Vascular endothelial (VE)-cadherin is a cell-cell adhesion molecule involved in endothelial barrier function. Here, we show that initial circumferential actin bundling induced by cyclic AMP-Epac-Rap1 signal and its linkage to VE-cadherin through α- and β-catenins lead to the stabilization of VE-cadherin at cell-cell contacts.
Abstract
Vascular endothelial (VE)-cadherin is a cell–cell adhesion molecule involved in endothelial barrier functions. Previously, we reported that cAMP-Epac-Rap1 signal enhances VE-cadherin–dependent cell adhesion. Here, we further scrutinized how cAMP-Epac-Rap1 pathway promotes stabilization of VE-cadherin at the cell–cell contacts. Forskolin induced circumferential actin bundling and accumulation of VE-cadherin fused with green fluorescence protein (VEC-GFP) on the bundled actin filaments. Fluorescence recovery after photobleaching (FRAP) analyses using VEC-GFP revealed that forskolin stabilizes VE-cadherin at cell–cell contacts. These effects of forskolin were mimicked by an activator for Epac but not by that for protein kinase A. Forskolin-induced both accumulation and stabilization of junctional VEC-GFP was impeded by latrunculin A. VE-cadherin, α-catenin, and β-catenin were dispensable for forskolin-induced circumferential actin bundling, indicating that homophilic VE-cadherin association is not the trigger of actin bundling. Requirement of α- and β-catenins for forskolin-induced stabilization of VE-cadherin on the actin bundles was confirmed by FRAP analyses using VEC-GFP mutants, supporting the classical model that α-catenin could potentially link the bundled actin to cadherin. Collectively, circumferential actin bundle formation and subsequent linkage between actin bundles and VE-cadherin through α- and β-catenins are important for the stabilization of VE-cadherin at the cell–cell contacts in cAMP-Epac-Rap1 signal-activated cells.
INTRODUCTION
Endothelial cells lining blood vessels regulate endothelial barrier function, which restricts the passage of plasma proteins and circulating cells across the endothelium. Compromising vascular integrity leads to an increase in vascular permeability, which is associated with chronic inflammation, edema, and tumor angiogenesis (Dejana et al., 2008; Wallez and Huber, 2008; Dejana et al., 2009). Endothelial cells have two specialized junctional domains, adherens junctions (AJs) and tight junctions. AJs are constituted by vascular endothelial (VE)-cadherin (also known as cadherin-5 and CD144) and nectin, whereas tight junctions are composed of members of junctional adhesion molecule, claudins, and occludins (Dejana, 2004; Ebnet et al., 2004; Wallez and Huber, 2008).
Interendothelial AJs are dynamic structures, and their adhesive property is finely controlled by various signaling molecules (Dejana et al., 2008; Vestweber et al., 2009). Inflammatory mediators such as thrombin and histamine induce intercellular gap formation leading to an increase in endothelial permeability (Andriopoulou et al., 1999; Gavard, 2009). Vascular endothelial growth factor also weakens interendothelial cell junctions, which is thought to be a key initiation step of angiogenesis (Paul et al., 2001; Weis et al., 2004; Gavard and Gutkind, 2006; Dejana et al., 2008). In contrast, angiopoietin-1 and sphingosine-1-phosphate stabilize endothelial barrier integrity (Thurston et al., 1999; Gamble et al., 2000; Garcia et al., 2001; Fukuhara et al., 2009; Augustin et al., 2009). Furthermore, it is widely recognized that an increase in intracellular cAMP level in endothelial cells strengthens barrier function and attenuates endothelial permeability both in vitro and in vivo (Fukuhra et al., 2006; Kooistra et al., 2007; Adamson et al., 2008; Pannekoek et al., 2009). Consistently, cAMP-elevating G protein-coupled receptor agonists, such as adrenomedullin, prostacyclin, prostaglandin E2, and β-adrenergic agonists, reduce endothelial hyperpermeability induced by inflammatory stimuli (Langeler and van Hinsbergh, 1991; Farmer et al., 2001; Hippenstiel et al., 2002).
The mechanism by which cAMP enhances endothelial barrier function is thought to involve two cAMP effectors, protein kinase A (PKA) and exchange protein directly activated by cAMP (Epac) (Yuan, 2002; Fukuhara et al., 2005; Cullere et al., 2005; Kooistra et al., 2005; Pannekoek et al., 2009). Although we could not find a significant role for PKA in cAMP-induced barrier function previously (Fukuhara et al., 2005), several reports have suggested that PKA stabilizes endothelial cell–cell junctions through reduction of myosin light chain phosphorylation, leading to relaxation of actomyosin complex, inhibition of Rho, and activation of Rac (Liu et al., 2001; Qiao et al., 2003; Birukova et al., 2004, 2007). Lorenowicz et al. (2008) have also reported that PKA activation by N6-benzoyl-cAMP (6-Bnz), a specific cAMP analogue for PKA, promotes endothelial barrier function in vitro. However, they also observed increased stress fiber formation in 6-Bnz–stimulated cells, which is a hallmark of Rho activation leading to disruption of endothelial cell–cell junctions. Thus, the role of PKA in cAMP-enhanced endothelial barrier function still remains elusive.
Epac is a guanine nucleotide exchange factor for Rap1 small GTPase (Kooistra et al., 2007; Pannekoek et al., 2009). 8-pCPT-2′-O-methl-cAMP (hereafter referred to as 007), a cAMP analogue specific for Epac, enhances endothelial barrier functions in vitro and in vivo (Fukuhara et al., 2005; Cullere et al., 2005; Kooistra et al., 2005; Adamson et al., 2008). Previously, we and others have shown that a cAMP–Epac–Rap1 pathway promotes endothelial barrier function by potentiating VE-cadherin–mediated cell–cell adhesions (Fukuhara et al., 2005; Cullere et al., 2005; Kooistra et al., 2005). Consistently, Rap1 is involved in E-cadherin–based cell–cell adhesions in epithelial cells (Hogan et al., 2004; Price et al., 2004). In endothelial cells, 007 induces cortical actin bundle formation along the cell–cell junctions, which is thought to be required for Rap1-enhanced barrier function (Fukuhara et al., 2005; Kooistra et al., 2005; Lorenowicz et al., 2008). Several signaling molecules, including Rac, K-Rev Interaction Trapped gene-1 (also known as CCM1), and AF-6, have been reported to act downstream of Rap1 to regulate actin cytoskeleton and barrier integrity (Boettner et al., 2000; Boettner et al., 2003; Arthur et al., 2004; Birukova et al., 2007; Glading et al., 2007). However, it remains elusive how a cAMP–Epac–Rap1 pathway enhances VE-cadherin-dependent cell adhesions.
In the classical model, cadherin-β-catenin complexes are statically linked to bundled actin filaments via α-catenin to maintain AJs. Cytoplasmic region of cadherin binds to three armadillo-family proteins, β-, γ-, and p120-catenins (Ozawa and Kemler, 1992; Kemler, 1993; Reynolds et al., 1994). α-Catenin associates with not only β- and γ-catenins but also the actin cytoskeleton (Nagafuchi et al., 1994; Rimm et al., 1995; Watabe-Uchida et al., 1998; Sako et al., 1998; Imamura et al., 1999). However, the Weiss and Nelson groups have recently suggested a new dynamic model that α-catenin does not stably connect actin to cadherin by showing that α-catenin does not bind simultaneously to both the cadherin-β–catenin complex and actin filaments (Yamada et al., 2005; Drees et al., 2005; Gates and Peifer, 2005). However, Abe and Takeichi have recently shown that epithelial protein lost in neoplasm (EPLIN) is able to mediate a stable linkage between the cadherin–catenin complex and the actin cytoskeleton (Abe and Takeichi, 2007). Thus, it is still controversial how α-catenin functions in cadherin-based cell–cell adhesions.
In the present study, we demonstrate that initial circumferential actin bundling induced by the cAMP–Epac–Rap1 signal and its linkage to VE-cadherin-β-catenin by α-catenin is essential for the stabilization of VE-cadherin at the cell–cell contacts.
MATERIALS AND METHODS
Reagents and Antibodies
Materials were purchased as follows: forskolin (FSK) and latrunculin A (Lat.A) were from Calbiochem (San Diego, CA), Epac-specific activator 007 was from Tocris Bioscience (Bristol, United Kingdom), PKA-specific activator 6-Bnz was from Biolog Life Science Institute (Bremen, Germany), H89 was from Sigma-Aldrich (St. Louis, MO), and Cellmatrix type I-C was from Nitta Gelatin (Osaka, Japan). Antibodies used here were purchased as follows: anti-VE-cadherin was from Santa Cruz Biotechnology (Santa Cruz, CA), BD Biosciences (San Jose, CA), and Cell Signaling Technology (Danvers, MA); anti-α-catenin was from Zymed Laboratories (South San Francisco, CA), anti-β-catenin and anti-p120-catenin were from BD Bioscience, anti-cAMP response element-binding protein (CREB) and anti-phospho-CREB (Ser133) were from Cell Signaling Technology; anti-Rap1 was from Santa Cruz Biotechnology; anti-β-actin and anti-β-tubulin were from Sigma-Aldrich; rhodamine-phalloidin, Alexa 488-labeled goat anti-mouse immunoglobulin G (IgG), Alexa 633-labeled goat anti-mouse IgG, and Alexa 546-labeled goat anti-rabbit IgG were from Invitrogen (Carlsbad, CA); horseradish peroxidase-coupled goat anti-mouse and horseradish peroxidase-coupled goat anti-rabbit IgG were from GE Healthcare (Piscataway, NJ); and horseradish peroxidase-coupled donkey anti-goat IgG was from Santa Cruz Biotechnology.
