Abstract
Little is known about costs related to the surveillance of patients that have undergone curative resection of colorectal cancer. The aim of this study was to calculate the observed surveillance costs for 385 patients followed-up over a 3-year period, to estimate surveillance costs if French guidelines are respected, and to identify the determinants related to surveillance costs to derive a global estimation for France, using a linear mixed model. The observed mean surveillance cost was € 713. If French recommendations were strictly applied, the estimated mean cost would vary between € 680 and € 1 069 according to the frequency of abdominal ultrasound. The predicted determinants of the cost were: age, recurrence, duration of surveillance since diagnosis, and adjuvant treatments. For France, the surveillance cost represented 4.4% of the cost of colorectal cancer management. The cost of surveillance should now be balanced with its effectiveness and compared with surveillance alternatives.
Keywords: Aged, Colorectal Neoplasms, epidemiology, Costs and Cost Analysis, Female, France, epidemiology, Humans, Male, Mass Screening, economics, Middle Aged, Population Surveillance, Registries
Keywords: economic evaluation, cost prediction, colorectal cancer, recurrence, surveillance
1. Introduction
Colorectal cancer is a major health problem. It is one of the most frequent cancers in both sexes in France with approximately 37,500 new cases in 2005 [7]. Data collected by the Burgundy Registry of Digestive Cancers in the administrative area of Côte d’Or between 1976 and 1995 indicate that about 80% of the patients undergo primary resection for cure. Nonetheless, about 9% of these patients will develop a loco-regional recurrence, and 21% of patients will develop metastases following their initial surgical resection. Almost 80% of these recurrences will appear within the first three years after surgery [22].
Current French guidelines for post-surgery management of colorectal cancer issued following the 1998 French Consensus Conference [1] are:
clinical examination every 3 months for the first 2 years, every 6 months for the next 3 years;
abdominal ultrasound every 3 to 6 months for the first 3 years, then yearly for 2 years;
chest x-ray yearly for 5 years;
colonoscopy after three years, or, if the initial colonoscopy detected at least three adenomas with one >1 cm diameter or presenting a villous component, a colonoscopy performed after 1 year.
In contrast with economic burden associated with the diagnosis and the initial therapeutic management of colorectal cancer, little is known about the costs related to the long-term surveillance of this cancer and their possible variation depending on whether guidelines are respected or not. A recent study performed on a data set of the Burgundy Registry of Digestive Cancers revealed that adherence to French guidelines for surveillance was relatively poor with a large proportion of patients followed-up below the recommendations (47%), and smaller proportions followed-up either in respect of (24%) or above the guidelines (29%) [6]. Therefore, due to the variability of surveillance patterns, there is uncertainty on the cost associated with surveillance practices. The possibility of a difference between the observed cost and the expected cost of surveillance if guidelines were respected can thus be questioned. The estimation of surveillance cost is of great interest for the French health care insurance system, which reimburses, in a context of scare resources, all medical procedures performed during the surveillance period. Moreover, better knowledge of the cost of surveillance may contribute to the discussion between decision makers and the scientific community on the need to reconsider the content of guidelines, or if the diffusion of these guidelines should be improved.
Therefore, the aim of our population-based study was to determine the cost of surveillance patterns and to compare it with the expected cost of surveillance if French guidelines had been respected. The secondary objective was to identify cost determinants in order to derive a global estimation of surveillance costs for France, and determine what proportion of the total cost of colorectal cancer management is taken up by surveillance.
2. Materials and methods
2.1. Study population
This study included all patients living in two French administrative areas (Côte d’Or and Saône-et-Loire), with a total population of 1,631,100 according to the 2005 census, who were diagnosed with a first colorectal cancer between January and December 1998. These patients were identified by the Burgundy Registry of Digestive Cancer which has recorded all digestive tract cancers occurring in Côte d’Or and Saône-et-Loire since 1976. Cancers were classified according to the TNM classification, T indicating the size or direct extent of the primary tumour, N indicating the degree of spread to regional lymph nodes and M indicating the presence of metastases. TNM stages I, II, III and IV correspond respectively to stages 1 to 4 of the Dukes classification [25].
2.1.1. Inclusion criteria
All patients diagnosed with TNM I, II and III colorectal cancer which could be resected with curative intent were included. In addition, patients with curatively treated stage IV tumours with a surgically removed single liver metastasis were also eligible. Finally 473 patients matched these inclusion criteria.
2.1.2. Exclusion criteria
Patients with evidence of progressive second cancer (n=3), and patients followed for less than 6 months because of recurrence or death after initial surgery (n=63) were excluded. Twenty-two patients with incomplete information on the management of their surveillance were also excluded, leading to a final study population of 385 patients (Figure 1).
Figure 1.
