Npap60 (Nup50) is a nucleoporin that binds directly to importin α. In humans, there are two Npap60 isoforms: the long (Npap60L) and short (Npap60S) forms. Our results demonstrate that Npap60S stabilizes the binding of importin α to classical NLS-cargo, whereas Npap60L promotes the release of NLS-cargo from importin α.
Abstract
Npap60 (Nup50) is a nucleoporin that binds directly to importin α. In humans, there are two Npap60 isoforms: the long (Npap60L) and short (Npap60S) forms. In this study, we provide both in vitro and in vivo evidence that Npap60L and Npap60S function differently in nuclear protein import. In vitro binding assays revealed that Npap60S stabilizes the binding of importin α to classical NLS-cargo, whereas Npap60L promotes the release of NLS-cargo from importin α. In vivo time-lapse experiments showed that when the Npap60 protein level is controlled, allowing CAS to efficiently promote the dissociation of the Npap60/importin α complex, Npap60S and Npap60L suppress and accelerate the nuclear import of NLS-cargo, respectively. These results demonstrate that Npap60L and Npap60S have opposing functions and suggest that Npap60L and Npap60S levels must be carefully controlled for efficient nuclear import of classical NLS-cargo in humans. This study provides novel evidence that nucleoporin expression levels regulate nuclear import efficiency.
INTRODUCTION
In eukaryotic cells, nuclear proteins containing a classical nuclear localization signal (NLS) are recognized by importin (karyopherin) α (Chook and Blobel, 2001; Goldfarb et al., 2004). Subsequently, importin (karyopherin) β binds to importin α, forming a ternary complex. This complex translocates from the cytoplasm into the nucleus through the nuclear pore complex (NPC). In the nucleus, a small GTPase, Ran, which exists predominantly in a GTP-bound form (RanGTP), binds to importin β, thereby releasing importin β from the import complex. After releasing the NLS-cargo, importin α is exported into the cytoplasm by the cellular apoptosis susceptibility gene product, CAS (also referred to as exportin 2), in conjunction with RanGTP (Kutay et al., 1997; Künzler and Hurt, 1998; Solsbacher et al., 1998). Although this fundamental mechanism of nuclear protein import is generally understood (Pemberton and Paschal, 2005; Sorokin et al., 2007; Stewart, 2007; Terry et al., 2007), the regulatory mechanisms that govern both the import pathway and machinery remain unknown.
The NPC is a supramolecular complex that mediates trafficking between the cytoplasm and nucleus. In mammalian cells, NPCs are composed of multiple copies of ∼30 different proteins termed nucleoporins (Cronshaw et al., 2002). Most nucleoporins contain multiple phenylalanine-glycine (FG) repeats. Interactions between the FG repeats of nucleoporins and importin β are essential for the nuclear import of classical NLS-cargo (Bayliss et al., 2000; Strawn et al., 2004; Zeitler and Weis, 2004). In contrast, the interaction between nucleoporin and importin α is thought to be less important for nuclear protein import. However, recently it has been revealed that the interaction between importin α and Npap60 (also referred to as Nup50), which is an FG repeat-containing nucleoporin, plays an important role in nuclear import, as described below. Npap60 is a mobile nucleoporin (Rabut et al., 2004; Tran and Wente, 2006) that is localized primarily in the nuclear basket of the NPC and in the nucleoplasm (Guan et al., 2000; Smitherman et al., 2000).
Using a semi-intact cell transport assay, Lindsay et al. first showed that Npap60 promotes nuclear import of classical NLS-cargo (Lindsay et al., 2002; Moore, 2003). In addition, Matsuura and Stewart (2005) demonstrated that the N-terminal region of Npap60 contains two importin α-binding segments, termed BS1 and BS2, both of which are involved in releasing NLS-cargo from importin α (see Figure 1A). In their model, the binding of BS2 to the C-terminus of importin α provides a scaffold that promotes the binding of BS1 to the NLS-binding domain of importin α. When BS1 binds to importin α, the NLS-cargo is released. Then, CAS binds to the C-terminus of importin α, in conjunction with RanGTP, to release Npap60 from importin α and transport importin α back to the cytoplasm. However, Sun et al. (2008) used single-molecule observations in semi-intact cells to demonstrate that Npap60 alone is insufficient to dissociate importin α from the cargo complex. Thus, although the function of Npap60 has been analyzed in vitro, its functional role in vivo is still unknown.
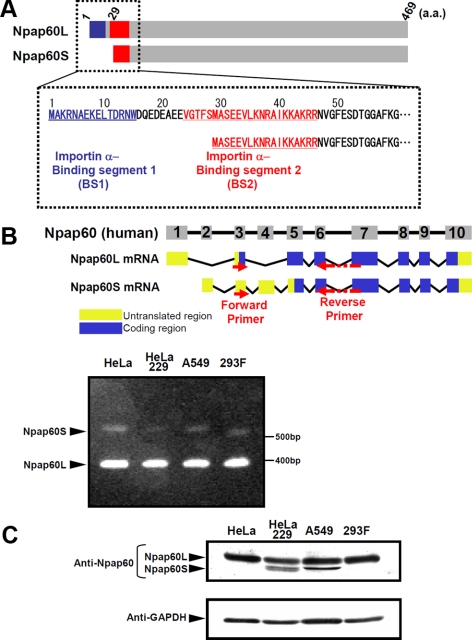
Figure 1.
Expression of the Npap60 isoforms. (A) Diagrams of the Npap60 isoforms. The importin α-binding segments 1 (BS1; 1-15 aa; blue) and 2 (BS2; 23-46 aa; red) of Npap60L are indicated. BS1 is thought to be involved in releasing NLS-cargo from importin α, whereas BS2 is believed to function as a scaffold (Matsuura et al., 2005). Npap60S lacks 1-28 aa of Npap60L, which includes BS1. (B) Diagrams of the genomic structure and mRNAs of Npap60L (NM_007172,3) and Npap60S (NM_153645,2). Numbered boxes indicate the exons. The human Npap60 gene is composed of 10 exons. Yellow boxes and blue boxes indicate the untranslated regions and coding regions, respectively. Arrows indicate the locations of the RT-PCR primers. Total RNA (100 ng) from HeLa, HeLa229, A549, and 293F cells was used as a template. Bands of ∼350 and 540 base pairs corresponding to Npap60L mRNA and Npap60S mRNA, respectively, were detected in all cell lines. (C) Protein expression of the endogenous Npap60 isoforms in cultured human cells. Npap60 proteins in lysates from HeLa, HeLa229, A549, and 293F cells were examined by Western blot analysis with an anti-Npap60 antibody (1:1000). The total amount of protein was normalized to GAPDH expression in each sample. All samples were visualized with alkaline phosphatase–conjugated secondary antibodies (1:1000).
