Abstract
Between 1980 and 2001, the United Kingdom Medical Research Council Childhood Leukemia Working Party has conducted 4 clinical trial in acute lymphoblastic leukemia, which have recruited a total of 6516 patients. UKALL VIII examined the role of daunorubicin in induction chemotherapy, and UKALL X examined the role of post-induction intensification. Both resulted in major improvement in the outcomes. UKALL XI examined the efficacy of different methods of CNS-directed therapy and the effects of an additional intensification. ALL97, which was initially based on the UKALL X D template (two intensification phases), examined the role of different steroids in induction and different thiopurines through continuing chemotherapy. A reappraisal of results from UKALL XI compared to other cooperative group results led to a redesign in 1999, which subsequently resulted in a major improvement in outcomes. Additionally, ALL97 and 97/99 demonstrated a significant advantage for the use of dexamethasone rather than prednisolone; although the use of 6-thioguanine resulted in fewer relapses, this advantage was offset by an increased incidence of deaths in remission. Over the era encompassed by these four trials there has been a major improvement in both event-free and overall survival for children in the UK with ALL.
Keywords: acute leukemia, therapy, clinical trial
Introduction
Since 1970, the UK Medical Research Council (MRC) Working Party on Childhood Leukaemia has conducted a series of therapeutic trials for acute lymphoblastic leukaemia (ALL). In the time period 1980–2001, 80–95% of all patients presenting with acute lymphoblastic leukaemia in the ages 0–15 were entered into the four consecutive trials described in this paper. Reasons for non-entry included: individual centres piloting succeeding trials, an alternative national trial existing for specific sub-types of leukaemia, (for example infant leukaemia post-1992, and B cell ALL from 1985), and following individual family or physician preference.
By 1972, the broad principles of therapy were established, and in a series of trials (UKALL II-VII) various aspects of the basic protocol were tested. The results were generally disappointing; fewer than 50% of the 1470 patients entered into the MRC studies between 1972 and 1979 remained in first remission after 4 years1. UKALL VII (1979–1980) gave somewhat better results for a small group of standard-risk patients2, but was still less impressive than results emerging at that time from the Berlin/Frankfurt/Munster group (BFM)3, and certain American trials4. These disappointing results led to collaboration with the United States Children’s Cancer Group who gave permission for the UK Working Party to use protocol CCG 162 (specifically arm 1A) as the standard MRC therapeutic protocol. The initial phase was designated the UKALL VIII study; after the first year two randomizations were introduced and this latter phase was designated the UKALL VIII trial5. The trial reported the single greatest improvement in event-free survival, compared to the historical groups, seen in the UK up to that time1. The results emphasised the value of international sharing of information and collaboration.
The subsequent MRC study, UKALL X6, evaluated the effects of a short very intensive module of treatment given early, or late, or both, or omitted completely. Ultimately, this trial demonstrated that there was a major advantage to having both early and late intensification. UKALL XI attempted to extend this philosophy by examining the contribution of a third intensification7, which was of the prolonged, reinduction-reconsolidation, style developed by the BFM group. It also attempted to evaluate the role of different forms of CNS directed therapy8. Randomization to receive or not the third intensification was carried over into the following trial, ALL 97, which also examined the roles of different steroids9 and thiopurines10 by randomizing prednisolone against dexamethasone, and mercaptopurine against thioguanine. Ultimately, although the third intensification provided an advantage7, the overall results of UKALL XI were not an improvement over UKALL X11. In addition, it was clear that the outcome for children in the UK, especially those with high-risk disease, were lagging behind the results being obtained by other cooperative groups. As a result, the UKALL treatment programme was revised to mimic more closely that used by the Children’s Oncology Group, with a second, revised, phase of ALL97, referred to as ALL97/9912. This study was closed in 2001 and its results are included in this updated long-term follow-up report.
Patients, materials and methods
From 1980 to 2001, 6516 consecutive patients aged 15 years or younger with de novo ALL were enrolled into four successive treatment protocols (UKALL VIII, X, XI; and ALL97; UKALL IX being for adult patients only). Fifty-four patients were not eligible because of wrong diagnosis, or sub-type of leukaemia, for example mature B cell, for which an alternative UK strategy was available. Thus there were 6462 eligible patients treated in the four trials (UKALL VIII, 825; UKALL X, 1612; UKALL XI, 2090; ALL97, 1935).
Diagnosis in all four trials was based on standard local hospital morphological review by an expert haematologist, supported by a centralised review panel which also reviewed early response and all marrows from reported non-remitting patients13. Early response was defined by percentage of marrow blasts at day 15 in UKALL VIII and X, at day 8 in UKALLXI, and at day 8 or 15, depending on the treatment arm, in ALL97/99.
A standard immunophenotyping panel has been used in all MRC trials, so that patients have been classified into the following groups:
Early preB or null, based on CD10 negativity, CD19 positivity, tdt positivity, and T cell marker negativity.
Common ALL: CD10 positivity, CD19 positivity, tdt positivity or T cell marker negativity.
Pre B, the same as common, but with also cytoplasmic immunoglobulin positivity.
T cell disease was classified by CD2 and/or CD7 positivity, but CD19 and HLA-DR negativity.
An unusual category has always been identified by patients who expressed so-called myeloid antigens, either CD13 or CD33.
Finally, other patients whose antibody profile did not fit into any of the above five categories.
It has been consistently possible to divide the great majority of ALL cases into either B lineage or T cell lineage, and it is these broad categories which have been used in a uniform fashion for consistency in this series of trials14. Only mature B cell ALL patients (surface immunoglobulin positive) have been excluded from any of the trials on the basis of immunophenotype.
With an increasing recognition of its importance, the development of chromosomal banding techniques, the introduction of fluorescence in situ hybridisation (FISH) to identify specific translocations and the review and central collection of cytogenetic/FISH data into a bespoke cytogenetics database15, the rate of successful cytogenetic analysis requested and performed has improved over the 21 year period. Cytogenetic analysis was performed successfully on 281 (34%) in UKALL VIII5, 830 (51%) in UKALL X16, 1658 (79%) in UKALL XI7 and 1728 (91%) in ALL9717. In the UK, FISH was the chosen molecular method for the routine detection of chromosomal abnormalities of prognostic significance, which was implemented in ALL97. There was specific emphasis on the poor risk abnormalities: t(9;22)(q34;q11.1)/BCR-ABL1 fusion and rearrangements of the MLL gene, particularly t(4;11)(q21;q23), used for risk stratification in this trial15
The four Medical Research Council protocols were designed by a working group of coordinators, modified and approved by the full Childhood Working Party and then subjected to independent peer review, firstly by a leukaemia trials steering committee and then by a separate data monitoring and ethical committee. Approval for individual patient entry was subject to local research ethical committee review for each hospital centre participating. Parent or patient consent was obtained prior to trial entry as required by the ethical procedures relevant at the time the trial was in progress.
Treatment
The four studies have all previously been described elsewhere 5-12, but are summarized briefly here so that the continuing themes and changes in strategy can be highlighted.
UKALL VIII (1980–1984)5
This study aimed to reproduce directly the more favourable results being reported by CCG. All children with ALL aged 0–14 years inclusive were eligible. Eight hundred and twenty-five patients were analysed. In the first year (designated UKALL VIIIS) running from September 1980 to October 1981, all patients irrespective of their initial prognostic features received a three-drug induction regimen of weekly vincristine (1.5mg/m2/dose) × 5, 28 days of oral Prednisolone (40mg/m2 per day) and nine intramuscular injections of asparaginase (6000 IU/m2/dose) given three times per week for 3 weeks. Initially Escherichia coli asparaginase (Merck, Sharp, Dohm) as per the CCG162 protocol4 was used, and subsequently Erwinia asparaginase in the same dosage18.
From November 1981 until the trial closed in December 1984, a randomisation was introduced, for patients to receive two doses of Daunorubicin 45mg/m2 per dose intravenously on days 1 and 2 of induction (arm B) or not (arm A which was, consequently, identical to UKALL VIIIS). Central nervous system-directed therapy for all patients consisted of intrathecal methotrexate (dosage based on age)19 at days 0, 15 and 28 and then weekly × 3 during cranial irradiation (18 Gy in 10 fractions) given immediately after achievement of remission. 6-Mercaptopurine was given at a dose of 75 mg/m2 per day orally throughout CNS irradiation therapy and continued along with oral weekly methotrexate 20mg/m2 in continuing therapy together with monthly pulses of intravenous vincristine (1.5mg/m2) and 5 days of oral Prednisolone (40mg/m2/day).
Between September 1980 and December 1980, the duration of therapy was 2 years from the time of remission, but subsequently from January 1981 to the close of the trial in December 1984 there was a second randomisation for those patients still in remission at 2 years, between 2 or 3 years of continuing therapy from achievement of remission. Those patients not in remission at day 29 were given 2 further weeks of vincristine and Prednisolone, but thereafter were considered off-protocol if not in complete remission.
UKALL X (1985–1990)6
This trial was designed to test the benefit of post-remission intensification, and was open to all children aged 0–14 years except for those with surface membrane immunoglobulin positive B cell ALL. Sixteen hundred and twelve eligible patients were enrolled. Induction therapy was identical to UKALL VIIIB (vincristine, Prednisolone, Daunorubicin and Erwinia asparaginase, (6000 iu/m2 3 × week × 9) together with intrathecal methotrexate on days 1, 15 and 29). Bone marrow response was again centrally assessed at day 15.