Cell Culture, Transfection, Small Interfering RNA (siRNA)-mediated Protein Knockdown, and Adenovirus Infection
Human umbilical vein endothelial cells (HUVECs) were purchased from Kurabo (Kurashiki, Japan), maintained as described previously (Fukuhara et al., 2008), and used for the experiments before passage 9. 293T cells were cultured in DMEM (Nissui, Tokyo, Japan) supplemented with 10% fetal bovine serum and antibiotics (100 μg streptomycin/ml and 100 U penicillin/ml). HUVECs and 293T cells were transfected using Lipofectamine 2000 and 293 fectin reagents (Invitrogen), respectively. Stealth siRNAs targeted to human VE-cadherin (HSS101682), human α-catenin (HSS102451 and 5′-UUAUUAGAGGGCCCUUUACUAUUGG-3′), human β-catenin (VHS50819 and VHS50822), and human p120-catenin (HSS102463 and HSS102465) were purchased from Invitrogen. As a control, siRNA duplexes with irrelevant sequences were used. HUVECs were transfected with 20 nM siRNA duplexes using Lipofectamine RNAi MAX reagent (Invitrogen). After incubation for 48 h, the cells were replated, cultured for additional 24 h, and were used for the experiments.
Recombinant adenoviruses encoding Rap1GAP and LacZ were obtained from S. Hattori (The Institute of Medical Science, University of Tokyo, Tokyo, Japan) and M. Matsuda (Research Institute for Microbial Disease, Osaka University, Osaka, Japan), respectively. HUVECs were infected with adenoviruses at the appropriated multiplicities of infection as described in the figure legends.
Plasmids
cDNAs for human VE-cadherin and human platelet/endothelial cell adhesion molecule (PECAM)1 were amplified from human heart cDNAs by reverse transcription-polymerase chain reaction (PCR) and cloned into pEGFP-N1 vector (Clontech, Mountain View, CA) to construct pEGFP-N1-VEC encoding VE-cadherin carboxy-terminally tagged with green fluorescence protein (VEC-GFP) and pEGFP-N1-PECAM1 encoding PECAM1 carboxy-terminally tagged with GFP (PECAM1-GFP), respectively. To generate the plasmid encoding VEC-GFP lacking β-catenin binding domain (VECΔβ-GFP) and that encoding VEC-GFP lacking cytoplasmic domain (VECΔC-GFP), amino acids 1-700 and 1-631 fragments of VE-cadherin were amplified by PCR and subcloned into pEGFP-N1 vector, namely, pEGFP-N1-VECΔβ and pEGFP-N1-VECΔC, respectively. To generate pEGFP-N1-VECΔC-α vector encoding VEC-GFP mutant in which cytoplasmic domain of VE-cadherin is replaced with α-catenin (VECΔC-α-GFP), a cDNA encoding full-length α-catenin was amplified by PCR using an expression vector for α-catenin (a gift from A. Nagafuchi, Kumamoto University, Kumamoto, Japan) as a template and inserted into the site immediately upstream of GFP in pEGFP-N1-VECΔC vector. Similarly, a cDNA encoding amino acids 327–906 fragment of α-catenin was amplified by PCR and inserted into the same site of pEGFP-N1-VECΔC vector to construct the plasmid expressing VECΔC-αΔN-GFP. An siRNA-insensitive version of pEGFP-N1-VECΔC-α plasmid, namely, pEGFP-N1-VECΔC-α-in vector, was generated using QuickChange Site-directed mutagenesis kit (Stratagene, La Jolla, CA). A cDNA fragment encoding PECAM1 lacking cytoplasmic region was amplified by PCR and inserted into pEGFP-N1-vector to generate the pEGFP-N1-PECAM1ΔC plasmid encoding PECAM1-GFP mutant lacking the cytoplasmic region of PECAM1 (PECAM1ΔC- GFP). To construct the pEGFP-N1-PECAM1ΔC-α plasmid encoding PECAM1-GFP mutant in which cytoplasmic region is replaced with α-catenin (PECAM1ΔC-α-GFP), a cDNA encoding full-length α-catenin amplified by PCR was inserted into the site immediately upstream of GFP in pEGFP-N1-PECAM1ΔC vector. Similarly, a cDNA encoding cytoplasmic region of VE-cadherin was also inserted into the same site of pEGFP-N1-PECAM1ΔC vector to construct the pEGFP-N1-PECAM1ΔC-VEC/C plasmid encoding PECAM1-GFP mutant in which cytoplasmic region is replaced with that of VE-cadherin (PECAM1ΔC-VEC/C-GFP).
Fluorescence Recovery after Photobleaching Analysis
HUVECs plated on a 35-mm-diameter collagen-coated glass-base dish (Asahi Techno Glass, Chiba, Japan) were transfected with the expression plasmids encoding VEC-GFP, PECAM1-GFP, and their mutants and cultured for 24 h at confluent cell density. The cells were then starved in medium 199 containing either 1 or 0.1% bovine serum albumin (BSA) for 3 h, and stimulated with vehicle, 10 μM FSK, 0.2 mM 007, and 0.2 mM 6-Bnz for 30 min. Fluorescence recovery after photobleaching (FRAP) experiments were performed on a FV1000 laser-scanning confocal microscope (Olympus, Tokyo, Japan) with a 60× objective lens, and GFP fluorescence was imaged by the excitation with 473-nm diode laser. All experiments were performed at 37°C with 5% CO2 using a heating chamber (Tokai Hit, Shizuoka, Japan). GFP-positive cells surrounded by GFP-negative cells were selected and subjected to FRAP analysis. GFP fluorescence at the cell–cell contacts was bleached for 5 s using 405-nm diode laser set at full power. To monitor fluorescence recovery, images were acquired every 90 s over a period of 50–60 min using the FluoView version 1.7c software (Olympus). Using Excel software (Microsoft, Redmond, WA), data were corrected for the overall loss in total fluorescence intensity as a result of the imaging scans. The fluorescence intensity of the bleached region over time was normalized with the prebleached fluorescence intensity. Recovery measurements were quantified by fitting normalized fluorescence intensities of bleached areas to a one-phase exponential association by using Prism 5 software (GraphPad Software, San Diego, CA). This program was also used for plotting of the data and statistical analysis.
Immunocytochemistry
Monolayer-cultured HUVECs grown on a collagen-coated glass-base dish were starved in medium 199 containing either 0.5 or 0.1% BSA for 3 h and subsequently stimulated with vehicle, 10 μM FSK, 0.2 mM 007, or 0.2 mM 6-Bnz for 30 min. After stimulation, the cells were fixed in phosphate-buffered saline (PBS) containing 2% formaldehyde for 30 min at 4°C, permeabilized with 0.05% Triton X-100 for 30 min at 4°C, and blocked with PBS containing 4% BSA for 1 h at room temperature. The cells were then stained with rhodamine-phalloidin for 20 min and with anti-VE-cadherin, anti-α-catenin, anti-β-catenin, and anti-p120-catenin antibodies for 60 min at room temperature. Protein reacting with antibody was visualized with species-matched Alexa 488-, Alexa 546- or Alexa 633-labeled secondary antibodies. Fluorescence images of GFP, rhodamine, Alexa 488, Alexa 546, and Alexa 633 were recorded with an Olympus IX-81 inverted fluorescence microscope (Olympus) equipped with pE-1 LED excitation system (CoolLED, Andover, United Kingdom) with a cooled charge-coupled device camera CoolSNAP-HQ (Roper Scientific, Trenton, NJ) and appropriate filter sets for GFP, Alexa 488, Alexa 546, and Alexa 633, and with a FluoView FV1000 confocal microscope with 60× and 100× oil immersion objective lens. To quantify the levels of F-actin at cell–cell contacts, fluorescence intensity of rhodamine along the 5-pixel-width lines randomly drawn on the rhodamine images was determined by line intensity scanning using MetaMorph software (Molecular Devices, Sunnyvale, CA). Peak fluorescence intensity at the points across the cell–cell contacts was taken as the value of F-actin at cell–cell contacts. A minimum of 80 contacts were analyzed per experiment, and experiments were repeated three times.
Detection of GTP-bound Form of Rap1 and Phosphorylated CREB
Rap1 activity and phosphorylation of CREB were assessed as described previously (Fukuhara et al., 2005). In brief, HUVECs starved in medium 199 containing 1% BSA for 6 h were stimulated with vehicle, 10 μM FSK, 0.2 mM 007, or 0.2 mM 6-Bnz for 15 min and lysed at 4°C in a pull-down lysis buffer containing 20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 1 mM EGTA, 1 mM dithiothreitol, 1 mM Na3VO4, and 1× protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). GTP-bound Rap1 was collected on the glutathione transferase-tagged Rap1 binding domain of RalGDS precoupled to glutathione-Sepharose beads and subjected to Western blot analysis with anti-Rap1 antibody. Aliquots of total cellular lysates were also subjected to Western blot analysis with anti-Rap1, anti-phospho-CREB, anti-CREB, and anti-β-actin antibodies.
Fluorescence-activated Cell Sorting (FACS) Analysis
Expression levels of VEC-GFP, PECAM1-GFP, and their mutants were analyzed by FACS analysis using FACSAria cell-sorting system (BD Biosciences).
Statistical Analysis
Data are expressed as either mean ± SD or mean ± SE as indicated in figure legends. Statistical significance was determined using Student's t test for paired samples or one-way analysis of variance and nonparametric tests for multiple groups. Data were considered statistically significant if p values <0.05.
