurite causes axon formation and leads to the molecular polarization of the neuron. Our measurements of microtubule stability based on the fluorescence decay of photoconverted dendra2-α-tubulin do not support this hypothesis. Although it remains possible that there are subtle differences in the stability of axons and minor neurites that cannot be detected by this method, our data indicate that the shafts of axons and minor neurites contain roughly equivalent populations of stable microtubules. Microtubule stability may well be required for axon formation, but our work indicates that the presence of a significant population of stable microtubules is not sufficient to specify axonal identity. It may be that the nocodazole-resistance of microtubules near the tip of the axon (Witte et al., 2008) is not an indicator of microtubule stability all along the neurite, but rather reflects other unique properties of microtubules in this region (Bielas et al., 2007). Because our measurements were made only at stage 3 of development, they do not pertain to the issue of microtubule stability in mature neurons (Baas et al., 1991).
Our previous work indicated that specification of the axon may depend on the selective translocation of Kinesin-1 (Jacobson et al., 2006). We provide support for this possibility by demonstrating that both Taxol treatment and inhibition of GSK3β in stage 3 cells cause an increase in tubulin PTMs in minor neurites, allow Kinesin-1 to accumulate at minor neurite tips, and lead to the formation of multiple axons. Because these changes in tubulin PTMs and Kinesin-1 selectivity occur very rapidly in response to drug treatment, they could well participate in the process that converts a minor neurite into an axon. Although Taxol treatment can lead to a series of pleiomorphic changes in microtubules, our results indicate that the effects of Taxol on the development of neuronal polarity are probably not limited to its effects on microtubule dynamics in the growth cone. That the ability of Kinesin-1 to undergo selective translocation is required for specification of the axon is also supported by recent work showing that a disruption of Kinesin-1 selectivity results in reduced neuronal polarization (Konishi and Setou, 2009).
Our work on microtubule PTMs and the selectivity of Kinesin-1 transport during axonal specification is also relevant to studies that examined the consequences of severing the axons of cultured neurons. Previous work (Dotti and Banker, 1987; Goslin and Banker, 1989; Gomis-Ruth et al., 2008) demonstrated that after distal transections, axons regenerate without any alteration in neuronal polarity, whereas axonal transections near the base of the axon led to a loss of polarity and the formation of a new axon from a different neurite. Thus, the signaling machinery concentrated at the axonal growth cone, which includes JIP1, the Par 3/Par6 complex, Rap1B, collapsin response mediator protein (CRMP)-2, and shootin-1 (Toriyama et al., 2006; Arimura and Kaibuchi, 2007; Barnes et al., 2008; Dajas-Bailador et al., 2008), is not required for maintaining axonal identity. Rather, the critical machinery resides near the base of the axon where the enrichment of microtubule PTMs begins. Distal transections would not disrupt these microtubule modifications nor the directed transport of kinesins and their cargoes, allowing restoration of axonal signaling components to the growth cone and permitting regeneration.
The recent identification of molecules that regulate tubulin PTMs in neurons (Janke et al., 2005; Ikegami et al., 2006; van Dijk et al., 2007; Ohkawa et al., 2008; Chang et al., 2009; Creppe et al., 2009) opens up avenues to address how different PTMs occur in different compartments in the same cell despite the similar microtubule stability in these compartments. Further investigation of the signaling pathways that function upstream and regulate microtubule PTMs will enable us to understand the machinery for axon specification and maintenance of axon identity.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Verhey and Banker laboratories for discussions. We are particularly grateful to Vladimir Gelfand, who provided the dendra2-α-tubulin construct and shared his findings on microtubule dynamics before publication. This work was supported by National Institutes of Health grants GM-070862 (to K.J.V.) and MH-66179 (to G. B.) and National Science Foundation predoctoral fellowship F017154 (to J.W.H.).
Abbreviations used:
- CA
constitutively active
- CRMP
collapsin response mediator protein
- DMSO
dimethyl sulfoxide
- GFP
green fluorescent protein
- GSK
glycogen synthase kinase
- HDAC
histone deacetylase
- JIP
JNK-interacting protein
- MAP
microtubule-associated protein
- mCit
monomeric citrine
- PTM
posttranslational modification
- TSA
trichostatin A.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-01-0044) on December 23, 2009.