Patients were randomised to receive no intensification block (arm A); an early block at week 5 (arm B); or a late block at week 20 (arm C); or both an early and late block (arm D). The philosophy of the MRC group has been to carry forward the best arm of each trial as the standard arm of the next. Therefore, arm A was the same as UKALL VIIIB. The composition of the two intensification blocks was identical and consisted of a 5-day course of cytarabine (10 doses of 100mg/m2 given 12 hourly intravenously), etoposide (100mg/m2 daily intravenously × 5), thioguanine (80mg/m2 orally daily for 5 days) plus two further doses of Daunorubicin (45mg/m2/dose on days 1 and 2), one dose of vincristine (1.5mg/m2 i.v. day 1) and 5 further days of prednisolone (40mg/m2/day tapering over 3–4 days after the early block). Intrathecal methotrexate was given on day 1 of each block. Moderate to severe pancytopenia was observed at 7–10 days and recovery adequate to permit resumption of therapy did not occur until 3–4 weeks later20.
The CNS-directed therapy phase was the same as in UKALL VIII (18 Gy cranial irradiation plus three intrathecal injections of methotrexate). Oral 6-mercaptopurine was given daily (75mg/m2/day) during irradiation, given to all patients.
Exceptions to the randomisation process included:
Patients with CNS disease at diagnosis as defined by more than five blast cells per μL in the CSF, which were recognisable blast cells who received arm B (early intensification) followed by 24 Gy cranial irradiation, and 12 Gy to the spine, and then continuation therapy with no further intensification modules.
Children with a white count greater than 100 × 109/l at diagnosis were allocated to arm D, and received cranial irradiation to a dose of 24 Gy unless they had a matched sibling donor when cranial irradiation was replaced by cyclophosphamide and total body irradiation as conditioning for bone marrow transplantation in first remission21.
From January 1985 until April 1988 females aged 2–9 years with an initial leukocyte count <20 × 109/l were allocated to arm A (that is no intensification). From April 1988 onwards, these patients were entered into the randomised trial.
Continuing therapy was similar to that used in UKALL VIII.
UKALL XI (1990–1997)7,8,11
This trial recruited 2090 eligible patients aged 1–14 years inclusive (up to their 15th birthday). Induction therapy was identical to UKALL X until May 1992, when the two doses of Daunorubicin were dropped from induction to reduce total anthracycline exposure to 180 mg/m2 because of anxiety about late cardiotoxicity then being reported22. Initially all patients except those with CNS disease at diagnosis (who were treated with modified UKALLXB and from 1994 on a modified UKALL XD regimen) were randomised between regimens UKALL XC and UKALL XD.
In May 1991, an interim analysis showed benefit for two intensification modules, and so all new patients were allocated to the UKALLX arm D (two intensification modules at weeks 5 and 20), and from May 1992 all new patients were randomised between two and three intensification modules, this change coinciding with the omission of Anthracycline from the induction regimen. The third intensification block was given between weeks 35 and 42 and differed from the previously used pulses in being a BFM style phase with reinduction and reconsolidation. Between intensification pulses and for a total of 2 years oral daily 6-mercaptopurine (75 mg/m2/day), weekly oral methotrexate (20 mg/m2/dose) and monthly pulses of 5 days of prednisolone (40 mg/m2/day) and a dose of vincristine (1.5 mg/m2/dose) was given as continuing therapy.
Patients with an initial peripheral blood white cell count <50 × 109/l were randomized to receive either a long course of intrathecal methotrexate alone or high-dose systemic methotrexate in a dose of 6–8g/m2 (those under 4 having 8 g/m2, those older 6 g/m2) in weeks 6, 8 and 1023, with four doses of intrathecal methotrexate as well as continuing 3-monthly throughout continuing therapy. Patients with an initial white count ≥50 × 109/l, were randomized between high-dose systemic methotrexate (6–8 g/m2) with a long course of intrathecal methotrexate, and cranial irradiation to a dose of 24 Gy (in 15 fractions of 1.6 Gy per fraction) with a short course of intrathecal methotrexate (7 doses). All intrathecal doses were calculated on the basis of age.
ALL 97 and 97/99 9-12
These linked trials ran from April 1997 until June 2001 and were to determine the possible benefits of Dexamethasone over Prednisolone, and of 6-Thioguanine over 6-Mercaptopurine. The treatment schedule for the ALL97 phase was based on that of UKALL XI, with two intensification blocks, and continued with the UKALL XI randomisation to a third intensification, as in the latter stage of UKALL XI. This randomisation was discontinued when it became clear that although the third intensification offered an advantage, the outcomes for patients in UKALL XI, and particularly those patients at highest risk of relapse, had not improved in the way seen by other cooperative groups. In 1999, the treatment schedule was modified to mimic that used in the USA by the Children’s Oncology Group, and this phase was named ALL 97/99. In essence, this change meant discontinuing the short (5 day), very intensive blocks used first in UKALL X and substituting prolonged (7 week) BFM style of intensification. Other changes included initial stratification by age and WCC into standard (age < 10 years or WCC < 50 × 109 cells/l) and high-risk groups (age ≥ 10 years or WCC ≥ 50 × 109 cells/l). Standard-risk patients received three-drug induction, whereas those at high risk, in addition, received daunorubicin. Early assessment of response by examination of the bone marrow at day 15 (standard risk) and day 8 (high risk) was adopted, so that patients with >25% blasts at the assessment point were deemed to be slow early responders (SER) and were transferred to a more intensive regimen incorporating augmented BFM consolidation and extended intensification phases. In addition, boys were given three years of maintenance therapy, rather than two, as had previously been the case, while girls continued to receive a total of two years of treatment, from the start of interim maintenance 1. CNS-directed therapy in both phases was a long course of intrathecal methotrexate. Only patients with CNS disease at the time of diagnosis were irradiated.
Patients were randomised at the time of diagnosis to receive either Dexamethasone (6.5mg/m2) or prednisolone (40mg/m2) during induction and during all subsequent treatment phases where prednisolone would conventionally have been included. They were also randomised to receive either 6-Mercaptopurine (75mg/m2) or 6-Thioguanine (40mg/m2) during consolidation and maintenance phases. These randomisations were continued throughout both phases of the trial until, in June 2001, the independent Data Monitoring Committee recommended that the trial should close because of the advantage of dexamethasone over prednisolone, consistent with confidentially supplied results from the parallel US COG trial.
During the ALL97 phase there was a separate non-randomised study – HR124 -for patients at high risk of relapse. Eligible patients were calculated to have a risk of relapse in excess of 30% on the Oxford Hazard score25, or to have high-risk cytogenetic features. This study was based on arm D of UKALL X, but with high dose methotrexate inserted between the two intensification blocks. Thus it had no induction Anthracycline and used only 9 doses of Erwinia asparaginase.
Statistical analyses
In these analyses, overall survival (OS) is defined as the time from diagnosis until death, censoring at date of last contact, whilst event-free survival (EFS) is defined as the time from diagnosis until either relapse (haematological, CNS or other site or a combination thereof) or death due to any cause, whether or not the patient entered remission. Time to an isolated CNS relapse was defined as the time from diagnosis to a CNS relapse without relapse at any other site, excluding patients who did not enter remission. Time to any CNS relapse includes both time to any isolated CNS relapse and to a CNS relapse in conjunction with relapse at any other site, occurring within 30 days of the CNS relapse. Actuarial event-free survival, overall survival and time to isolated/any CNS relapse curves were calculated by the method of Kaplan and Meier. Results are given as % (standard error). Approximate confidence intervals can be calculated by subtracting and adding twice the standard error. Results were looked at in randomised patients aged 1 year or older at diagnosis divided by cell lineage (Null cell or pre-B, T) and Rome/NCI risk (standard risk, age 1–9 years with WBC <50×109/dl; high risk, all other patients aged 10 years or more regardless of initial white cell count, and all patients with initial white cell count ≥ 50×109/dl).
Patient follow-up on these trials was carried out by the individual participating physicians in their own centre with an annual request for up-dated information for all trial entrants, carried out by the Clinical Trial Service Unit each October for at least 10 years after trial entry. The current analyses are to the most recent completed follow-ups (UKALLVIII – 2001, UKALLX – 2007, UKALLXI – 2007, ALL97 – 2008). In addition, information on deaths is obtained from the UK national registration system. When a death is reported through this system for a patient not reported as ever having relapsed, the treating centre is contacted to determine whether the patient relapsed prior to death. Fewer than 1% of patients are lost to follow-up before 10 years.
