Abstract
Central neurons develop and maintain molecularly distinct synaptic specializations for excitatory and inhibitory transmitters, often only microns apart on their dendritic arbor. Progress towards understanding the molecular basis of synaptogenesis has come from several recent studies using a coculture system of non-neuronal cells expressing molecules that generate presynaptic or postsynaptic ‘hemi-synapses’ on contacting neurons. Together with molecular properties of these protein families, such studies have yielded interesting clues to how glutamatergic and GABAergic synapses are assembled. Other clues come from heterochronic cultures, manipulations of activity in subsets of neurons in a network, and of course many in vivo studies. Taking into account these data, we consider here how basic parameters of synapses –competence, placement, composition, size and longevity – might be determined.
Introduction
Synaptogenesis involves a complex series of events, spanning neuronal differentiation, cell–cell contact and localized induction of presynaptic and postsynaptic differentiation. Synaptic specificity is determined by the developmental status of both partner cells, by neuronal and glial cues that influence competence for synaptogenesis, by long-range and local axon and dendrite guidance cues, by cell-adhesion molecules that mediate contact, and by local presentation of differentiation-inducing molecules. Although activity is a major force in sculpting circuitry during development [1] and regulates synaptic composition and strength [2–4], it is not essential for the basic assembly of synapses. Synapses form normally when neurotransmitter release is chronically blocked using clostridial neurotoxins or genetic methods [5–7]. We focus here on molecular cues involved in the later stages of synaptogenesis, once appropriate axons and dendrites are brought into proximity. Studies of several major synaptogenic molecules identified for glutamatergic and/or GABAergic synapses are summarized in Table 1, and partial molecular linkages are shown in Figure 1 [8–10]. We also focus on aspects of recent studies that particularly illuminate how basic parameters of synapses are shaped.
Table 1.
Major secreted or cell adhesion proteins implicated in genesis of glutamatergic and GABAergic synapsesa
| Synaptogenic protein [Refs] | Key binding partner | Family members and related proteins | Effect of bath addition, coculture, bead presentation or overexpression in neurons | Effect of targeted deletion in vivo, or of knock-down or dominant-negative forms in culture |
|---|---|---|---|---|
| Wnt7a [18] | Frizzled | >16 Wnts 10 Frizzleds |
Addition induced axon morphological change and synaptic vesicle clustering | Wnt7a−/− transiently reduced complexity and synapsin I content of glomerular rosettes between cerebellar mossy fibers at P8–P9 but not ≥P10 |
| FGF22 [21] | FGFR2 | >20 FGFRs 4 FGFs |
Addition induced axon branching and presynaptic specializations | FGFR2−/− reduced number and size of synapsin aggregates in the cerebellar granule layer FGFR2 soluble blocking reagents reduced number and size of synaptophysin-YFP aggregates in the cerebellar granule layer |
| TSPs 1 and 2 [22] | Several possible | 5 TSPs | Addition to glia-free culture induced postsynaptically silent synapses | (TSP1,2)−/− reduced numbers of SV2 puncta and opposed SAP102–bassoon puncta in cortex |
| N-cadherin [65,138,139] | N-cadherin | 5 Classical cadherins 12 Atypical cadherins >52 Protocadherins |
Overexpression had no effect on spine morphology or density in culture | Dominant-negative form reduced numbers of synapsin, FM-dye, GAD and PSD95 puncta more effectively at 1 week than at 3 weeks in hippocampal culture β-Catenin−/− reduced presynaptic content of non-docked vesicles and response to prolonged repetitive lowfrequency stimulation at hippocampal CA1 synapses; increased length of synaptophysin–GFP but not bassoon or PSD95–GFP puncta in hippocampal culture α-N-catenin−/− increased dendritic protrusion length and motility |
| Neuroligins [41,55,59–61] | Neurexins | 4 Neuroligins | Coculture or beads induced local glutamatergic and GABAergic presynaptic specializations Overexpression increased presynaptic input Moderate-level expression increased functional synapses |
RNAi knock-down of Nlg-1,2,3 in hippocampal culture reduced numbers of spines, VGlut1, GluR1 and VGAT puncta, and reduced mPSC amplitude and frequency; presumed mIPSC reduced most Mislocalized overexpression of Nlg-2 in hippocampa lculture reduced number and size of gephyrin, GABAAR, PSD95 and NMDAR puncta, and reduced mIPSC and mEPSC frequency and amplitude |
| β-Neurexin1 [60,62,66] | Neuroligins | 3β-Neurexins 3α-Neurexins Extensive alternative splicing |
Coculture or beads induced local glutamatergic and GABAergic postsynaptic specializations (clustering of PSD95, NMDAR, gephyrin and GABAAR) | (α-Neurexin-1, −2, −3)−/− (without disruption of β-neurexins) reduced numbers of GABAergic but not glutamatergic synapses, reduced N-type Ca2+ channel function, reduced spontaneous transmitter release, and greatly reduced evoked transmission in neonatal brainstem and cultured cortical slices |
| SynCAM1 [29,140] | SynCAMs | 4 SynCAMs 4 Nectins |
Coculture induced local glutamatergic presynaptic specializations Overexpression increased functional synaptic inputs |
Dominant-negative construct against SynCAMs and neurexins reduced number and size of FM-dye puncta in hippocampal culture |
| Ephrin-B1 [71,74,116,141] | EphBs | 5 A-ephrins 3 B-ephrins 9 EphA receptors 5 EphB receptors |
Addition of clustered B-ephrins induced clustering of EphB and NMDAR, and enhanced spine morphogenesis | Ephrin-B2−/− had no effect on number of synapses or NMDAR content in hippocampal CA1 region EphB2−/− reduced NMDAR content and NMDAR-mediated currents but had no effect on spine density in Hippocampus (EphB-1, −2, −3)−/− reduced number and size of spines and PSD area in hippocampal CA3region; increased length of dendritic protrusions, caused loss of spine heads and reduced NMDAR and AMPAR content in hippocampal culture |
| NARP [67–69] | AMPA receptors | NP1, NPR | Coculture induced local AMPAR clustering and cell-type-specific NMDAR clustering Overexpression increased cell-type-specific glutamatergic synaptogenesis |
Dominant-negative form reduced number of AMPAR and synaptophysin clusters but not gephyrin of GAD clusters in spinal cord culture; reduced number of AMPAR and NMDAR clusters on hippocampal interneurons but not pyramidal neurons |
Abbreviations: AMPAR, AMPA receptor; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; GABAAR, GABAA receptor; mEPSC, miniature excitatory postsynaptic current; mIPSC, miniature inhibitory postsynaptic current; mPSC, miniature postsynaptic current; NARP, neuronal activity-regulated pentraxin; NMDAR, NMDA receptor; NPR, neuronal pentraxin receptor; P, postnatal day; TSPs, thombospondins; VGAT, vesicular GABA transporter; VGlut1, vesicular glutamate transporter1; YFP, yellow fluorescent protein.
Figure 1.
Molecular components of glutamatergic (a) and GABAergic (b) synapses. Only some of the components are shown, emphasizing cleft and transmembrane proteins and their interacting partners. Solid lines indicate reported protein–protein interactions; broken lines indicate presumed indirect interactions. For references, see main text.