Humans reportedly have two isoforms of Npap60, Npap60L (1-469 aa), and its alternatively spliced isoform, Npap60S (29-469 aa of Npap60L; Trichet et al., 1999; Patre et al., 2006; Figure 1A). Npap60L is widely expressed from vertebrates to yeast. Nup2p, which is the yeast counterpart of human Npap60L, releases NLS-cargo from Srp1p (yeast importin α; Gilchrist et al., 2002; Matsuura et al., 2003; Liu and Stewart, 2005) and is important for the efficient nuclear import of NLS-cargo and export of Srp1p (Solsbacher et al., 2000; Dilworth et al., 2001; Gilchrist and Rexach, 2003).
In contrast, Npap60S has been found only in humans. Npap60S lacks the BS1 portion of Npap60L, which is involved in releasing NLS-cargo from importin α, suggesting that Npap60S and Npap60L have different functions in nuclear protein import. However, if or how Npap60S acts on importin α to affect nuclear protein import has not been examined in vitro or in vivo.
MATERIALS AND METHODS
Expression and Purification of Recombinant Proteins
Glutathione S-transferase (GST)-importin α, GST-IBB (1-69 aa), GST-ΔIBB-importin α (70-529 aa), GST-Npap60LN (1-207 aa), GST-Npap60SN (29-207 aa of Npap60L), and GST-CAS were cloned into the pGEX-6P-1 vector. GST-NLS-green fluorescent protein (GFP) was cloned into the pGEX-2T vector. Transformation of expression vectors, protein expression, bacterial cell lysis, and protein purification were performed as described previously (Imamoto et al., 1995; Miyamoto et al., 2002). GTP-bound Q69LRan was purified as described previously (Sekimoto et al., 1996).
Plasmids
pmRFP-N3-Npap60L, pmRFP-N3-Npap60S, and pmRFP-N3-Npap60ΔN were constructed by inserting full-length Npap60L, full-length Npap60S, or an Npap60L N-terminal deletion mutant (47-469 aa), Npap60ΔN, into the pmRFP-N3 vector. Then, pmRFP-N3-Npap60L and pmRFP-N3-Npap60S were further modified by introducing silent mutations in the siRNA 60-2 targeting sequence: 5′-GGAGGACGCTTTTCTGGAT-3′→ 5′-GGTGGTCGTTTCTCAGGTT-3′. Likewise, pIRES-EGFP2-Npap60L, pIRES-EGFP2-Npap60S, and pIRES-EGFP2-Npap60ΔN were constructed by inserting the above coding regions into the pIRES EGFP2 vector (Clontech, Palo Alto, CA). pcDNA 4/HisMax C-CAS was constructed by inserting full-length CAS into the pcDNA 4/HisMax C vector (Invitrogen, Carlsbad, CA).
Small Interfering RNA
The following small interfering RNA (siRNA) duplexes were purchased from NIPPON EGT: siRNA 60-1 (5′-CCACCUUGGUUGAUAAAGUTT-3′), siRNA 60-2 (5′-GGAGGACGCUUUUCUGGAUTT-3′), and siRNA DsRed, as a negative control (5′-CACCGUGAAGCUGAAGGUGTT-3′).
RT-PCR
Total RNA was extracted from cell lines using TRIzol Reagent (Invitrogen) following the manufacturer's protocol. Total RNA of normal human tissues was purchased from Ambion (Austin, TX; Cat. no. AM6000). RT-PCR was performed using a forward primer that binds to exon 3 (5′-ATAGGATCCACATGGCCAAAAGAAATGCC-3′) and a reverse primer that spans exons 6 and 7 (5′-GATAAGCTTGCAGCAAGAGAACCAAAGGCTA-3′) of Npap60 using SuperScript III one-step RT-PCR with Platinum Taq DNA Polymerase kit (12574-018; Invitrogen).
Antibodies
Rabbit anti-human Npap60 antiserum was produced against recombinant full-length human Npap60S. Goat anti-karyopherin α2 (C-20; Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-GFP (M048-3; MBL International, San Diego, CA), mouse anti-GST (Z-5; Santa Cruz Biotechnology), mouse mAb414 (MMS-120P; Covance, Madison, WI), mouse anti-karyopherin α/Rch-1 (610485; BD Biosciences, San Diego, CA), and sheep anti-Nup153 (NBP 1-00620; Novus Biologicals, Littleton, CO; Smythe et al., 2000) antibodies were purchased commercially.
Preparation of Whole-Cell Extracts
To prepare whole-cell protein extracts, the cells were harvested, washed with PBS, resuspended in lysis buffer (50 mM Tris/HCl, pH 8.0, 0.5% SDS, 1 mM DTT), and heated at 95°C for 10 min. Then, the samples were centrifuged at 20,000 × g for 20 min.
Transfection
Plasmids were transfected into cells as described previously (Miyamoto et al., 2004). siRNA transfection was performed using RNAi MAX (Invitrogen) according to the manufacturer's protocol for reverse transfection.
Binding Assay
The proteins were added to transport buffer (20 mM HEPES/NaOH, pH 7.3, 110 mM potassium acetate, 2 mM magnesium acetate, 5 mM sodium acetate, 0.5 mM EGTA/NaOH, pH 7.3, 2 mM DTT) containing 1% BSA and 0.1% Tween 20, and mixed with glutathione Sepharose beads. The mixtures were incubated at 4°C for 1 h and then washed with transport buffer containing 0.1% Tween 20.
Permeabilization of HeLa Cells
After washing with ice-cold transport buffer, HeLa cells were incubated with 40 μg/ml digitonin in transport buffer on ice for 5 min. After washing with transport buffer, the cells were incubated for 10 min with ice-cold transport buffer and then fixed with 3.7% formaldehyde in PBS.
Cell Culture
HeLa, HeLa229, A549, and 293F cells were cultured in Dulbecco's modified MEM (Sigma-Aldrich, St. Louis, MO) supplemented with 10% heat-inactivated FBS at 37°C in a 10% CO2 atmosphere.
