Abstract
Platelet-activating factor (PAF) plays an important role in the pathogenesis of sepsis, and the level of plasma PAF acetylhydrolase (pPAF-AH), which inactivates PAF, decreases in sepsis patients except for the sepsis caused by severe leptospirosis. Usually, increase of pPAF-AH activity was observed in lipopolysaccharide (LPS)-induced Syrian hamster and rat sepsis models, while contradictory effects were reported for mouse model in different studies. Here, we demonstrated the in vivo effects of LPS upon the change of pPAF-AH activity in C57BL/6 mice and Mongolian gerbils. After LPS-treatment, the clinical manifestations of Mongolian gerbil model were apparently similar to that of C57BL/6 mouse sepsis model. The pPAF-AH activity increased in C57BL/6 mice after LPS induction, but decreased in Mongolian gerbils, which was similar to that of the human sepsis. It thus suggests that among the LPS-induced rodent sepsis models, only Mongolian gerbil could be used for the study of pPAF-AH related to the pathogenesis of human sepsis. Proper application of this model might enable people to clarify the underline mechanism accounted for the contradictory results between the phase II and phase III clinical trials for the administration of recombinant human pPAF-AH in the sepsis therapy.
Introduction
Platelet-activating factor (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine, PAF), a potent proinflammatory phospholipid mediator, has remarkably diverse biological effects in diseases [1], including sepsis, which arises through body's inflammation response to infection and is a leading cause of death and disability for patients in an intensive care unit [2]. PAF synthesis is up-regulated in response to bacterial endotoxins both in vivo and in vitro [3], [4]. Although it was recently reported that PAF may protect mice against lipopolysaccharide (LPS)-mediated sepsis [5], many studies indicated that increased concentrations of PAF may contribute to the deleterious effects of systemic inflammation in the pathogenesis of severe sepsis [2].
The inactivation of PAF is mediated by PAF acetylhydrolase (PAF-AH), a calcium-independent phospholipases A2 with specificity for hydrolysis of this lipid mediator [6]. The plasma form of PAF-AH (pPAF-AH) is a secreted protein in the blood that serves to inactivate PAF and PAF-like phospholipids [6]. This enzyme accounts for all of the PAF-inhibitory activity found in human serum, limiting the normal serum half-life of PAF to only a few minutes [1], [7]. Except for the sepsis caused by severe leptospirosis [8], the activity of pPAF-AH is diminished in human sepsis [7], [9], [10], [11] as a consequence of endotoxin and cytokine-induced reduction of the pPAF-AH encoding gene transcription and possible inactivation by oxidant injury [11], [12].
A potential therapeutic strategy for sepsis is to facilitate the inactivation of PAF with the supplement of pPAF-AH. The results of the clinical trials of recombinant human pPAF-AH in patients with severe sepsis were controversial (Table 1). In 2006, Gomes et al. reported that the administration of exogenous recombinant pPAF-AH reduced mortality and inflammatory injury relevant to the clinical syndrome (Table 1), based on the depressed pPAF-AH activity in C57BL/6 and Swiss mouse models induced by LPS or cecal ligation and puncture (CLP) (Table 2). However, this result is partly in contradictory to the previous studies in rodents challenged with LPS, which showed an increase of pPAF-AH activity in Syrian hamsters, rats, and C57BL/6 mice (Table 2). Therefore, it would be important to clarify the response of pPAF-AH against LPS-treatment in mice, and/or explore alternative animals suitable for simulating the role of pPAF-AH in human sepsis.
Table 1. Therapeutic effects of the administration of recombinant human pPAF-AH in human sepsis patients and mouse models.
| Status | Year | Effect | Reference | |
| Human | Phase IIa | 1999 | Improvements in oxygenation and multiple organ dysfunction | [20] |
| Phase IIb | 2003 | Striking survival advantage and other positive effects | [21] | |
| Phase III | 2004 | No effect on decreasing mortality | [22] | |
| Mouse | LPS/CLP model | 2006 | Reduction of inflammatory injury and mortality | [9] |
Mouse represented the C57BL/6 mouse.
Table 2. Response of pPAF-AH in LPS and CLP sepsis models.