RESULTS
cAMP Stabilizes VE-Cadherin at Cell–Cell Contacts through Epac–Rap1 Pathway, but Not PKA Pathway
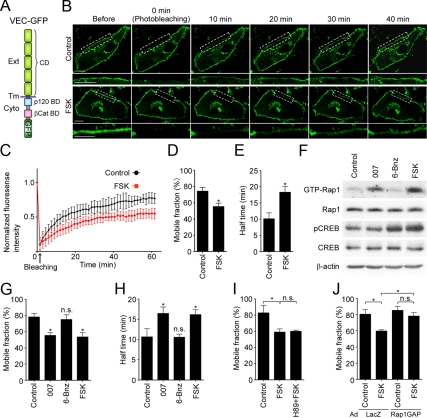
To examine the dynamics of VE-cadherin in living cells, we constructed the plasmid encoding VE-cadherin carboxy-terminally tagged with GFP (VEC-GFP; Figure 1A). VEC-GFP, but not GFP, expressed in confluent HUVECs localized at the cell–cell contacts and seemed to form the zipper-like structures, as did endogenous VE-cadherin (Supplemental Figure 1A). On stimulation with FSK, VEC-GFP as well as endogenous VE-cadherin was linearly accumulated at the cell–cell contacts (Supplemental Figure 1A). Coimmunoprecipitation study using 293T cells revealed the ability of VEC-GFP to associate with α-catenin, β-catenin, and p120-catenin (Supplemental Figure 1B). These results indicate that VEC-GFP behaves similarly to endogenous VE-cadherin. Thus, we decided to use VEC-GFP as a tool to analyze the dynamics of VE-cadherin in living cells.
Figure 1.
cAMP stabilizes VE-cadherin at cell–cell contacts through Epac, but not PKA. (A) Schematic illustration of VEC-GFP in which a GFP tag is fused to the carboxy terminus of full-length VE-cadherin. VE-cadherin consists of an extracellular region (Ext) consisting of five cadherin domains (CD), a transmembrane region (Tm), and a conserved cytoplasmic region (Cyto) containing p120–catenin-binding domain (p120 BD) and β-catenin-binding domain (βCat BD). (B) Confluent HUVECs plated on a collagen-coated glass-base dish were transfected with the plasmid encoding VEC-GFP. After 24 h, the cells were starved in medium 199 containing 1% BSA for 3 h and stimulated with vehicle (top, control) or 10 μM FSK (bottom) for 30 min. To measure the mobility of VEC-GFP at cell–cell junctions, GFP-positive cells surrounded by GFP-negative cells were selected and immediately subjected to FRAP analysis as described in Materials and Methods. Representative GFP images before and at the indicated time points after photobleaching are shown. Photobleached areas are marked by dotted rectangles and enlarged at the bottom of each image. Bar, 20 μm. (C) Quantitative analysis of FRAP experiments in B. Plot of normalized fluorescence intensity of VEC-GFP expressed in the cells stimulated with vehicle (control, black circles) or FSK (red squares) versus time (minutes) after photobleaching. Data are expressed as mean ± SD of five independent experiments. (D and E) The mobile fraction (D) and the recovery half-time (E) of VEC-GFP expressed in the cells stimulated with vehicle (control) or FSK were calculated from the fluorescence recovery curves shown in C. Data are expressed as mean ± SE of five independent experiments. (F) HUVECs starved in 0.5% BSA-containing medium 199 for 6 h were stimulated with either vehicle (control), 0.2 mM 007, 0.2 mM 6-Bnz, or 10 μM FSK for 15 min as indicated at the top. GTP-bound Rap1 was collected as described in Materials and Methods and subjected to Western blot analysis with anti-Rap1 antibody (GTP-Rap1). Aliquots of cell lysates were also subjected to Western blot analysis with anti-Rap1 (Rap1), anti-phospho-CREB (pCREB), anti-CREB (CREB), and anti-β-actin (β-actin) antibodies. (G and H) Confluent HUVECs expressing VEC-GFP were stimulated with vehicle (control), 0.2 mM 007, 0.2 mM 6-Bnz, or 10 μM FSK for 30 min and subjected to FRAP analysis as described in B. The mobile fraction of VEC-GFP (G) and its recovery half-time (H) were calculated similarly to D and E. Data are expressed as mean ± SE of six independent experiments. (I) Confluent HUVECs expressing VEC-GFP were starved in medium 199 containing 0.1% BSA for 3 h, treated with or without 10 μM H89 for 30 min, and subsequently stimulated with vehicle (control) or 10 μM FSK for 30 min as indicated at the bottom of each graph. The cells were then subjected to FRAP analysis as described in B. The mobile fraction of VEC-GFP was calculated similarly to D. (J) Confluent HUVECs plated on collagen-coated glass-base dish were transfected with the plasmid encoding VEC-GFP and infected with adenoviruses encoding either LacZ or Rap1GAP. After 24 h, the cells were starved, stimulated with FSK, and subsequently subjected to FRAP analysis as described in B. The mobile fraction of VEC-GFP was calculated similarly to D. Data are expressed as mean ± SE of four to five independent experiments. Significant differences from the control (D, E, G, and H) or between two groups (I and J) are indicated as *p < 0.05. n.s. indicates no significance between two groups.
To investigate whether intracellular cAMP stabilizes VE-cadherin at the endothelial cell–cell contacts, we performed the FRAP analysis of VEC-GFP. We examined the fluorescence recovery at the photobleached region of the cell–cell junctions in the confluent HUVECs expressing VEC-GFP using a confocal time-lapse microscope. In control cells, fluorescence of VEC-GFP recovered to 74.3% of the original level (Figure 1, B–D), indicating that the mobile and immobile fractions of VEC-GFP at the cell–cell contacts are 74.3 and 25.7%, respectively (Figure 1D). Among the mobile fraction, the half-time of fluorescence recovery for VEC-GFP was 10.1 min (Figure 1E). On stimulation with FSK, the mobile fraction of VEC-GFP at cell–cell contacts was reduced to 55.3%, whereas the half-time of the fluorescence recovery was prolonged to 18.3 min (Figure 1, B–E). These results suggest that elevation of intracellular cAMP level induces the stabilization of VE-cadherin at the cell–cell contacts.
Intracellular cAMP regulates diverse cellular functions mainly through two downstream effectors; Epac and PKA. Therefore, we investigated which effectors are involved in cAMP-induced VE-cadherin stabilization at cell–cell contacts by using specific activators for PKA and Epac, 6-Bnz and 007, respectively. 007 but not 6-Bnz induced activation of Rap1, a small GTPase acting downstream of Epac, whereas 6-Bnz but not 007 induced phosphorylation of CREB, a direct PKA substrate, confirming their specificity (Figure 1F). The mobile fraction of VEC-GFP at the cell–cell contacts was decreased by 007 to the level observed in FSK-stimulated cells (Figure 1G). 007 prolonged the half-time of the fluorescence recovery of VEC-GFP (Figure 1H). In contrast, 6-Bnz did not affect the mobile fraction and recovery rate of junctional VEC-GFP (Figure 1, G and H). In addition, H89, a PKA-specific inhibitor, did not affect FSK-reduced mobile fraction of VEC-GFP, although it blocked FSK-induced CREB phosphorylation (Figure 1I and Supplemental Figure 2A). However, increased recovery rate of VEC-GFP observed in either FSK- or 007-stimulated cells was partially inhibited by H89 (Supplemental Figure 2B; data not shown). Thus, basal PKA activity may influence the mobility of VE-cadherin at cell–cell contacts. Furthermore, overexpression of Rap1GAP, a GTPase-activating protein for Rap1, not only prevented FSK-induced Rap1 activation but also inhibited FSK-decreased mobile fraction of junctional VEC-GFP and the FSK-prolonged half-time of its fluorescent recovery (Figure 1J and Supplemental Figure 2, C and D). Collectively, these results indicate that cAMP stabilizes VE-cadherin at cell–cell contacts through Epac–Rap1 pathway, but not PKA pathway.
Circumferential Actin Bundles Induced by cAMP Are Required for Stabilization of VE-Cadherin at the Endothelial Cell–Cell Contacts
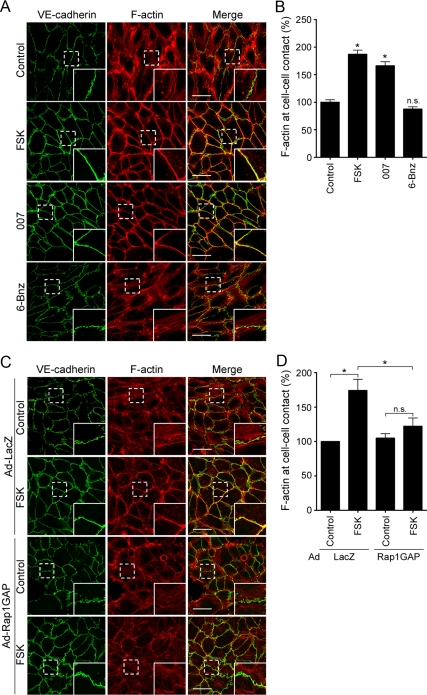
Previously, we and others have reported that cAMP induces accumulation of polymerized actin at the endothelial cell–cell contacts (Fukuhara et al., 2005; Kooistra et al., 2005; Lorenowicz et al., 2008). Thus, we decided to investigate whether reorganization of actin cytoskeleton is required for cAMP-mediated stabilization of VE-cadherin at cell–cell contacts. In control HUVECs, VE-cadherin formed zipper-like structures along the cell–cell junctions (Figure 2A). Staining with rhodamine-phalloidin revealed that actin stress fibers terminating at VE-cadherin-based cell–cell contacts were distributed through the cytoplasm (Figure 2A). Stimulation with either FSK or 007 reduced the central stress fibers and induced the formation of circumferential actin bundles along the cell–cell junctions (Figure 2, A and B). In these cells, VE-cadherin clearly concentrated along the circumferential actin bundles (Figure 2A). In contrast, PKA activation induced by 6-Bnz did not affect the organization of actin cytoskeleton and the localization of VE-cadherin (Figure 2, A and B). Furthermore, H89 inhibited neither FSK-induced formation of circumferential actin bundles nor FSK-induced accumulation of VE-cadherin at the cell–cell contacts (Supplemental Figure 3). However, overexpression of Rap1GAP in HUVECs prevented FSK-induced circumferential actin bundling (Figure 2, C and D). Collectively, these findings suggest that a cAMP–Epac–Rap1 pathway induces circumferential actin bundling and accumulation of VE-cadherin on the bundled actin filaments.