Results
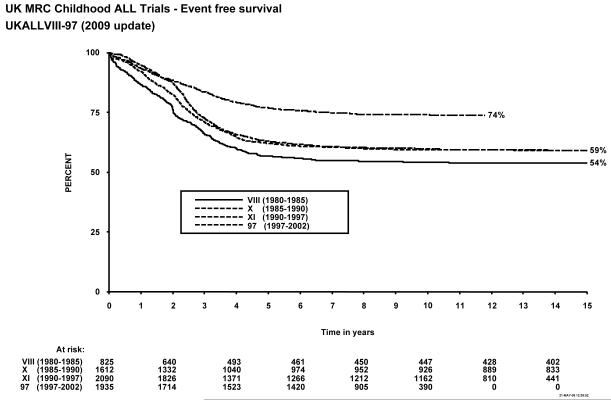
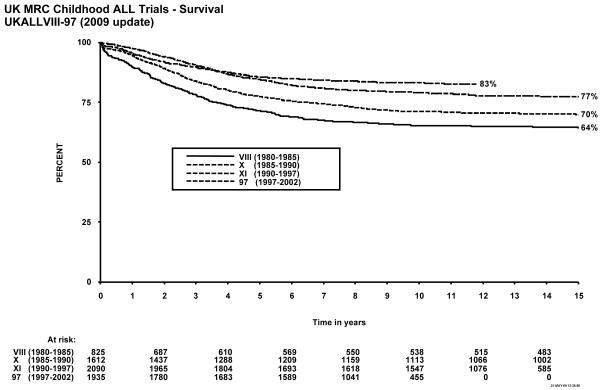
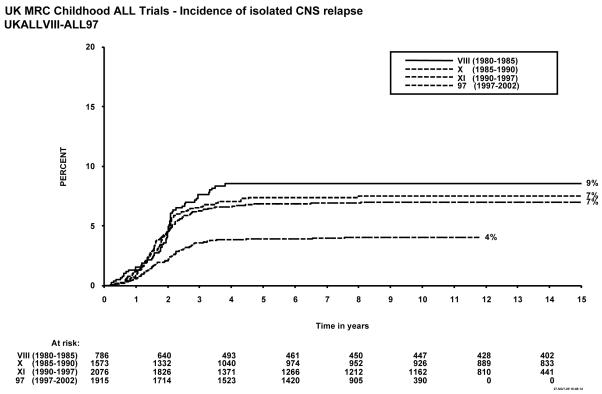
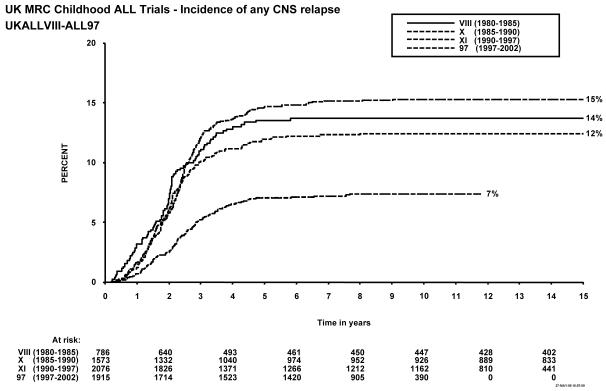
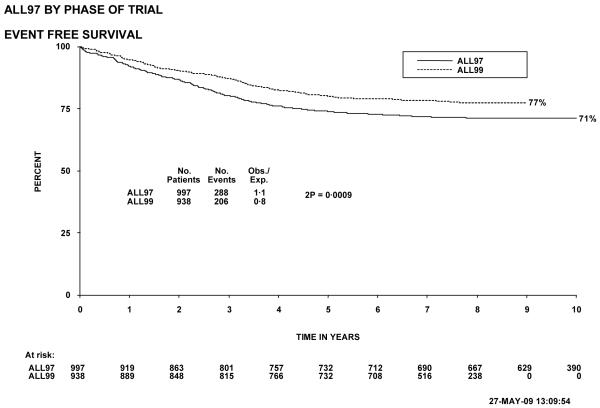
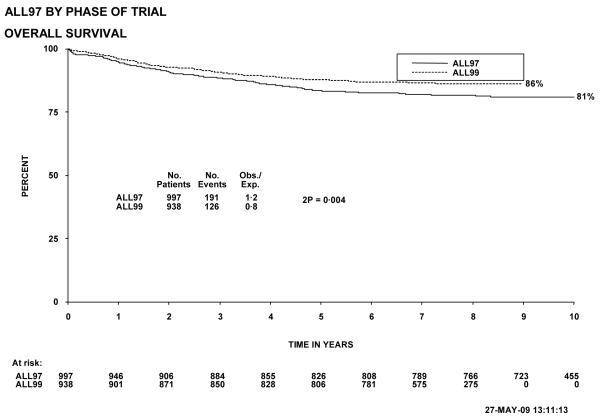
Figure 1 shows event-free survival by trial, and overall survival is shown in figure 2. Figure 3 shows cumulative isolated CNS relapse risk and figure 4 shows rates for any CNS relapse. Figures 5 and 6 show event-free and overall survival in ALL97 and ALL97/99 by phase of trial.
Figure 1.
Event free survival, MRC Childhood ALL trials, UKALL VIII – ALL97. The number of children at risk is indicated along the time axis.
Figure 2.
Overall survival, MRC Childhood ALL trials, UKALL VIII – ALL97. The number of children at risk is indicated along the time axis.
Figure 3.
Incidence of isolated CNS relapse, MRC Childhood ALL trials, UKALL VIII – ALL97. The number of children at risk is indicated along the time axis.
Figure 4.
Incidence of any CNS relapse, MRC Childhood ALL trials, UKALL VIII – ALL97. The number of children at risk is indicated along the time axis.
Figure 5.
Event free survival, ALL97 and 97/99, by phase of trial. The number of children at risk is indicated along the time axis.
Figure 6.
Overall survival, ALL97 and 97/99, by phase of trial. The number of children at risk is indicated along the time axis.
Table 1 shows the EFS at 5, and 10 years, for each trial, overall and according to presenting features. There are few events beyond 10 years. Table 2 shows the EFS by NCI risk group, and by immunophenotype.
Table 1. Diagnostic features of patients in four successive trials.
| Group | UKALLVIII |
UKALLX |
UKALLXI |
ALL97 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 5yrEFS | 10yrEFS | N | 5yrEFS | 10yrEFS | N | 5yrEFS | 10yrEFS | N | 5yrEFS | 10yrEFS | |
| Overall | 825 | 56.7 (1.7)* | 54.3 (1.7) | 1612 | 62.2 (1.2) | 59.5 (1.2) | 2090 | 63.0 (1.1) | 59.9 (1.1) | 1935 | 76.9 (1.0) | 74.1 (1.0) |
| Age at diagnosis | ||||||||||||
| <1 | 20 | 30.0(10.2) | 30.0(10.2) | 26 | 26.9 (8.7) | 26.9 (8.7) | 1 | - | - | 1 | - | - |
| 1–9 | 672 | 59.2 (1.9) | 56.8(1.9) | 1349 | 64.7 (1.3) | 62.0(1.3) | 1773 | 65.2 (1.1) | 62.0 (1.2) | 1534 | 79.9 (1.0) | 77.2 (1.1) |
| 10+ | 133 | 48.1 (4.3) | 45.1 (4.3) | 237 | 51.5 (3.2) | 49.3 (3.2) | 316 | 51.0(2.8) | 47.8(2.8) | 400 | 65.4 (2.4) | 62.0 (2.5) |
| WBCx109/l | ||||||||||||
| <10 | 424 | 60.6 (2.4) | 57.5 (2.4) | 756 | 71.1 (1.7) | 67.9 (1.7) | 970 | 68.3 (1.5) | 64.5 (1.5) | 887 | 81.8 (1.3) | 78.6 (1.4) |
| 10–49 | 259 | 58.3 (3.1) | 56.4 (3.1) | 514 | 59.5 (2.2) | 57.1 (2.2) | 658 | 65.9 (1.9) | 62.1 (1.9) | 600 | 78.0 (1.7) | 75.8 (1.8) |
| 50–99 | 62 | 46.8 (6.3) | 46.8 (6.3) | 130 | 45.4 (4.4) | 43.8 (4.4) | 210 | 53.8 (3.4) | 52.4 (3.4) | 191 | 70.1 (3.3) | 66.6 (3.6) |
| 100+ | 80 | 38.8 (5.4) | 36.3 (5.4) | 212 | 47.2 (3.4) | 45.3 (3.4) | 252 | 43.1 (3.1) | 42.3 (3.1) | 257 | 62.6 (3.0) | 60.2 (3.1) |
| NCI risk group | ||||||||||||
| Standard | 571 | 61.6 (2.0) | 59.0 (2.1) | 1090 | 67.9 (1.4) | 65.0 (1.4) | 1396 | 69.3 (1.2) | 65.6 (1.3) | 1196 | 83.1 (1.1) | 80.6 (1.2) |
| High | 234 | 47.0 (3.3) | 44.9 (3.3) | 496 | 51.4 (2.2) | 49.2 (2.2) | 693 | 50.5 (1.9) | 48.5 (1.9) | 738 | 66.9 (1.7) | 63.5 (1.8) |
| Gender | ||||||||||||
| Male | 452 | 50.4 (2.4) | 47.1 (2.3) | 922 | 56.2 (1.6) | 53.1 (1.6) | 1189 | 58.1 (1.4) | 54.3 (1.4) | 1084 | 77.4 (1.3) | 73.8 (1.4) |
| Female | 373 | 64.3 (2.5) | 63.0 (2.5) | 690 | 70.2 (1.7) | 68.1 (1.8) | 901 | 69.5 (1.5) | 67.3 (1.6) | 851 | 76.2 (1.5) | 74.5 (1.5) |
| Lineage | ||||||||||||
| Null, C/pre-B | 553 | 59.0 (2.1) | 56.2 (2.1) | 1349 | 63.5 (1.3) | 60.8 (1.3) | 1731 | 65.0 (1.1) | 61.7 (1.2) | 1676 | 77.8 (1.0) | 74.8 (1.1) |
| T | 58 | 39.7 (6.4) | 37.9 (6.4) | 139 | 47.5 (4.2) | 46.8 (4.2) | 205 | 50.6 (3.5) | 50.1 (3.5) | 210 | 68.6 (3.2) | 66.6 (3.3) |
| CNS disease | ||||||||||||
| Yes | 9 | 66.7 (15.7) | 66.7 (15.7) | 29 | 51.7 (9.3) | 48.3 (9.3) | 31 | 61.3 (8.7) | 54.8 (8.9) | 38 | 62.9 (7.9) | 62.9 (7.9) |
| No | 784 | 56.8 (1.8) | 54.6 (1.8) | 1583 | 62.4 (1.2) | 59.7 (1.2) | 2059 | 63.1 (1.1) | 59.9 (1.1) | 1897 | 77.2 (1.0) | 74.3 (1.0) |
| t(9;22) | ||||||||||||
| Yes | 11 | 27.3 (13.4) | 27.3 (13.4) | 26 | 23.1(8.3) | 19.2 (7.7) | 43 | 43.9 (7.6) | 38.8 (7.5) | |||
| No | 536 | 61.2 (2.1) | 58.5 (2.1) | 1630 | 63.6 (1.2) | 60.3 (1.2) | 1791 | 78.1 (1.0) | 75.4 (1.0) | |||
| t(1:19) | ||||||||||||
| Yes | 16 | 87.5 (8.3) | 87.5 (8.3) | 47 | 72.3 (6.5) | 72.3 (6.5) | 51 | 80.4 (5.6) | 80.4 (5.6) | |||
| No | 814 | 61.1 (1.7) | 58.6 (1.7) | 1611 | 62.6 (1.2) | 59.3 (1.2) | 1546 | 77.2 (1.1) | 74.3 (1.2) | |||
| t(4;11) | ||||||||||||
| Yes | 12 | 33.3 (13.6) | 33.3 (13.6) | 15 | 13.3 (8.8) | 13.3 (8.8) | 17 | 64.7 (11.6) | 64.7 (11.6) | |||
| No | 818 | 62.0 (1.7) | 59.5 (1.7) | 1643 | 63.4(1.2) | 60.1 (1.2) | 1813 | 77.3 (1.0) | 74.5 (1.1) | |||
Event-free survival (standard error)
Table 2.