Flow chart
2. Data collection
2.2.1. Demographic and clinical data
Most initial data were extracted from the registry database. Demographic data included: age, gender, administrative area. Place of residence was also recorded: patients living in towns with more than 2,000 inhabitants were considered as urban residents. Those living in towns with less than 2,000 inhabitants were considered as rural residents. Tumours were characterized according to TNM stage (I, II, III, IV) and location (rectum, colon) defined according the International Classification of diseases, 9th Revision. Clinical modifications (ICD-9-CM), and adjuvant treatment (radiotherapy, chemotherapy) were also extracted. The registry database did not provide any information on other clinical baseline data such as comorbidities defined by the Charlson index [9], preoperative complications (occlusion and perforation), recurrence and date of recurrence of the primary tumour, and on surveillance procedures. We thus collected data from all of the physicians involved in the diagnosis and management of patients during the three first years after curative surgery. The names of surgeons, gastroenterologists, general practitioners were first identified from the registry database. Their medical records were systematically reviewed in order to collect information about surveillance procedures and to identify other physicians (oncologists or other specialists) involved in the surveillance of patients through correspondence and radiological, ultrasound, biological and endoscopic reports. Data on geographical access to medical care were also recorded. These data referred to the distance that a patient had to travel from his/her place of residence to consult a general practitioner or a gastroenterologist. Distances are expressed in kilometres. Calculations were computed using ‘Michelin road network tables’ [2]. When a patient’s medical record contained no names of general practitioners or gastroenterologists, a theoretical distance was computed from the patient’s residence to the nearest general practitioner and gastroenterologist (distance was zero when there was a practice in the patient’s town). The decision to include distance to the general practitioner and gastroenterologist was justified by the fact that these doctors performed most of the routine clinical examinations for surveillance [13].
2.2.2. Surveillance data
The medical records from the physicians were systematically searched for information on all surveillance procedures recommended by the 1998 French Consensus Conference for management after curative colic surgery [1]. These surveillance procedures were: clinical examination, abdominal ultrasound, chest x-ray, and colonoscopy. All other surveillance tests were also recorded: tumour markers such as carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA19-9), other blood tests (cell blood count, liver enzymes, gammaglutamyltranspeptidase, bilibirubin, lactate dehydrogenase, alcaline phosphatase, glutamic-oxaloacetic transaminase, ionogram and creatinine), and other radiographic or endoscopic examinations such as computed tomography, magnetic resonance imaging, scintigraphy and endorectal ultrasonography.
For each patient, all of the surveillance procedures were collected for every quarter (i.e. every 3 months) during the period related to the surveillance. The surveillance period started at the date of curative surgery and ended at the date of the procedure showing the first sign of recurrence, or at the end of the 3-year surveillance period if no recurrence occurred.
2.3. Economic analysis
2.3.1. Economic data
The economic analysis was performed from the French national health insurance perspective. Only direct medical costs, expressed in Euros (€) were included. Reimbursement prices were used as a proxy of costs. These prices were obtained from three fixed-price scales commonly used in France. Until 2005, the only way to calculate the cost of examinations reimbursed by the French national health insurance system was to use the “Nomenclature Générale des Actes Professionnels” (NGAP), and the “Nomenclature des Actes de Biologie Médicale” (NABM), two price scales of medical and biological procedures. In 2005, a new price scale was introduced: the “Classification Commune des Actes Medicaux” (CCAM), which should take the place of the NGAP and NABM. At the moment however, three scales are used simultaneously. In this study, the most recent versions of the NGAP, NABM and CCAM were used, the NGAP for the year 2005 to estimate the cost of clinical examinations, the NABM for the year 2005 to estimate the cost of tumour markers, and the CCAM for the year 2007 to estimate the cost of all radiographic and endoscopic examinations. All reimbursement prices used in the cost analysis are given in Table 1. The cost of biological tests was not taken into account in the analysis because 75% of this cost was found among patients treated by chemotherapy. Thus it was assumed that it was related to the specific follow-up of chemotherapy patients, and could therefore not be included in surveillance costs related to colorectal cancer.
Table 1.
Unit costa of procedures related to surveillance
| Procedures related to surveillance | Unit price (€) | Source |
|---|---|---|
| Medical consultations | ||
| General practitioner | 20 | NGAPb |
| Specialist (surgeon, oncologist, gastroenterologist) | 23 | NGAP |
| Medical examinations | ||
| Abdominal ultrasound | 75.6 | CCAMb |
| Chest x-ray | 21.3 | CCAM |
| Colonoscopy | 153.6 | CCAM |
| Rectosigmoidoscopy | 57.6 | CCAM |
| Rectoscopy | 20.6 | CCAM |
| Abdominal computed tomography scan | 111.8 | CCAM |
| Thoracic computed tomography scan | 86.5 | CCAM |
| Magnetic resonance imaging | 152.9 | CCAM |
| Scintigraphy | 193.2 | CCAM |
| Endorectal ultrasound endoscopy | 128.6 | CCAM |
| Tumour markers | ||
| Carcinoembryonic antigen (CEA) | 18.9 | NABMb |
| Cancer antigen 19-9 (CA19-9) | 18.9 | NABM |
Reimbursement prices were used as a proxy of costs
NGAP, NABM and CCAM are French price scales defining amounts to be reimbursed by the French National Insurance. NGAP (year 2005) = « Nomenclature Générale des Actes Professionnels »; NABM (year 2005) = « Nomenclature des Actes de Biologie Médicale »; CCAM (year 2007) = « Classification Commune des Actes Médicaux ».
2.3.2. Observed costs
Observed surveillance costs were calculated by summing up, quarter by quarter, the costs of all of the recorded medical procedures performed (consultations, radiographic and endoscopic examinations, and tumour markers).