Figure 2.
cAMP induces circumferential actin bundle formation and accumulation of VE-cadherin on the bundled actin filaments through an Epac-Rap1 pathway. (A) Monolayer-cultured HUVECs starved in 0.5% BSA-containing medium 199 for 3 h were stimulated with vehicle (top, control), 10 μM FSK (second panel), 0.2 mM 007 (third panel), and 0.2 mM 6-Bnz (bottom) for 30 min. After stimulation, the cells were fixed, immunostained with anti-VE-cadherin antibody and visualized with Alexa 488-conjugated secondary antibody. The cells were also stained with rhodamine-phalloidin to visualize F-actin. Alexa 488 and rhodamine images were obtained through a confocal microscope. Alexa 488 (VE-cadherin, green), rhodamine (F-actin, red) and the merged (merge) images are shown as indicated at the top of each column. The boxed areas marked by dotted line in the images are enlarged in the bottom right corner of each image. (B) Levels of F-actin at cell–cell contacts observed in A were quantified as described in Materials and Methods. Values are expressed as a percentage relative to that in the control cells and shown as mean ± SE of >80 contacts. Similar results were obtained in three independent experiments. (C) Confluent HUVECs were infected with adenoviruses encoding either LacZ (Ad-LacZ) or Rap1GAP (Ad-Rap1GAP) as indicated at the left. After 24 h, the cells were starved in 0.5% BSA-containing medium 199 for 3 h and stimulated with vehicle (control) or 10 μM FSK for 30 min. The cells were then stained with anti-VE-cadherin antibody and visualized with Alexa 488-conjugated secondary antibody as described in A. The cells were also stained with rhodamine-phalloidin to visualize F-actin. Alexa 488 (VE-cadherin, green), rhodamine (F-actin, red) and the merged (merge) images are shown as indicated at the top of each column. The boxed areas marked by dotted line in the images are enlarged in the bottom right corner of each image. (D) Levels of F-actin at cell–cell contacts observed in C were quantified similarly to B. Values are expressed as a percentage relative to that in the control cells infected with adenoviruses encoding LacZ and are shown as mean ± SE of >100 contacts. Similar results were obtained in three independent experiments. Bars, 50 μm (A and C). Significant differences from the control (B) or between two groups (D) are indicated as *p < 0.05. n.s. indicates no significance between two groups.
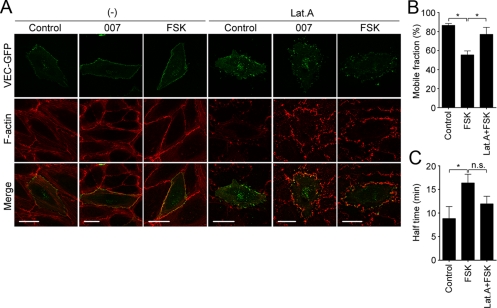
It has been reported that circumferential actin filament networks are required for the maintenance of E-cadherin-based cell–cell contacts in epithelial cells (Quinlan and Hyatt, 1999; Kobielak and Fuchs, 2004; Mege et al., 2006). Therefore, we investigated whether circumferential actin bundle formation is responsible for cAMP-induced accumulation of VE-cadherin at the cell–cell contacts. Treatment with 200 nM Lat.A, an inhibitor of actin polymerization (Spector et al., 1989), resulted in not only disruption of central stress fibers in the control HUVECs expressing VEC-GFP but also in fragmentation of circumferential actin bundles formed upon stimulation with either FSK or 007 (Figure 3A). In these cells, VEC-GFP could not accumulate at the cell–cell junctions even when stimulated with FSK and 007 (Figure 3A). We further performed the FRAP analysis of VEC-GFP in the presence or absence of Lat.A; 100 nM Lat.A less suppressed FSK-induced circumferential actin bundling and less decreased the junctional localization of VEC-GFP than 200 nM Lat.A (Supplemental Figure 4). Therefore, we tested the effect of Lat.A. on the stabilization of VE-cadherin at the cell–cell contacts by using the cells treated with 100 nM Lat.A. FSK-decreased mobile fraction of VEC-GFP at the cell–cell junctions and the FSK-prolonged half-time of its fluorescent recovery were partially inhibited by 100 nM Lat.A (Figure 3, B and C). These results suggest that the circumferential actin bundles induced by cAMP-Epac-Rap1 signal are required for stabilization of VE-cadherin at the cell–cell contacts.
Figure 3.
Circumferential actin bundle formation is responsible for cAMP-induced stabilization of junctional VE-cadherin. (A) Confluent HUVECs transfected with the plasmid encoding VEC-GFP were starved in 0.5% BSA-containing medium 199 for 3 h and incubated with vehicle [left, (−)] or 200 nM Lat.A (right, Lat.A) for 30 min. The cells were then stimulated with vehicle (control), 0.2 mM 007 (007), and 10 μM FSK (FSK) for 30 min as indicated at the top and stained with rhodamine-phalloidin as described in legend of Figure 2A. GFP and rhodamine images were obtained through a confocal microscope. GFP (VEC-GFP), rhodamine (F-actin), and the merged (merge) images are shown as indicated at the left. Bars, 30 μm. (B and C) Confluent HUVECs expressing VEC-GFP were starved in medium 199 containing 1% BSA for 3 h, treated with or without 100 nM Lat.A for 30 min, and stimulated with vehicle (control) or 10 μM FSK for 30 min as indicated at the bottom of each graph. The cells were then subjected to FRAP analysis as described in the legend of Figure 1B. The mobile fraction of VEC-GFP (B) and its recovery half-time (C) were calculated as described in the legend of Figure 1, D and E. Data are expressed as mean ± SE of five to seven independent experiments. Significant differences between two groups are indicated as *p < 0.05. n.s. indicates no significance between two groups.
The Circumferential Actin Bundling upon cAMP Stimulation Does Not Need Homophilic VE-Cadherin-based Cell–Cell Adhesions
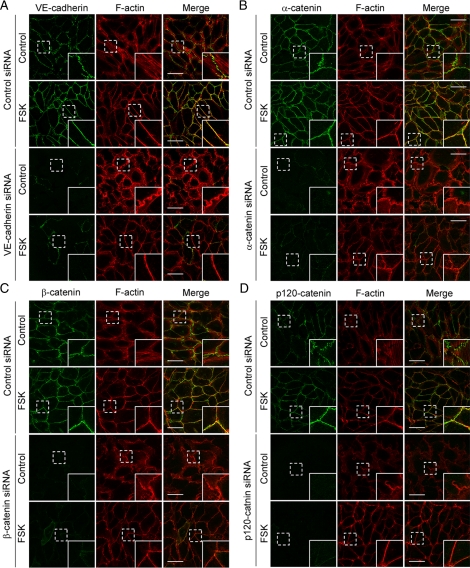
The cytoplasmic region of cadherin binds β-catenin, which in turn associates with α-catenin (Ozawa and Kemler, 1992; Kemler, 1993). It has been shown that α-catenin coordinates actin dynamics at the sites of cadherin-based cell–cell contacts (Kobielak and Fuchs, 2004; Mege et al., 2006; Weis and Nelson, 2006). Cadherin associates with p120-catenin through its juxtamembrane domain as well as β-catenin (Reynolds et al., 1994). p120-catenin regulates actin cytoskeleton by controlling the activity of Rho family small GTPases (Reynolds, 2007). Thus, we hypothesized that a cAMP-Epac-Rap1 pathway initially promotes VE-cadherin–based cell–cell adhesions, which subsequently induce circumferential actin bundling through catenins. To test this hypothesis, we examined the effect of depletion of either VE-cadherin, α-catenin, β-catenin, or p120-catenin on actin reorganization. Depletion of either of them resulted in the disruption of central stress fibers and the induction of membrane ruffle formation close to the cell–cell contacts (Figure 4, A–D). These results suggest that central stress fiber formation depends on VE-cadherin/catenin complexes. However, unexpectedly FSK-induced circumferential actin bundling was not affected by depletion of VE-cadherin/catenin complexes (Figure 4, A–D, and Supplemental Figure 5, A–D). Furthermore, we examined the effect of extracellular Ca2+ chelation on FSK-induced circumferential actin bundling, because cadherin-dependent cell adhesion requires extracellular Ca2+. FSK apparently induced formation of circumferential actin bundles even in the presence of EGTA (Supplemental Figure 6), although VE-cadherin disappeared from the cell–cell border in the presence of EGTA even when the cells were stimulated with FSK (Supplemental Figure 6). These results clearly indicate that cAMP-induced circumferential actin bundling does not require VE-cadherin/catenin complex-dependent reorganization of actin cytoskeleton.