Outcome by lineage/NCI risk group for four trials
| Trial | Lineage | Standard risk |
High risk |
||||
|---|---|---|---|---|---|---|---|
| N | 5yr EFS | 10yr EFS | N | 5yr EFS | 10yr EFS | ||
| UKALLVIII | Null, C/preB | 407 | 61.9 (2.4) | 59.0 (2.4) | 135 | 51.9 (4.3) | 49.6 (4.3) |
| T | 12 | 41.7(14.2) | 41.7 (14.2) | 46 | 39.1 (7.2) | 37.0 (7.1) | |
| UKALLX | Null, C/preB | 984 | 68.2 (1.5) | 65.5 (1.5) | 344 | 52.3 (2.7) | 49.7 (2.7) |
| T | 27 | 59.3 (9.5) | 59.3 (9.5) | 111 | 45.0 (4.7) | 44.1 (4.7) | |
| UKALLXINull, | C/preB 1 | 257 | 70.1 (1.3) | 66.8 (1.3) | 474 | 51.3 (2.3) | 48.7 (2.3) |
| T | 41 | 51.2 (7.8) | 48.8 (7.8) | 163 | 50.8 (3.9) | 50.8 (3.9) | |
| ALL97 | Null, C/preB | 1134 | 82.9 (1.1) | 80.5 (1.2) | 541 | 66.8 (2.0) | 63.3 (2.1) |
| T | 39 | 84.6 (5.8) | 81.9 (6.2) | 171 | 64.9 (3.6) | 63.1 (3.7) | |
Protocol specific treatment outcome
UKALLVIII (1980–1984, 17–21 years of follow-up)
Forty-one of the 825 patients enrolled failed induction (non-remitters + deaths in induction), giving a 95% remission rate. There have been 56 (7%) deaths in remission; six from second malignancies and the EFS rates were 57% (1.7) at 5 years, and 54% (1.7) at 10 years (see Table 1). A total of 14 patients suffered a second malignancy as a first event. Overall survival was 71.5% (1.6) at 5, and 65% (1.7) at 10 years. The cumulative risk of isolated CNS relapse at 5 years was 9% (1.1) and of any CNS relapse 14% (1.3). For the 20 infants under 1 year of age at diagnosis treated on this protocol, the EFS was 30% (10.2) at 10 years.
Patients who received induction Daunorubicin (arm B) had a 6% rate of induction failure (non-remitters + deaths), which was double that of arm A (3%). However, there were reductions in marrow, testicular and combined relapses (30% of all relapses arm B, 38% arm A; P = 0.06 odds ratio 0.8 with 95% confidence intervals of 0.6–1.1), but not of isolated CNS relapse. Deaths in early remission were also higher in those receiving Daunorubicin (8% vs. 4% of remitters). As a consequence of these contrasting risks and benefits, there was no significant event-free or overall survival benefit for receipt of Daunorubicin26. A meta-analysis of all trials of the addition of an anthracycline shows a small, non-significant, EFS benefit for the inclusion of daunorubicin, but about 2/3 of the data are from UKALLVIII27. For the late randomisation between 2 and 3 years of continuing therapy more relapses were seen after stopping therapy at 2 years (P value for relapse-free survival 0.04), but this advantage was counterbalanced by a 4% death rate in remission with the longer course of therapy, and a higher retrieval rate for patients relapsing after 2 years only of treatment. Consequently, there was no significant survival benefit for those receiving 3 years of continuing therapy on this trial28.
Patients in this trial were not stratified for therapy-based on any initial presenting features. Although the event-free survival was superior to all previous UKALL protocols and comparable to results of the CCG 160 series, there was a persistent adverse impact of gender (male sex), age under 1 year, age ≥10 years, excess blasts (>5%) at day 15 and presenting white count ≥ 50 (especially above 100), and apparently of T-cell lineage, although in this trial that feature was not independent of the presenting white count.
UKALL X (1985 – 1990; 17–22 years follow-up)
Forty of the 1612 patients enrolled failed induction (non-remitters + deaths) giving a remission rate of 98%. There were 68 deaths in remission (4%), 18 after a second malignancy. A total of 30 patients suffered second malignancy as a first event. The EFS rates for the whole trial were 62% (1.2) at 5 years and 60% (1.2) at 10 years. Overall survival was 77% (1.0) at 5 years and 71% (1.1) at 10 years and 59.1% at 15 years. The cumulative rate of isolated CNS relapse was 7% (0.7) at 5, and 10 years, and 12% (0.9) for any CNS relapse. For the 26 infants under 1 year of age at diagnosis EFS was 27% (8.7) at 10 years.
1171 children were randomised to receive intensification at 5 weeks (arm B), 20 weeks (arm C), both (arm D) or neither (arm A). The 5-year EFS was 71% (2.7) for those receiving two blocks, compared with 62% (2.8) on arm C, 63% (2.8) on arm B and 57% (2.9) on arm A (without any intensification). At 10 years the EFS on arms D, C, B, A were 67% (2.8), 58% (2.9), 62% (2.8), 55% (2.9), respectively.
The benefits of intensification therapy were seen irrespective of any clinical presenting features previously shown to influence outcome such as age, gender and initial leukocyte count, with a 12% improvement in long term EFS for those patients randomised to receive two courses of intensification therapy. Further analyses showed that all subgroups of children, without exception, benefited from double intensification.
In this study, age, leukocyte count and gender were the most significant prognostic features. Response to therapy was also important, as assessed by day 15 bone marrow blast percentages. The most significant adverse effect was noted for the 31 patients with M3 marrows at day 15 (32% EFS compared with 63% for those with an M1 marrow). There was little difference between patients with M1 and M2 marrows at day 15. As in UKALL VIII, children with common or pre-B ALL appeared to fare better than those with T-cell immunophenotype on univariate analysis, but after stratification for age, gender and leukocyte count, the immunophenotype was no longer significant.
CNS disease at diagnosis did not confer an overall adverse prognosis on the 29 patients so identified in this trial. Fifty-one per cent of patients had successful cytogenetic analyses and the group of patients with high hyperdiploidy had a more favourable outcome. There was a worse prognosis for those with t(4;11) and for t(9;22). The 16 patients with t(1;19)(23;p13), thought at the time to be an unfavourable characteristic, had a more favourable outcome15.
UKALL XI (1990 – 1997; 10–17 years follow-up)
Fourteen of the 2090 children enrolled failed induction (non-remitters + deaths) giving a remission rate of 99%. There were 42 deaths in remission (2%), of which twelve were patients who had second malignancies. A total of 16 patients suffered a second malignancy as their first event.
