Abstract
Background
We test whether the phenotypic variance of symbionts (Symbiodinium) in corals is closely related with the capacity of corals to acclimatize to increasing seawater temperatures. Moreover, we assess whether more specialist symbionts will increase within coral hosts under ocean warming. The present study is only applicable to those corals that naturally have the capacity to support more than one type of Symbiodinium within the lifetime of a colony; for example, Montastraea annularis and Montastraea faveolata.
Methodology/Principal Findings
The population dynamics of competing Symbiodinium symbiont populations were projected through time in coral hosts using a novel, discrete time optimal–resource model. Models were run for two Atlantic Ocean localities. Four symbiont populations, with different environmental optima and phenotypic variances, were modeled to grow, divide, and compete in the corals under seasonal fluctuations in solar insolation and seawater temperature. Elevated seawater temperatures were input into the model 1.5°C above the seasonal summer average, and the symbiont population response was observed for each location. The models showed dynamic fluctuations in Symbiodinium populations densities within corals. Population density predictions for Lee Stocking Island, the Bahamas, where temperatures were relatively homogenous throughout the year, showed a dominance of both type 2, with high phenotypic variance, and type 1, a high-temperature and high-insolation specialist. Whereas the densities of Symbiodinium types 3 and 4, a high-temperature, low-insolation specialist, and a high-temperature, low-insolation generalist, remained consistently low. Predictions for Key Largo, Florida, where environmental conditions were more seasonally variable, showed the coexistence of generalists (types 2 and 4) and low densities of specialists (types 1 and 3). When elevated temperatures were input into the model, population densities in corals at Lee Stocking Island showed an emergence of high-temperature specialists. However, even under high temperatures, corals in the Florida Keys were dominated by generalists.
Conclusions/Significance
Predictions at higher seawater temperatures showed endogenous shuffling and an emergence of the high-temperature Symbiodinium specialists, even though their phenotypic variance was low. The model shows that sustaining these “hidden” specialists becomes advantageous under thermal stress conditions, and shuffling symbionts may increase the corals' capacity to acclimatize but not adapt to climatechange–induced ocean warming.
Introduction
The ubiquity of modern reef-building corals in the shallow, low-nutrient tropical environments stems from their capacity to house unicellular dinoflagellates [1]. This mutually beneficial relationship depends on photosynthates that are released by the symbionts and utilized by the coral host; corals, in turn, produce organic wastes upon which the symbionts thrive [2], [3]. Coral symbionts, or Symbiodinium species, were once thought to consist of only one species [4]. However technological advances show potentially hundreds of symbiont types [5]–[9], and some preliminary research has shown that coral physiology is highly dependent on the type of symbionts present in the host [10]–[13].
Most corals seem very specific in the type of Symbiodinium they support, and most corals only support one Symbiodinium type over time [14]–[16]. Still, some coral species are capable of simultaneously supporting more than one Symbiodinium population, which are spread across coral colonies in accordance with down-welling irradiance [17], [18]. Symbiodinium population densities are not, however, in a steady state. Population densities vary in accordance with seasonal temperature, irradiance and nutrient concentrations [19]–[24]. Recently, Chen et al. [23] demonstrated seasonal dynamics in the relative densities of different Symbiodinium types, presumably upregulating the high-light, high-heat tolerant species in summer. Several key studies have also shown seasonal declines in photosynthetic efficiency that is related to high seawater temperature and irradiance [25], [26]. Corals pale, or bleach, when temperatures exceed seasonal averages for extended periods [27]. An extreme case-in-point is the 1997–98 global thermal stress event, which was an extreme manifestation of a more general impact of the El Niño-Southern Oscillation cycle. This event led to extreme coral bleaching and extensive coral mortality worldwide [28].
Symbiotic scleractinian corals live close to their thermal tolerance levels. The last two decades have seen an increase in the frequency and severity of symbiotic dysfunction (i.e., coral bleaching) in response to anomalous sea-surface temperature increases [29]–[33]. Yet, symbiont responses vary in accordance with the type of stress [3]. If temperature and irradiance stresses are of moderate intensity and duration, corals are capable of regaining pigmentation, both through increases in Symbiodinium pigment and population densities [34]. If stress exceeds a critical threshold, which varies among coral species and geographic locality [35], [36], [10], bleaching is inevitable, often leading to partial or whole-colony mortality [37]–[39].