Figure 4.
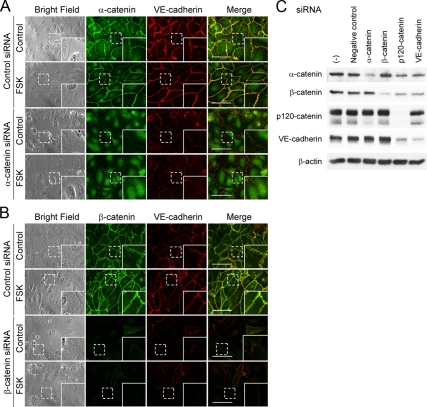
cAMP-induced circumferential actin bundling does not depend upon VE-cadherin–based cell–cell adhesions. (A–D) HUVECs were transfected with control siRNA (top panels of each figure) or with siRNAs targeting against VE-cadherin (A), α-catenin (B), β-catenin (C), and p120-catenin (D) (bottom panels of each figure), cultured for 48 h, and replated onto the collagen-coated glass-base dish. After 24 h, the cells were starved in medium 199 containing 0.5% BSA for 3 h and stimulated with vehicle (control) or 10 μM FSK for 30 min as indicated at the left of each figure. The cells were stained with anti-VE-cadherin (A), anti-α-catenin (B), anti-β-catenin (C), and anti-p120-catenin (D) antibodies and visualized with Alexa 488-conjugated secondary antibody as described in Figure 2A. The cells were also stained with rhodamine-phalloidin to visualize F-actin. Alexa 488 and rhodamine images were obtained through a confocal microscope. Alexa 488 images for VE-cadherin (A), α-catenin (B), β-catenin (C), and p120-catenin (D) are shown at the left column. Rhodamine (F-actin) and the merged (merge) images are shown at the middle and right columns, respectively. The boxed areas marked by dotted line in the images are enlarged in the bottom right corner of each image. Bars, 50 μm.
α- and β-Catenins Are Required for cAMP-induced Accumulation of VE-Cadherin at Cell–Cell Contacts
It had been believed that cadherin–β-catenin complex is physically linked with actin fibers via α-catenin, although this model is recently challenged by the report that α-catenin dose not bind simultaneously to both the cadherin–β-catnin complex and the actin fibers (Yamada et al., 2005; Drees et al., 2005; Gates and Peifer, 2005). To test the requirement of α- and β-catenins in cAMP-induced accumulation of VE-cadherin at the cell–cell contacts, we examined the effect of depletion of catenins on the accumulation of VE-cadherin. Depletion of either α- or β-catenin weakened not only the junctional localization of VE-cadherin in control HUVECs but also the FSK-induced accumulation of VE-cadherin at cell–cell contacts without affecting the expression level of VE-cadherin (Figure 5, A–C, and Supplemental Figure 7, A and B). Knockdown of p120-catenin by siRNA also resulted in disappearance of VE-cadherin at cell–cell contacts in either control- or FSK-stimulated cells but possibly due to the primarily down-regulated expression of VE-cadherin (Figure 5C and Supplemental Figure 8). These results indicate that α- and β-catenins are responsible for cAMP-induced accumulation of VE-cadherin at the cell–cell contacts and suggest that α- and β-catenins mediate the physical link between actin and VE-cadherin.
Figure 5.
α- and β-Catenins are essential for cAMP-induced accumulation of VE-cadherin at cell–cell contacts. (A and B) HUVECs were transfected with control siRNA (top panels of each figure) or with siRNAs targeting against α-catenin (A) and β-catenin (B) (bottom panels of each figure) and stimulated with vehicle (control) or FSK as described in the legend of Figure 4. The cells were immunostained with either anti-α-catenin (A) or anti-β-catenin (B) antibody and with anti-VE-cadherin antibody and then visualized with Alexa 488- and Alexa 546-conjugated secondary antibodies, respectively. Phase contrast, Alexa 488 and Alexa 546 images were obtained using an IX81 inverted microscope (Olympus). Phase contrast (bright field), Alexa 488 (α-catenin in A and β-catenin in B), Alexa 546 (VE-cadherin), and the merged (merge) images are shown as indicated at the top of each column. The boxed areas marked by dotted line in the images are enlarged in the lower right corner of each image. Bars, 30 μm. (C) HUVECs were transfected without [(−)] or with control siRNA (negative control) or siRNAs targeting α-catenin, β-catenin, p120-catenin, and VE-cadherin as indicated at the top and cultured for 72 h. Cell lysates were subjected to Western blot analysis with anti-α-catenin, anti-β-catenin, anti-p120-catenin, anti-VE-cadherin, and anti-β-actin antibodies as indicated at the left.
cAMP Induces Formation of Circumferential Actin Bundles, Which Anchor Junctional VE-Cadherin through α- and β-Catenins
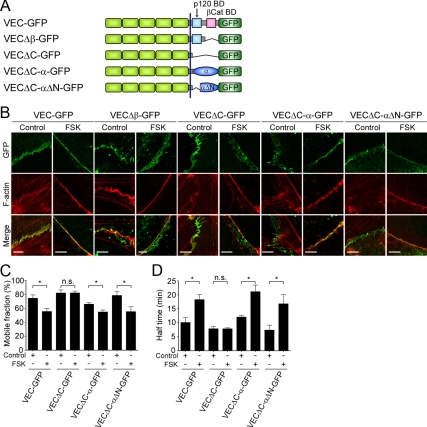
To confirm that α- and β-catenins mediate physical link between actin and VE-cadherin in cAMP–Epac–Rap1 signal-activated endothelial cells, we decided to examine the localization and dynamics of the mutants of VE-cadherin in which the cytoplasmic domain was modified. We also constructed the plasmids encoding the mutants of VEC-GFP (Figure 6A and Supplemental Figure 9, A and B): VECΔβ-GFP in which the β-catenin binding domain is deleted, VECΔC-GFP in which the cytoplasmic domain is deleted, VECΔC-α-GFP in which the cytoplasmic domain is replaced with α-catenin, and VECΔC-αΔN–GFP in which the cytoplasmic domain is replaced with α-catenin lacking N-terminal β-catenin binding domain. When these expression plasmids were transfected into HUVECs, all VEC-GFP mutants as well as wild type VEC-GFP localized at cell–cell contacts (Figure 6B and Supplemental Figure 9C). FACS analysis revealed that their expression levels are similar (Supplemental Figure 9D). Stimulation with FSK resulted in accumulation of wild-type VEC-GFP on the circumferential actin bundles (Figure 6B). When the cells expressing either VECΔβ-GFP or VECΔC-GFP were stimulated with FSK, circumferential actin bundling occurred. However, neither of them could accumulate on the bundled actin filaments and was broadly distributed around the cell–cell junctions (Figure 6B). In contrast, VECΔC-α-GFP clearly concentrated on the circumferential actin bundles formed upon the stimulation with FSK (Figure 6B). Similarly, VECΔC-αΔN-GFP also accumulated on the bundled actin filaments in FSK-stimulated cells (Figure 6B). Together with the evidence that VECΔC-αΔN-GFP was incapable of associating with β-catenin (Supplemental Figure 9E), these results indicate that the ability of VE-cadherin/α-catenin chimera to concentrate on the circumferential actin bundles is not due to α-catenin acting as a β-catenin binding site. Consistently, VECΔC-α-GFP, but not VEC-GFP, could accumulate on the bundled actin filaments even in the β-catenin–depleted cells (Supplemental Figure 10, B and D). Furthermore, depletion of α-catenin by siRNA did not affect the accumulation of VECΔC-α-GFP on the bundled actin filaments, although α-catenin was required for the localization of VEC-GFP on the actin bundles (Supplemental Figure 10, A and C). Collectively, these results indicate that α- and β-catenins localize VE-cadherin to the circumferential actin bundles.
Figure 6.
α- and β-Catenins locate and stabilize VE-cadherin at the circumferential actin bundles. (A) Schematic illustrations of VEC-GFP and its mutants. VEC-GFP, VE-cadherin carboxy-terminally tagged with GFP; VEC-GFPΔβ-GFP, a VEC-GFP mutant lacking the β-catenin binding domain of VE-cadherin; VEC-ΔC-GFP, a VEC-GFP mutant lacking the cytoplasmic region of VE-cadherin; VECΔC-α-GFP, a VEC-GFP mutant in which the cytoplasmic region of VE-cadherin is replaced with α-catenin; and VECΔC-αΔN-GFP, a VEC-GFP mutant in which the cytoplasmic region of VE-cadherin is replaced with α-catenin lacking N-terminal β-catenin binding domain. (B) HUVECs were transfected with the plasmid encoding either VEC-GFP or its mutant as indicated at the top. The cells were starved in medium 199 containing 0.5% BSA for 3 h and stimulated with vehicle (control) or 10 μM FSK (FSK) for 30 min. The cells were stained with rhodamine-phalloidin to visualize F-actin as described in the legend of Figure 2A. GFP and rhodamine images were obtained through a confocal microscope. GFP, rhodamine (F-actin), and the merged (merge) images are shown as indicated at the left. The border between the untransfected cell and the cell expressing GFP tagged-VE-cadhein is shown. Bars, 5 μm. (C and D) Confluent HUVECs plated on collagen-coated glass-base dish were transfected with the plasmid encoding VEC-GFP, VECΔC-GFP, VECΔC-α-GFP, or VECΔC-αΔN-GFP as indicated at the bottom of each figure. The cells were starved, stimulated with vehicle (control) or 10 μM FSK for 30 min, and subjected to FRAP analysis as described in the legend of Figure 1B. The mobile fraction of VEC-GFP and its mutants (C) and their recovery half-time (D) were calculated as described in the legend of Figure 1, D and E. Data are expressed as mean ± SE of six to eight independent experiments. Significant differences between two groups are indicated as *p < 0.05. n.s. indicates no significance between two groups.