The EFS rate for the whole trial was 63% (1.1) at 5 years, and 60% at 10 years, with an overall survival rate of 85% (0.8) at 5 and 79% (0.9) at 10 years. The cumulative risk of isolated CNS relapse was 7% (0.6) at 5 and 10 years and 15% (0.8) for any CNS relapse.
Following the change in protocol after May 1992, when the induction Daunorubicin was dropped, there was a retrospective analysis on the effects of induction Daunorubicin. The first 1419 children in the trial were previously reported, of whom 342 received Daunorubicin26, 29. Forty-four percent of the recipients of Daunorubicin completely cleared their marrow of blast cells after 8 days, compared with only 13% of the non-recipients (X2= 158.2, P value <0.0001). In addition, 9% of patients who received Daunorubicin, but 15% of those who did not, had more than 80% blasts at day 8 (X2 = 7.7, P value 0.006). In both groups independently, the rate of disease clearance correlated with disease-free survival, but there was no significant difference in overall outcome when comparing the two groups with each other, either for disease-free or relapse-free survival.
UKALL XI demonstrated a disease-free survival benefit for those allocated the third block of intensification at week 35: 69% (1.7) versus 60% (1.8) at 5 years and 66% (1.7) v 56% (1.8) at 10 years. The difference arose principally because of fewer bone marrow relapses amongst patients who received three intensification pulses (10 year bone marrow relapse rate 25% vs 34%). The third block did not significantly affect overall survival (82% (1.4) versus 79% (1.5)), since there appeared to be better salvage in patients who had not received it. There was no evidence for any difference in EFS benefit endowed by the third block of intensification for specific sub-groups defined by age, white count, immunophenotype or international risk groups. The addition of data for patients in the succeeding trial ALL 97, who were also randomised between three and two blocks, gave the same outcome7.
1513 patients with initial white cell counts of <50×109/dl were randomised between a long course of intrathecal methotrexate and high-dose methotrexate with subsequent intrathecal therapy. There was no significant difference in EFS or OS for the two groups, nor was there any significant heterogeneity of treatment effect of groups by age, gender, immunophenotype, or cytogenetic features. High dose methotrexate was significantly better than IT methotrexate alone in preventing isolated CNS relapse (p=0.008; 3% (0.7) versus 7% (1.0) at 10 years), and preventing isolated plus combined CNS relapses (p=0.04; 11% (1.2) versus 15% (1.4)). Non-CNS relapses and deaths in remission were similar with either treatment. In this group of patients, those with T-cell disease fared worse than those with a pre-B phenotype (53% (6.9) compared to 65% (1.3)) The worse outcome was due to an increase in both CNS and non-CNS relapses, which occurred with both treatments and which remained significant after adjustment for age, gender, and initial white cell count (P = 0.03)8. For the group of patients with initial white cell counts of ≥50×109/l, who were randomised between high dose methotrexate with continuing intrathecal chemotherapy and cranial radiotherapy with a short course of intrathecal chemotherapy, there was no significant difference in EFS. All CNS relapses in this high risk group, both isolated and combined, were reduced in the radiotherapy arm (P = 0.08) with a cumulative actuarial incidence at 10 years of 19% (3.5) for radiotherapy, compared to 28% (4.0) for high-dose methotrexate. There were fewer isolated CNS relapses in the radiotherapy group, although this difference was not significant, but this advantage was offset by a non-significant increase in the number of non-CNS relapses. As a consequence there was no significant difference in EFS and OS between the treatment arms, nor was there significant heterogeneity of effect by age, gender, immunophenotype, or cytogenetic features.
In contrast to the T-cell patients with white cell counts less than 50×109/l, T-cell patients with initial white cell counts in excess of 50×109/l fared as well as those with a pre-B phenotype, regardless of the treatment received.
In the low white cell group the incidence of isolated testicular relapse was 6.3% (1.0), with one relapse occurring beyond 10 years, and in the high white cell group 6.4% (2.1) with no evidence of randomised treatment effect in either group.
The adverse prognostic significance of male sex, age ≥10 years and white count ≥50 × 109/l persisted in this trial, as did the presence of t(9;22) and t(4;11) (see table 1). T-cell lineage per se was significant in univariate analysis, but not when stratified by white count, age and gender. CNS disease at diagnosis was not of prognostic significance.
ALL97 (1997–2002; (6–11 years follow-up)
A total of 1948 patients were registered in the trial between January 1997 and June 2002, 13 of whom were subsequently excluded because of incorrect diagnoses, leaving 1935 for further analyses. There were 24 induction failures, giving a remission rate of 99%, and 66 deaths in remission (4%), of which 5 were from secondary tumours. A total of 11 patients suffered a secondary tumour as their first event. EFS for the whole trial was 77% (1.0) at 5 years and 74% (1.0) at 10 years, and overall survival was 86% (0.8) and 83% (0.9) respectively. The isolated CNS relapse rate was 4% (0.5) and the rate of any CNS relapse was 7% (0.6) at 5 and 10 years9.
This trial was the first to use cytogenetics in risk stratification, thus routine FISH screening for the important chromosomal abnormalities with prognostic significance was introduced. This approach ensured that patients with the high risk abnormalities, BCR-ABL1 fusion and t(4;11) were identified in all cases, including those with a normal karyotype and failed cytogenetic result. The good risk associated with high hyperdiploidy was confirmed and patients with the favourable risk abnormality: t(12;21)(p13;q22)/ETV6-RUNX1 (TEL-AML1) fusion, were accurately detected in 22% of patients.
Nine hundred and ninety-seven patients were treated in the ALL97 phase (846 treated on ALL97 and 151 very high risk treated on HR124), and 938 were in the ALL97/99 phase. Of the 938 patients in ALL97/99, 578 (62%) were initially treated with regimen A and 360 (38%) with regimen B. Twenty-one regimen A patients and 133 regimen B patients were transferred to regimen C because of slow early response (SER) or unfavourable cytogenetic abnormality, so that overall 557 were treated with regimen A (standard risk), 227 with regimen B (high risk) and 154 with regimen C (very high risk). Of this latter group, 112 were considered to be very high risk solely because of a slow early response to induction chemotherapy; 40 patients had high-risk cytogenetic abnormalities, 13 of whom also had a slow early response. Two patients had M2 marrows at day 29, but were not slow early responders and did not have high-risk cytogenetics.
Diagnostic characteristics were similar between the 2 phases but a greater proportion of patients in ALL97 were not randomised for steroid (20% versus 13%; p<0.0001), and of these, only one patient in ALL97 received dexamethasone by choice compared with 15 in ALL97/99.
Among 1603 randomised patients, those allocated dexamethasone had half the risk of an isolated CNS relapse (p=0.001; 2.2% (0.5) versus 5.3% (0.8)). Both CNS relapse, and non-CNS relapses (p=0.005; 11% (1.2) versus 16% (1.4)) were reduced. Event-free survival was significantly improved with dexamethasone (p=0.0002; 83% (1.2) and 81% (1.4) versus 76% (1.5) and 73% (1.6) at five and ten years, respectively), with no evidence of differing effects in any subgroup of patients. The use of dexamethasone throughout treatment led to a decrease in the risk of relapse for all risk-groups of patients9.
1493 patients were randomised between Thioguanine and Mercaptopurine during interim maintenance and continuing therapy, with all patients receiving Thioguanine in the intensification phases. There was no difference in event-free or overall survival between the two arms. Although 6-Thioguanine halved the risk of isolated CNS relapse compared to 6-Mercaptopurine (p=0.02; 2.5% (0.6) versus 5% (0.8)), the benefit was offset by an increased risk of death in remission (p=0.01; 4% (0.7) versus 2% (0.5)), mainly due to infections during continuing therapy. Additionally, 95 patients developed veno-occlusive disease of the liver. Of this group, 85 were randomly assigned 6-Thioguanine representing 11% of all 6-Thioguanine recipients. Longer term follow-up revealed that 5% of 6-Thioguanine recipients had evidence of non-cirrhotic portal hypertension due to periportal liver fibrosis or nodular regenerative hyperplasia10.
Outcome by phase of trial
Both event-free (figure 5; p=0.0009) and overall survival (figure 6; p=0.004) were significantly better in ALL97/99 than in ALL97 (80.0% (1.3) versus 74.0% (1.4) and 88.0% (1.1) versus 83.5% (1.2), respectively at 5 years). ALL97 showed improvement in EFS, but not in overall survival, compared with the previous trial, UKALLXI. The risk of isolated CNS relapse for patients in ALL97/99 was significantly lower than for patients in ALL97 (p=0.04), with a 5 year isolated CNS relapse rate of 3.0% (0.6) for the former compared to 4.9% (0.7) for the latter. The overall CNS relapse rate was also significantly less for ALL97/99 (p<0.00005; 4.4% (0.7) versus 9.6%(1.0) at 5 years). There were no significant differences in the rates for non-CNS relapse, induction deaths or deaths in remission between the 2 phases of the trial12.
The use of two phases of differing albeit related types of treatment permits a non-randomised comparison of the effects of various presenting features such as gender, age group, WCC group, immunophenotype, and thiopurine type, to see if there were significant differences between the relative effects within these subgroups. For all disease-related outcomes there was no evidence of a difference in effect, with one possible exception. There was a suggestion that the relative risk reduction for isolated CNS relapse with ALL97/99 might be greatest for those aged 10 years and above (p-value for heterogeneity=0.03); however, because of the large number of statistical tests carried out this result cannot be regarded as statistically significant and should be interpreted as a chance effect.