Contemporary molecular-ecology research is interested in the dynamics of Symbiodinium in corals, their response to thermal stress events [40]–[42], and what role the Symbiodinium might play in acclimatization and adaptation of reef corals [43]. We note that the present study is only applicable to those corals, approximately 25% of corals worldwide [16], that naturally have the capacity to support more than one type of Symbiodinium within the lifetime of a colony, for example Montastraea annularis and Montastraea faveolata.
Models
Ware et al. [44] devised a mathematical model to examine Symbiodinium population growth during and after thermal stress events using generalized Lotka-Volterra competition equations. Although Ware's model predicts the superior Symbiodinium type, the system is set such that the differential equation that governs Symbiodinium type 1 (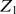 ), the first equation in the set, will ultimately dominate the entire system. The model does not consider resources for which Symbiodinium species compete. We sought to examine the response of Symbiodinium population densities to the seasonal dynamics of solar insolation (a resource) and seawater temperature.
), the first equation in the set, will ultimately dominate the entire system. The model does not consider resources for which Symbiodinium species compete. We sought to examine the response of Symbiodinium population densities to the seasonal dynamics of solar insolation (a resource) and seawater temperature.
Recent research on adaptation to climate change and increasing thermal stresses has emphasized the need to assess phenotypic variance of organisms in general [45], [46] and corals in particular [43], [47]. We test whether the phenotypic variance of symbionts may be closely related with the capacity of Montastraea corals to acclimatize to increasing seawater temperatures. Moreover, we assess whether more specialist symbionts are lost from the coral (holobiont) under a warming ocean. The objectives are to obtain accurate time-course predictions of Symbiodinium population densities in Montastraea corals, and make valid estimates, of Symbiodinium densities, under seasonal dynamics of solar insolation and seawater temperature, and through thermal stress events.
Materials and Methods
Symbiont-Population Growth
Growth of each Symbiodinium population can be modeled by considering specific growth rates relative to specific loss rates. Population flux can be theoretically estimated (following Jones and Yellowlees [48]) using the difference equation:
| (1) |
where 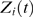 is the population density of Symbiodinium (or zooxanthellae) type i,
is the population density of Symbiodinium (or zooxanthellae) type i, 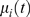 is the specific growth rate of
is the specific growth rate of 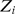 , and
, and 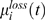 is the specific loss rate of
is the specific loss rate of 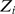 from the host coral at time
from the host coral at time  (Table 1). An assumption of the model is that the resources allocated to each Symbiodinium population influences
(Table 1). An assumption of the model is that the resources allocated to each Symbiodinium population influences 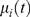 , and that down-welling solar insolation is the primary resource limiting symbiont population densities (see Table 2 for other assumptions). We note that high insolation, in early summer, leads to photoinhibition and reductions in symbiont population densities. Furthermore, increasing nutrients can have the opposite effect of increasing symbiont densities [49], [22], [14]. But nutrient concentrations are far less predictable than insolation and temperature [50], and are therefore not input into our model. Where nutrient concentrations (X) are available, then X can be defined as a function of time f(t), and inserted as a resource in Equation 3 (below).
, and that down-welling solar insolation is the primary resource limiting symbiont population densities (see Table 2 for other assumptions). We note that high insolation, in early summer, leads to photoinhibition and reductions in symbiont population densities. Furthermore, increasing nutrients can have the opposite effect of increasing symbiont densities [49], [22], [14]. But nutrient concentrations are far less predictable than insolation and temperature [50], and are therefore not input into our model. Where nutrient concentrations (X) are available, then X can be defined as a function of time f(t), and inserted as a resource in Equation 3 (below).
Table 1. Notations and abbreviations used in the optimal-resource model.
| Notation | Unit | Interpretation |
| t | day | time |
| Z | cells cm−2 | zooxanthellae density |
| μi | dimensionless | proliferation rate of zooxanthella type i |
| μloss | dimensionless | zooxanthellae loss rate from host |
| rpro | kWm−2d−1 | resource for zooxanthellae proliferation |
| Ri | resource dependent | required resource for proliferation of zooxanthella type i |
| a, b, c | dimensionless | coefficients of environmental parameters [51] |
| SI | kWm−2d−1 | solar insolation |
| SIiopt | kWm−2d−1 | optimal SI for zooxanthella type i |
| hSIopt | kWm−2d−1 | optimal SI for host |
| SST | °C | sea surface temperature |
| SSTiopt | °C | optimal SST for zooxanthella type i |
| hSSTopt | °C | optimal SST for host |
| α | kWm−2d−1 | optimal SI range for zooxanthella type i |
| β | °C | optimal SST range for zooxanthella type i |
| γ | kWm−2d−1 | optimal SI range for host |
| K | cells cm−2 | carrying capacity - host dependent |
| Kc | cells cm−2 | environmentally dependent carrying capacity |
| ∑ Zi | cells cm−2 | total number of zooxanthellae cm−2 |
Table 2. Assumptions used in the optimal-resource model.