Furthermore, we performed FRAP analyses of VEC-GFP mutants. FSK significantly reduced the mobile fractions of VEC-GFP, VECΔC-α-GFP, and VECΔC-αΔN-GFP at the cell–cell contacts and prolonged the half-time of their fluorescence recovery (Figure 6, C and D). Effect of FSK on the stability of VECΔC-α-GFP as well as VEC-GFP at cell–cell contacts was canceled by treatment with 100 nM Lat.A (Figure 3, B and C, and Supplemental Figure 11, A and B). In clear contrast, the mobile fraction of junctional VECΔC-GFP and its recovery rate were unaffected by stimulation with FSK (Figure 6, C and D). Collectively, these results strongly suggest that anchoring of VE-cadherin to circumferential actin bundles through α- and β-catenins results in the stabilization of VE-cadherin at cell–cell junctions.
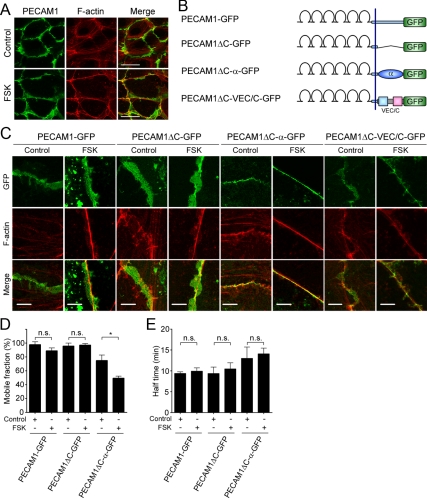
PECAM1 is an immunoglobulin-family cell adhesion molecule expressed in endothelial cells and localized at cell–cell junctions (Woodfin et al., 2007). However, unlike VE-cadherin, localization of PECAM1 is not confined to the AJs because it is incapable of associating with actin cytoskeleton. Indeed, PECAM1 did not accumulate on the circumferential actin bundles in FSK-stimulated HUVECs (Figure 7A), which is reminiscent of the localization of VECΔC-GFP and VECΔβ-GFP. Thus, we assumed that the importance of the link between actin and VE-cadherin through α- and β-catenins for the stabilization of VE-cadherin can be claimed by comparing the localization and dynamics of VE-cadherin to those of PECAM1. To this end, we constructed the plasmid encoding PECAM1 carboxy-terminally tagged with GFP (PECAM1-GFP) and those encoding its mutants (Figure 7B and Supplemental Figure 9, A–D); PECAM1ΔC-GFP in which the cytoplasmic region is deleted, PECAM1ΔC-α-GFP in which the cytoplasmic region is replaced with α-catenin and PECAM1ΔC-VEC/C-GFP in which the cytoplasmic region is replaced with that of VE-cadherin. As expected, PECAM1-GFP and PECAM1ΔC-GFP were broadly localized around the cell–cell junctions, irrespective of the presence or absence of circumferential actin bundles upon stimulation with FSK (Figure 7C). Consistently, FSK did not affect the mobile fractions of PECAM1-GFP and PECAM1ΔC-GFP at the cell–cell contacts and their recovery rate (Figure 7, D and E). In contrast, PECAM1ΔC-α-GFP and PECAM1ΔC-VEC/C-GFP clearly accumulated on the circumferential actin bundles in the FSK-stimulated cells (Figure 7C). FSK-induced concentration of PECAM1ΔC-α-GFP on the bundled actin filaments occurred even in the β-catenin–depleted cells (Supplemental Figure 12). Furthermore, the mobile fraction of PECAM1ΔC-α-GFP was significantly reduced by stimulation with FSK, although FSK did not affect its recovery rate (Figure 7, D and E), possibly due to the interaction with endogenous PECAM1. These findings indicate that α- and β-catenins have potential to locate VE-cadherin to the circumferential actin bundles induced by a cAMP–Epac–Rap1 signal and to stabilize VE-cadherin at the cell–cell contacts.
Figure 7.
PECAM1 that is incapable of associating with actin cytoskeleton is not stabilized on the circumferential actin bundles. (A) Monolayer-cultured HUVECs were stimulated with vehicle (top, control) or 10 μM FSK (bottom) for 30 min as described in the legend of Figure 2A. After stimulation, the cells were immunostained with anti-PECAM1 antibody and visualized with Alexa 488-conjugated secondary antibody. The cells were also stained with rhodamine-phalloidin to visualize F-actin. Alexa 488 and rhodamine images were obtained through a confocal microscope. Alexa 488 (PECAM1), rhodamine (F-actin), and the merged (merge) images are shown as indicated at the top of each column. (B) Schematic illustrations of PECAM1-GFP and its mutants. PECAM1-GFP, PECAM1 carboxy-terminally tagged with GFP; PECAM1ΔC-GFP, a PECAM1-GFP mutant lacking the cytoplasmic region of PECAM1; PECAM1ΔC-α-GFP, a PECAM1-GFP mutant in which the cytoplasmic region of PECAM1 is replaced with α-catenin; and PECAM1ΔC-VEC/C-GFP, a PECAM1-GFP mutant in which the cytoplasmic region of PECAM1 is replaced with that of VE-cadherin. (C) HUVECs were transfected with the plasmid encoding either PECAM1-GFP or its mutant as indicated at the top. The cells were stimulated with vehicle (control) or 10 μM FSK and stained with rhodamine-phalloidin similarly to the legend of Figure 6B. GFP and rhodamine images were obtained through a confocal microscope. GFP, rhodamine (F- actin), and the merged (merge) images are shown as indicated at the left. The border between the untransfected cell and the cell expressing GFP tagged-PECAM1 is shown. (D and E) Confluent HUVECs plated on a collagen-coated glass-base dish were transfected with the plasmid encoding PECAM1-GFP, PECAM1ΔC-GFP, or PECAM1ΔC-α-GFP as indicated at the bottom of each graph. The cells were starved, stimulated with vehicle (control) or 10 μM FSK for 30 min, and subjected to FRAP analysis as described in the legend of Figure 1B. The mobile fraction of PECAM1-GFP and its mutants (D) and their recovery half-time (E) were calculated as described in the legend of Figure 1, D and E. Data are expressed as mean ± SE of five to six independent experiments. Significant differences between two groups are indicated as *p < 0.05. n.s. indicates no significance between two groups. Bars, 30 μm (A) and 5 μm (C).
DISCUSSION
We found that a cAMP-Epac–Rap1 signal initially induces circumferential actin bundle formation independently of VE-cadherin/catenin complexes and that α- and β-catenins have potential to locate VE-cadherin to the bundled actin and to stabilize VE-cadherin at the cell–cell contacts. cAMP is a well-known intracellular second messenger that is capable of promoting endothelial barrier function through both PKA and Epac (Yuan, 2002; Cullere et al., 2005; Fukuhara et al., 2005; Kooistra et al., 2005; Pannekoek et al., 2009). Previously, we and others have reported that cAMP potentiates VE-cadherin–based cell–cell adhesions through an Epac–Rap1 pathway (Cullere et al., 2005; Fukuhara et al., 2005; Kooistra et al., 2005). Thus, circumferential actin bundling induced by cAMP–Epac–Rap1 signal is essential for endothelial barrier function mediated by VE-cadherin.
The mechanism how stabilization of VE-cadherin is regulated in cAMP–Epac–Rap1 signal-activated endothelial cells fits the classical static model: Catenin tethers cadherin to actin cytoskeleton. The cadherin–β-catenin complex has been believed to be physically linked with actin cytoskeleton directly or indirectly through α-catenin, which is responsible for maintenance of AJs (Nagafuchi et al., 1994; Rimm et al., 1995; Sako et al., 1998; Watabe-Uchida et al., 1998; Imamura et al., 1999). However, the Weis and Nelson groups have recently suggested a new dynamic model in which α-catenin does not statically link cadherin to actin but directly regulates actin dynamics at the cell–cell contacts by demonstrating that α-catenin does not associate with the cadherin–β-catenin complex and with cytoskeleton simultaneously (Drees et al., 2005; Gates and Peifer, 2005; Yamada et al., 2005). By performing FRAP analysis of GFP-tagged E-cadherin (E-cadherin-GFP), they showed that ∼80% of E-cadherin-GFP is immobilized at cell–cell contacts in epithelial cells. However, the mobility of junctional E-cadherin-GFP was not affected by disruption of actin cytoskeleton. In addition, a mutant of E-cadherin-GFP lacking its cytoplasmic domain had a mobility fraction similar to that of the full-length E-cadherin-GFP. These observations suggest that anchoring to actin cytoskeleton through α- and β-catenins does not contribute to the stabilization of E-cadherin at cell–cell junctions. In clear contrast to their findings, we found that >70% of VE-cadherin is mobile at the cell–cell contacts in unstimulated endothelial cells under confluent culture and that elevation of intracellular cAMP level reduced the mobile fraction of junctional VEC-GFP, which was significantly inhibited by disruption of actin cytoskeleton. Furthermore, elevation of intracellular cAMP level did not affect the mobile fractions of VECΔC-GFP but decreased that of VECΔC-α-GFP. These results indicate that a stable linkage between VE-cadherin and actin bundles mediated by α- and β-catenins is responsible for cAMP-induced stabilization of VE-cadherin at cell–cell contacts.