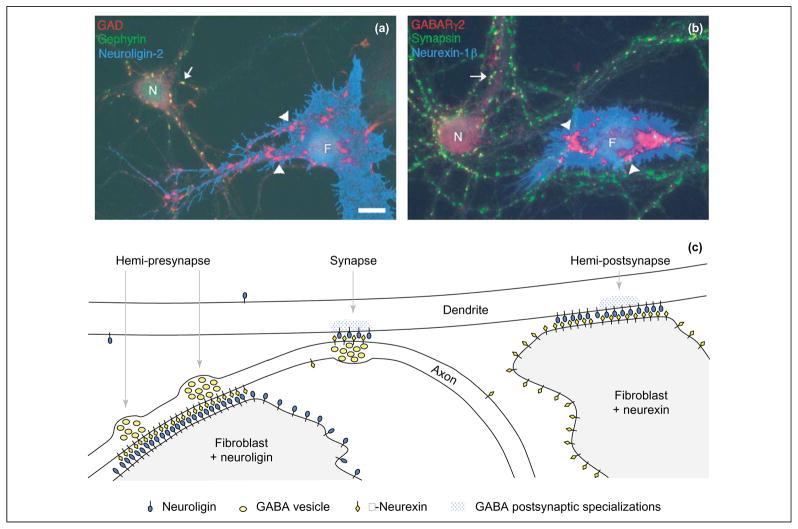
For this review, we consider a ‘synapse’ to mean a functional synapse (noting that other more limited definitions of synapses, based on structure or molecular composition, can be useful in many circumstances) (Box 1). We use ‘hemi-presynapse’ or ‘presynaptic differentiation’ to refer to clusters of release-competent synaptic vesicles, and ‘hemi-postsynapse’ or ‘postsynaptic differentiation’ to refer to clusters of surface neurotransmitter receptors and associated signaling and scaffolding molecules. These hemi-synaptic elements can be combined in a bona fide synapse or induced in isolated axons or dendrites by individual synaptogenic molecules (Figure 2).
Box 1. Working definitions of a synapse.
The ideal assay for building a functional synapse is one that tests both presynaptic and postsynaptic function, such as local input stimulation resulting in a local dendritic potential change. However, such assays are not yet possible at the single-synapse level for most CNS synapses. Thus, other working definitions of synapses must be considered. Ultrastructural features consisting of presynaptic vesicles apposed to a postsynaptic density via a uniform cleft mark a synapse [133]. Apposition of immunoreactivity for molecular components (i.e. clusters of postsynaptic receptors or scaffolding proteins apposed to synaptic vesicle proteins) also marks a synapse. Ultrastructurally or molecularly defined synapses might be presynaptically or postsynaptically silent. Less conventional assays are particularly important when studying early development [134] because synapses might acquire their fully mature properties over time.
A working definition of a hemi-synapse is essential when testing the ability of candidate synaptogenic molecules to induce presynaptic or postsynaptic differentiation in isolated axons or dendrites (Figure 2). The same ultrastructural features and combinations of molecular markers can be used as for the corresponding half of a conventional synapse [55,60]. In addition, isolated presynaptic function can be probed by activity-stimulated uptake andreleaseof FM dyes [29,55,57]. Isolated postsynaptic functionis moredifficulttoassay,but couldbeprobed by precise mapping of transmitter response [135]. An interesting twist using the coculture system is the generation of miniature postsynaptic current (mPSC)-like events from induced presynaptic specializations by engineering the non-neuronal cell to respond to transmitter [29,136]. It might be interesting to try the converse – that is, to see whether induced postsynaptic specializations can be assayed functionally by engineering transmitter release from the contacting non-neuronal cell [137].
Figure 2.
Hemi-synapse induction by neuroligins or neurexins presented to isolated axons or dendrites on the surface of fibroblasts. (a) Fibroblasts (F) expressing neuroligins induce clusters of presynaptic components, including GAD, at contact sites with axons of cultured neurons (N). These induced clusters of GAD (arrowheads) lack the normal postsynaptic proteins such as gephyrin, in contrast to endogenous synapses (arrow). (b) Fibroblasts (F) expressing neurexins induce clusters of postsynaptic components,including the GABAA receptor γ2 subunit (GABARγ2), at contact sites with dendrites of cultured neurons (N). These induced clusters of GABARγ2 (arrowheads) lack the normal presynaptic proteins such as synapsin, in contrast to endogenous synapses (arrow). (c) Hemi-presynapse (left) and hemi-postsynapse (right) formation in isolated axons and dendrites compared with bona fide synapses at axon–dendrite contacts (centre). Neuroligins, SynCAM, soluble FGF22 and soluble Wnt7a induce hemi-presynapses [18,21,29,55]; neurexins, NARP and ephrins induce full or partial hemi-postsynapses [60,67,71]. Scale bar, 10 μm.
Competence
Neurons acquire the ability to form synapses as part of a developmental maturation process. Intrinsic limitations in competence to form synapses have been demonstrated in cell culture studies, where a difference in experience of two days can be crucial. For example, hippocampal neurons from embryonic day (E)18 rats form functional synapses in culture but, under the same conditions, E16 neurons form morphological synapses that are largely presynaptically silent, regardless of how long they are maintained in culture [11]. Thus, E16 neurons can be considered as lacking in competence to form fully functional synapses. In other heterochronic culture experiments, axons and dendrites were found to mature at different rates. Axons of E18 neurons can form presynaptic specializations within one day in vitro, whereas the target dendrites require three days in vitro to promote such presynaptic development in contacting axons [12].
Mechanisms promoting synaptogenic competence involve both intrinsic programs of differentiation and exposure to neuron-target-derived and glia-derived factors. As in the example of E16 hippocampal neurons in culture, functional synapses can be induced by addition of neurotrophins [11]. Brain-derived neurotrophic factor (BDNF) promotes formation of functional synapses through multiple but distinct mechanisms for glutamatergic and GABAergic synapses. For hippocampal and neocortical neurons, BDNF alters properties of both synapse types and also selectively promotes interneuron axon and dendrite growth and glutamic acid decarboxylase (GAD) gene expression [11,13–16]. Other factors that might be considered to promote synaptogenic competence include neuron-target-derived factors such as Wnts [17,18] and fibroblast growth factors (FGFs) [19–21] and glia-derived factors such as thrombospondins [22] and cholesterol [23]. These factors were identified as promoters of synaptogenesis when added to cultured neuron media, and in most cases were further supported by in vivo knockout analyses. These factors do not necessarily exert their effects directly at the developing synapse. The target-derived factors could diffuse some distance to promote localized axon branching and varicosity formation, in addition to changes in gene expression. These priming factors might promote synaptogenesis in a temporal rather than spatial window, acting as permissive factors rather than local instructive factors controlling exactly which synapses form where. Manipulations of priming factor expression in subsets of neurons or glia will be required to test how widespread or localized are the actions of these synaptogenic molecules.
Placement
Matching cellular partners
For synapse assembly to occur, the plasma membranes of the appropriate presynaptic and postsynaptic cells must be brought into contact. Members of the cadherin and immunoglobulin (Ig) superfamilies are thought to mediate this function. Cadherin expression patterns and some function-blocking studies support the idea that cadherins have a key role in mediating selective adhesion leading to formation of synapses between the appropriate partners [24,25]. In Drosophila, single-cell mosaic analyses revealed that N-cadherin is required in both individual photoreceptor neurons and their targets to mediate appropriate synaptic contacts [26,27]. Although many defects resulting from perturbation of cadherins might be explained by altered axon and dendrite targeting (e.g. when axons fail to reach their targets), these single-cell analyses of the roles of cadherins in both presynaptic and postsynaptic partners strengthen the idea that cadherins directly mediate axon–target adhesion leading to synaptogenesis. However, in this system at least, different classical cadherin isoforms do not instruct partner choice [27]. Whether partner choice is determined by temporal regulation of N-cadherin function, by other molecules, or by a different and perhaps competitive patterning mechanism is not yet clear.
Evidence also implicates Ig superfamily members, including neural cell-adhesion molecules (NCAMs), nectins, synaptic cell-adhesion molecules (SynCAMs), Sidekicks and neurofascin, in mediating synaptic target recognition [10]. Nectins and SynCAMs (also known as Necls) are closely related, exhibit homophilic and heterophilic interactions and, like cadherins, function at multiple classes of cell–cell junctions including synapses [28,29]. Sidekick-1 and sidekick-2, two large transmembrane proteins containing multiple Ig and fibronectin-type-III domains and a PDZ-domain-binding site, exclusively mediate homophilic interactions and promote lamina-specific connectivity in chick retina [30]. A striking example of the role of Ig-domain proteins in mediating synaptic specificity comes from Caenorhabditis elegans. Binding of the transmembrane Ig superfamily protein SYG-1 on HSNL motoneurons to SYG-2 on vulval epithelial guidepost cells is necessary for correct placement of the motoneuron synapses [31,32]. The guidepost cells are thought to function as transient postsynaptic partners. Such a mechanism involving non-neuronal partners greatly increases the potential diversity of molecules involved in synapse placement.