REFERENCES
- Arimura N., Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 2007;8:194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- Baas P. W., Black M. M., Banker G. A. Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. J. Cell Biol. 1989;109:3085–3094. doi: 10.1083/jcb.109.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Slaughter T., Brown A., Black M. M. Microtubule dynamics in axons and dendrites. J. Neurosci. Res. 1991;30:134–153. doi: 10.1002/jnr.490300115. [DOI] [PubMed] [Google Scholar]
- Barnes A. P., Solecki D., Polleux F. New insights into the molecular mechanisms specifying neuronal polarity in vivo. Curr. Opin. Neurobiol. 2008;18:44–52. doi: 10.1016/j.conb.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielas S. L., Serneo F. F., Chechlacz M., Deerinck T. J., Perkins G. A., Allen P. B., Ellisman M. H., Gleeson J. G. Spinophilin facilitates dephosphorylation of doublecortin by PP1 to mediate microtubule bundling at the axonal wrist. Cell. 2007;129:579–591. doi: 10.1016/j.cell.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M. M. Taxol interferes with the interaction of microtubule-associated proteins with microtubules in cultured neurons. J. Neurosci. 1987;7:3695–3702. doi: 10.1523/JNEUROSCI.07-11-03695.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D., McEwen D. P., Martens J. R., Meyhofer E., Verhey K. J. Single molecule imaging reveals differences in microtubule track selection between kinesin motors. PLoS Biol. 2009;7:e1000216. doi: 10.1371/journal.pbio.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Baloh R. H., Milbrandt J. The NIMA-family kinase Nek3 regulates microtubule acetylation in neurons. J. Cell Sci. 2009;122:2274–2282. doi: 10.1242/jcs.048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creppe C., et al. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136:551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador F., Jones E. V., Whitmarsh A. J. The JIP1 scaffold protein regulates axonal development in cortical neurons. Curr. Biol. 2008;18:221–226. doi: 10.1016/j.cub.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dompierre J. P., Godin J. D., Charrin B. C., Cordelieres F. P., King S. J., Humbert S., Saudou F. Histone deacetylase 6 inhibition compensates for the TRANSPORT deficit in Huntington's disease by increasing tubulin acetylation. J. Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti C. G., Banker G. A. Experimentally induced alteration in the polarity of developing neurons. Nature. 1987;330:254–256. doi: 10.1038/330254a0. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Sullivan C. A., Banker G. A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S., Morrison E. E., Liverpool T. B., Molina-Paris C., Cross R. A., Alonso M. C., Peckham M. Differential trafficking of Kif5c on tyrosinated and detyrosinated microtubules in live cells. J. Cell Sci. 2008;121:1085–1095. doi: 10.1242/jcs.026492. [DOI] [PubMed] [Google Scholar]
- Eng C. H., Huckaba T. M., Gundersen G. G. The formin mDia regulates GSK3beta through novel PKCs to promote microtubule stabilization but not MTOC reorientation in migrating fibroblasts. Mol. Biol. Cell. 2006;17:5004–5016. doi: 10.1091/mbc.E05-10-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner A., Huang X., Hall A. Neuronal polarity is regulated by glycogen synthase kinase-3 (GSK-3beta) independently of Akt/PKB serine phosphorylation. J. Cell Sci. 2006;119:3927–3934. doi: 10.1242/jcs.03159. [DOI] [PubMed] [Google Scholar]
- Gomis-Ruth S., Wierenga C. J., Bradke F. Plasticity of polarization: changing dendrites into axons in neurons integrated in neuronal circuits. Curr. Biol. 2008;18:992–1000. doi: 10.1016/j.cub.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Goslin K., Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J. Cell Biol. 1989;108:1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurskaya N. G., Verkhusha V. V., Shcheglov A. S., Staroverov D. B., Chepurnykh T. V., Fradkov A. F., Lukyanov S., Lukyanov K. A. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat. Biotechnol. 2006;24:461–465. doi: 10.1038/nbt1191. [DOI] [PubMed] [Google Scholar]
- Haggarty S. J., Koeller K. M., Wong J. C., Grozinger C. M., Schreiber S. L. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc. Natl. Acad. Sci. USA. 2003;100:4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J. W., Cai D., Verhey K. J. Tubulin modifications and their cellular functions. Curr. Opin. Cell Biol. 2008;20:71–76. doi: 10.1016/j.ceb.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X. F., Yao T. P. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Ikegami K., et al. Loss of alpha-tubulin polyglutamylation in ROSA22 mice is associated with abnormal targeting of KIF1A and modulated synaptic function. Proc. Natl. Acad. Sci. USA. 2007;104:3213–3218. doi: 10.1073/pnas.0611547104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K., Mukai M., Tsuchida J., Heier R. L., Macgregor G. R., Setou M. TTLL7 is a mammalian beta-tubulin polyglutamylase required for growth of MAP2-positive neurites. J. Biol. Chem. 2006;281:30707–30716. doi: 10.1074/jbc.M603984200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson C., Schnapp B., Banker G. A. A change in the selective translocation of the Kinesin-1 motor domain marks the initial specification of the axon. Neuron. 2006;49:797–804. doi: 10.1016/j.neuron.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Janke C., et al. Tubulin polyglutamylase enzymes are members of the TTL Domain protein family. Science. 2005;308:1758–1762. doi: 10.1126/science.1113010. [DOI] [PubMed] [Google Scholar]