α- and β-Catenins might indirectly tether VE-cadherin to bundled actin. α-catenin is known to interact with various actin binding proteins including vinculin, α-actinin, spectrin, zonula occludins-1, and afadin (Kobielak and Fuchs, 2004). Therefore, these proteins may mediate linkage of the cadherin–catenin complex to actin cytoskeleton. Consistently, Abe and Takeichi (2007) have recently reported that EPLIN binds α-catenin and actin filaments simultaneously, thereby linking the cadherin–catenin complex to the actin cytoskeleton. Because EPLIN is expressed not only in epithelial cells but also in endothelial cells (our unpublished data), VE-cadherin–β-catenin–α-catenin complex may be anchored to circumferential actin bundles through EPLIN.
We noticed that homophilic VE-cadherin–based cell–cell adhesion is not the trigger for promoting circumferential actin bundling in cAMP–Epac–Rap1 signal-activated cells. It has been reported that α-catenin directly regulates actin dynamics instead of simply linking the cadherin–β-catenin complex to actin cytoskeleton (Kobielak and Fuchs, 2004; Mege et al., 2006; Weis and Nelson, 2006). The Weis and Nelson groups have shown that α-catenin homodimer competes with the Arp2/3 complex for binding to actin filaments, thereby inhibiting branching nucleation of actin filaments, instead promoting formation of linear actin bundles (Drees et al., 2005; Gates and Peifer, 2005). EPLIN also acts not only in linking the cadherin–catenin complex to actin bundles but also in actively stabilizing these bundles (Abe and Takeichi, 2007). Furthermore, it has been reported that α-catenin locates several actin regulators such as formin-1, vinculin and Ena/VASP to the nascent AJs, thereby inducing formation of radial actin cables (Watabe-Uchida et al., 1998; Weiss et al., 1998; Vasioukhin et al., 2001; Kobielak and Fuchs, 2004; Kobielak et al., 2004). These results indicate that cadherin-based cell–cell adhesions coordinate actin dynamics at cell–cell junctions through α-catenin. Consistently, we found that VE-cadherin–based cell–cell adhesions are responsible for formation of central stress fibers in unstimulated cells. However, FSK-induced circumferential actin bundling was not inhibited by the depletion of either VE-cadherin, β-catenin, α-catenin, or p120-catenin or by disruption of cadherin-dependent cell adhesions by extracellular Ca2+ chelation. This result is consistent with the previous report that VE-cadherin–specific blocking antibody does not affect 007-induced actin remodeling in endothelial cells (Kooistra et al., 2005). Thus, these findings demonstrate that circumferential actin bundling induced by cAMP signal does not require VE-cadherin–based cell–cell adhesions.
How does cAMP-Epac-Rap1 signal induce reorganization of actin cytoskeleton at endothelial cell–cell contacts? It has been reported that Rap1 promotes cell spreading by localizing a subset of Rac guanine nucleotide exchange factors such as Vav2 and Tiam1 to sites of active lamellipodia extension (Arthur et al., 2004). Consistently, Rac has been shown to be involved in cAMP-induced endothelial barrier function (Birukova et al., 2007; Baumer et al., 2008). Thus, cAMP-induced Rap1 activation at cell–cell contacts may lead to the local activation of Rac, thereby inducing circumferential actin bundling. Indeed, our previous report revealed that Rap1 is activated at endothelial cell–cell contacts (Sakurai et al., 2006). In addition, other Rap1 effectors such as K-Rev Interaction Trapped gene-1 and AF-6 may also be involved in cAMP-induced actin bundling (Boettner et al., 2000, 2003; Glading et al., 2007). Furthermore, it still remains unknown how cAMP signal locally regulates actin dynamics at cell–cell junctions. Because VE-cadherin–based cell–cell adhesions are dispensable for cAMP-induced actin bundling, other cell adhesion molecules may be involved in local regulation of actin dynamics. One of the candidates is nectin that is involved in formation of AJs in epithelial cells (Takai et al., 2008), although its function in endothelial cells is still unknown. Thus, further studies are required to elucidate the molecular mechanisms by which a cAMP–Epac–Rap1 pathway induces circumferential actin bundling.
Intracellular cAMP activates two downstream effectors Epac and PKA, both of which are known to enhance endothelial barrier functions. In this study, we clearly indicate that PKA activation by 6-Bnz does not induce circumferential actin bundling and does not enhance stability of VE-cadherin at cell–cell contacts. Recently, Lorenowicz et al. (2008) have reported that Epac promotes the formation of cortical actin bundles, whereas PKA induces formation of stress fibers, although activation of either cAMP effector decreases endothelial permeability. Therefore, Epac and PKA may regulate endothelial barrier function through distinct signaling mechanisms.
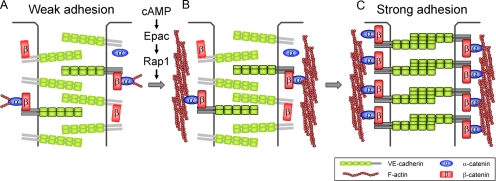
In conclusion, we have demonstrated that circumferential actin bundle formation and subsequent linkage between actin bundles and VE-cadherin through α- and β-catenins are responsible for the stabilization of VE-cadherin at the cell–cell contacts in cAMP–Epac-Rap1 signal-activated cells (Figure 8). These findings strongly support the classical model that α-catenin is required for statically linking cadherin–β-catenin complexes to bundled actin filaments to maintain AJs.
Figure 8.
Schematic representation of our proposed model that accounts for how VE-cadherin is stabilized at the cell–cell contacts in cAMP–Epac–Rap1 signal-activated endothelial cells. (A) In unstimulated endothelial cells, the majority of VE-cadherin molecules (∼75%) is mobile at cell–cell junctions, possibly due to the lack of circumferential actin bundles. VE-cadherin that does not associate with actin cytoskeleton has weak cell–cell adhesion activity. (B) When cAMP-Epac-Rap1 signal is activated, circumferential actin bundling occurs independently of VE-cadherin-based cell–cell adhesions. (C) Subsequently, α- and β-catenins link VE-cadherin to the bundled actin filaments, thereby stabilizing VE-cadherin at cell–cell contacts. Stabilization of VE-cadherin on the bundled actin filaments results in strong cell–cell adhesions. VE-cadherin that associates with bundled actin filaments through α- and β-catenins is outlined. α- and β-Catenins which link VE-cadherin to the bundled actin filaments are also outlined.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to A. Nagafuchi (Kumamoto University) for the expression plasmid encoding α-catenin and helpful advice; to S. Hattori (University of Tokyo) and M. Matsuda (Kyoto University) for adenoviruses; to K. Matsuo, M. Sone, M. Minamimoto, and Y. Matsuura for technical assistance; and to J. T. Pearson for critical reading of the manuscript. This work was supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan (to S. F. and N. M.); the Ministry of Health, Labor, and Welfare of Japan (to N. M.); the Program for the Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (to S. F. and N. M.); the Naito Foundation (to S. F.); Takeda Science Foundation (to S. F. and N. M.); the Sagawa Foundation for Promotion of Cancer Research (to S. F.); Mochida Memorial Foundation for Medical and Pharmaceutical Research (to S. F.); Kowa Life Science Foundation (to S. F.); Kanae Foundation for the Promotion of Medical Science (to S. F.); The Novartis Foundation (Japan) for the Promotion of Science (to S. F.); Senri Life Science Foundation (to S. F.); the Mitsubishi Foundation (to N. M.); and AstraZeneca (to N. M.).
Abbreviations used:
- 007
8-pCPT-2′-O-methl-cAMP
- 6-Bnz
N6-benzoyl-cAMP
- AJ
adherens junction
- CREB
cAMP response element-binding protein
- E-cadherin-GFP
green fluorescent protein-tagged E-cadherin
- Epac
exchange protein directly activated by cAMP
- EPLIN
epithelial protein lost in neoplasm
- FRAP
fluorescence recovery after photobleaching
- FSK
forskolin
- GFP
green fluorescent protein
- HUVEC
human umbilical vein endothelial cell
- Lat.A
latrunculin A
- PECAM
platelet/endothelial cell adhesion molecule
- PECAM1-GFP
platelet/endothelial cell adhesion molecule 1 carboxy-terminally tagged with green fluorescent protein
- PKA
protein kinase A
- VE
vascular endothelial
- VEC-GFP
vascular endothelial-cadherin carboxy-terminally fused with green fluorescent protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-07-0580) on December 23, 2009.