2.3.3. Expected costs of surveillance according to the French guidelines
The expected surveillance costs were estimated for each patient, quarter by quarter, taking into account the duration of the surveillance period and the French Conference Consensus guidelines (medical procedures recommended and intervals between medical procedures). First, the expected number of clinical examinations, abdominal ultrasounds, chest x-rays, and colonoscopies as recommended by the Conference Consensus was estimated for each patient according to the duration of the surveillance (until recurrence or until the end of the three-year surveillance period if no recurrence was detected). Then the expected cost of surveillance was calculated using the NGAP, NABM and CCAM for each patient and for each quarter of their surveillance period. Because abdominal ultrasound could be performed either every 3 or every 6 months according to the French guidelines, two expected surveillance costs were estimated. The first corresponded to the situation where abdominal ultrasound was performed every 3 months; the second corresponded to the situation where abdominal ultrasound was performed every 6 months.
Discounting was not be applied because observed surveillance costs and expected surveillance costs were estimated over the same period of time, ie 3 years.
2.4. Statistical analysis
The z-test was used to determine the statistical difference between the observed surveillance cost and the two estimated surveillance costs. The difference between surveillance costs was considered significant when the p value was lower than 0.05.
In order to identify independent determinants of the observed surveillance cost, a multivariable linear mixed model analysis of variance for repeated data was used [21]. The cost related to the surveillance, log-transformed to reduce its skewness, was used as the dependent variable. Variables introduced into the model were: the quarter, defined as a time variable from 1 to 12 and assumed to have a linear effect on the cost; recurrence categorised into three categories: absence of recurrence, recurrence detected during a medical visit for symptoms, recurrence detected during a routine medical visit; age categorised as: <65, 65–74, ≥75; gender; administrative area (Saône-et-Loire, Côte d’Or); urban or rural area of residence; tumour location (rectum, colon); TNM stage at diagnosis (I, II, III+IV), preoperative complications (No, Yes), comorbidities (No, Yes); adjuvant chemotherapy (No, Yes); adjuvant radiotherapy (No, Yes), distance to general practitioner (0 kilometers, > 0 kilometers) and distance to the gastroenterologist (<15 kilometers, ≥ 15 kilometers). A coefficient above 0 for a given dummy indicator meant that the surveillance cost was higher for a subgroup compared to the reference subgroup.
In the multivariable linear mixed model analysis of variance for repeated data, we stipulated Yi as the ni dimensional cost vector for subject i, 1≤i≤N, N being the number of subjects. Therefore the linear mixed-effects model with serial correlation could be written as:
Xi and Zi were (ni x p) and (ni x q) known design matrices, β was the p-dimensional vector containing the fixed effects and bi ~ N(0,D) was the q-dimensional vector containing the random effects. εi ~N(0, σ2 Ini) was a ni -dimensional vector of measurement error components. Serial correlation was captured by using a Gaussian stochastic process, Wi, which is assumed to follow an N(0, τ2 Hi) law. The serial covariance matrix Hi only depends on i through the number ni of observations and through the time points tij at which measurements are taken. The fixed-effects parameters captured the influence of explanatory variables on the mean structure, exactly as in the standard linear model. However, the occurrence of random effects and a structured covariance matrix distinguished the linear mixed model from the standard linear model by taking into account repeated measurements of the same experimental unit, with spatially correlated data. Part of the covariance structure arises from so-called “random effects” (i.e., additional covariate effects with random parameters). These are effects that arise from the characteristics of individual subjects. A compound symmetry covariance structure and an autoregressive variance structure were tested. The choice of the covariance structure was based on the Akaike information criteria. The analysis was conducted using SAS package version 9.1.
2.5. Model validation
The model was validated by comparing the observed average cost per patient to the predicted average surveillance cost for nine hypothetical patient profiles with different age (<65, 65–74, ≥75), and TNM stage of the tumour (I, II, III+IV). The “smearing estimator” developed by Duan, et al, was used to back-transform the log of cost in order to allow cost predictions [16]. Stratification of the surveillance cost according to the age and the TNM stage distribution was justified by the fact that stage is known to be a major prognostic factor. Moreover, age and stage are two basic parameters characterizing the clinical status of the patient at the time of diagnosis and may strongly influence the intensity of surveillance.
3. Results
3.1. Sample characteristics
Overall, 385 patients were included in this study. Among them, 98 (25%) presented a recurrence before the end of the 3-year period. The remaining 287 patients were followed until the end of the 3-year period. (Table 2). Characteristics of the study population are detailed in Table 3. The mean age was 70.4 years (standard deviation 11.3 years). Sixty-three percent of patients were living in a rural area (n=242). The tumour was located in the colon for 255 patients (66%). Only 17% patients were diagnosed with stage I cancer (n=65). Most of the patients had neither comorbidities (n=273), nor preoperative complications (n=344). Adjuvant chemotherapy and radiotherapy was given to 105 (27%) and 58 (15%) patients respectively.
Table 2.