Based on studies of the Drosophila neuromuscular system [33], we might expect synaptic target recognition to involve combinatorial codes of attractive and repulsive cues from several gene families. Neurexins and their binding partners neuroligins are another family of neuron-specific surface molecules that might bring together the correct cellular partners [34] (Dean and Dresbach, in this issue). Although recent focus has been on their role in recruiting synaptic components, as will be discussed later, neurexins and neuroligins can also mediate cell adhesion [35]. Furthermore, based on the thousands of possible splice variants [36], it has been suggested that neurexins could contribute to specifying synaptic circuitry [34]. For many of these mammalian synaptic adhesion molecules, inducible cell-specific knockouts combined with single-cell analyses might be necessary to define their precise role in shaping circuitry.
Determining synapse spacing
To some degree, spacing between synapses is controlled by axon and dendrite guidance, which define potential contact sites. Based on differences in axon tortuosity and branch layout relative to the positions of postsynaptic partners, fine-scale axon guidance seems to be more important in determining synaptic partnerships for GABAergic interneurons than for pyramidal cells in the neocortex [37]. Several lines of evidence also argue for intrinsic control of inter-synapse spacing within both axons and dendrites. Mean inter-varicosity distance varies among cell types (e.g. 3.7 μm for axons of CA3 hippocampal cells projecting to CA1, and 5.2 μm for cerebellar parallel fibers [38]). This relatively large spacing between presynaptic sites could be explained by the development of non-synaptogenic regions devoid of key synaptogenic molecules, bordering each presynaptic specialization. This could occur by active mechanisms or more simply by passive sequestration of limited resources over the length of an axon. Imaging studies in culture suggest that mobile units of recycling vesicles are abundant in developing axons [39,40], but the abundance of molecules with the ability to recruit vesicles to the membrane is not known. Further studies determining the distribution of synaptogenic recruiting molecules such as neurexins [41] and SynCAM [29] during axon maturation, and testing whether their overexpression might reduce synapse spacing, could be useful. The induced hemi-synapse preparations could also be useful for determining whether increasing hemi-presynapse density locally results in reduced inter-synapse spacing in other regions of an axon. In vivo, spacing patterns over small axon distances are consistent with a random distribution of synapses [38,42], but spacing can exhibit greater variability including proximodistal gradients over larger distances [43], indicating additional mechanisms of control.
Spacing between postsynaptic elements is generally much less than that between presynaptic elements, with densities reaching up to 17 spine synapses per μm for Purkinje dendrites [44]. Spacing can be minimal between synapses that are chemically alike or chemically distinct, suggesting much less control of synapse spacing by dendrites than by axons. However, live imaging studies in culture reveal periods of addition and removal of glutamatergic postsynaptic sites that are synchronized among several dendrites within a cell but not synchronized among cells, therefore suggesting regulation of synapse spacing by a cellular sensor [45]. In addition, considerable differences in density of excitatory and inhibitory synapses along dendrites and in the form of excitatory synapses exist among cell types and among dendrite regions within one cell [46,47]. For example, CA1 radiatum thick primary dendrites exhibit a striking proximal-to-distal gradient with increasing excitatory synapse density and decreasing inhibitory synapse density [47]. Such a gradient is not observed on thin dendrites in the same region, suggesting some degree of intrinsic control by the postsynaptic neurons. Perhaps such gradients in synapse density arise in part from demonstrated gradients of the hyperpolarization-activated cation current Ih or of KA K+ currents, which both mediate local excitability [48], or from gradients of functional cell-adhesion molecules, which might be generated by a common mechanism.
Targeting synapses to subcellular domains
The phenomenon of targeting inputs to specific subcellular domains is best exemplified by distinct classes of interneurons that preferentially form inhibitory synapses on dendritic spines, the dendritic shaft, the soma or the axon initial segment (AIS) [49]. Recent experiments suggest that molecular cues have a crucial role in this process. The selective perisomatic versus distal dendritic innervation of cortical pyramidal cells by parvalbumin-expressing versus somatostatin-expressing interneurons develops properly in organotypic slice cultures, indicating that sensory experience is not required for such selective placement of synapses [50]. A direct role for the cytoskeletal protein ankyrin G and the cell-adhesion molecule neurofascin 186 in targeting basket-cell synapses to the Purkinje AIS was reported recently [51]. Although neurofascin is normally enriched at the AIS in Purkinje cells, deletion of ankyrin G resulted in a more dispersed localization of neurofascin and a corresponding spread in the zone of basket-cell-axon contact. Even more strikingly, the synaptic differentiation of basket-cell terminals was severely compromised in ankyrin-G-mutant mice and in transgenic mice expressing a dominant-negative form of neurofascin [51]. Thus, the ankyrin-G–neurofascin complex contributes to localized axon contact and is essential for localized synaptic differentiation.
These results indicate a possible role for subcellular-domain-specific cytoskeletal specialization in selective synapse formation. The cell-adhesion activity of neurofascin is enhanced by its association with the cytoskeleton through ankyrin G, probably via focal concentration and stabilization of neurofascin [52]. Such enhancement of adhesive activity via cytoskeletal association, as has also been described for cadherin [53,54], could stabilize transient contacts between input and target domains, prolonging the duration of contact and enabling other synaptogenic molecules to induce local synaptic specialization. It would be interesting to determine whether the basket-cell-axon contacts to Purkinje cells are less stable in the ankyrin-G mutant than in wild-type mice, perhaps leading to the severe defect in synaptic differentiation.
Composition
Recruiting presynaptic components
Once the cell membranes are brought into contact, the next step is to recruit the molecular assemblies that mediate transmitter release and response. Several studies in the past few years have demonstrated the surprising ability of a handful of isolated molecules to induce focal aggregation of release-competent synaptic vesicles when presented to axons of cultured neurons. Neuroligins were the first of these molecules to be identified [55] and appear to be the most potent inducers of presynaptic specializations. This recent work builds on the seminal studies of Sudhof and colleagues identifying neuroligins as binding partners for neurexins, which themselves were first identified as presynaptic receptors for the spider toxin α-latrotoxin [34,56] (Dean and Dresbach, in this issue). SynCAM [29] also induces local presynaptic specializations. These induced hemi-presynapses undergo activity-dependent recycling and mimic release properties of bona fide synapses [57], and thus must have fairly complete molecular composition. Rapid recruitment of a complex presynaptic apparatus can be facilitated by delivery of components in prepackaged transport packets [58]. Whereas SynCAM appears to affect only excitatory synapses [57], neuroligins induce and function at both excitatory and inhibitory synapses and can affect the balance of synaptic inputs [59–61]. Additional factors can induce aspects of presynaptic specialization along the length of axons when added to neuron culture bath, including FGF22 [21] and Wnt7a [18], as already mentioned. Studies determining the effects of focal presentation of FGFs and Wnts could help in determining whether these proteins function more in promoting regional competence for synaptogenesis or in mediating localized differentiation.
These inductive studies raise a host of questions for future work, such as: why do multiple proteins appear to perform the same basic function for inducing presynaptic differentiation? They might act at different types of synapses or induce subtly different synaptic composition and function. However, neuroligins, FGF22 and Wnt7a have all been identified at the same cerebellar mossy-fiber to granule-cell synapse type [18,21,55], suggesting a redundancy in function. Functional interference studies support a role for each of these proteins, but not an absolute requirement for any (Table 1). Interestingly, although a complete neurexin-knockout mouse has not yet been made, knockout of α-neurexins, which leaves the shorter neuroligin-binding β-neurexins intact, results in a dramatic loss of functional presynaptic Ca2+ channels [62,63]. Thus, these individual synaptogenic molecules might contribute some unique properties to synapse assembly. Although cadherin alone is not sufficient to induce synaptic vesicle clustering, function-blocking studies and catenin-knockout mice also support a role for cadherin interactions in mediating organization of synaptic components [64]. A clear example is the dispersal of synaptic vesicles and reduction in reserve pool in the β-catenin knockout [65]. Future studies are likely to focus on how these multiple presynaptic inducing factors cooperate and on their sequence of action in vivo.