Microscopy
All imaging was performed using a LSM510 microscope (Carl Zeiss, Thornwood, NY). Indirect immunofluorescence was performed as described previously (Miyamoto et al., 2002) using a 63 × 1.4 NA Plan Apochromat objective (Carl Zeiss) at room temperature. The nuclear-to-total fluorescence ratio of endogenous importin α was calculated using the image analysis software associated with the LSM510 microscope. Time-lapse analysis was performed as follows. The media was replaced with CO2-independent DMEM (21063; Invitrogen). The temperature of the stage was maintained at 37°C using a heater. From 1 min after microinjection, images were collected at 10-s intervals using a 40 × 0.6 NA Long Distance Achroplan objective (Carl Zeiss). Import efficiency was analyzed using the image analysis software associated with the LSM510 microscope.
Estimation of Dissociation Constants
Glutathione Sepharose beads were incubated with 1 μM GST-SV40T NLS and various concentrations of importin α in the absence or presence of either 3 μM Npap60LN or Npap60SN at 4°C for 1 h. The amount of importin α bound to GST-SV40T NLS was detected by Coomassie Brilliant Blue (Supplemental Figure S2), and the dissociation constants were estimated.
RESULTS
Both Npap60S and Npap60L Are Expressed in Human Cells
Previous reports showed that human Npap60 mRNA has multiple splice-variants (Trichet et al., 1999), yielding several Npap60 isoforms at the protein level (Patre et al., 2006). According to databases, the human Npap60 gene consists of 10 exons and produces two alternatively-spliced mRNA isoforms. The Npap60L mRNA contains exons 1, 3, and 5–10, and the Npap60S mRNA consists of exons 2–10 (Figure 1B). To confirm that these two Npap60 isoforms are expressed in human cells, RT-PCR analysis was performed on total RNA isolated from a variety of human cell lines, such as HeLa, HeLa229, A549, and 293F cells. The primers used in this study allowed both of the Npap60 isoforms to be simultaneously PCR amplified. As shown in Figure 1B, bands of ∼350 and 540 base pairs were detected in all of the examined cell lines. DNA sequencing confirmed that these PCR products corresponded to Npap60L and Npap60S, respectively. We further examined the expression of the Npap60 isoforms in human tissues and found that both isoforms were expressed in the brain, liver, lung, kidney, and testis (Supplemental Figure S1). These results indicate that the mRNA of both isoforms is expressed in human cells. Like Npap60L mRNA, Npap60S mRNA contains an AUG start codon in exon 3. Therefore, cap-dependent translation of Npap60S mRNA could start at the AUG codon in exon 3. However, exon 4 has a very short open reading frame and translation cannot proceed. In this study, we could not confirm whether translation of the Npap60S mRNA initiates only in exon 5, as is predicted in databases.
Npap60S protein expression was originally detected in HeLa229 cells, a subline of HeLa cells (Patre et al., 2006). To examine Npap60S and Npap60L protein expression in other cell lines, whole-cell lysates from four cell lines, including HeLa229, were examined by Western blotting using an anti-Npap60 antibody (Figure 1C). Npap60L was detected in the lysates of all cell lines, whereas Npap60S was detected only in HeLa229 and A549 cell lysates. Thus, the Npap60S protein is expressed in some human cell lines, despite the fact that Npap60S mRNA is expressed in all of the examined cell lines. However, the reason the Npap60S protein was not detected in all cell lines is still unknown.
Npap60S Stabilizes the Binding of Importin α to a Classical NLS In Vitro
Because the two Npap60 isoforms differ only in their N-terminal region, the role of Npap60S was examined using recombinant proteins of the N-terminal region of these isoforms, i.e., Npap60LN (1-207 aa) and Npap60SN (29-207 aa of Npap60L). We then examined the effects of these recombinant N-terminal regions on the binding of importin α to the SV40 T antigen-NLS. As shown in Figure 2A, Npap60LN strongly inhibited the binding of importin α to GST-NLS-GFP, which is consistent with the results reported by Matsuura et al. (2005). The dissociation constant of the importin α/GST-NLS-GFP complex in the absence or presence of Npap60LN was ∼1.1 and 3.7 μM, respectively (Supplemental Table S1 and Supplemental Figure S2). In contrast, we found that the binding of importin α to GST-NLS-GFP was significantly increased in the presence of Npap60SN (Figure 2B), with a dissociation constant of ∼2.8 nM (Supplemental Table S1 and Supplemental Figure S2). These results indicate that Npap60S stabilizes the importin α/NLS-cargo complex, whereas Npap60L destabilizes the interaction between importin α and NLS-cargo. Consistent with the model proposed by Matsuura et al. (2005), it is likely that Npap60S forms a complex with importin α/NLS-cargo via its BS2 domain, but cannot release the NLS-cargo from importin α because Npap60S lacks a BS1 domain.
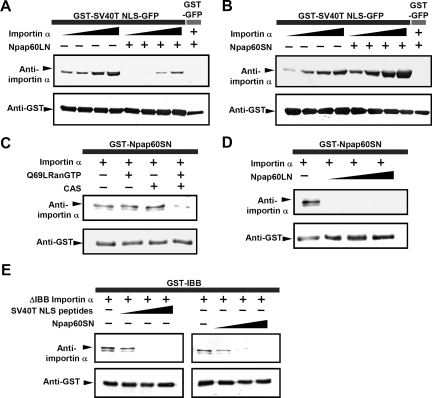
Figure 2.
The Npap60 isoforms function differently in the binding between importin α and NLS-cargo. (A) Importin α alone at concentrations of 50, 100, 200 or 400 nM or in combination with 1 μM of Npap60LN was incubated with 1 μM of GST-NLS-GFP immobilized on glutathione beads, and the bound proteins were analyzed by Western blotting. (B) Importin α alone at concentrations of 50, 100, 200 or 400 nM or in combination with 1 μM Npap60SN was incubated with 1 μM immobilized GST-NLS-GFP. (C) A mixture of 50 nM of importin α and either 2 μM Q69LRan or 1 μM of CAS, or both was incubated with 500 nM of immobilized GST-Npap60SN. (D) A mixture of 50 nM importin α alone or in combination with 1, 2, or 4 μM Npap60LN was incubated with 1 μM of immobilized GST-Npap60SN. (E) ΔIBB-importin α (250 nM) and either the NLS peptide or Npap60SN were incubated with 1 μM immobilized GST-IBB. GST-fusion proteins, GFP-fusion proteins, and importin α mutants were detected using anti-GST (1:1000), anti-GFP (1:1000), and anti-karyopherin α2 (1:1000) antibodies, respectively. All samples were visualized with alkaline phosphatase–conjugated secondary antibodies (1:1000).