| 1) | Host corals may possess multiple Symbiodinium types at any given time and exogenous Symbiodinium do not contribute to any population densities; |
| 2) | Symbiodinium proliferation rate is driven by the dynamic resource solar insolation; |
| 3) | Solar insolation and seawater and temperature covary [66]; |
| 4) | Symbiodinium density is a balance between specific growth and loss rates; |
| 5) | The growth response function of each Symbiodinium type follows a Gaussian distribution [52]. |
Symbiodinium population densities were predicted for corals at Lee Stocking Island (23°N, 76°W) and Key Largo (24°N, 80°W) using solar insolation (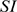 ) (kW m−2 d−1) as a primary resource at each location using the general equation:
) (kW m−2 d−1) as a primary resource at each location using the general equation:
| (2) |
where 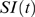 is solar insolation at time t, and ai, bi, and
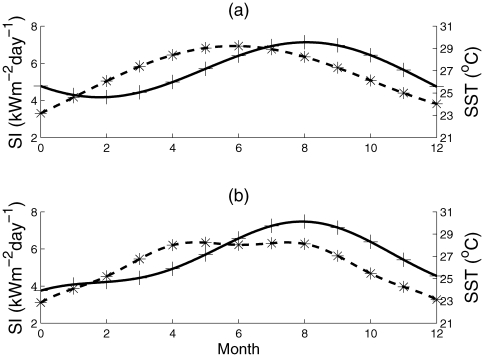
is solar insolation at time t, and ai, bi, and 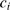 were locality specific coefficients, while temperature is not a resource, but rather a condition. From satellite data [51], ten-year averages of solar insolation and sea surface temperature were used to derive functions with respect to time (Figure 1). For simplicity, annual change in Sea Surface Temperature (
were locality specific coefficients, while temperature is not a resource, but rather a condition. From satellite data [51], ten-year averages of solar insolation and sea surface temperature were used to derive functions with respect to time (Figure 1). For simplicity, annual change in Sea Surface Temperature (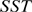 in °C) followed the same general construct, replacing
in °C) followed the same general construct, replacing 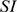 with
with 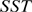 in Equation 2 and the parameters were changed appropriately for each location.
in Equation 2 and the parameters were changed appropriately for each location.
Figure 1. Dynamics of 10-year seasonal means of Sea Surface Temperature (SST) (solid line) and Solar Insolation (SI) (dashed line).
Panel (a) shows the dynamics for Lee Stocking Island, the Bahamas, and panel (b) shows the dynamics for Key Largo, Florida.
Competition for Resources
An average Symbiodinium is generally no more than 10 µm diameter, and 106
Symbiodinium cells can fit in 1 cm2 of coral tissue depending on tissue thickness, which can vary from 0.3 to 10 mm depending on the coral species under examination. Deeper Symbiodinium receive less light than surface Symbiodinium. Since solar insolation is the primary resource considered here, the resource becomes limiting with an increase in Symbiodinium density. Therefore, Symbiodinium proliferation rate, 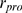 , (following Tilman et al. 1997 [52]) can be described as a function of time:
, (following Tilman et al. 1997 [52]) can be described as a function of time:
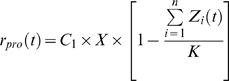 |
(3) |
where 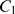 is a constant coefficient;
is a constant coefficient; 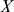 is the primary resource (here solar insolation (t));
is the primary resource (here solar insolation (t)); 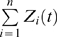 is the total number of Symbiodinium cm−2; and
is the total number of Symbiodinium cm−2; and 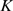 is the carrying capacity within the host corals. While this model examines changes in symbiont dynamics over time, it is equally appropriate to examine micro-environmental profiles, such as those reported in Rowan et al. [17]. Partitioning coral colonies into different micro-irradiance environments, for example, would be equally valid.
is the carrying capacity within the host corals. While this model examines changes in symbiont dynamics over time, it is equally appropriate to examine micro-environmental profiles, such as those reported in Rowan et al. [17]. Partitioning coral colonies into different micro-irradiance environments, for example, would be equally valid.