Recruiting postsynaptic components
Neurexins and neuroligins are so far unique among synaptogenic factors because each partner can induce a complementary hemi-synapse. Whereas neuroligins induce presynaptic differentiation in isolated axons, neurexins induce postsynaptic differentiation in isolated dendrites [60,66]. β-Neurexin presented on non-neuronal cells or beads induces aggregates of the glutamatergic postsynapse components PSD95 and NMDA receptors, and separate aggregates of the GABAergic postsynapse components gephyrin and GABAA receptors [60]. RNA interference (RNAi) knock-down and mislocalized expression studies further support an essential function for neurexins and their binding partners neuroligins at glutamatergic and, perhaps more significantly, at GABAergic synapses [59–61].
Although neurexins are the only factors reported to induce GABAergic postsynaptic differentiation, two other factors can induce aspects of glutamatergic postsynaptic differentiation. In one of the earliest CNS coculture experiments, O’Brien et al. [67] demonstrated that neuronal activity-regulated pentraxin (NARP) presented on the surface of non-neuronal cells induces local aggregation of AMPA receptors in contacting dendrites in spinal cord culture. Interestingly, although NARP can bind and aggregate AMPA receptors on any cell type, endogenous localization and dominant-negative inhibition experiments suggest that NARP drives glutamate synaptogenesis on aspiny neurons but not on pyramidal neurons [68,69]. On aspiny neurons, NARP-mediated clustering of AMPA receptors also drives clustering of NMDA receptors, probably via a PSD95 and stargazin link [70].
Another intriguing direct interaction is binding of the NMDA receptor NR1 subunit and EphB2 extracellular domains [71], which modulates Ca2+ influx through NMDA receptors [72]. Presentation of ephrin-B1 to cultured neurons induces coclustering of EphB and NMDA receptors, suggesting that ephrins might drive aspects of glutamatergic synapse assembly [71]. Whether ephrins selectively regulate the local density of NMDA receptors or promote assembly of a more complete glutamatergic postsynaptic specialization is not yet known. Many studies indicate that ephrins regulate spine morphology and synaptic plasticity [73]. Analyses of EphB1−/−; EphB2−/−; EphB3−/− mice revealed alterations in spine morphology and postsynaptic-density (PSD) length in vivo and even more severe defects in synapse assembly in vitro [74].
Although initial assembly can be rapid, development of a mature synapse is generally prolonged and can require sequential signals. The induced hemi-synapse preparations could be useful for delineating synaptogenic signals that act sequentially. A recent experiment highlighting this idea is the finding that NMDA receptor activation induces local insertion of AMPA receptors at β-neurexin-induced hemi-synapses [66], as it does at bona fide silent synapses. The development of spine morphology at specific classes of glutamatergic synapses is another aspect of synapse maturation regulated by multiple signals [75,76].
For GABAergic synapses, the sheer diversity of GABAA receptor subunits [77], the selective targeting of subunit combinations to specific synaptic or extrasynaptic locations [78,79] and the differential dependence on gephyrin for synaptic localization [80,81] suggest the existence of multiple signals and pathways for recruitment of different receptor complexes. Compelling rescue experiments expressing chimeric subunits in cultures from mice lacking the GABAA receptor γ2 subunit (GABAARγ2) indicate a surprising function of the fourth transmembrane domain of GABAARγ2 in mediating synaptic localization [82], through unidentified interacting partners. In addition to GABAA receptors, gephyrin and neuroligin-2, the dystroglycan–dystrophin complex is another identified component of some mature GABAergic postsynaptic sites, but its function there is poorly understood [83,84]. Dystroglycan is a major binding partner of neurexins [85] but it does not mediate the neurexin-induced aggregation of GABAA receptors and gephyrin in the coculture experiments [60]. Dystroglycan is recruited to GABA synapses by a mechanism independent of gephyrin or GABAARγ2, by signals unique to GABAergic terminals [84,86].
Matching postsynaptic and presynaptic components
Complexity of synapse development arises from the presence of multiple neuron types in the CNS, each forming molecularly and functionally distinct synaptic specializations. Differentiation of neurons into glutamatergic or GABAergic subtypes occurs early, before neurons extend axonal processes; thus, the type of neurotransmitter released from a given presynaptic active zone is predetermined well before synapse formation. Consequently, the question arises of how a dendrite can specifically cluster glutamatergic and GABAergic postsynaptic specializations opposite glutamatergic and GABAergic presynaptic contacts, respectively. A likely possibility is that local signals from the axon at nascent contact sites direct the aggregation of appropriate postsynaptic proteins at these sites.
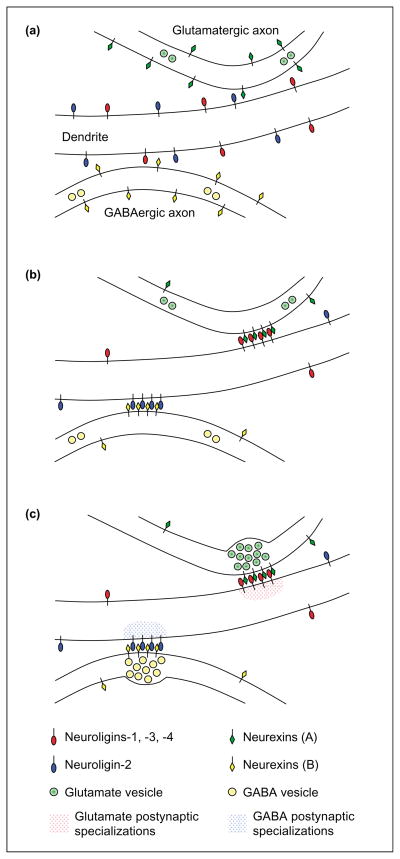
Recent evidence suggests that β-neurexins could be good candidates for such signals. As already summarized, exogenous focal application of neurexin-1β induces the clustering of glutamatergic and GABAergic postsynaptic receptors, scaffolding proteins and signaling proteins via neuroligins [60]. The localization of neuroligin-1 to glutamatergic synapses [87] and neuroligin-2 to GABAergic synapses [60,88], and the apparent linkage of neuroligins-1, -3, and -4 to proteins of glutamatergic postsynapses and of neuroligin-2 to proteins of GABAergic postsynapses [60], suggests that neurexins can influence postsynaptic differentiation by aggregating the proper neuroligin isoforms at nascent contact sites. Based on these results, one might predict that differential expression of neurexin isoforms by glutamatergic versus GABAergic neurons could contribute to local induction of glutamatergic versus GABAergic postsynaptic specializations (Figure 3).
Figure 3.
Potential role for neurexins in matching postsynaptic and presynaptic composition. (a) It is possible that glutamatergic axons express neurexin isoforms (A) that bind most strongly to neuroligins-1, -3 and -4, whereas GABAergic axons express neurexins (B) that bind most strongly to neuroligin-2. Neurexins (A) versus (B) represent different forms that could result from differential gene usage, promoter usage, alternative splicing and/or glycosylation. Before synapse formation, all neuroligin isoforms might be diffusely distributed over the dendritic surface, whereas neurexins might be distributed ubiquitously over the axonal surface. (b) Synapse formation could be triggered when presynaptic neurexins interact with the appropriate postsynaptic neuroligins, stabilizing and aggregating both at nascent contact sites. (c) By specifically aggregating neuroligins-1, -3 and -4, glutamatergic neurexins could then cause the subsequent clustering of glutamate postsynaptic proteins. Likewise, by binding to and aggregating neuroligin-2, GABAergic neurexins could thereby influence the clustering of GABAergic postsynaptic proteins. In a complementary manner, the interaction of neuroligins with neurexins would also stabilize axon contacts and induce presynaptic specializations.