Next, we examined the mechanism by which Npap60S stabilizes the importin α/NLS-cargo complex. Previous studies have shown that after RanGTP releases importin β from importin α, the internal NLS-like sequence in the N-terminal importin β-binding (IBB) domain of importin α partially releases the NLS-cargo by directly binding to its own NLS-binding domain (i.e., auto-inhibition; Kobe, 1999). Therefore, we speculated that Npap60S prevents this autoinhibition of importin α by binding to the C-terminal region of importin α via the BS2 domain, thereby suppressing the release of NLS-cargo from the NLS-binding domain of importin α. As shown in Figure 2E, we confirmed that the NLS peptides competitively inhibited GST-IBB (1–69 aa of importin α) from binding to ΔIBB-importin α (70–529 aa of importin α), indicating that GST-IBB was correctly bound to the NLS-binding domain of ΔIBB-importin α. Under these conditions, Npap60SN inhibited the binding of GST-IBB to ΔIBB-importin α (Figure 2E), although GST-NLS-GFP efficiently bound to importin α in the presence of Npap60SN (Figure 2B). Collectively, we concluded that Npap60S causes steric hindrance to the interaction between the IBB domain and NLS binding domain, leading to the stabilization of the importin α/NLS-cargo complex.
Next, to determine what causes the dissociation of the Npap60S/importin α/NLS-cargo complex, we focused on Npap60L and CAS, which bind to the C-terminal domain of importin α. It is likely that Npap60S binds to importin α via its BS2 portion, and may compete with the BS2 portion of Npap60L. Therefore, to determine if either CAS or Npap60L releases importin α from Npap60S, GST-Npap60SN, and importin α were coincubated in the presence of CAS together with GTP-bound Q69LRan (where Gln69 is replaced by Leu), a GTPase-deficient Ran mutant, or Npap60LN. The binding of importin α to Npap60SN was significantly reduced in the presence of CAS/Q69LRanGTP (Figure 2C) or Npap60LN (Figure 2D). These results indicate that either CAS or Npap60L can lead to the disassembly of the Npap60S/importin α/NLS-cargo complex.
Overexpression of Both Isoforms Affects Intracellular Localization of Importin α
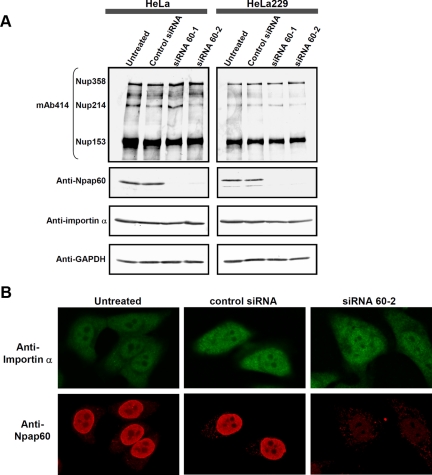
To determine if siRNA-mediated knockdown of the Npap60 isoforms (siRNA) affects the localization of endogenous importin α, we prepared two siRNAs targeting different sites in the region where the Npap60S and Npap60L mRNAs overlap and designated these as siRNA 60-1 and siRNA 60-2. To confirm knockdown of the Npap60 isoforms and examine the effects on the expression of importin α and several nucleoporins, whole-cell lysates from cells transfected with either a control siRNA, siRNA 60-1, or siRNA 60-2 were analyzed by Western blotting. As shown in Figure 3A, expression of the Npap60 isoforms was efficiently decreased in both HeLa and HeLa229 cells. Furthermore, the expression of endogenous importin α, and several nucleoporins including Nup358, Nup214, and Nup153, which are major NPC components, did not change. Under these assay conditions, we examined the localization of endogenous importin α and the Npap60 isoforms using indirect immunofluorescence. In untransfected cells, endogenous Npap60 localized mainly to the nucleus and partially at the nuclear rim (Figure 3B), which is consistent with a previous study (Hase and Cordes, 2003). In Npap60-knockdown cells, endogenous Npap60 was undetected, and there were no significant changes in the localization of endogenous importin α (Figure 3B).
Figure 3.
siRNA-mediated knockdown of the Npap60 isoforms does not affect the localization of endogenous importin α. (A) HeLa and HeLa229 cells were transfected with control siRNA, siRNA 60-1, or siRNA 60-2. After 24 h, whole-cell lysates were prepared. The endogenous Npap60 isoforms, importin α, Nup153, Nup214, and Nup358 were detected by Western blot analysis. The Npap60 isoforms and importin α were detected with an anti-Npap60 antibody (1:1000) and anti-karyopherin α/Rch-1 antibody (1:200), respectively. Other nucleoporins were detected with mAb 414, which recognizes FG nucleoporins, including Nup153, Nup214, and Nup358. The total protein levels were normalized to GAPDH expression in each sample. (B) HeLa cells were either untransfected or transfected with a control siRNA or siRNA 60-2. After fixing the cells, endogenous Npap60 and importin α were detected with an anti-Npap60 antibody (1:100) and anti-karyopherin α/Rch-1 antibody (1:200), followed by Alexa 568– and Alexa 488–conjugated secondary antibodies, respectively.