Vital status of the study population during the 3-year surveillance period (n=385)
| Status | n | % |
|---|---|---|
| Recurrence occurring during the 3-year period | 98 | 25% |
| Alive at the end of the 3-year period | 47 | |
| Dead from colorectal cancer recurrence | 49 | |
| Dead from another cause after recurrence | 2 | |
| Alive without recurrence at the end of the 3-year period | 287 | 75% |
Table 3.
Initial characteristics of the study population (n=385)
| Characteristic | n | (%) |
|---|---|---|
| Age | Mean 70.4 | |
| < 65 years | 108 | 28% |
| 65–74 years | 133 | 35% |
| ≥ 75 years | 144 | 37% |
| Gender | ||
| Male | 210 | 55% |
| Female | 175 | 45% |
| Urban area | ||
| Urban | 143 | 37% |
| Rural | 242 | 63% |
| Administrative area | ||
| Saône-et-Loire | 238 | 62% |
| Côte d’Or | 147 | 38% |
| Tumour location | ||
| Rectum | 130 | 34% |
| Colon | 255 | 66% |
| TNM stage at diagnosis | ||
| I | 65 | 17% |
| II | 204 | 53% |
| III + IV | 116 | 30% |
| Preoperative complications | ||
| No | 344 | 89% |
| Yes | 41 | 11% |
| Comorbidities | ||
| None | 273 | 71% |
| At least one | 112 | 29% |
| Adjuvant chemotherapy | ||
| No | 280 | 73% |
| Yes | 105 | 27% |
| Radiotherapy | ||
| No | 327 | 85% |
| Yes | 58 | 15% |
| Distance to the general practitioner | ||
| 0 kilometers | 244 | 63% |
| > 0 kilometers | 141 | 37% |
| Distance to the gastroenterologist | ||
| < 15 kilometers | 208 | 54% |
| ≥ 15 kilometers | 177 | 46% |
3.2. Observed and expected cost of surveillance
3.2.1. Observed cost
The total observed cost of the 3 years of surveillance was € 274,421 for the 385 patients included in the study (Table 4). The average cost per patient was thus € 713. Eighty-six percent of the total surveillance cost was associated with medical procedures recommended by French guidelines (€ 235,976). The cost of recommended procedures was mostly composed of clinical consultations and abdominal ultrasounds. Fourteen percent of the total cost of the 3 years of surveillance was associated with medical procedures that were not recommended by French guidelines (€ 38,445), mainly analyses for tumour markers.
Table 4.
Costa of recommended and non recommended medical procedures performed during the surveillance period (n=385)
| Procedures | Observed total cost (€) | Relative cost of medical procedures |
|---|---|---|
| Recommended procedures | ||
| Clinical examination | 92,878 | 39% |
| General Practitioner | 36,620 | 35% |
| Specialistb | 56 258 | 65% |
| Abdominal ultrasound | 91,382 | 39% |
| Chest X-ray | 14,939 | 6% |
| Colonoscopy | 36,778 | 16% |
| Total cost of recommended procedures | 235,976 | 86% |
| Non recommended procedures | ||
| CT scans | 10,709 | 17% |
| MRI | 918 | 1% |
| Scintigraphy | 773 | 1% |
| Endorectal ultrasound | 1,930 | 3% |
| Carcinoembryonic antigen (CEA) | 16,348 | 25% |
| Cancer antigen 19-9 (CA19-9) | 7,768 | 12% |
| Total cost of non recommended procedures | 38,445 | 14% |
| Total surveillance cost | 274,421 | |
| Average surveillance cost per patient | 713 | |
Reimbursement prices were used as a proxy of costs
Specialist included gastroenterologists, oncologists, surgeons and other specialists
3.2.2. Expected cost
The two expected costs of the surveillance period were estimated according to intervals for abdominal ultrasound recommended by French guidelines. They were compared to the observed surveillance cost (table 5). Results showed that the observed surveillance cost was close to the expected cost if abdominal ultrasound was performed every 6 months (respectively € 713 and € 680 on average per patient (p=0.04)). If abdominal ultrasound was performed every 3 months, the expected cost was clearly higher than the observed cost (respectively € 713 and € 1,069 on average per patient (p<0.001)).
Table 5.
Estimation of the total expected surveillance costa if French guidelines are respected
| Observed cost related to the surveillance (€) | Expected cost related to the surveillance (€) | ||
|---|---|---|---|
| 6-months abdominal ultrasound | 3-months abdominal ultrasound | ||
| Recommended procedures | |||
| Clinical consultations | 92,878 | 76,521 | |
| Abdominal ultrasound | 91,382 | 146,286 | 296,125 |
| Chest X-ray | 14,939 | 19,876 | |
| Colonoscopy | 36,778 | 19,134 | |
| Non recommended procedures | 38,445 | - | - |
| Total surveillance cost | 274,421 | 261,817 (p=0.04) b | 411,656 (p<0.001) b |
| Average surveillance cost | 713 | 680 | 1,069 |
Reimbursement prices were used as a proxy of costs
Comparison between the observed surveillance cost and the estimated surveillance cost was performed using a z-test at a significance level of 0.05.