REFERENCES
- Abe K., Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc. Natl. Acad. Sci. USA. 2007;105:13–19. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson R. H., Ly J. C., Sarai R. K., Lenz J. F., Altangerel A., Drenckhahn D., Curry F. E. Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1188–H1196. doi: 10.1152/ajpheart.00937.2007. [DOI] [PubMed] [Google Scholar]
- Andriopoulou P., Navarro P., Zanetti A., Lampugnani M. G., Dejana E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler. Thromb. Vasc. Biol. 1999;19:2286–2297. doi: 10.1161/01.atv.19.10.2286. [DOI] [PubMed] [Google Scholar]
- Arthur W. T., Quilliam L. A., Cooper J. A. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J. Cell Biol. 2004;167:111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin H. G., Young K. G., Thurston G., Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Baumer Y., Drenckhahn D., Waschke J. cAMP induced Rac 1-mediated cytoskeletal reorganization in microvascular endothelium. Histochem. Cell Biol. 2008;129:765–778. doi: 10.1007/s00418-008-0422-y. [DOI] [PubMed] [Google Scholar]
- Birukova A. A., Liu F., Garcia J. G., Verin A. D. Protein kinase A attenuates endothelial cell barrier dysfunction induced by microtubule disassembly. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L86–L93. doi: 10.1152/ajplung.00441.2003. [DOI] [PubMed] [Google Scholar]
- Birukova A. A., Zagranichnaya T., Fu P., Alekseeva E., Chen W., Jacobson J. R., Birukov K. G. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp. Cell Res. 2007;313:2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner B., Govek E. E., Cross J., Van Aelst L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc. Natl. Acad. Sci. USA. 2000;97:9064–9069. doi: 10.1073/pnas.97.16.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner B., Harjes P., Ishimaru S., Heke M., Fan H. Q., Qin Y., Van Aelst L., Gaul U. The AF-6 homolog canoe acts as a Rap1 effector during dorsal closure of the Drosophila embryo. Genetics. 2003;165:159–169. doi: 10.1093/genetics/165.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullere X., Shaw S. K., Andersson L., Hirahashi J., Luscinskas F. W., Mayadas T. N. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- Dejana E. Endothelial cell-cell junctions: happy together. Nat. Rev. Mol. Cell. Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- Dejana E., Tournier-Lasserve E., Weinstein B. M. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev. Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Dejana E., Orsenigo F., Lampugnani M. G. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- Drees F., Pokutta S., Yamada S., Nelson W. J., Weis W. I. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K., Suzuki A., Ohno S., Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J. Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- Farmer P. J., Bernier S. G., Lepage A., Guillemette G., Regoli D., Sirois P. Permeability of endothelial monolayers to albumin is increased by bradykinin and inhibited by prostaglandins. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L732–L738. doi: 10.1152/ajplung.2001.280.4.L732. [DOI] [PubMed] [Google Scholar]
- Fukuhara S., Sako K., Minami T., Noda K., Kim H. Z., Kodama T., Shibuya M., Takakura N., Koh G. Y., Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat. Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- Fukuhara S., Sako K., Noda K., Nagao K., Miura K., Mochizuki N. Tie2 is tied at the cell-cell contacts and to extracellular matrix by angiopoietin-1. Exp. Mol. Med. 2009;41:133–139. doi: 10.3858/emm.2009.41.3.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S., Sakurai A., Sano H., Yamagishi A., Somekawa S., Takakura N., Saito Y., Kangawa K., Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol. Cell. Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S., Sakurai A., Yamagishi A., Sako K., Mochizuki N. Vascular endothelial cadherin-mediated cell-cell adhesion regulated by a small GTPase, Rap1. J. Biochem. Mol. Biol. 2006;39:132–139. doi: 10.5483/bmbrep.2006.39.2.132. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Drew J., Trezise L., Underwood A., Parsons M., Kasminkas L., Rudge J., Yancopoulos G., Vadas M. A. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ. Res. 2000;87:603–607. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- Garcia J. G., Liu F., Verin A. D., Birukova A., Dechert M. A., Gerthoffer W. T., Bamberg J. R., English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates J., Peifer M. Can 1000 reviews be wrong? Actin, alpha-catenin, and adherens junctions. Cell. 2005;123:769–772. doi: 10.1016/j.cell.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Gavard J. Breaking the VE-cadherin bonds. FEBS Lett. 2009;583:1–6. doi: 10.1016/j.febslet.2008.11.032. [DOI] [PubMed] [Google Scholar]
- Gavard J., Gutkind J. S. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- Glading A., Han J., Stockton R. A., Ginsberg M. H. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J. Cell Biol. 2007;179:247–254. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenstiel S., Witzenrath M., Schmeck B., Hocke A., Krisp M., Krull M., Seybold J., Seeger W., Rascher W., Schutte H., Suttorp N. Adrenomedullin reduces endothelial hyperpermeability. Circ. Res. 2002;91:618–625. doi: 10.1161/01.res.0000036603.61868.f9. [DOI] [PubMed] [Google Scholar]
- Hogan C., Serpente N., Cogram P., Hosking C. R., Bialucha C. U., Feller S. M., Braga V.M.M., Birchmeier W., Fujita Y. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol. Cell. Biol. 2004;24:6690–6700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y., Itoh M., Maeno Y., Tsukita S., Nagafuchi A. Functional domains of α-catenin required for the strong state of cadherin-based cell adhesion. J. Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Kobielak A., Pasolli H. A., Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat. Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A., Fuchs E. α-Catenin: at the junction of intercellular adhesion and actin dynamics. Nat. Rev. Mol. Cell Biol. 2004;5:614–625. doi: 10.1038/nrm1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra M. R., Corada M., Dejana E., Bos J. L. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- Kooistra M. R., Dube N., Bos J. L. Rap 1, a key regulator in cell-cell junction formation. J. Cell Sci. 2007;120:17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- Langeler E. G., van Hinsbergh V. W. Norepinephrine and iloprost improve barrier function of human endothelial cell monolayers: role of cAMP. Am. J. Physiol. 1991;260:C1052–C1059. doi: 10.1152/ajpcell.1991.260.5.C1052. [DOI] [PubMed] [Google Scholar]
- Liu F., Verin A. D., Borbiev T., Garcia J. G. Role of cAMP-dependent protein kinase A activity in endothelial cell cytoskeleton rearrangement. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L1309–L1317. doi: 10.1152/ajplung.2001.280.6.L1309. [DOI] [PubMed] [Google Scholar]
- Lorenowicz M. J., Fernandez-Borja M., Kooistra M. R., Bos J. L., Hordijk P. L. PKA and Epac1 regulate endothelial integrity and migration through parallel and independent pathways. Eur. J. Cell Biol. 2008;87:779–792. doi: 10.1016/j.ejcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Mege R. M., Gavard J., Lambert M. Regulation of cell-cell junctions by the cytoskeleton. Curr. Opin. Cell Biol. 2006;18:541–548. doi: 10.1016/j.ceb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Ishihara S., Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-alpha catenin fusion molecules. J. Cell Biol. 1994;127:235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Kemler R. Molecular organization of the uvomorulin-catenin complex. J. Cell Biol. 1992;116:989–996. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek W. J., Kooistra M. R., Zwartkruis F. J., Bos J. L. Cell-cell junction formation: the role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim. Biophys. Acta. 2009;1788:790–796. doi: 10.1016/j.bbamem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Paul R., Zhang Z. G., Eliceiri B. P., Jiang Q., Boccia A. D., Zhang R. L., Chopp M., Cheresh D. A. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat. Med. 2001;7:222–227. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- Price L. S., Hajdo-Milasinovic A., Zhao J., Zwartkruis F. J., Collard J. G., Bos J. L. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J. Biol. Chem. 2004;279:35127–35132. doi: 10.1074/jbc.M404917200. [DOI] [PubMed] [Google Scholar]
- Qiao J., Huang F., Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284:L972–L980. doi: 10.1152/ajplung.00429.2002. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Hyatt J. L. Establishment of the circumferential actin filament network is a prerequisite for localization of the cadherin-catenin complex in epithelial cells. Cell Growth Differ. 1999;10:839–854. [PubMed] [Google Scholar]
- Reynolds A. B. p120-catenin: past and present. Biochim. Biophys. Acta. 2007;1773:2–7. doi: 10.1016/j.bbamcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. B., Daniel J., McCrea P. D., Wheelock M. J., Wu J., Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol. Cell. Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm D. L., Koslov E. R., Kebriaei P., Cianci C. D., Morrow J. S. α1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc. Natl. Acad. Sci. USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako Y., Nagafuchi A., Tsukita S., Takeichi M., Kusumi A. Cytoplasmic regulation of the movement of E-cadherin on the free cell surface as studied by optical tweezers and single particle tracking: corralling and tethering by the membrane skeleton. J. Cell Biol. 1998;140:1227–1240. doi: 10.1083/jcb.140.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A., Fukuhara S., Yamagishi A., Sako K., Kamioka Y., Masuda M., Nakaoka Y., Mochizuki N. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol. Biol. Cell. 2006;17:966–976. doi: 10.1091/mbc.E05-07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector I., Shochet N. R., Blasberger D., Kashman Y. Latrunculins–novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil. Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- Takai Y., Miyoshi J., Ikeda W., Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat. Rev. Mol. Cell Biol. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- Thurston G., Suri C., Smith K., McClain J., Sato T. N., Yancopoulos G. D., McDonald D. M. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V., Bauer C., Degenstein L., Wise B., Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of α-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Winderlich M., Cagna G., Nottebaum A. F. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2009;19:8–15. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Wallez Y., Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M., Uchida N., Imamura Y., Nagafuchi A., Fujimoto K., Uemura T., Vermeulen S., van Roy F., Adamson E. D., Takeichi M. α-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J. Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S., Cui J., Barnes L., Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis W. I., Nelson W. J. Re-solving the cadherin-catenin-actin conundrum. J. Biol. Chem. 2006;281:35593–35597. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. E., Kroemker M., Rudiger A. H., Jockusch B. M., Rudiger M. Vinculin is part of the cadherin-catenin junctional complex: complex formation between α-catenin and vinculin. J. Cell Biol. 1998;141:755–764. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin A., Voisin M. B., Nourshargh S. PECAM-1, a multi-functional molecule in inflammation and vascular biology. Arterioscler. Thromb. Vasc. Biol. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- Yamada S., Pokutta S., Drees F., Weis W. I., Nelson W. J. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S. Y. Protein kinase signaling in the modulation of microvascular permeability. Vasc. Pharmacol. 2002;39:213–223. doi: 10.1016/s1537-1891(03)00010-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.