- Jiang H., Guo W., Liang X., Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Jordan M. A., Wilson L. Use of drugs to study role of microtubule assembly dynamics in living cells. Methods Enzymol. 1998;298:252–276. doi: 10.1016/s0076-6879(98)98024-7. [DOI] [PubMed] [Google Scholar]
- Kaech S., Banker G. Culturing hippocampal neurons. Nat. Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kim W. Y., Zhou F. Q., Zhou J., Yokota Y., Wang Y. M., Yoshimura T., Kaibuchi K., Woodgett J. R., Anton E. S., Snider W. D. Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron. 2006;52:981–996. doi: 10.1016/j.neuron.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi Y., Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat. Neurosci. 2009;12:559–567. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- Larcher J. C., Boucher D., Lazereg S., Gros F., Denoulet P. Interaction of kinesin motor domains with alpha- and beta-tubulin subunits at a tau-independent binding site. Regulation by polyglutamylation. J. Biol. Chem. 1996;271:22117–22124. doi: 10.1074/jbc.271.36.22117. [DOI] [PubMed] [Google Scholar]
- Liao G., Gundersen G. G. Kinesin is a candidate for cross-bridging microtubules and intermediate filaments. Selective binding of kinesin to detyrosinated tubulin and vimentin. J. Biol. Chem. 1998;273:9797–9803. doi: 10.1074/jbc.273.16.9797. [DOI] [PubMed] [Google Scholar]
- Matsuyama A., et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21:6820–6831. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D., Liu A., Margolis B. Interaction of c-Jun amino-terminal kinase interacting protein-1 with p190 rhoGEF and its localization in differentiated neurons. J. Biol. Chem. 1999;274:35113–35118. doi: 10.1074/jbc.274.49.35113. [DOI] [PubMed] [Google Scholar]
- Nakata T., Hirokawa N. Microtubules provide directional cues for polarized axonal TRANSPORT through interaction with kinesin motor head. J. Cell Biol. 2003;162:1045–1055. doi: 10.1083/jcb.200302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa N., Sugisaki S., Tokunaga E., Fujitani K., Hayasaka T., Setou M., Inokuchi K. N-Acetyltransferase ARD1-NAT1 regulates neuronal dendritic development. Genes Cells. 2008;13:1171–1183. doi: 10.1111/j.1365-2443.2008.01235.x. [DOI] [PubMed] [Google Scholar]
- Owen R., Gordon-Weeks P. R. Inhibition of glycogen synthase kinase 3beta in sensory neurons in culture alters filopodia dynamics and microtubule distribution in growth cones. Mol. Cell. Neurosci. 2003;23:626–637. doi: 10.1016/s1044-7431(03)00095-2. [DOI] [PubMed] [Google Scholar]
- Reed N. A., Cai D., Blasius T. L., Jih G. T., Meyhofer E., Gaertig J., Verhey K. J. Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 2006;16:2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Samsonov A., Yu J. Z., Rasenick M., Popov S. V. Tau interaction with microtubules in vivo. J. Cell Sci. 2004;117:6129–6141. doi: 10.1242/jcs.01531. [DOI] [PubMed] [Google Scholar]
- Toriyama M., Shimada T., Kim K. B., Mitsuba M., Nomura E., Katsuta K., Sakumura Y., Roepstorff P., Inagaki N. Shootin1, A protein involved in the organization of an asymmetric signal for neuronal polarization. J. Cell Biol. 2006;175:147–157. doi: 10.1083/jcb.200604160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk J., Rogowski K., Miro J., Lacroix B., Edde B., Janke C. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol. Cell. 2007;26:437–448. doi: 10.1016/j.molcel.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Verhey K. J., Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–2160. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- Verhey K. J., Meyer D., Deehan R., Blenis J., Schnapp B. J., Rapoport T. A., Margolis B. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J. Cell Biol. 2001;152:959–970. doi: 10.1083/jcb.152.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte H., Neukirchen D., Bradke F. Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 2008;180:619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff A., de Nechaud B., Chillet D., Mazarguil H., Desbruyeres E., Audebert S., Edde B., Gros F., Denoulet P. Distribution of glutamylated alpha and beta-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur J. Cell Biol. 1992;59:425–432. [PubMed] [Google Scholar]
- Yoshimura T., Kawano Y., Arimura N., Kawabata S., Kikuchi A., Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.