3.3. Independent factors associated with the observed cost of 3 years of surveillance
The model was finally based on an autoregressive structure. Results of the multivariable analysis are shown in Table 6. After adjusting for the other covariates, adjuvant treatments, age, recurrence, and quarter were found to be significant determinants of surveillance costs. Both chemotherapy and radiotherapy were associated with higher surveillance costs compared to the absence of adjuvant treatment (p<0.0001 and p=0.03 respectively) even after adjustment for stage at diagnosis and after exclusion of costs of biological tests, which were assumed to be related to specific post-chemotherapy follow-up. Patients whose recurrence was detected during either a medical visit due to symptoms or during routine medical visit, were found to have a higher mean surveillance cost than patients with no recurrence during the 3-year period (p=0.005 and p<0.0001 respectively). Age was also related to surveillance costs: patients aged over 75 years had a lower cost than patients less than 65 years (p=0.007). Time since diagnosis was also found to be a determinant of surveillance costs. The longer the surveillance period, the lower the mean surveillance cost (p<0.0001).
Table 6.
Independent predictors of the observed surveillance costa. Multivariate analysis.
| Coeff | SD b | p-value c | |
|---|---|---|---|
| Quarter | −0.13 | 0.01 | <0.0001 |
| Recurrence versus absence of recurrence | |||
| Recurrence detected during medical visit for symptoms | 0.85 | 0.29 | 0.005 |
| Recurrence detected during routine medical visit | 1.42 | 0.30 | <0.0001 |
| Gender | |||
| Men versus women | 0.004 | 0.07 | 0.96 |
| Age at diagnosis | |||
| 65–74 years versus <65 years | −0.15 | 0.09 | 0.09 |
| ≥ 75 years versus <65 years | −0.25 | 0.09 | 0.007 |
| Urban area | |||
| Rural versus urban | 0.07 | 0.08 | 0.40 |
| Administrative area | |||
| Côte d’Or versus Saône et Loire | −0.09 | 0.07 | 0.20 |
| Tumour location | |||
| Colon versus rectum | −0.07 | 0.09 | 0.45 |
| TNM stage at diagnosis | |||
| II versus I | 0.18 | 0.10 | 0.06 |
| III +IV versus I | 0.22 | 0.12 | 0.07 |
| Preoperative complications | |||
| Yes versus no complications | −0.23 | 0.12 | 0.06 |
| Comorbidities | |||
| Yes versus no comorbidities | 0.05 | 0.08 | 0.48 |
| Adjuvant chemotherapy | |||
| Yes versus no chemotherapy | 0.93 | 0.10 | <0.0001 |
| Adjuvant radiotherapy | |||
| Yes versus no radiotherapy | 0.26 | 0.12 | 0.03 |
| Distance to the General Practitioner | |||
| > 0 kilometers versus 0 kilometers | 0.02 | 0.07 | 0.83 |
| Distance to the gastroenterologist | |||
| ≥ 15 kilometers versus <15 kilometers | −0.03 | 0.08 | 0.83 |
| Intercept | 3.39 | ||
Reimbursement prices were used as a proxy of costs
SD = standard deviation;
a p-value lower than 5% showed significant results.
3.4. Validation of the model
The model was validated by comparing the average observed surveillance cost to the average predicted surveillance cost for patients with colorectal cancer, alternative combinations of age category (<65, 65–74, ≥75) and stage at diagnosis (I, II, III+IV). Comparisons between predicted costs and observed costs are illustrated in Figure 2. This comparison is represented by the difference between observed and predicted cost quarter by quarter over the 3-year period related to the surveillance. Globally, predicted and observed costs are relatively concordant, even though a greater difference can be noticed among patients with stage III or IV cancer and those younger than 65 years. Patients younger than 65 years had predicted surveillance costs of € 642, € 1,140 and € 2,646, respectively, depending on the stage (I, II, III+IV). The corresponding observed costs were € 617, € 883 and € 996, respectively. For patients aged 65–74, average predicted costs were € 523, € 921 and € 1,574 respectively, while the observed costs were € 568, € 774 and € 847. For patients over 75 years old, predicted costs were € 489, € 604 and € 867, versus mean observed costs of € 660, € 526 and € 606, respectively.
Figure 2.