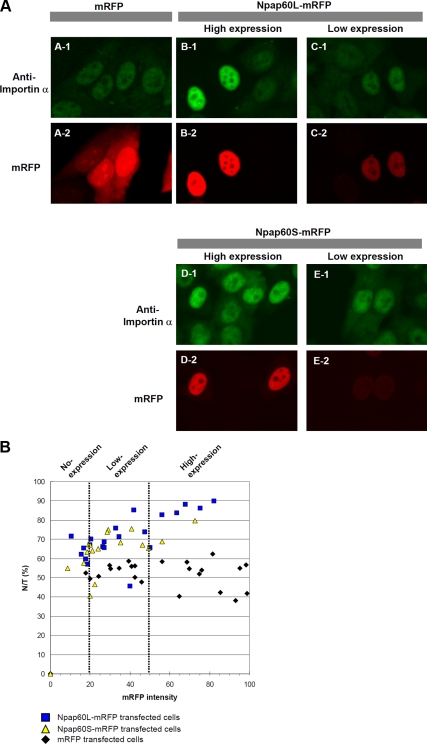
Next, to determine if the individual Npap60 isoforms affect the nucleocytoplasmic recycling of importin α, we examined the effects of transiently expressing the C-terminal monomeric red fluorescent protein (mRFP)-fused Npap60L or Npap60S on the localization of endogenous importin α in HeLa cells. Both Npap60L-mRFP and Npap60S-mRFP localized predominantly in the nucleus (Figure 4A), similar to the localization of endogenous Npap60 (Figure 3B). Interestingly, localization of endogenous importin α was dependent on the level of Npap60L-mRFP or Npap60S-mRFP expression (Figure 4, A and B). In cells expressing high levels of these proteins, endogenous importin α was localized exclusively in the nucleus. Therefore, we sorted cells expressing Npap60L-mRFP or Npap60S-mRFP into three groups, i.e., no-expression, low-expression, and high-expression, based on the relative intensity of mRFP (Figure 4B). Cells in the no-expression group and control mRFP-transfected cells had nuclear-to-total ratios (N/Ts) for endogenous importin α of ∼40–60%. However, the N/Ts of the low- and high-expression groups were predominantly 60–80% and 70–90%, respectively. To exclude the possibility that the mRFP moiety of the fusion protein influenced the function of the Npap60 isoforms, we examined the effects of the untagged Npap60 isoforms and found that the untagged isoforms similarly affected the localization of endogenous importin α (Supplemental Figure S3). Furthermore, the localization of importin α did not change in cells that were transfected with Npap60ΔN, which lacks both BS1 and BS2. These results suggest that both of the Npap60 isoforms affect the nucleocytoplasmic recycling of importin α in vivo in a dose-dependent manner.
Figure 4.
Overexpression of the Npap60 isoforms induces the nuclear accumulation of endogenous importin α. (A) HeLa cells were transfected with expression vectors encoding mRFP, Npap60L-mRFP, or Npap60S-mRFP. Twenty-four hours after transfection, the cells were fixed and examined by immunofluorescence using an anti-karyopherin α/Rch-1 antibody, followed by an Alexa 488–conjugated secondary antibody (A-1–E-1). A-2 shows cells expressing mRFP. B-2 and D-2 show cells expressing high levels of the mRFP-fused Npap60 isoforms, whereas C-2 and E-2 show cells expressing low levels of the mRFP-fused Npap60 isoforms. Importin α accumulated in nuclei depending on the expression levels of the exogenous Npap60 isoforms (B-1–E-1). (B) The nuclear-to-total ratios of endogenous importin α were plotted relative to the mRFP intensity (0–100). Based on the mRFP intensity, the cells were sorted into the no-expression group (0–20), low-expression group (20–50), or high-expression group (50–100). Black diamonds, blue squares, and yellow triangles indicate mRFP-, Npap60L-mRFP–, and Npap60S-mRFP–transfected cells, respectively.
Import Efficiency Is Regulated by Npap60
On the basis of a series of in vitro binding assays, we concluded that Npap60L promotes the release of NLS-cargo from importin α, whereas Npap60S stabilizes the interaction between importin α and NLS-cargo. Npap60L is thought to localize predominantly in the nuclear basket of the NPC (Guan et al., 2000). However, as shown in Figures 3B and 4A, both the endogenous and exogenous Npap60 isoforms were localized mainly in the nucleus. In addition, Npap60L is thought to be one of the most mobile nucleoporins (Rabut et al., 2004) and shuttles between the nucleus and cytoplasm (Lindsay et al., 2002). If the Npap60 isoforms function in the cytoplasm, Npap60S is expected to promote importin α/β-dependent import, whereas Npap60L would inhibit this activity. In contrast, if the isoforms function in the nucleoplasm, Npap60S should inhibit nuclear import, while Npap60L should promote nuclear import.
To determine if or how the Npap60 isoforms are involved in nuclear import in vivo, we used time-lapse analysis to examine the nuclear import of GST-NLS-GFP, as a model of NLS-cargo. After either overexpressing the individual Npap60 isoforms or knocking down Npap60, GST-NLS-GFP was microinjected into the cytoplasm, and the kinetics of the nuclear accumulation of GST-NLS-GFP was examined by calculating the N/T at each time point.
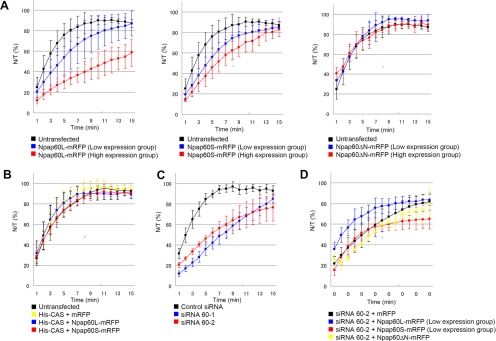
In the overexpression experiments, HeLa cells were transfected with either Npap60L-mRFP or Npap60S-mRFP. The cells were classified into high- and low-expression groups based on the fluorescence intensities of mRFP because the subcellular localization of endogenous importin α was dependent on the level of Npap60 expression (Figure 4). Import efficiency was decreased in cells transfected with either Npap60 isoform (Figure 5A). Import efficiency was down-regulated to a greater degree in the high-expression group than the low-expression group. On the other hand, the expression of Npap60ΔN-mRFP, which lacks both the BS1 and BS2 domains of Npap60, did not affect the import efficiency. These results indicate that overexpressing either of the exogenous Npap60 isoforms negatively regulates nuclear import through the BS1 or BS2 domain in a dose-dependent manner.
Figure 5.
In vivo time-lapse analysis. A mixture of 4 mg/ml GST-SV40T NLS-GFP and 0.5 mg/ml Alexa 647–conjugated anti-mouse IgG, as the injection marker, was injected into the cytoplasm of HeLa cells. The nuclear-to-total ratios of GST-NLS-GFP (N/T [%]) were calculated based on the intensity of GFP fluorescence at each time point. (A) Import efficiencies of cells transfected with Npap60L-mRFP, Npap60S-mRFP, or Npap60ΔN-mRFP. HeLa cells were transfected with Npap60L-mRFP (left), Npap60S-mRFP (middle), or Npap60ΔN-mRFP (right). After 24 h, time-lapse analyses were performed. The expression levels of the mRFP-fused proteins were used to classify cells into low- or high-expression groups based on the mRFP intensities (see Materials and Methods). The black line indicates the average rates of untransfected cells (n = 6). Blue and red lines indicate the rates of low-expression cells (Npap60L: n = 15, Npap60S: n = 13, Npap60ΔN: n = 5) and high-expression cells (Npap60L: n = 4, Npap60S: n = 3, Npap60ΔN: n = 3), respectively. (B) Import efficiencies of cells cotransfected with both His-tagged CAS and either mRFP (yellow line, n = 8), Npap60L-mRFP (blue line, n = 5), or Npap60S-mRFP (red line, n = 6). The black line indicates the average rates of untransfected cells. (C) Import efficiencies of Npap60-knockdown cells. HeLa cells were transfected with either siRNA 60-1 (blue line, n = 8), siRNA 60-2 (red line, n = 7), or a negative control siRNA (black line, n = 7). After 48 h, time-lapse analyses were performed. siRNA 60-1 and siRNA 60-2 target different sites that are conserved between the Npap60L and Npap60S mRNAs. (D) HeLa cells were transfected with siRNA 60-2. After 24 h, the cells were further transfected with either Npap60L-mRFP (blue line, n = 5), Npap60S-mRFP (red line, n = 4), Npap60ΔN-mRFP (yellow line, n = 3), or mRFP (black line, n = 6). After 24 h, time-lapse analyses were performed. In this assay, only the low-expression groups were included in the statistical analysis. All data in the graphs are the means ± SD.