However, such a model of postsynaptic specification based solely on neurexins is probably too simplistic [60]. Additional proteins that interact with neurexins and neuroligins and/or that act independently, such as NARP [67] and ephrins [71], are probably needed to specify appropriate postsynaptic differentiation. Interestingly, N-cadherin is found at hippocampal glutamatergic and GABAergic synapses early in development and then lost from GABAergic synapses [89]. Thus, cadherin isoforms could also contribute to maintaining aspects of specificity. Further studies of the roles of candidate molecules, and simply identifying additional components of inhibitory synapses, could be the next steps towards understanding the basis for matching postsynaptic and presynaptic components. The finding that a single protein family, that of the neurexins and neuroligins, functions at both excitatory and inhibitory synapses had been previously predicted based on the existence of mismatched appositions in neurons that lack one type of input [90]. Although there could be an evolutionary reason for using a single protein family to build both glutamatergic and GABAergic synapses, there could also be a functional reason. Sets of neuroligins and neurexins with differential affinities of interaction coupled with variations in expression level of synapse-specific binding proteins such as PSD95 could be used to control the balance of excitatory and inhibitory inputs [59] (Dean and Dresbach, in this issue).
Size
At a typical CNS synapse composed of a single active zone, the areas of the active zone and postsynaptic density, the numbers of synaptic and docked vesicles and the volumes of the axon varicosity and spine head are all highly correlated [91–93]. This size correlation suggests a coordinated regulation of presynaptic active zone and bouton size with PSD and spine size via connecting transmembrane and cytoskeletal proteins. Active zone and PSD sizes range over about two orders of magnitude [92,93]. However, rather than the size of a contiguous active zone and apposed PSD increasing indefinitely, strong synapses are split into multiple separate active zones and PSD units, suggesting an inherent limit in the size that an individual active zone and apposed PSD can maintain. A classic example is the calyx of Held, a giant glutamatergic terminal in the auditory brainstem that maintains hundreds of active zones apposed to individual PSDs on the soma of its target cell [94].
Remarkably, this size limit appears to hold even for induced hemi-synapses. For example [60] (Figure 2b), when neurexins are presented to a dendrite at high concentration over the entire surface of a COS cell, they can induce an apposed concentration of neuroligins over a large surface area of a dendrite (more than tens of square microns). Yet within this area, neurotransmitter receptors and scaffolding proteins do not form one large continuous aggregate but, rather, numerous smaller aggregates, typical of the size of a synapse (≤1 μm2). Even direct aggregation of neuroligins on the dendrite surface using beads of 3-μm diameter results in aggregation of smaller clusters of postsynaptic receptors and scaffolding proteins [60]. Likewise, induction of presynaptic hemi-synapses with neuroligins or SynCAMs presented to axons over a large area results in numerous separate active zones [29,55] (Figure 2a).
Why is active zone and PSD size limited, and how is the size maintained? Active zone size might be limited to enable efficient retrieval of components from the edge of an active zone following vesicle exocytosis. Similarly, on the postsynaptic side, a limited PSD size might enable rapid regulated insertion or removal of specific components mediating functional regulation. Experimental evidence supports the idea that endocytosis occurs at the edge of both active zones and PSDs [95,96]. Mechanistically, size could be self-limiting. Considering the synaptic unit of an active zone plus a PSD as a dynamic assembly of protein–protein interactions, size could be determined by the concentrations and affinities of interaction of the molecular players. Following an initial nucleating event, the synaptic unit could rapidly reach a steady-state size. The inverse correlation of synaptic strength and density of innervation in hippocampal cultures [97] supports the idea that neurons that have to partition molecular players to a greater number of synapses make smaller synapses.
Mechanisms that regulate the size of individual active zone plus PSD units or the number of such units made between partners are poorly understood for mammalian CNS synapses. Such questions have been more extensively studied in Drosophila and C. elegans, where identified regulatory molecules include the cell-adhesion molecule FasII, DLiprin-α/Syd-2 and its binding partner DLar receptor tyrosine phosphatase, the guanine nucleotide exchange factor and E3 ubiquitinating enzyme Hiw/RPM-1, and the microtubule-affinity-regulating kinase Sad-1 [98,99]. Mammalian homologs also function in nervous system development, although perhaps not in a directly analogous manner: Liprin and LAR are involved in AMPA receptor trafficking and postsynaptic assembly [100], and mouse Sad-1 mutants have defective neuronal polarity [101]. An interesting twist is the finding that loss of GAD, the GABA synthetic enzyme, regulates postsynaptic glutamate receptor fields through alterations in glutamate levels at the Drosophila neuromuscular junction [102]. At mammalian CNS synapses, size is also regulated by activity [103]. There is further evidence that certain key linking molecules, such as PSD95, could be limiting and thus their cellular concentration might directly regulate synapse size [104].
Another mechanism that might be used developmentally to control synapse size could involve a specific molecular border. Cadherins are concentrated at most if not all developing synapses [89] and are generally concentrated at one edge of many mature synapses [105–107]. The molecular dimensions of trans-interacting cadherins appear by structural studies and electron tomography to generate an inter-membrane spacing of ~25 nm [108,109]. In addition, surface-force measurements between opposing cadherin monolayers reveal an attractive energy minimum at a distance of 25 nm, with repulsive forces increasing significantly as the cadherin monolayers are brought to within 20 nm [110]. These data are inconsistent with inclusion of classical cadherins within the width of a typical synaptic cleft (~20 nm) [44]. By contrast, based on the structures of their major domains, the bond lengths of the β-neurexin–neuroligin pair and the ephrin-B2–EphB2 pair might be considerably smaller (Figure 4). Studies of the immune synapse could provide some clues as to how the extracellular domains of cell-adhesion molecules define the spacing between the two cell membranes and create distinct zones at a cellular junction [111,112]. At the immune synapse, the LFA-1–ICAM-1 pair (41-nm bond length) mediates initial adhesion between the membranes. As the junction develops, the T-cell-receptor–MHC pair (14-nm bond length) occupies the central zone of contact while the LFA-1–ICAM-1 pair segregates into a peripheral zone. Whether neurexins and neuroligins or other cell-adhesion molecules define synaptic cleft dimensions remains to be determined. Interestingly, the slightly smaller cleft width of inhibitory compared with excitatory synapses [113] suggests a difference in composition of key cell-adhesion molecules.
Figure 4.
Comparison of predicted size differences between synaptic adhesion molecule pairs. The classical cadherin trans-dimer is modeled after an experimentally determined structure [108]. In this model, homophilic interaction between cadherin molecules located on opposing membrane surfaces is mediated by a strand exchange between the two extracellular cadherin 1 (EC1) domains. Cis-interactions (not shown) also contribute to defining the inter-membrane spacing of ~25 nm. The other structures are shown relative to a 20 nm inter-membrane spacing typical of a synaptic cleft [44]. The neurexin 1-β LNS domain structure is oriented so that the loops involved in interactions with neuroligins are oriented away from the membrane. Based on sequence homology, the crystal structure of acetylcholinesterase and the tandem fibronectin type III (FNIII) repeats of Drosophila neuroglian are used here to represent the size of the neuroligin ectodomain and the tandem EphB2 FNIII repeats, respectively. Ephrin-B2, EphB2, β-neurexins and neuroligins also have additional extracellular peptide sequence that cannot be modeled or predicted. Secondary structural elements are coded by color: red, α-helix; orange, 3–10 helix; green, β-sheet; yellow, turn; blue, coil. The structures were visualized using the Visual Molecular Dynamics (VMD) program [142] (http://www.ks.uiuc.edu/Research/vmd/). Protein Databank (PDB) files: C-cadherin (1L3W) [108], neurexin 1-β (1C4R) [143], mouse acetylcholinesterase (1MAA) [144], EphB2–Ephrin-B2 complex (1KGY) [145], Drosophila neuroglian (1CFB) [146].