127 patients had CNS relapses, of which 72 were isolated CNS relapses. The actuarial isolated CNS relapse rate in ALL97/99 patients randomised to dexamethasone was only 1.8% (0.7) at 5 years compared to 3.7% (1.0) in patients randomised to prednisolone. These rates are much lower than reported in previous trials which used cranial radiotherapy for CNS directed therapy. The incidence of isolated CNS relapse in ALL97/99 was reassuringly low, even for sub-groups perceived to be at higher risk of CNS relapse, such as those patients with WCC > 100 × 109/l (4.8%) or T-cell phenotype (3.8% (2.2)), despite restricting the use of cranial radiotherapy only to patients with overt CNS disease (CNS 3, <5% of all patients).
There were 38 patients presenting with CNS disease at diagnosis, 18 (1.8%) in ALL97 and 20 (2.1%) in ALL97/99. Six of the 18 patients in ALL97 with CNS disease at diagnosis were treated on ALL-HR1, i.e. they had other high-risk features at the time of diagnosis. Five of the 20 ALL97/99 patients were standard risk and 15 were high risk. By the end of the follow up period (31st October 2008), 7 of the 18 ALL97 patients with CNS disease at diagnosis had died and 2 further patients had received a BMT (1 related donor, 1 unrelated donor). Out of the 20 ALL97/99 patients, 7 had died, and 1 patient had suffered an isolated bone marrow relapse followed by an unrelated donor BMT.
Patients in ALL97/99 who transferred to regimen C, whether because of slow early response or because of high risk cytogenetics only still had a relatively poor prognosis, with an EFS at 5 years of 58.8% (4.0) (SER 58.9% (4.4), high risk cytogenetics 58.6% (9.1)).
In the ALL97/99 phase of the trial, there was no significant difference in the proportion with slow early response by randomised steroid. Excluding patients who transferred to regimen C solely because of high risk cytogenetics or M2 marrow at day 29, 46 (12%) of the patients randomised to dexamethasone, compared with 58 (15%) of those randomised to prednisolone, were transferred to C because of a slow early response, p=0.2.
Treatment results by Rome/NCI criteria and lineage in each era
Table 2 shows the event-free survival at 5 and 10 years by Rome/NCI criteria and lineage for all patients treated in each time period on the relevant trial. Patients with indeterminate lineage are also excluded from this table.
The proportion of patients defined as standard risk by the NCI criteria in these trials is 65–70% and high risk 30–35%. The 5-year and 10-year event-free survival has progressively improved in each consecutive trial for standard risk B cell lineage ALL. The NCI criteria have clearly delineated those with B cell lineage and a more adverse outcome, but for those high-risk patients survival did not improve during the period of 1980–1997, but improved with the advent of ALL97/99. For T-cell ALL only in UKALL X did the criteria clearly define a less favourable prognostic group. There is not a consistent improvement with time for the small numbers of standard risk T cell patients, whereas there does appear to be a more favourable trend for high risk T cell patients. Overall in UKALL X and XI high-risk T cell patients do not appear to fare significantly worse than high risk-B lineage patients, whilst standard-risk T cell patients do appear to experience more events than those with B-lineage ALL and standard risk features.
ALL97 and ALL97/99 ran from 1997 up until 2001, and subsequent follow-up takes this era to 2005. The comparisons within subgroups by NCI risk group/lineage are shown in table 4. The benefit of dexamethasone and the lack of difference between thioguanine and mercaptopurine seem to be consistent across groups.
Table 4.
ALL97 randomised treatment results
| Allocation | Lineage | Standard risk |
High risk |
||||
|---|---|---|---|---|---|---|---|
| N | 5yr EFS | 10yr EFS | N | 5yr EFS | 10yr EFS | ||
| Prednisolone | Null, C/pre-B | 517 | 79.6 (1.8) | 76.7 (1.9) | 203 | 66.9 (3.3) | 62.9 (3.4) |
| T | 17 | 82.4 (9.2) | 76.5 (10.3) | 38 | 68.4 (7.5) | 65.8 (7.7) | |
| Dexamethasone | Null, C/pre-B | 521 | 86.7 (1.5) | 84.9 (1.6) | 208 | 74.4 (3.0) | 69.4 (3.4) |
| T | 15 | 86.7 (8.8) | 86.7 (8.8) | 46 | 69.6 (6.8) | 67.3 (6.9) | |
| MP | Null, C/pre-B | 500 | 83.2 (1.7) | 80.7 (1.8) | 164 | 73.2 (3.4) | 69.9 (3.6) |
| T | 13 | 84.6 (10.0) | 84.6 (10.0) | 40 | 70.0 (7.2) | 70.0 (7.2) | |
| TG | Null, C/pre-B | 483 | 84.8 (1.6) | 82.6 (1.7) | 176 | 70.4 (3.4) | 65.6 (3.6) |
| T | 22 | 86.4 (7.3) | 81.3 (8.5) | 42 | 73.8 (6.8) | 69.0 (7.2) | |
| 3rd block | Null, C/pre-B | 105 | 75.2 (4.2) | 70.4 (4.5) | 38 | 65.8 (7.7) | 65.8 (7.7) |
| T | 4 | 100 | 75.0 (21.7) | 4 | 25.0 (21.7) | 25.0 (21.7) | |
| No 3rd block | Null, C/pre-B | 103 | 85.4 (3.5) | 82.5 (3.7) | 35 | 74.3 (7.4) | 65.7 (8.0) |
| T | 5 | 80.0 (17.9) | 80.0 (17.9) | 5 | 60.0 (21.9) | 40.0 (21.9) | |
| High-dose MTX | Null, C/pre-B | - | 21 | 76.2 (9.3) | 76.2 (9.3) | ||
| T | - | 8 | 62.5 (17.1) | 62.5 (17.1) | |||
| Cranial irrad | Null, C/pre-B | - | 22 | 81.8 (8.2) 77.3 (8.2) | |||
| T | - | 9 | 77.8 (13.9) | 66.7 (15.7) | |||
Overall conclusions on prognostic factors
Throughout the time period 1980–2001, features consistently associated with a more favourable prognosis include female gender, age between 1 and 9 years, white count under 50 × 109/l, M1 status (5% marrow blasts) achieved by either day 8 or day 15 and more recently, a high hyperdiploid karyotype and the presence of the ETV6-RUNX1 fusion.
DISCUSSION
The improvement in outcome seen in the UK in the early 1980s owed greatly to collaboration with the then US Children’s Cancer Group (CCG), and adoption of one of its standard risk protocols for all UK patients. This decision led to an 11% improvement in disease free survival compared with the previous standard treatment in UKALL trials II to VII and were at least as good for standard risk patients as the result reported by the CCG using the same regimen4,5. The subsequent trial, UKALL X, demonstrated a continuing improvement in disease control, particularly for patients who received two intensification modules, though even patients treated with regimen A, with no intensification, had better overall survival than patients who received identical treatment on arm B of UKALL VIII, largely due to reductions in induction and remission death rates6. In UKALL XI, disease control, especially prevention of bone marrow relapse was better in patients who received three intensification modules compared to patients who received only two modules, but this advantage did not translate into improved overall survival7. It appears those patients who relapse after only two intensification modules were more salvageable than patients relapsing after three. The result of the UKALL R1 relapse study demonstrated improvement in retrieving patients who relapsed more than 6 months off therapy with chemotherapy alone (5 year EFS of 57%), compared to other published series; this finding might in part at least, explain why overall but not event-free survival in the UK during the era 1990–1997 was comparable to that reported by the CCG30. However, event-free survival in standard risk and more markedly in high-risk patients clearly lagged behind CCG and other cooperative group results. Although patients were being retrieved following relapse, the price of this extra therapy was greater toxicity.
In its initial guise, ALL97, which ran from 1997 to 2001 was a direct descendent of the UKALL X and UKALL XI regimens, the innovations being the randomisations to examine the potential benefits of dexamethasone over prednisolone; and of Thioguanine over Mercaptopurine. Patients at very high risk of relapse, identified by chromosomal abnormalities or the Oxford Hazard score24 were treated on a different regimen, HR124 which was, again, a direct descendant of the UKALL X and XI regimens, although it did not include the steroid or thiopurine randomisations.
As a consequence of the comparisons of UK results29 with the results obtained by the BFM31 and CCG32 groups, which confirmed that both UKALL X and XI gave unsatisfactory results for high risk patients, the UK group decided to adapt US Children’s Cancer Group (CCG) style regimens CCG 1991 and 1961, which included stratification not only by initial risk factors (age, white cell count) but also by early response to therapy. This phase of the trial was designated ALL97/99. The event free survivals (EFS) for both ALL97 and ALL97/99 were better than previous UKALL trials, as was the overall survival (OS) for ALL97/99. Both EFS and OS were significantly better in ALL97/99 than in ALL97.
The cumulative risk of isolated CNS relapse did not increase with the replacement of cranial irradiation in UKALL VIII and X by long-course intrathecal methotrexate or by high-dose methotrexate with intrathecal therapy in UKALL XI; or the adoption of long-course intrathecal methotrexate for all patients in ALL 97 and ALL 97/99. Isolated CNS relapse was reduced with the combination of high-dose methotrexate and intrathecal methotrexate in UKALL XI for patient with an initial white cell count of 50 or higher, but there was no advantage for disease free or overall survival compared to intrathecal therapy only.