Previously, we found that Mongolian gerbil has its normal pPAF-AH level similar to that of human, and the patterns of the change of PAF-AH level in serum during the course of severe leptospirosis in gerbil model are similar to that of severe leptospirosis patients, including the levels of elevation [8]. These findings were consistent with the fact that LPS of Leptospira interrogans is much less virulent than that of Escherichia coli, and has little effect on the sepsis caused by leptospirosis [13]. Therefore, among experimental rodents and rabbits, gerbil is likely to be a good candidate to develop an animal model to mimic the role of pPAF-AH in human diseases, particularly, the LPS-induced sepsis [8]. In this study, we examined the in vivo effects of LPS on pPAF-AH activity in C57BL/6 mice and gerbils.
Materials and Methods
Ethics Statement
All animals were handled in strict accordance with good animal practice as defined by the relevant local animal welfare bodies, and all animal work was approved by the Animal Research Committee of the Chinese National Human Genome Center at Shanghai.
Animal Study
Male C57BL/6 mice (Shanghai Laboratory Animal Center, China), one month of age (18 to 22 g), and male gerbils (Zhejiang Laboratory Animal Center, China), two months of age (45 to 60 g), were given a standard laboratory diet and water ad libitum and housed under controlled environmental conditions. LPS from Escherichia coli (serotype 0111:B4) was purchased from Sigma Chemical Company and was freshly diluted to desired concentrations in pyrogen-free 0.9% saline. After a minimum 3-day acclimation period, animals were intraperitoneally injected with either saline (control) or LPS (3 or 5 mg/kg body weight).
pPAF-AH Activity Assay
pPAF-AH activity was determined by using a commercially available assay kit (Cayman Chemical) according to manufacturer's instructions. The assay uses 2-thio-PAF, which serves as a substrate for pPAF-AH. On hydrolysis of the acetyl thioester bond by pPAF-AH, free thiols are detected using 5, 5′-dithio-bis-(2-nitrobenzoic acid) (DTNB, Ellman's reagent). The absorbance is read at 405 nm over a period of time using an ELISA plate reader.
Statistics
Data were analyzed with Graph-Pad Prism, version 2.0 (GraphPad Software). Data were presented as mean values ± SEM. Statistical analyses were performed using one way analysis of variance (ANOVA).
Results and Discussion
Compared to the control group (0 mg/kg body weight, saline only), the C57BL/6 mice and gerbils with LPS-treatment (3 or 5 mg/kg body weight) appeared acutely ill and displayed signs of lethargy, and then they were euthanized while the animals appeared moribund after LPS-treatment (Table 3). Most of the animals died after LPS injection, and autopsy showed the volume increase of spleen in all the animals of both C57BL/6 mice and gerbils (Table 3). Therefore, the clinical manifestations of LPS-induced gerbil model were apparently similar to that of C57BL/6 mouse sepsis model [9].
Table 3. Clinical manifestations of C57BL/6 mice and gerbils induced by LPS.
| Dose (mg/kg) | Clinical observation | Death/total | Time to death (hr) | |
| Mouse | 0a | Normal | 0/10 | ND |
| 3 | Lethargy, diarrhea and spleen volume increase | 10/10 | 36-45 | |
| 5 | Lethargy, diarrhea and spleen volume increase | 10/10 | 15-26 | |
| Gerbil | 0a | Normal | 0/10 | ND |
| 3 | Lethargy, diarrhea and spleen volume increase | 9/10 | 36-49 | |
| 5 | Lethargy, diarrhea and spleen volume increase | 10/10 | 13-27 |
Mouse represented the C57BL/6 mouse.
Control, intraperitoneally injected with saline only.
ND, no animal death determined.
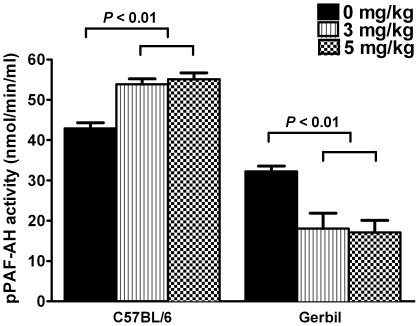
Blood was collected by cardiac puncture and pPAF-AH activity was measured. We found that both 3 and 5 mg/kg body weight LPS induced the elevation of the pPAF-AH activity in C57BL/6 mice, and the elevated levels were similar in these two dose group (Figure 1). In contrast, after LPS-treatment (3 or 5 mg/kg body weight), the pPAF-AH activity of gerbils decreased compared to that of the control, and the decreased levels were similar in the doses of 3 and 5 mg/kg body weight (Figure 1).