Longevity and plasticity
The majority of spine synapses in the mature brain are stable for months [114,115], presumably through continual replenishment of the synaptogenic signals. However, as exemplified by studies of ephrins and Eph receptors [116] and cadherins [117], the same molecules that function centrally in synaptogenesis also contribute to activity-dependent synaptic plasticity in more mature systems. Such plasticity can occur through synapse assembly or disassembly and through altering the strength of existing synapses. Experimental alterations in levels of synaptogenic signals, particularly neuroligins, can directly drive synapse stabilization and disassembly in neuronal cultures [59,61]. Altering the levels of neuroligins and/or PSD95 alters the density of both glutamatergic and GABAergic synapses. Perhaps such mechanisms are utilized during activity-dependent developmental sculpting of the nervous system. It would be interesting to know whether the neurexin–neuroligin system contributes to fine-scale balancing of excitatory and inhibitory inputs within dendritic branches [118]. As already mentioned, activity-dependent release of BDNF from pyramidal neurons and subsequent BDNF promotion of GABAergic interneuron development is another mechanism regulating the balance of excitation and inhibition.
Although it is uniformly accepted that activity sculpts circuitry during development, the overall rules governing this process in the CNS are not well understood. In one elegant genetic study manipulating transmitter release or excitability in subsets of olfactory neurons, axonal misrouting and selective loss of defective neurons were observed [119]. However, in simple culture systems, neurons defective in release are not always at a competitive disadvantage for maintaining synaptic terminals [120]. In multiple systems, reducing excitability by expression of the K+ channel Kir2.1 appears to have a more drastic effect than blocking transmitter release by expression of tetanus toxin light chain [119–121]. Further studies into the nature of the activity sensor mediating competitive reorganization, and consideration of possible balancing effects of the limited resources of individual neurons, are warranted.
Concluding remarks
A key question is whether any single factor or even single family of proteins is essential for synaptogenesis. At present, mutant mice for individual synaptogenic proteins mentioned here are viable and form synapses. Moreover, directed screens in C. elegans and Drosophila have not revealed any proteins essential for basic synapse assembly – that is, mutants completely lacking synapses have not been found [98,99]. These results, or lack of them, suggest either that the key synaptogenic factors have yet to be found and have additional functions earlier in development, thus requiring different screening strategies, or more likely that there is a high degree of redundancy or compensation inherent in the process of synaptogenesis. Furthermore, isolated motor neurons and isolated muscles form hemi-synapses in vivo if deprived of their targets [122,123]. Therefore, perhaps all of these synaptogenic proteins are more involved in defining the precise timing, spatial location and composition of synapses than in determining whether synapses form.
Reconstructing aspects of synaptogenesis in cocultures of neurons with non-neuronal cells and eventually in purely non-neuronal cells and lipid bilayers, along with complementary studies probing the in vivo requirements for each of these synaptogenic molecules, will be key tools for future research on the molecular basis of synapse formation. Studies of the endogenous distributions and local concentrations of the various synaptogenic molecules before and during synaptogenesis will also be necessary for understanding the synapse assembly process. For example, the coculture studies indicate that a given molecule can induce aspects of presynaptic or postsynaptic differentiation, but it still needs to be determined when that molecule is present at the appropriate location and concentration to have a similar effect in vivo. As previously mentioned, owing to the presence of multiple family members for most of these synaptogenic proteins, potential redundancy in function among families, and participation of these proteins in other aspects of development, in vivo knockout analyses to define the precise functions of synaptogenic proteins will be challenging. Such studies could be facilitated by the development of better tools for quantitative pathway-specific assays of synapse formation and stability, such as mouse subset lines expressing tagged presynaptic and postsynaptic markers [45,124,125].
A true understanding of the processes underlying synapse assembly, leading to the ability to predict synaptic competence, placement, size and composition, will require mathematical modeling of the molecular interactions involved within the context of an appropriate morphological structure. Approaching such a goal will first require studies to determine the absolute number [126,127], structure and orientation [108,128], ultrastructural localization [129], affinities and strength of interactions [110,130] of each molecule at glutamatergic and GABAergic synapses. Models based on current information about synaptic assemblies are already providing insights about modes of synaptic transmission [131,132]; such models could be extended in the future for a better understanding of the process of synapse assembly itself.
Acknowledgments
We thank Huaiyang Wu for preparation of cultures used in Figure 2, and YunHee Kang and other members of the Craig laboratory for helpful comments. Supported by grants from NIH, CIHR and MSFHR, and by Canada Research Chair (AMC) and NSF Predoctoral Fellow (ERG) awards.
References
- 1.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 2.Craig AM, Boudin H. Molecular heterogeneity of central synapses: afferent and target regulation. Nat Neurosci. 2001;4:569–578. doi: 10.1038/88388. [DOI] [PubMed] [Google Scholar]
- 3.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 4.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 5.Verhage M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 6.Varoqueaux F, et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harms KJ, Craig AM. Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. J Comp Neurol. 2005;490:72–84. doi: 10.1002/cne.20635. [DOI] [PubMed] [Google Scholar]
- 8.Waites CL, et al. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- 9.Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- 10.Yamagata M, et al. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 11.Vicario-Abejon C, et al. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher TL, et al. Synaptogenesis in hippocampal cultures: evidence indicating that axons and dendrites become competent to form synapses at different stages of neuronal development. J Neurosci. 1994;14:6695–6706. doi: 10.1523/JNEUROSCI.14-11-06695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marty S, et al. Neurotrophins and activity-dependent plasticity of cortical interneurons. Trends Neurosci. 1997;20:198–202. doi: 10.1016/s0166-2236(96)01026-0. [DOI] [PubMed] [Google Scholar]
- 14.Rutherford LC, et al. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang ZJ, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 16.Jin X, et al. Brain-derived neurotrophic factor mediates activity-dependent dendritic growth in nonpyramidal neocortical interneurons in developing organotypic cultures. J Neurosci. 2003;23:5662–5673. doi: 10.1523/JNEUROSCI.23-13-05662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krylova O, et al. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- 18.Hall AC, et al. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 19.Dai Z, Peng HB. Presynaptic differentiation induced in cultured neurons by local application of basic fibroblast growth factor. J Neurosci. 1995;15:5466–5475. doi: 10.1523/JNEUROSCI.15-08-05466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li AJ, et al. Fibroblast growth factor-2 increases functional excitatory synapses on hippocampal neurons. Eur J Neurosci. 2002;16:1313–1324. doi: 10.1046/j.1460-9568.2002.02193.x. [DOI] [PubMed] [Google Scholar]
- 21.Umemori H, et al. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Christopherson KS, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Mauch DH, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro L, Colman DR. The diversity of cadherins and implications for a synaptic adhesive code in the CNS. Neuron. 1999;23:427–430. doi: 10.1016/s0896-6273(00)80796-5. [DOI] [PubMed] [Google Scholar]
- 25.Benson DL, et al. Molecules, maps and synapse specificity. Nat Rev Neurosci. 2001;2:899–909. doi: 10.1038/35104078. [DOI] [PubMed] [Google Scholar]