A major improvement in CNS relapse rate was seen in ALL97/99, where the rate was nearly halved, for both standard and high risk patients, regardless of which steroid – prednisolone or dexamethasone -was given, so that for all groups together there was a highly significant reduction in CNS relapse risk. The best result was seen in ALL97/99 patients randomised to dexamethasone, where the actuarial isolated CNS relapse rate in was only 1.8% at 5 years (95% CI: 0.4%–3.2%) compared to 3.7% (1.7%–5.7%) in patients randomised to prednisolone. These rates are much lower than reported in previous trials that used cranial radiotherapy for CNS directed therapy9, 33. The incidence of isolated CNS relapse in ALL97/99 was reassuringly low, even for sub-groups perceived to be at higher risk of CNS relapse, such as those patients with WCC > 100 × 10 9/l (4.8%) or T-cell phenotype (3.8%), despite restricting the use of cranial radiotherapy only to patients with overt CNS disease (CNS 3, <5% of all patients).
The reasons for the failure of UKALL trials to significantly improve event-free survival in a fashion seen by other cooperative groups in the period 1980–1997 are not clear. Possible factors include the use of prednisolone at a dose of 40mg/m2 rather than 60mg/m2 or the use of dexamethasone; omission of Daunorubicin from induction; sub-optimal route of administration dosage and scheduling of asparaginase, the necessity of a lengthy gap (often over 3 weeks) in therapy to allow marrow recovery after the week 5 and week 20 intensifications in UKALL X and XI; the form of the UKALL X and XI intensifications and the possible lack of strict and intensive compliance with the delivery of continuation therapy. The adoption of ALL97/99 resolved many of these potential issues, but in an uncontrolled fashion so that it is now possible only to speculate on which of these factors might have been most relevant. The importance of adequate delivery of continuation therapy was addressed by Chessells et al34 who were able to show in UKALL X that patients who had one or more episodes of neutropenia (absolute neutrophil count <0.05×109/L) during the continuation phase had a better outcome than patients who had no documented periods of neutropenia.
The current study, ALL2003, has built on the foundation provided by ALL97/99. The only substantial change in therapy from the latter trial has been the adoption of pegylated asparaginase throughout the induction and intensification phases. The trial is examining the role of minimal residual disease detection as a means of characterising an additional group of patients who might benefit from augmented therapy, as has previously been demonstrated for morphological slow early responders35; and a group of patients who can maintain a high expectation of cure even after therapy reduction which in this instance is the omission of the second delayed intensification module. This trial should conclude in 2010, and its successor is now being designed.
During the periods of ALL97, ALL 97/99 and ALL2003 it has become apparent that both short and longer-term toxicity, particularly associated with the use of dexamethasone has become a major concern, as has the incidence of treatment related mortality. These factors have been accentuated by the very high survival rates now being achieved. Additionally, it seems possible to identify, early in therapy, a small group of patients for whom current regimens offer only a small prospect of cure and who might therefore be considered for additional early treatment and first remission bone marrow transplant. Thus the next UK trial will most likely focus on toxicity reduction for the great majority of patients, who appear to be readily curable using relatively simple, conventional, chemotherapy regimens, and intensification of therapy for a minority of patients who might yet prove curable if the benefits of very early identification and intensification, including bone marrow transplantation, can be realized.
Table 3.
UKALLXI randomised treatment results
| Allocation | Lineage | Standard risk |
High risk |
||||
|---|---|---|---|---|---|---|---|
| N | 5yr EFS | 10yr EFS | N | 5yr EFS | 10yr EFS | ||
| C (late block) | Null, C/pre-B | 46 | 60.9 (7.2) | 58.6 (7.3) | 16 | 62.5 (12.1) | 56.3 (12.4) |
| T | 1 | - | - | 4 | 75.0 (21.7)75.0 (21.7) | ||
| D (both) | Null, C/pre-B | 47 | 74.5 (6.4) | 74.5 (6.4) | 18 | 61.1 (11.5) | 50.0 (11.8) |
| T | 0 | 2 | 50.0 (35.4)50.0 (35.4) | ||||
| i.t. MTX | Null, C/pre-B | 596 | 68.3 (1.9) | 64.8 (2.0) | 83 | 59.0 (5.4) | 52.9 (5.5) |
| T | 21 | 61.9 (10.6) | 57.1 (10.8) | 4 | 25.0 (21.7) | 25.0 (21.7) | |
| High-dose MTX | Null, C/pre-B | 588 | 72.2 (1.8) | 68.4 (1.9) | 74 | 52.7 (5.8) | 51.3 (5.8) |
| T | 19 | 42.1 (11.3) | 42.1 (11.3) | 9 | 77.8 (13.9) | 77.8 (13.9) | |
| High-dose MTX | Null, C/pre-B | - | 99 | 49.5 (5.0) | 49.5 (5.0) | ||
| T | - | 52 | 46.2 (6.9) | 46.2 (6.9) | |||
| Cranial irrad | Null, C/pre-B | - | 105 | 54.3 (4.9) | 50.5 (4.9) | ||
| T | - | 42 | 59.5 (7.6) | 59.5 (7.6) | |||
| 3rd block | Null, C/pre-B | 461 | 74.0 (2.0) | 70.9 (2.1) | 153 | 59.5 (4.0) | 56.9 (4.0) |
| T 14 | 50.0 (13.4) | 50.0 (13.4) | 44 | 47.7 (7.5) | 47.7 (7.5) | ||
| No 3rd block | Null, C/pre-B | 466 | 67.3 (2.2) | 63.1 (2.2) | 161 | 44.7 (3.9) | 42.8 (3.9) |
| T | 19 | 47.4 (11.5) | 47.4 (11.5) | 38 | 55.3 (8.1) | 55.3 (8.1) | |
Acknowledgement
The authors acknowledge the many co-investigators who contributed to the design, conduct and previous reporting of the four trials that are the subject of this updated report.
This work was supported by Medical Research Council grant number G8223452.
Footnotes
Conflict of interest: None of the four authors has any competing financial interest in the work and results included in this report.
REFERENCES
- 1.Medical Research Council UKALL Trials 1972–84. Improvement in treatment for children with acute lymphoblastic leukaemia. Report to the Council by the Working Party on Leukaemia inChildhood. Lancet. 1986;i:408–411. [PubMed] [Google Scholar]
- 2.Lilleyman JS, Richards S, Rankin A, Medical Research Council Leukaemia Trial UKALL VII Archives of Diseases in Childhood. 1985;60:1050–1054. doi: 10.1136/adc.60.11.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riehm H, Gadner H, Henze G, Kornhuber B, Langermann HJ, Muller-Weinrich S, Schellong G. Acute lymphoblastic leukaemia. Treatment results in 3 BFM studies (1970–81) In: Murphy SB, Gilbert JR, editors. Leukaemia Research: Advances in Cell Biology and Treatment. Elsevier Biomedical; New York: 1998. pp. 251–263. [Google Scholar]
- 4.Coccia PF. Development and preliminary findings of the Children’s Cancer Study Group protocols (161,162 and 163) for low, average and high risk acute lymphoblastic leukaemia in children. In: Murphy SB, Gilbert J, editors. Leukaemia Research: Advances in Cell Biology and Treatment. Elsevier Biomedical; New York: 1998. pp. 240–250. [Google Scholar]
- 5.Eden OB, Lilleyman JS, Richards S, Shaw MP, Peto J. Results of the MRC Leukaemia Trial UKALL VIII. British Journal of Haematology. 1991;78:187–196. doi: 10.1111/j.1365-2141.1991.tb04415.x. [DOI] [PubMed] [Google Scholar]
- 6.Chessells JM, Bailey CC, Richards SM, Medical Research Council Working Party on Childhood Leukaemia Intensification of treatment and survival in all children with lymphoblastic leukaemia: results of the UK MRC Trial UKALL X. Lancet. 1995;345:143–148. doi: 10.1016/s0140-6736(95)90164-7. for the. [DOI] [PubMed] [Google Scholar]
- 7.Hann I, Vora A, Richards SM, Hill F, Gibson B, Lilleyman J, et al. UK Medical Research Council Working Party on Childhood Leukaemia Benefit of intensified treatment for all children with acute lymphoblastic leukaemia: results from MRC UKALL XI and MRC ALL97 randomised trials. Leukemia. 2000;14:356–363. doi: 10.1038/sj.leu.2401704. on behalf of the. [DOI] [PubMed] [Google Scholar]
- 8.Hill F, Hann I, Gibson B, Eden OB, Richards S. Comparison of high dose methotrexate with continuing intrathecal methotrexate vs intrathecal methotrexate alone in low white blood count childhood acute lymphoblastic leukaemia: preliminary results from the UKALL XI randomised trial. Blood. 1998;92(Suppl. 1):398a. [Google Scholar]