Why was the nuclear import efficiency similarly decreased when either of the Npap60 isoforms was overexpressed? Because CAS together with RanGTP competes with Npap60 for binding to the C-terminal region of importin α (Figure 2C), we hypothesized that overexpression of the exogenous Npap60 isoforms competitively inhibits access of CAS to importin α, thereby inducing the nuclear accumulation of importin α (Figure 4A). As a result, importin α–mediated nuclear import is suppressed due to the depletion of cytoplasmic importin α. To test this hypothesis, we determined if cotransfecting CAS rescues the down-regulation of nuclear import that is induced by overexpressing the Npap60 isoforms. As shown in Figure 5B, cotransfection of CAS restored the nuclear import efficiency, even though the Npap60 isoforms were highly expressed. These results indicate that an imbalance between the abundance of Npap60 and CAS negatively affects nuclear protein import. Consequently, for efficient import of NLS-cargo, the relative abundance of the Npap60 isoforms should be maintained at an appropriate level so that CAS can efficiently disassemble the importin α/Npap60 complex in vivo.
Next, we examined the effects of knocking down both Npap60 isoforms in vivo. In Npap60-knockdown cells, import efficiency was markedly decreased, indicating that Npap60 is necessary for efficient nuclear protein import (Figure 5C). It is likely that the decline in import efficiency observed in this experiment is predominantly due to Npap60L knockdown, as endogenous Npap60S is minimally expressed in HeLa cells.
To examine the effects of the individual Npap60 isoforms on nuclear import in detail, we transfected Npap60 knockdown cells with either of the mRFP-fused Npap60 isoforms and performed in vivo time-lapse analyses. For this assay, Npap60-mRFP isoform expression plasmids containing silent mutations in the siRNA 60-2 target sequence were constructed. Twenty-four hours after transfection with siRNA 60-2, the cells were transfected with either Npap60L-mRFP or Npap60S-mRFP. After an additional 24 h, the transfected cells were classified into high- and low-expression groups, and time-lapse analysis was performed. As shown in Figure 5D, cells that express low Npap60L-mRFP levels had a higher import efficiency than cells transfected with mRFP alone. By contrast, the decline in import efficiency was greater in cells transfected with Npap60S-mRFP, even in cells expressing the protein at low levels. These results clearly indicate that Npap60L and Npap60S up- and down-regulate nuclear import efficiency, respectively, under physiological conditions in vivo. Furthermore, these functions of the Npap60 isoforms are derived from BS1 and BS2, because expression of Npap60ΔN-mRFP did not affect the import efficiency.
DISCUSSION
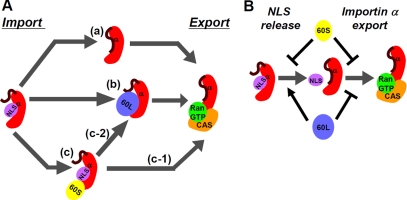
On the basis of our results, we propose the following model (Figure 6A): In the nucleus, importin α can partially release NLS-cargo by auto-inhibition (a), and Npap60L binds to importin α, facilitating the efficient release of NLS-cargo (b). Subsequently, CAS, together with RanGTP, releases importin α from Npap60L. When Npap60S binds to importin α/NLS-cargo, there are two possible pathways (Figure 6Ac). Npap60S can be released from importin α/NLS-cargo upon CAS/RanGTP binding to importin α (c-1). Then, importin α can release the NLS-cargo by auto-inhibition, and importin α can release NLS-cargo when Npap60S is replaced with Npap60L (c-2). After the NLS-cargo is released, Npap60L bound to importin α can be replaced with CAS/RanGTP. In addition to these mechanisms, CAS/RanGTP can release NLS-cargo from importin α (Matsuura and Stewart, 2004). After these events, CAS/RanGTP can export importin α from the nucleus.
Figure 6.
Model of the nucleocytoplasmic recycling of importin α mediated by the Npap60 isoforms. (A) After importin β is released by RanGTP, importin α may be recycled via the following pathways in the nucleus. (a) Importin α releases NLS-cargo by auto-inhibition. Then, CAS/RanGTP exports cargo-free importin α. (b) Npap60L binds to the importin α/NLS-cargo complex and releases the NLS-cargo from importin α. Next, Npap60L is replaced by CAS/RanGTP. (c) Npap60S binds to importin α/NLS-cargo and stabilizes this complex. (c-1) CAS/RanGTP binds to importin α and releases Npap60S. Then, importin α releases NLS-cargo by auto-inhibition. (c-2) Npap60S is replaced by Npap60L. (B) Npap60S inhibits both the release of NLS-cargo and the export of importin α. On the other hand, Npap60L promotes the release of NLS-cargo and inhibits the export of importin α. Therefore, when the expression levels of the Npap60 isoforms are high relative to CAS, the import efficiency of NLS-cargo decreases. When the expression levels of the Npap60 isoforms are appropriate for CAS to bind importin α and release the Npap60 isoforms, Npap60L increases the import efficiency while Npap60S decreases the import efficiency.
In addition to this model, the opposite properties of the Npap60 isoforms control the import efficiency; Npap60L promotes the release of NLS-cargo from importin α, whereas Npap60S inhibits this release. Furthermore, because both Npap60 isoforms compete with CAS/RanGTP for binding to importin α, they inhibit the export of importin α by CAS/RanGTP. Therefore, when the expression levels of the Npap60 isoforms are too high, both Npap60S and Npap60L can inhibit import (Figure 6B). Only when the Npap60 isoforms and CAS are expressed at the appropriate levels can Npap60L and Npap60S promote and inhibit import, respectively.