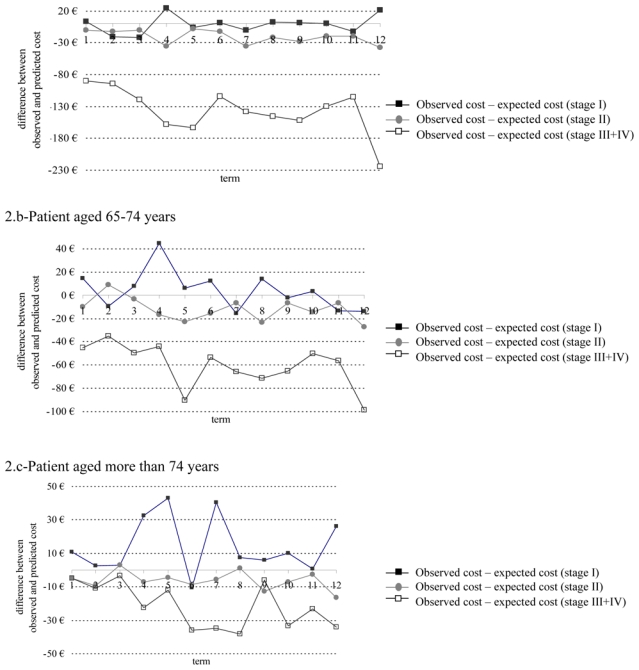
Difference between predicted and observed surveillance costs quarter by quarter over the 3-year period related to the surveillance for three profiles of patients (duration of quarter: 3 months, reimbursement prices were used as a proxy of costs)
3.5. Estimation of the economic burden of colorectal cancer surveillance in France
In order to assess surveillance costs in France, average age- and TNM stage-specific costs predicted by the linear mixed model were applied to the 36,000 new cases of colorectal cancer diagnosed in France in 2000 [24]. The distribution of these 36,000 colorectal cancers according to age and TNM stage at diagnosis was estimated using data from the Association of French Cancer Registries (Francim network), which covers 12 areas in France and provides French national estimations of cancer incidence and mortality from data of all French cancer registries [3]. As indicated in Table 7, the cost of surveillance in France was estimated to be € 42,357,068. To assess the economic burden of colorectal cancer surveillance in France, the surveillance cost was related to the total cost of treatment for colorectal cancer in France, estimated from the 36,000 new cases diagnosed in 2000, and the average costs of treatment of colorectal cancer per patient according to TNM stage, estimated with the database of the Burgundy Cancer Registry [11]. The cost of treatment of colorectal cancer in France was estimated at € 930,828,696. Therefore, the estimated cost of surveillance in France accounts for 4.4% of the total management cost for colorectal cancer (i.e. treatment cost and surveillance).
Table 7.
Estimation of surveillance costsa of colorectal cancer in France over a 3-year period according to age and TNM stage
| Age-and stage- specific distribution of colorectal cancers in 12 areas in France b | Distribution of the 36 000 new cases of colorectal cancers in France according to age-and stage for the year 2000 | Predicted surveillance cost per patient according to age and stage (mixed model) | Annual surveillance cost in France (36 000 colorectal cancers) | |
|---|---|---|---|---|
| Stage I | ||||
| < 65 years | 6.4% | 2 312 | 642 € | 1 484 110 € |
| 65–74 years | 6.7% | 2 424 | 523 € | 1 267 835 € |
| > 74 years | 6.3% | 2 274 | 489 € | 1 112 089 € |
| Stage II | ||||
| < 65 years | 7.7% | 2 787 | 1 140 € | 3 176 647 € |
| 65–74 years | 9.1% | 3 261 | 921 € | 3 003 720 € |
| > 74 years | 12.3% | 4 423 | 604 € | 2 671 772 € |
| Stage III + IV | ||||
| < 65 years | 14% | 5 198 | 2 646 € | 13 754 424 € |
| 65–74 years | 17% | 6 135 | 1 574 € | 9 657 072 € |
| > 74 years | 20% | 7 185 | 867 € | 6 229 400 € |
| Total | 100% | 36 000 | - | 42 357 068 € |
Reimbursement prices were used as a proxy of costs
Francim data (The Association of the French Cancer Registries)
4. Discussion
The first originality of this work lied in the estimation of the economic burden of observed surveillance practices compared to recommended surveillance guidelines. The study showed that the observed surveillance cost was near to the lowest boundary of the expected surveillance cost if French guidelines were respected. This result can be explained by the fact that almost two thirds of patients underused or respected the guidelines [6]
As far as we know, most of previously published international studies on the topic of colorectal cancer surveillance only assessed adherence to recommended guidelines. These studies showed that most of the time recommendations were not strictly followed, and suggested either that the content of existing guidelines be reconsidered or that practitioner should receive further training [17,18,19,10,12,20,23,26]. However, none of them compared the observed cost of surveillance with the expected costs if guidelines had been respected. To our knowledge, the only study that could be really compared to our work is French. This study estimated the cost of a surveillance period following curative resection for colorectal cancer using data from the Herault Tumor Registry [4,5]. Their results showed that the cost of the examinations not recommended by the Consensus Conference accounted for 30% of the total observed surveillance expenditures. The average cost per patient was estimated to be € 843 over a 5-year period. Our study showed some different results: the cost of non-recommended surveillance procedures accounted for 14% of the total cost of surveillance, and the average cost per patient was estimated to be € 713. This difference can be explained by the choice of the surveillance period (5 years versus 3 years in our study). The choice of a 3-year period in our study was justified by the fact that 80% of recurrences appear within the first three years after surgery [22]. The difference can also be explained by the exclusion of the costs of biological tests from the calculation of the observed surveillance cost. We estimated that three-quarters of the cost of biological tests was related to patients treated with chemotherapy. Therefore this cost could not really be considered as the cost related to the surveillance of colorectal cancer after curative surgery. One last explanation could be that our study was performed after the Consensus Conference whereas the Herault’ study was performed before. Indeed, patients included in the Herault’ study underwent a potentially curative resection of colorectal cancer in 1992.