- 26.Lee CH, et al. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 27.Prakash S, et al. Drosophila N-cadherin mediates an attractive interaction between photoreceptor axons and their targets. Nat Neurosci. 2005;8:443–450. doi: 10.1038/nn1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irie K, et al. Roles and modes of action of nectins in cell-cell adhesion. Semin Cell Dev Biol. 2004;15:643–656. doi: 10.1016/j.semcdb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Biederer T, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 30.Yamagata M, et al. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- 31.Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 2003;112:619–630. doi: 10.1016/s0092-8674(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 32.Shen K, et al. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 33.Winberg ML, et al. Genetic analysis of the mechanisms controlling target selection: complementary and combinatorial functions of netrins, semaphorins, and IgCAMs. Cell. 1998;93:581–591. doi: 10.1016/s0092-8674(00)81187-3. [DOI] [PubMed] [Google Scholar]
- 34.Missler M, Sudhof TC. Neurexins: three genes and 1001 products. Trends Genet. 1998;14:20–26. doi: 10.1016/S0168-9525(97)01324-3. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen T, Sudhof TC. Binding properties of neuroligin 1 and neurexin 1β reveal function as heterophilic cell adhesion molecules. J Biol Chem. 1997;272:26032–26039. doi: 10.1074/jbc.272.41.26032. [DOI] [PubMed] [Google Scholar]
- 36.Tabuchi K, Sudhof TC. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002;79:849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- 37.Stepanyants A, et al. Class-specific features of neuronal wiring. Neuron. 2004;43:251–259. doi: 10.1016/j.neuron.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Shepherd GM, et al. General and variable features of varicosity spacing along unmyelinated axons in the hippocampus and cerebellum. Proc Natl Acad Sci U S A. 2002;99:6340–6345. doi: 10.1073/pnas.052151299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraszewski K, et al. Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J Neurosci. 1995;15:4328–4342. doi: 10.1523/JNEUROSCI.15-06-04328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krueger SR, et al. The presynaptic release apparatus is functional in the absence of dendritic contact and highly mobile within isolated axons. Neuron. 2003;40:945–957. doi: 10.1016/s0896-6273(03)00729-3. [DOI] [PubMed] [Google Scholar]
- 41.Dean C, et al. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hellwig B, et al. Synapses on axon collaterals of pyramidal cells are spaced at random intervals: a Golgi study in the mouse cerebral cortex. Biol Cybern. 1994;71:1–12. doi: 10.1007/BF00198906. [DOI] [PubMed] [Google Scholar]
- 43.Pichitpornchai C, et al. Morphology of parallel fibres in the cerebellar cortex of the rat: an experimental light and electron microscopic study with biocytin. J Comp Neurol. 1994;342:206–220. doi: 10.1002/cne.903420205. [DOI] [PubMed] [Google Scholar]
- 44.Peters A, et al. The Neurons and Supporting cells. Oxford University Press; 1990. The Fine Structure of the Nervous System. [Google Scholar]
- 45.Ebihara T, et al. Synchronized formation and remodeling of postsynaptic densities: long-term visualization of hippocampal neurons expressing postsynaptic density proteins tagged with green fluorescent protein. J Neurosci. 2003;23:2170–2181. doi: 10.1523/JNEUROSCI.23-06-02170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gulyas AI, et al. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Megias M, et al. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- 48.Migliore M, Shepherd GM. Emerging rules for the distributions of active dendritic conductances. Nat Rev Neurosci. 2002;3:362–370. doi: 10.1038/nrn810. [DOI] [PubMed] [Google Scholar]
- 49.Somogyi P, et al. Salient features of synaptic organisation in the cerebral cortex. Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 50.Di Cristo G, et al. Subcellular domain-restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat Neurosci. 2004;7:1184–1186. doi: 10.1038/nn1334. [DOI] [PubMed] [Google Scholar]
- 51.Ango F, et al. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Tuvia S, et al. The phosphorylation state of the FIGQY tyrosine of neurofascin determines ankyrin-binding activity and patterns of cell segregation. Proc Natl Acad Sci U S A. 1997;94:12957–12962. doi: 10.1073/pnas.94.24.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988;7:3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chu YS, et al. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol. 2004;167:1183–1194. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheiffele P, et al. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 56.Brose N. Synaptic cell adhesion proteins and synaptogenesis in the mammalian central nervous system. Naturwissenschaften. 1999;86:516–524. doi: 10.1007/s001140050666. [DOI] [PubMed] [Google Scholar]
- 57.Sara Y, et al. Selective capability of SynCAM and neuroligin for functional synapse assembly. J Neurosci. 2005;25:260–270. doi: 10.1523/JNEUROSCI.3165-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci. 2004;5:385–399. doi: 10.1038/nrn1370. [DOI] [PubMed] [Google Scholar]
- 59.Prange O, et al. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Graf ER, et al. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chih B, et al. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 62.Missler M, et al. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W, et al. Extracellular domains of α-neurexins participate in regulating synaptic transmission by selectively affecting N- and P/Q-type Ca2+ channels. J Neurosci. 2005;25:4330–4342. doi: 10.1523/JNEUROSCI.0497-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salinas PC, Price SR. Cadherins and catenins in synapse development. Curr Opin Neurobiol. 2005;15:73–80. doi: 10.1016/j.conb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Bamji SX, et al. Role of β-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nam CI, Chen L. Postsynaptic assembly induced by neurexin–neuroligin interaction and neurotransmitter. Proc Natl Acad Sci U S A. 2005;102:6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Brien RJ, et al. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 68.O’Brien R, et al. Synaptically targeted NARP plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J Neurosci. 2002;22:4487–4498. doi: 10.1523/JNEUROSCI.22-11-04487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mi R, et al. Differing mechanisms for glutamate receptor aggregation on dendritic spines and shafts in cultured hippocampal neurons. J Neurosci. 2002;22:7606–7616. doi: 10.1523/JNEUROSCI.22-17-07606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mi R, et al. AMPA receptor-dependent clustering of synaptic NMDA receptors is mediated by Stargazin and NR2A/B in spinal neurons and hippocampal interneurons. Neuron. 2004;44:335–349. doi: 10.1016/j.neuron.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 71.Dalva MB, et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 72.Takasu MA, et al. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- 73.Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004;16:580–589. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 74.Henkemeyer M, et al. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. J Cell Biol. 2003;163:1313–1326. doi: 10.1083/jcb.200306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 76.Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75:161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 77.Whiting PJ, et al. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann N Y Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- 78.Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiolo-gical implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 79.Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 80.Fischer F, et al. Reduced synaptic clustering of GABA and glycine receptors in the retina of the gephyrin null mutant mouse. J Comp Neurol. 2000;427:634–648. doi: 10.1002/1096-9861(20001127)427:4<634::aid-cne10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 81.Levi S, et al. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alldred MJ, et al. Distinct γ2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knuesel I, et al. Short communication: altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice) Eur J Neurosci. 1999;11:4457–4462. doi: 10.1046/j.1460-9568.1999.00887.x. [DOI] [PubMed] [Google Scholar]
- 84.Levi S, et al. Dystroglycan is selectively associated with inhibitory GABAergic synapses but is dispensable for their differentiation. J Neurosci. 2002;22:4274–4285. doi: 10.1523/JNEUROSCI.22-11-04274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sugita S, et al. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brunig I, et al. GABAergic terminals are required for postsynaptic clustering of dystrophin but not of GABAA receptors and gephyrin. J Neurosci. 2002;22:4805–4813. doi: 10.1523/JNEUROSCI.22-12-04805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song JY, et al. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Varoqueaux F, et al. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- 89.Benson DL, Tanaka H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J Neurosci. 1998;18:6892–6904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rao A, et al. Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons. J Neurosci. 2000;20:8344–8353. doi: 10.1523/JNEUROSCI.20-22-08344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pierce JP, Mendell LM. Quantitative ultrastructure of Ia boutons in the ventral horn: scaling and positional relationships. J Neurosci. 1993;13:4748–4763. doi: 10.1523/JNEUROSCI.13-11-04748.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci. 1997;17:5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taschenberger H, et al. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron. 2002;36:1127–1143. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- 95.Brodin L, et al. Sequential steps in clathrin-mediated synaptic vesicle endocytosis. Curr Opin Neurobiol. 2000;10:312–320. doi: 10.1016/s0959-4388(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 96.Racz B, et al. Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- 97.Liu G, Tsien RW. Properties of synaptic transmission at single hippocampal synaptic boutons. Nature. 1995;375:404–408. doi: 10.1038/375404a0. [DOI] [PubMed] [Google Scholar]