Figure 1. Effects of LPS on pPAF-AH activity in C57BL/6 mice and gerbils.
Data were presented as means ± SEM; n = 10 for each dose group.
Our result showed that LPS caused the elevation of pPAF-AH activity in C57BL/6 mice (Figure 1), which was the same with that reported by Memon et al. [14] (Table 2), but different from the study of Gomes et al. [9] (Table 2). Although the reason for the different effects of LPS in C57BL/6 mice was yet to be elucidated, the present study, together with the studies in Syrian hamsters, rats, and C57BL/6 mice challenged by LPS [14], [15], [16], [17] (Table 2), showed that, among rodent species, only the gerbil demonstrated the decrease of pPAF-AH activity under the exposure of LPS (Figure 1), which was similar to the response of pPAF-AH measured in sepsis patients [7], [9], [10], [11]. Therefore, the LPS-induced sepsis in gerbil could be used to study the pharmacological effect of recombinant pPAF-AH in sepsis, and may provide pre-clinical evaluation of pPAF-AH in sepsis for the additional clinical trials in the future.
LPS is a relatively pure compound that can be stably stored in lyophilized form. Therefore, the LPS model is much easier to be established than the surgical CLP model, which is the “gold standard” in sepsis research [18]. However, the LPS model is known to be different from the sepsis in human and CLP model with respect to the profile of cytokine release [18], [19]. PAF is a phospholipid cytokine implicated in a wide range of biological and pathologic responses [1], and thus, the different responses of pPAF-AH, the regulator of serum PAF [6], between sepsis patients and LPS models of Syrian hamster, rat and mouse (Table 2 and Figure 1) might be accounted by the differences in the profile of cytokine release. The similar responses of pPAF-AH between sepsis patients and gerbil LPS model (Figure 1), as well as between the severe leptospirosis patients and the gerbil leptospirosis model [8], may implicate the similar response of cytokine release between human and gerbil. This possible underline mechanism should be further tested in order to fully characterize this novel model, which is potentially advantageous in mimicking the cytokine response in sepsis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported in part by grants from the National Natural Science Foundation of China (No. 30830002, 30970077, 30900051, 30970125, 30770111 and 30770820), the National Key Program for Infectious Diseases of China (No. 2008ZX10004-002, 2008ZX10004-009 and 2009ZX10004-712), and Shanghai Leading Academic Discipline Project (T0206), Shanghai Natural Science Foundation of China (No. 062R14056), Shanghai Municipal Education Commission and Shanghai Education Development Foundation (“Chen Guang” project, 2007CGB06). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chao W, Olson MS. Platelet-activating factor: receptors and signal transduction. Biochem J. 1993;292 (Pt 3):617–629. doi: 10.1042/bj2920617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall JC. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat Rev Drug Discov. 2003;2:391–405. doi: 10.1038/nrd1084. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman GA, McIntyre TM, Prescott SM, Stafforini DM. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit Care Med. 2002;30:S294–301. doi: 10.1097/00003246-200205001-00020. [DOI] [PubMed] [Google Scholar]
- 4.Chang SW, Feddersen CO, Henson PM, Voelkel NF. Platelet-activating factor mediates hemodynamic changes and lung injury in endotoxin-treated rats. J Clin Invest. 1987;79:1498–1509. doi: 10.1172/JCI112980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong YI, Jung ID, Lee CM, Chang JH, Chun SH, et al. The novel role of platelet-activating factor in protecting mice against lipopolysaccharide-induced endotoxic shock. PLoS One. 2009;4:e6503. doi: 10.1371/journal.pone.0006503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet-activating factor acetylhydrolases. J Biol Chem. 1997;272:17895–17898. doi: 10.1074/jbc.272.29.17895. [DOI] [PubMed] [Google Scholar]