Another original contribution of our work was the identification of the determinants of costs using a mixed model analysis of variance for repeated measurements. This model is known to manage repeated data. It was appropriate for our study because the history of individual costs was available at each quarter of the 3-year period. The other main advantage of the model is to allow meaningful interpretation of coefficients. This is not the case for other methods such as the Cox model [14]. The Cox model requires no specific assumptions on the distribution of costs and allows cost-to-event to be taken into-account. However, hazard ratios provided by the Cox model are difficult to interpret in practice when applied to costs. Our model was validated by comparing the observed and the expected cost of profiles of patients differing by their age and the TNM stage at diagnosis of their colorectal tumour. The results showed a relatively close concordance, except for the youngest patients and those with stage III or IV cancer. This could be explained by the small size of these groups of patients.
The model showed that surveillance costs were higher among patients treated with chemotherapy or radiotherapy than among patients without adjuvant treatment. These results could suggest that patients undergoing adjuvant treatment are followed more closely than patients without adjuvant treatment, even after exclusion of costs of biological tests performed during the surveillance period. Certain sociological and psychological factors may also influence the intensity of follow-up. Another important result was the influence of age on surveillance costs. A more advanced age (i.e patients over 74) was associated with a lower surveillance cost than that for younger patients. Low costs can be due to either quantitative factors (smaller number of consultations and radiographic and endoscopic examinations), and/or monetary factors (low cost examinations). A previous analysis on the same population comes in support of the finding in our study, and showed that inclusion in the intensive follow-up pattern rather than in the minimal follow-up pattern was independently predicted by age < 65 years, advanced tumour stage, chemotherapy and, with a borderline significance level, by radiation therapy [6].
One last original contribution of our study was to use determinants of the costs in order to derive a global estimation of surveillance costs for France as a whole. We showed that surveillance costs represented less than 5% of the total cost of colorectal cancer management. Although we can not rule out possible variations in surveillance costs across geographical areas, we have reasons to believe that our cost estimation is relevant for the whole of France, even though registry data set covers only two geographical areas. This estimation was based on an unselected population from two districts that may be considered representative of France as a whole because their colorectal cancer incidence and mortality rates, as well as their public and private cancer care facilities are comparable to the national average. Moreover, age and TNM stage distribution of cancer cases were close to those observed in all French districts covered by the Association of French cancer registries [3], which covers about 16% of the French population. Our global estimation, however, could be criticized because it was based on two parameters only: the age and TNM stage distribution. Even though they can be considered major parameters to characterize the status of the patient at the time of diagnosis, other variables such as tumour location or adjuvant treatment could have been included.
This work can be considered as a cost-of-illness study. This approach has often been criticized because the only information it provides is a global cost and an average cost per patient associated with a health care strategy [8]. Therefore, despite the originality of the topic, our results are not sufficient to suggest reconsidering the content of French guidelines. In this context, a cost-effectiveness analysis comparing a conventional surveillance program as recommended by French guidelines with an intensive surveillance program based on the use of computed tomography, abdominal ultrasound and colonoscopy as suggested by the American Society of Clinical Oncology in 2005 [15] could be of interest and help in decision making. As far as we know, no cost-effectiveness analysis has been undertaken in the French context yet. Our work could provide a framework by providing economic data required by such an analysis.
In conclusion, this population-based study showed that despite imperfect adherence to French guidelines, surveillance cost accounted for a small part of the total cost of management of colorectal cancer. Moreover surveillance costs were close to the expected costs if guidelines had been respected. The decision to reconsider the content of guidelines now depends on either new medical evidence, or medico-economic information.
Acknowledgments
The authors would like to thank: Dr F. Sassi (London School of Economics) for his methodological help; N. Thomas, E. Couturier, D. Carel, E. Naudet, and E. Monnet for providing data on geographic access to medical care.
Funding: This work has been funded by a SNFGE grants (Société Nationale Française de Gastroentérologie).
Footnotes
Conflict of interest: none
JEL CODES: C1 - Econometric and Statistical Methods: General C10: General C13: Estimation
References
- 1.Consensus Conference. Prevention, screening and management of the colonic cancers. Gastroenterol Clin Biol. 1998;22:S275–S288. [PubMed] [Google Scholar]
- 2.Michelin road network tables. 2008. http://www.viamichelin.com/viamichelin/fra/dyn/controller/ItiWGHomePage.
- 3.Francim network. 2009. http://www.invs.sante.fr/surveillance/cancers/surveillance_cancers.htm.