- 98.Ackley BD, Jin Y. Genetic analysis of synaptic target recognition and assembly. Trends Neurosci. 2004;27:540–547. doi: 10.1016/j.tins.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 99.Broadie KS, Richmond JE. Establishing and sculpting the synapse in Drosophila and C. elegans. Curr Opin Neurobiol. 2002;12:491–498. doi: 10.1016/s0959-4388(02)00359-8. [DOI] [PubMed] [Google Scholar]
- 100.Dunah AW, et al. LAR receptor protein tyrosine phospha-tases in the development and maintenance of excitatory synapses. Nat Neurosci. 2005;8:458–467. doi: 10.1038/nn1416. [DOI] [PubMed] [Google Scholar]
- 101.Kishi M, et al. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307:929–932. doi: 10.1126/science.1107403. [DOI] [PubMed] [Google Scholar]
- 102.Featherstone DE, et al. Presynaptic glutamic acid decarbox-ylase is required for induction of the postsynaptic receptor field at a glutamatergic synapse. Neuron. 2000;27:71–84. doi: 10.1016/s0896-6273(00)00010-6. [DOI] [PubMed] [Google Scholar]
- 103.Murthy VN, et al. Inactivity produces increases in neuro-transmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- 104.El-Husseini AE, et al. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 105.Uchida N, et al. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fannon AM, Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- 107.Spacek J, Harris KM. Three-dimensional organization of cell adhesion junctions at synapses and dendritic spines in area CA1 of the rat hippocampus. J Comp Neurol. 1998;393:58–68. doi: 10.1002/(sici)1096-9861(19980330)393:1<58::aid-cne6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 108.Boggon TJ, et al. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- 109.He W, et al. Untangling desmosomal knots with electron tomography. Science. 2003;302:109–113. doi: 10.1126/science.1086957. [DOI] [PubMed] [Google Scholar]
- 110.Sivasankar S, et al. Direct measurements of multiple adhesive alignments and unbinding trajectories between cadherin extracellular domains. Biophys J. 2001;80:1758–1768. doi: 10.1016/S0006-3495(01)76146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Monks CR, et al. Three-dimensional segregation of supra-molecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 112.Krummel MF, Davis MM. Dynamics of the immunological synapse: finding, establishing and solidifying a connection. Curr Opin Immunol. 2002;14:66–74. doi: 10.1016/s0952-7915(01)00299-0. [DOI] [PubMed] [Google Scholar]
- 113.Akert K, et al. Freeze etching and cytochemistry of vesicles and membrane complexes in synapses of the central nervous system. In: Pappas GD, Purpura DP, editors. Structure and Function of Synapses. Raven Press; 1972. pp. 67–86. [Google Scholar]
- 114.Zuo Y, et al. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 115.Holtmaat AJ, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 116.Grunwald IC, et al. Hippocampal plasticity requires postsynaptic ephrinBs. Nat Neurosci. 2004;7:33–40. doi: 10.1038/nn1164. [DOI] [PubMed] [Google Scholar]
- 117.Bozdagi O, et al. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 118.Liu G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci. 2004;7:373–379. doi: 10.1038/nn1206. [DOI] [PubMed] [Google Scholar]
- 119.Yu CR, et al. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–566. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- 120.Harms KJ, et al. Synapse-specific regulation of AMPA receptor subunit composition by activity. J Neurosci. 2005;25:6379–6388. doi: 10.1523/JNEUROSCI.0302-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Burrone J, et al. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- 122.Prokop A, et al. Presynaptic development at the Drosophila neuromuscular junction: assembly and localization of presynaptic active zones. Neuron. 1996;17:617–626. doi: 10.1016/s0896-6273(00)80195-6. [DOI] [PubMed] [Google Scholar]
- 123.Lin W, et al. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- 124.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 125.De Paola V, et al. AMPA receptors regulate dynamic equilibrium of presynaptic terminals in mature hippocampal networks. Nat Neurosci. 2003;6:491–500. doi: 10.1038/nn1046. [DOI] [PubMed] [Google Scholar]
- 126.Chiu CS, et al. Number, density, and surface/cytoplasmic distribution of GABA transporters at presynaptic structures of knock-in mice carrying GABA transporter subtype 1–green fluor-escent protein fusions. J Neurosci. 2002;22:10251–10266. doi: 10.1523/JNEUROSCI.22-23-10251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sugiyama Y, et al. Determination of absolute protein numbers in single synapses by a GFP-based calibration technique. Nat Methods. 2005;2:677–684. doi: 10.1038/nmeth783. [DOI] [PubMed] [Google Scholar]
- 128.Nakagawa T, et al. Structure and different conformational states of native AMPA receptor complexes. Nature. 2005;433:545–549. doi: 10.1038/nature03328. [DOI] [PubMed] [Google Scholar]
- 129.Valtschanoff JG, Weinberg RJ. Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci. 2001;21:1211–1217. doi: 10.1523/JNEUROSCI.21-04-01211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Comoletti D, et al. Characterization of the interaction of a recombinant soluble neuroligin-1 with neurexin-1β. J Biol Chem. 2003;278:50497–50505. doi: 10.1074/jbc.M306803200. [DOI] [PubMed] [Google Scholar]
- 131.Coggan JS, et al. Evidence for ectopic neurotransmission at a neuronal synapse. Science. 2005;309:446–451. doi: 10.1126/science.1108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kennedy MB. Signal-processing machines at the postsyn-aptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- 133.Vaughn JE. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3:255–285. doi: 10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- 134.Ahmari SE, Smith SJ. Knowing a nascent synapse when you see it. Neuron. 2002;34:333–336. doi: 10.1016/s0896-6273(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 135.Cottrell JR, et al. Distribution, density, and clustering of functional glutamate receptors before and after synaptogenesis in hippocampal neurons. J Neurophysiol. 2000;84:1573–1587. doi: 10.1152/jn.2000.84.3.1573. [DOI] [PubMed] [Google Scholar]
- 136.Fu Z, et al. Functional excitatory synapses in HEK293 cells expressing neuroligin and glutamate receptors. J Neurophysiol. 2003;90:3950–3957. doi: 10.1152/jn.00647.2003. [DOI] [PubMed] [Google Scholar]
- 137.Morimoto T, et al. Calcium-dependent transmitter secretion from fibroblasts: modulation by synaptotagmin I. Neuron. 1995;15:689–696. doi: 10.1016/0896-6273(95)90156-6. [DOI] [PubMed] [Google Scholar]
- 138.Togashi H, et al. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 139.Abe K, et al. Stability of dendritic spines and synaptic contacts is controlled by α N-catenin. Nat Neurosci. 2004;7:357–363. doi: 10.1038/nn1212. [DOI] [PubMed] [Google Scholar]
- 140.Shingai T, et al. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell–cell adhesion and transmembrane protein localization in epithelial cells. J Biol Chem. 2003;278:35421–35427. doi: 10.1074/jbc.M305387200. [DOI] [PubMed] [Google Scholar]
- 141.Henderson JT, et al. The receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. Neuron. 2001;32:1041–1056. doi: 10.1016/s0896-6273(01)00553-0. [DOI] [PubMed] [Google Scholar]
- 142.Humphrey W, et al. VMD: visual molecular dynamics. J Mol Graph. 1996;33–38:27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 143.Rudenko G, et al. The structure of the ligand-binding domain of neurexin Iβ: regulation of LNS domain function by alternative splicing. Cell. 1999;99:93–101. doi: 10.1016/s0092-8674(00)80065-3. [DOI] [PubMed] [Google Scholar]
- 144.Bourne Y, et al. Crystal structure of mouse acetylcholinesterase. A peripheral site-occluding loop in a tetrameric assembly. J Biol Chem. 1999;274:2963–2970. doi: 10.1074/jbc.274.5.2963. [DOI] [PubMed] [Google Scholar]
- 145.Himanen JP, et al. Crystal structure of an Eph receptor–ephrin complex. Nature. 2001;414:933–938. doi: 10.1038/414933a. [DOI] [PubMed] [Google Scholar]
- 146.Huber AH, et al. Crystal structure of tandem type III fibronectin domains from Drosophila neuroglian at 2.0 Å Neuron 12, 717–731.0 Å. Neuron. 1994;12:717–731. doi: 10.1016/0896-6273(94)90326-3. [DOI] [PubMed] [Google Scholar]