- 7.Graham RM, Stephens CJ, Silvester W, Leong LL, Sturm MJ, et al. Plasma degradation of platelet-activating factor in severely ill patients with clinical sepsis. Crit Care Med. 1994;22:204–212. doi: 10.1097/00003246-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Zhang Y, Xu J, Geng Y, Chen X, et al. Serum activity of platelet-activating factor acetylhydrolase is a potential clinical marker for leptospirosis pulmonary hemorrhage. PLoS ONE. 2009;4:e4181. doi: 10.1371/journal.pone.0004181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes RN, Bozza FA, Amancio RT, Japiassu AM, Vianna RC, et al. Exogenous platelet-activating factor acetylhydrolase reduces mortality in mice with systemic inflammatory response syndrome and sepsis. Shock. 2006;26:41–49. doi: 10.1097/01.shk.0000209562.00070.1a. [DOI] [PubMed] [Google Scholar]
- 10.Claus RA, Russwurm S, Dohrn B, Bauer M, Losche W. Plasma platelet-activating factor acetylhydrolase activity in critically ill patients. Crit Care Med. 2005;33:1416–1419. doi: 10.1097/01.ccm.0000165807.26485.ed. [DOI] [PubMed] [Google Scholar]
- 11.Partrick DA, Moore EE, Moore FA, Biffl WL, Barnett CC. Reduced PAF-acetylhydrolase activity is associated with postinjury multiple organ failure. Shock. 1997;7:170–174. doi: 10.1097/00024382-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y, Stafforini DM, Zimmerman GA, McIntyre TM, Prescott SM. Expression of plasma platelet-activating factor acetylhydrolase is transcriptionally regulated by mediators of inflammation. J Biol Chem. 1998;273:4012–4020. doi: 10.1074/jbc.273.7.4012. [DOI] [PubMed] [Google Scholar]
- 13.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 14.Memon RA, Fuller J, Moser AH, Feingold KR, Grunfeld C. In vivo regulation of plasma platelet-activating factor acetylhydrolase during the acute phase response. Am J Physiol. 1999;277:R94–103. doi: 10.1152/ajpregu.1999.277.1.R94. [DOI] [PubMed] [Google Scholar]
- 15.Howard KM, Olson MS. The expression and localization of plasma platelet-activating factor acetylhydrolase in endotoxemic rats. J Biol Chem. 2000;275:19891–19896. doi: 10.1074/jbc.M001462200. [DOI] [PubMed] [Google Scholar]
- 16.Svetlov SI, Sturm E, Olson MS, Crawford JM. Hepatic regulation of platelet-activating factor acetylhydrolase and lecithin:cholesterol acyltransferase biliary and plasma output in rats exposed to bacterial lipopolysaccharide. Hepatology. 1999;30:128–136. doi: 10.1002/hep.510300122. [DOI] [PubMed] [Google Scholar]
- 17.Howard KM, Miller JE, Miwa M, Olson MS. Cell-specific regulation of expression of plasma-type platelet-activating factor acetylhydrolase in the liver. J Biol Chem. 1997;272:27543–27548. doi: 10.1074/jbc.272.44.27543. [DOI] [PubMed] [Google Scholar]
- 18.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 19.Fink MP. Animal models of sepsis and its complications. Kidney Int. 2008;74:991–993. doi: 10.1038/ki.2008.442. [DOI] [PubMed] [Google Scholar]
- 20.Robert Metzler MB, Daniel Schuster, Gus Slotman, John Michael, Janice Zimmerman, et al. An evaluation of recombinant human platelet activating factor acetylhydrolase (rPAF-AH) in patients at risk for developing acute respiratory distress syndrome. Critical Care Medicine Volume. 1999;27:p85A. [Google Scholar]
- 21.Schuster DP, Metzler M, Opal S, Lowry S, Balk R, et al. Recombinant platelet-activating factor acetylhydrolase to prevent acute respiratory distress syndrome and mortality in severe sepsis: Phase IIb, multicenter, randomized, placebo-controlled, clinical trial. Crit Care Med. 2003;31:1612–1619. doi: 10.1097/01.CCM.0000063267.79824.DB. [DOI] [PubMed] [Google Scholar]
- 22.Opal S, Laterre PF, Abraham E, Francois B, Wittebole X, et al. Recombinant human platelet-activating factor acetylhydrolase for treatment of severe sepsis: results of a phase III, multicenter, randomized, double-blind, placebo-controlled, clinical trial. Crit Care Med. 2004;32:332–341. doi: 10.1097/01.CCM.0000108867.87890.6D. [DOI] [PubMed] [Google Scholar]