Sun et al. (2008) recently used single-molecule measurements to demonstrate that when Npap60L alone is retained by NPCs, it cannot release NLS-cargo from importin α. The authors proposed that in NPCs, Npap60L tethers the importin α/NLS-cargo complex to the CAS/RanGTP complex, resulting in efficient dissociation of the cargo from importin α by CAS/RanGTP. In fact, they showed that permeabilized cells retained a significant fraction of Npap60 at NPCs. However, as shown in Supplemental Figure S4, permeabilization with digitonin caused a significant loss of Npap60 in the nucleoplasm, whereas other nucleoporins including Nup153, which is a highly mobile nucleoporin like Npap60 (Rabut et al., 2004), were less affected. This change in the localization of Npap60 is consistent with a previous study (Hase and Cordes, 2003). These results indicate that a portion of Npap60 is anchored at the nuclear baskets of NPCs, and the remaining Npap60 is localized in the nucleoplasm. However, in permeabilized cells, only the Npap60 molecules that are anchored at the nuclear baskets may be present because the soluble Npap60 molecules are washed out. Therefore, we speculated that the localization of the Npap60 isoforms in permeabilized cells only partially reflects that in living cells.
Although Npap60S mRNA was expressed in all of the cell lines examined in this study, the Npap60S protein was only detected in HeLa229 and A549 cells (Figure 1C). This implies that the posttranscriptional regulation of Npap60S may differ from that of Npap60L. It will be interesting to determine whether the expression patterns of the Npap60 isoforms are tissue-specific or are altered in response to various stimuli. Previous studies have shown that Npap60 is highly expressed in the testis (Fan et al., 1997; Trichet et al., 1999; Guan et al., 2000; Smitherman et al., 2000) and that the localization of some importin α family members changes during spermatogenic differentiation (Giarrè et al., 2002; Hogarth et al., 2005). Therefore, it will be interesting to examine the expression pattern of the Npap60 isoforms during spermatogenesis.
Thus, although regulation of the function of nuclear import factors has been reported previously, in this study, using living cells, we show novel evidence that nucleoporins are involved in regulating the efficiency of nuclear protein import.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. J. Katahira (Osaka University) and T. Miki (Kyoto University) for valuable discussions. This work was partly supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Takeda Science Foundation.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-05-0374) on December 16, 2009.
REFERENCES
- Bayliss R., Littlewood T., Stewart M. Structural basis for the interaction between FxFG nucleoporin repeats and importin-beta in nuclear trafficking. Cell. 2000;102:99–108. doi: 10.1016/s0092-8674(00)00014-3. [DOI] [PubMed] [Google Scholar]
- Chook Y. M., Blobel G. Karyopherins and nuclear import. Curr. Opin. Struct. Biol. 2001;11:703–715. doi: 10.1016/s0959-440x(01)00264-0. [DOI] [PubMed] [Google Scholar]
- Cronshaw J. M., Krutchinsky A. N., Zhang W., Chait B. T., Matunis M. J. Proteomic analysis of the mammalian nuclear pore complex. J. Cell Biol. 2002;158:915–927. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth D. J., Suprapto A., Padovan J. C., Chait B. T., Wozniak R. W., Rout M. P., Aitchison J. D. Nup2p dynamically associates with the distal regions of the yeast nuclear pore complex. J. Cell Biol. 2001;153:1465–1478. doi: 10.1083/jcb.153.7.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F., Liu C. P., Korobova O., Heyting C., Offenberg H. H., Trump G., Arnheim N. cDNA cloning and characterization of Npap60, a novel rat nuclear pore-associated protein with an unusual subcellular localization during male germ cell differentiation. Genomics. 1997;40:444–453. doi: 10.1006/geno.1996.4557. [DOI] [PubMed] [Google Scholar]
- Giarrè M., Török I., Schmitt R., Gorjánácz M., Kiss I., Mechler B. M. Patterns of importin-alpha expression during Drosophila spermatogenesis. J. Struct. Biol. 2002;140:279–290. doi: 10.1016/s1047-8477(02)00543-9. [DOI] [PubMed] [Google Scholar]
- Gilchrist D., Mykytka B., Rexach M. Accelerating the rate of disassembly of karyopherin-cargo complexes. J. Biol. Chem. 2002;277:18161–18172. doi: 10.1074/jbc.M112306200. [DOI] [PubMed] [Google Scholar]
- Gilchrist D., Rexach M. Molecular basis for the rapid dissociation of nuclear localization signals from karyopherin alpha in the nucleoplasm. J. Biol. Chem. 2003;278:51937–51949. doi: 10.1074/jbc.M307371200. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S., Corbett A. H., Mason D. A., Harreman M. T., Adam S. A. Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol. 2004;14:505–514. doi: 10.1016/j.tcb.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Guan T., Kehlenbach R. H., Schirmer E. C., Kehlenbach A., Fan F., Clurman B. E., Arnheim N., Gerace L. Nup50, a nucleoplasmically oriented nucleoporin with a role in nuclear protein export. Mol. Cell. Biol. 2000;15:5619–5630. doi: 10.1128/mcb.20.15.5619-5630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase M. E., Cordes V. C. Direct interaction with Nup153 mediates binding of Tpr to the periphery of the nuclear pore complex. Mol. Biol. Cell. 2003;14:1923–1940. doi: 10.1091/mbc.E02-09-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth C., Itman C., Jans D. A., Loveland K. L. Regulated nucleocytoplasmic transport in spermatogenesis: a driver of cellular differentiation? Bioessays. 2005;27:1011–1025. doi: 10.1002/bies.20289. [DOI] [PubMed] [Google Scholar]
- Imamoto N., Shimamoto T., Takao T., Tachibana T., Kose S., Matsubae M., Sekimoto T., Shimonishi Y., Yoneda Y. In vivo evidence for involvement of a 58 kDa component of nuclear pore-targeting complex in nuclear protein import. EMBO J. 1995;15:3617–3626. doi: 10.1002/j.1460-2075.1995.tb00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobe B. Autoinhibition by an internal nuclear localization signal revealed by the crystal structure of mammalian importin alpha. Nat. Struct. Biol. 1999;6:388–397. doi: 10.1038/7625. [DOI] [PubMed] [Google Scholar]