- 4.Borie F, Daures JP, Millat B, Folschveiller-Bruggeman M, Tretarre B. Surveillance du cancer colorectal opéré à visée curative dans le département de l’Hérault: Etude médico-économique. Gastroenterol Clin Biol. 2001;25:881–884. [PubMed] [Google Scholar]
- 5.Borie F, Daures JP, Millat B, Tretarre B. Cost and effectiveness of follow-up examinations in patients with colorectal cancer resected for cure in a French population-based study. J Gastrointest Surg. 2004;8:552–558. doi: 10.1016/j.gassur.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Boulin M, Lejeune C, Le Teuff G, Binquet C, Bouvier AM, Bedenne L, Bonithon-Kopp C. Patterns of surveillance practices after curative surgery for colorectal cancer in a French population. Dis Colon Rectum. 2005;48:1890–1899. doi: 10.1007/s10350-005-0096-7. [DOI] [PubMed] [Google Scholar]
- 7.Bouvier AM. Mass screening for colorectal cancer in France. BEH. 2009;2:14–16. [Google Scholar]
- 8.Byford S, Torgerson DJ, Raftery J. Economic note: cost of illness studies. Bmj. 2000;320:1335. doi: 10.1136/bmj.320.7245.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Cheung WY, Pond GR, Rother M, Krzyzanowska MK, Swallow C, Brierley J, Kaizer L, Myers J, Hajra L, Siu LL. Adherence to surveillance guidelines after curative resection for stage II/III colorectal cancer. Clin Colorectal Cancer. 2008;7:191–196. doi: 10.3816/CCC.2008.n.025. [DOI] [PubMed] [Google Scholar]
- 11.Clerc L, Jooste V, Lejeune C, Schmitt B, Arveux P, Quantin C, Faivre J, Bouvier AM. Cost of care of colorectal cancers according to health care patterns and stage at diagnosis in France [Epub ahead of print] Eur J Health Econ. 2007 doi: 10.1007/s10198-007-0083-0. [DOI] [PubMed] [Google Scholar]
- 12.Cooper GS, Kou TD, Reynolds HL., Jr Receipt of guideline-recommended follow-up in older colorectal cancer survivors: a population-based analysis. Cancer. 2008;113:2029–2037. doi: 10.1002/cncr.23823. [DOI] [PubMed] [Google Scholar]
- 13.Coriat R, Mahboubi A, Lejeune C, Bouvier AM, Bedenne L, Bonithon-Kopp C. How do gastroenterologists follow patients with colorectal cancer after curative surgical resection? A three-year population-based study. Gastroenterol Clin Biol. 2007;31:950–955. doi: 10.1016/s0399-8320(07)78303-3. [DOI] [PubMed] [Google Scholar]
- 14.Cox DR. Regression models and life tables. J R Statist Soc. 1972;34:187–220. [Google Scholar]
- 15.Desch CE, Benson AB, 3rd, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Pfister DG, Virgo KS, Petrelli NJ. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–8519. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 16.Duan N. Smearing estimate: A nonparametric retransformation method. J Am Stat Assoc. 1983;78:605–610. [Google Scholar]
- 17.Elston Lafata J, Simpkins J, Schultz L, Chase GA, Johnson CC, Yood MU, Lamerato L, Nathanson D, Cooper G. Routine surveillance care after cancer treatment with curative intent. Med Care. 2005;43:592–599. doi: 10.1097/01.mlr.0000163656.62562.c4. [DOI] [PubMed] [Google Scholar]
- 18.Giordano P, Efron J, Vernava AM, 3rd, Weiss EG, Nogueras JJ, Wexner SD. Strategies of follow-up for colorectal cancer: a survey of the American Society of Colon and Rectal Surgeons. Tech Coloproctol. 2006;10:199–207. doi: 10.1007/s10151-006-0280-3. [DOI] [PubMed] [Google Scholar]
- 19.Grossmann I, de Bock GH, van de Velde CJ, Kievit J, Wiggers T. Results of a national survey among Dutch surgeons treating patients with colorectal carcinoma. Current opinion about follow-up, treatment of metastasis, and reasons to revise follow-up practice. Colorectal Dis. 2007;9:787–792. doi: 10.1111/j.1463-1318.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 20.Harewood GC, Rathore O, Patchett S, Murray F. Assessment of adherence to published surveillance guidelines--opportunity to enhance efficiency of endoscopic practice. Ir Med J. 2008;101:248–250. [PubMed] [Google Scholar]
- 21.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 22.Manfredi S, Bouvier AM, Lepage C, Hatem C, Dancourt V, Faivre J. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg. 2006;93:1115–1122. doi: 10.1002/bjs.5349. [DOI] [PubMed] [Google Scholar]
- 23.Mulder SA, Ouwendijk RJ, van Leerdam ME, Nagengast FM, Kuipers EJ. A nationwide survey evaluating adherence to guidelines for follow-up after polypectomy or treatment for colorectal cancer. J Clin Gastroenterol. 2008;42:487–492. doi: 10.1097/MCG.0b013e31809e703c. [DOI] [PubMed] [Google Scholar]
- 24.Remontet L, Esteve J, Bouvier AM, Grosclaude P, Launoy G, Menegoz F, Exbrayat C, Tretare B, Carli PM, Guizard AV, Troussard X, Bercelli P, Colonna M, Halna JM, Hedelin G, Mace-Lesec’h J, Peng J, Buemi A, Velten M, Jougla E, Arveux P, Le Bodic L, Michel E, Sauvage M, Schvartz C, Faivre J. Cancer incidence and mortality in France over the period 1978–2000. Rev Epidemiol Sante Publique. 2003;51:3–30. [PubMed] [Google Scholar]
- 25.Sobin LH, Wittekind CH. International Union Against Cancer. 4. Springer-Verlag; Berlin: 1997. TNM Atlas. [Google Scholar]
- 26.Spratlin JL, Hui D, Hanson J, Butts C, Au HJ. Community compliance with carcinoembryonic antigen: follow-up of patients with colorectal cancer. Clin Colorectal Cancer. 2008;7:118–125. doi: 10.3816/CCC.2008.n.016. [DOI] [PubMed] [Google Scholar]