- 9.Mitchell CD, Richards SM, Kinsey SE, Lilleyman JE, Vora A, Eden TO. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL97 randomized trial. British Journal of Haematology. 2005;129(6):734–45. doi: 10.1111/j.1365-2141.2005.05509.x. [DOI] [PubMed] [Google Scholar]
- 10.Vora A, Mitchell CD, Lennard L, Eden TO, Kinsey SE, Lilleyman J, Richards SM. Toxicity and Efficacy of Thioguanine Compared With Mercaptopurine in Childhood Lymphoblastic Leukaemia: results of the UK Medical Research Council randomised trial ALL97. Lancet. 2006;368:1339–1348. doi: 10.1016/S0140-6736(06)69558-5. [DOI] [PubMed] [Google Scholar]
- 11.Chessells JM, Harrison G, Richards SM, Gibson BE, Bailey CC, Hill FGH, Hann IM. Failure of a new protocol to improve treatment results in paediatric lymphoblastic leukaemia: lessons from UK Medical Research Council trials UKALL X and UKALL XI. British Journal of Haematology. 2002;118:445–455. doi: 10.1046/j.1365-2141.2002.03647.x. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell CD, Payne J, Wade R, Vora A, Kinsey SE, Richards SM, Eden TOB. The impact of risk stratification by early bone-marrow response in childhood lymphoblastic leukaemia: results from the United Kingdom Medical Research Council trial ALL97 and ALL97/99. British Journal of Haematology. 2009;146:424–436. doi: 10.1111/j.1365-2141.2009.07769.x. [DOI] [PubMed] [Google Scholar]
- 13.Lilleyman JS, Hann IM, Stevens RF, Richards SM, Eden OB, Chessells JM, Bailey C. Cytomorphology of childhood lymphoblastic leukaemia: a prospective study of 2000 patients. British Journal of Haematology. 1992;81:52–57. doi: 10.1111/j.1365-2141.1992.tb08170.x. [DOI] [PubMed] [Google Scholar]
- 14.Hann IM, Richards SM, Eden OB, Hill FGH, Medical Research Council Childhood Leukaemia Working Party Analysis of the immunophenotype of children treated on the MRC UKALL Trial XI. Leukemia. 1998;12:1249–1255. doi: 10.1038/sj.leu.2401093. on behalf of the. [DOI] [PubMed] [Google Scholar]
- 15.Harrison CJ, Martineau M, Secker-Walker L. The Leukaemia Research Fund/United Kingdom Cancer Cytogenetics Group Karyotype Database in acute lymphoblastic leukaemia: a valuable resource for patient management. British Journal of Haematology. 2001;113(1):3–10. doi: 10.1046/j.1365-2141.2001.02643.x. [DOI] [PubMed] [Google Scholar]
- 16.Chessells JM, Swansbury GJ, Reeves B, Bailey LL, Richards S, Medical Research Council Working Party in Childhood Leukaemia Cytogenetics and prognosis in childhood lymphoblastic leukaemia: results of MRC UKALL X. British Journal of Haematology. 1997;99:93–100. doi: 10.1046/j.1365-2141.1997.3493163.x. [DOI] [PubMed] [Google Scholar]
- 17.Harrison CJ, Moorman AV, Barber KE, Broadfield ZJ, Cheung KL, Harris RL, et al. Interphase molecular cytogenetic screening for chromosomal abnormalities of prognostic significance in childhood acute lymphoblastic leukaemia: a UK Cancer Cytogenetics Group Study. British Journal of Haematology. 2005;129:520–530. doi: 10.1111/j.1365-2141.2005.05497.x. 1. [DOI] [PubMed] [Google Scholar]
- 18.Eden OB, Shaw MP, Lilleyman JS, Richards S. Non-randomised study comparing toxicity of Escherichia coli and Erwinia asparaginase in children with lymphoblastic leukaemia. Medical and Pediatric Oncology. 1990;18:497–502. doi: 10.1002/mpo.2950180612. [DOI] [PubMed] [Google Scholar]
- 19.Bleyer WA, Coccia PF, Sather HN. Reduction in central nervous system leukaemia with a pharmacokinetically derived intrathecal methotrexate dosage regimen. Journal of Clinical Oncology. 1983;1:317–325. doi: 10.1200/JCO.1983.1.5.317. [DOI] [PubMed] [Google Scholar]
- 20.Pinkerton CR, Bowman A, Holtzel H, Chessells JM. Intensive consolidation chemotherapy for acute lymphoblastic leukaemia(UKALL X pilot study) Archives of Diseases in Childhood. 1987;62:12–18. doi: 10.1136/adc.62.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chessells JM, Bailey CC, Wheeler K, Richards SM. Bone marrow transplantation for high risk childhood lymphoblastic leukaemia in first remission: experience in MRC UKALL X. Lancet. 1992;340:565–568. doi: 10.1016/0140-6736(92)92103-m. [DOI] [PubMed] [Google Scholar]
- 22.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukaemia in childhood. New England Journal of Medicine. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 23.Moe PJ, Wesenberg F, Kolmannskog S. Methotrexate infusions in poor prognosis ALL: 11 high dose methotrexate (HDM) in acute lymphoblastic leukaemia in childhood. A pilot study from 1981. Medical and Pediatric Hematology and Oncology. 1986;14:187–188. doi: 10.1002/mpo.2950140316. [DOI] [PubMed] [Google Scholar]
- 24.Kinsey SE, Mitchell CD, Harrison G, Richards S. Treatment of High Risk Leukaemia – Results of MRC Childhood Acute Lymphoblastic Leukaemia Study MRC HR-1. Blood (ASH Annual Meeting Abstracts) 2002;100:768a. [Google Scholar]
- 25.Chessells JM, Richards SM, Bailey CC, Lilleyman JS, Eden OB. Gender and treatment outcome in childhood lymphoblastic leukaemia: report from the MRC UKALL trials. British Journal of Haematology. 1995;89:364–372. doi: 10.1111/j.1365-2141.1995.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 26.Lilleyman JS, Gibson BS, Stevens RF, Will AM, Hann IM, Richards SM, Hill FGH. Clearance of marrow infiltration after 1 week of therapy for childhood lymphoblastic leukaemia: clinical importance and the effect of daunorubicin. British Journal of Haematology. 1997;97:603–606. doi: 10.1046/j.1365-2141.1997.1002914.x. [DOI] [PubMed] [Google Scholar]
- 27.Childhood Acute Lymphoblastic Leukaemia Collaborative Group (CALLCG) Beneficial and harmful effects of anthracyclines in the treatment of childhood acute lymphoblastic leukaemia: a systematic review and meta-analysis. British Journal of Haematology. 2009;145:376–388. doi: 10.1111/j.1365-2141.2009.07624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards S, Peto R, Gray R, Childhood ALL Collaborative Group Duration and intensity of maintenance chemotherapy in acute lymphoblastic leukaemia: overview of 42 trials involving 12,000 randomised children. Lancet. 1996;347:1783–1788. doi: 10.1016/s0140-6736(96)91615-3. [DOI] [PubMed] [Google Scholar]
- 29.Eden OB, Harrison G, Richards S, Lilleyman JS, Bailey CC, Chessells JM, et al. Long-term follow-up of the United Kingdom Medical Research Council Protocols for Childhood Acute Lymphoblastic Leukaemia 1980–97. Leukemia. 2000;14:2307–2320. doi: 10.1038/sj.leu.2401962. [DOI] [PubMed] [Google Scholar]
- 30.Lawson SE, Harrison G, Richards S, Oakhill A, Stevens RF, Eden OB, Darbyshire PJ. The UK experience in treating relapsed childhood acute lymphoblastic leukaemia: a report on the Medical Research Council UKALL R1 study. British Journal of Haematology. 2000;108:531–543. doi: 10.1046/j.1365-2141.2000.01891.x. [DOI] [PubMed] [Google Scholar]
- 31.Schrappe M, Reiter A, Zimmermann M, Harbott J, Ludwig WD, Henze G, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Leukaemia. 2000;14:2205–2222. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- 32.Gaynon PS, Trigg ME, Heerema NA, Sensel MG, Sather HN, Hammond GD, Bleyer WA. Children’s Cancer Group trials in childhood acute lymphoblastic leukaemia: 1983–1995. Leukemia. 2000;14:2223–2233. doi: 10.1038/sj.leu.2401939. [DOI] [PubMed] [Google Scholar]
- 33.Childhood ALL Collaborative Group. Clarke M, Gaynon P, Hann I, Harrison G, Masera G, Peto R, Richards S. CNS-directed therapy for childhood acute lymphoblastic leukaemia: overview of 43 randomised trials involving 13,162 children. Journal of Clinical Oncology. 2003;21:1798–1809. doi: 10.1200/JCO.2003.08.047. Writing committee. [DOI] [PubMed] [Google Scholar]
- 34.Chessells JM, Harrison G, Lilleyman JS, Bailey CC, Richards SM, MRC Working Party in Childhood Leukaemia Continuing (maintenance) therapy in lymphoblastic leukaemia: lessons from MRC UKALLX. British Journal of Haematology. 1997;98:945–951. doi: 10.1046/j.1365-2141.1997.3113127.x. on behalf of the. [DOI] [PubMed] [Google Scholar]
- 35.Nachman JB, Sather HN, Sensel MG, Trigg ME, Cherlow JM, Lukens JN, et al. Augmented post-induction therapy for children with high risk acute lymphoblastic leukaemia and a slow response to initial therapy. New England Journal of Medicine. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]