- Künzler M., Hurt E. C. Cse1p functions as the nuclear export receptor for importin alpha in yeast. FEBS Lett. 1998;433:185–190. doi: 10.1016/s0014-5793(98)00892-8. [DOI] [PubMed] [Google Scholar]
- Kutay U., Bischoff F. R., Kostka S., Kraft R., Görlich D. Export of importin alpha from the nucleus is mediated by a specific nuclear transport factor. Cell. 1997;90:1061–1071. doi: 10.1016/s0092-8674(00)80372-4. [DOI] [PubMed] [Google Scholar]
- Lindsay M. E., Plafker K., Smith A. E., Clurman B. E., Macara I. G. Npap60/Nup50 is a tri-stable switch that stimulates importin-α:β-mediated nuclear protein import. Cell. 2002;110:349–360. doi: 10.1016/s0092-8674(02)00836-x. [DOI] [PubMed] [Google Scholar]
- Liu S. M., Stewart M. Structural basis for the high-affinity binding of nucleoporin Nup1p to the Saccharomyces cerevisiae importin-beta homologue, Kap95p. J. Mol. Biol. 2005;349:515–525. doi: 10.1016/j.jmb.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Lange A., Harreman M. T., Corbett A. H., Stewart M. Structural basis for Nup2p function in cargo release and karyopherin recycling in nuclear import. EMBO J. 2003;22:5358–5369. doi: 10.1093/emboj/cdg538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura Y., Stewart M. Structural basis for the assembly of a nuclear export complex. Nature. 2004;432:872–877. doi: 10.1038/nature03144. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Stewart M. Nup50/Npap60 function in nuclear protein import complex disassembly and importin recycling. EMBO J. 2005;24:3681–3689. doi: 10.1038/sj.emboj.7600843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Hieda M., Harreman M. T., Fukumoto M., Saiwaki T., Hodel A. E., Corbett A. H., Yoneda Y. Importin alpha can migrate into the nucleus in an importin beta- and Ran-independent manner. EMBO J. 2002;21:5833–5842. doi: 10.1093/emboj/cdf569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Saiwaki T., Yamashita J., Yasuda Y., Kotera I., Shibata S., Shigeta M., Hiraoka Y., Haraguchi T., Yoneda Y. Cellular stresses induce the nuclear accumulation of importin alpha and cause a conventional nuclear import block. J. Cell Biol. 2004;165:617–623. doi: 10.1083/jcb.200312008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S. Npap60, a new player in nuclear protein import. Trends Cell Biol. 2003;13:61–64. doi: 10.1016/s0962-8924(02)00044-2. [DOI] [PubMed] [Google Scholar]
- Patre M., Tabbert A., Hermann D., Walczak H., Rackwitz H. R., Cordes V. C., Elisa F. M. Caspases target only two architectural components within the core structure of the nuclear pore complex. J. Biol. Chem. 2006;281:1296–1304. doi: 10.1074/jbc.M511717200. [DOI] [PubMed] [Google Scholar]
- Pemberton L. F., Paschal B. M. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic. 2005;6:187–198. doi: 10.1111/j.1600-0854.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Rabut G., Doye V., Ellenberg J. Mapping the dynamic organization of the nuclear pore complex inside single living cell. Nat. Cell Biol. 2004;6:1114–1121. doi: 10.1038/ncb1184. [DOI] [PubMed] [Google Scholar]
- Sekimoto T., Nakajima K., Tachibana T., Hirano T., Yoneda Y. Interferon-gamma-dependent nuclear import of Stat1 is mediated by the GTPase activity of Ran/TC4. J. Biol. Chem. 1996;271:31017–31020. doi: 10.1074/jbc.271.49.31017. [DOI] [PubMed] [Google Scholar]
- Smitherman M., Lee K., Swanger J., Kapur R., Clurman B. E. Characterization and targeted disruption of murine Nup50, a p27 (Kip1)-interacting component of the nuclear pore complex. Mol. Cell. Biol. 2000;20:5631–5642. doi: 10.1128/mcb.20.15.5631-5642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe C., Jenkins H. E., Hutchison C. J. Incorporation of the nuclear pore basket protein nup153 into nuclear pore structures is dependent upon lamina assembly: evidence from cell-free extracts of Xenopus eggs. EMBO J. 2000;19:3918–3931. doi: 10.1093/emboj/19.15.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solsbacher J., Maurer P., Bischoff F. R., Schlenstedt G. Cse1p is involved in export of yeast importin alpha from the nucleus. Mol. Cell. Biol. 1998;18:6805–6815. doi: 10.1128/mcb.18.11.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solsbacher J., Maurer P., Vogel F., Schlenstedt G. Nup2p, a yeast nucleoporin, functions in bidirectional transport of importin alpha. Mol. Cell. Biol. 2000;20:8468–8479. doi: 10.1128/mcb.20.22.8468-8479.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin A. V., Kim E. R., Ovchinnikov L. P. Nucleocytoplasmic transport of proteins. Biochemistry. 2007;72:1439–1457. doi: 10.1134/s0006297907130032. [DOI] [PubMed] [Google Scholar]
- Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat. Rev. Mol. Cell. Biol. 2007;8:195–208. doi: 10.1038/nrm2114. [DOI] [PubMed] [Google Scholar]
- Strawn L. A., Shen T., Shulga N., Goldfarb D. S., Wente S. R. Minimal nuclear pore complexes define FG repeat domains essential for transport. Nat. Cell Biol. 2004;6:197–206. doi: 10.1038/ncb1097. [DOI] [PubMed] [Google Scholar]
- Sun C., Yang W., Tu L. C., Musser S. M. Single-molecule measurements of importin alpha/cargo complex dissociation at the nuclear pore. Proc. Natl. Acad. Sci. USA. 2008;105:8613–8618. doi: 10.1073/pnas.0710867105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry L. J., Shows E. B., Wente S. R. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- Tran E. J., Wente S. R. Dynamic nuclear pore complexes: life on the edge. Cell. 2006;125:1041–1053. doi: 10.1016/j.cell.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Trichet V., Shkolny D., Dunham I., Beare D., McDermid H. E. Mapping and complex expression pattern of the human NPAP60L nucleoporin gene. Cytogenet. Cell Genet. 1999;85:221–226. doi: 10.1159/000015297. [DOI] [PubMed] [Google Scholar]
- Zeitler B., Weis K. The FG-repeat asymmetry of the nuclear pore complex is dispensable for bulk nucleocytoplasmic transport in vivo. J. Cell Biol. 2004;167:583–590. doi: 10.1083/jcb.200407156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.