The specific growth rate, 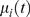 , of Symbiodinium
, of Symbiodinium
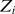 is given as:
is given as:
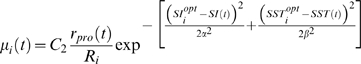 |
(4) |
where 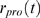 is the resource allocation to Symbiodinium proliferation at time
is the resource allocation to Symbiodinium proliferation at time  (derived in Equation 3);
(derived in Equation 3); 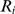 is the resource requirement for
is the resource requirement for 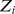 ;
; 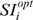 and
and 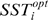 are optimal proliferation requirements (following Pulliam 2000 [53]) of
are optimal proliferation requirements (following Pulliam 2000 [53]) of 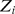 with regard to solar insolation (SI) and sea surface temperature (SST);
with regard to solar insolation (SI) and sea surface temperature (SST);  and
and 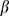 are standard deviations of SI and SST (Figure 2a); and C2 is a constant coefficient. For each Zi,
are standard deviations of SI and SST (Figure 2a); and C2 is a constant coefficient. For each Zi, 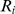 was set to 1, with all Symbiodinium showing equal competitive abilities for resources. If physiological studies find otherwise, Ri can be set hierarchically, with the most competitive Symbiodinium type set at i = 1, and the most inferior type set at i = n. Dynamics of the sustainable Symbiodinium density for each Zi in host corals are expressed by:
was set to 1, with all Symbiodinium showing equal competitive abilities for resources. If physiological studies find otherwise, Ri can be set hierarchically, with the most competitive Symbiodinium type set at i = 1, and the most inferior type set at i = n. Dynamics of the sustainable Symbiodinium density for each Zi in host corals are expressed by:
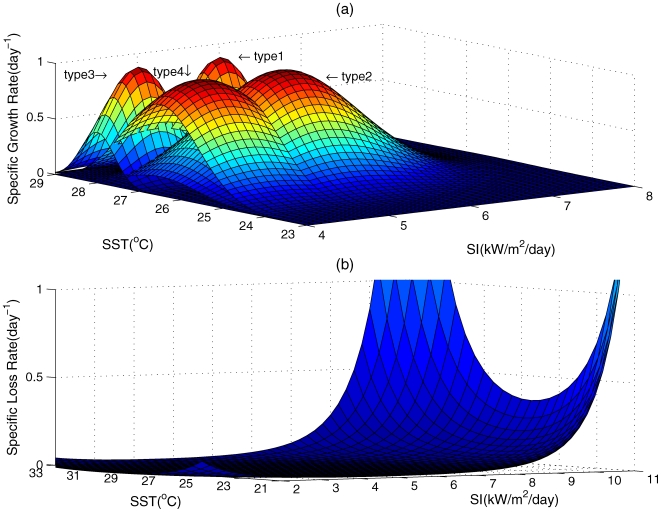
Figure 2. Symbiodinium growth and loss rate response curves in relation to Sea Surface Temperature (SST) and Solar Insolation (SI).
Panel (a) shows specific growth rates of four Symbiodinium types, and panel (b) shows specific loss rates.
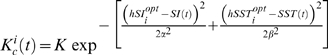 |
(5) |
Because no data are available to the contrary, excess symbionts relative to 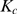 , are assumed to be lost from the host corals randomly and independent of Symbiodinium type. The specific Symbiodinium loss rate is:
, are assumed to be lost from the host corals randomly and independent of Symbiodinium type. The specific Symbiodinium loss rate is:
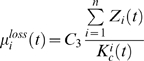 |
(6) |
where C3 is a constant coefficient (Figure 2b).
Population-Density Predictions
To predict Symbiodinium population densities, the following conditions were applied to the models:
values of
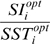 were 5.5/28, 5.5/26.5, 4.5/28, and 4.5/26.5, for each
were 5.5/28, 5.5/26.5, 4.5/28, and 4.5/26.5, for each 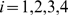 , respectively, which covers the range of solar insolation and seawater temperature probabilities for the Florida Keys and the Bahamas (using the units °C for sea surface temperature and kW m−2 d−1 for solar insolation) (Figure 2);
, respectively, which covers the range of solar insolation and seawater temperature probabilities for the Florida Keys and the Bahamas (using the units °C for sea surface temperature and kW m−2 d−1 for solar insolation) (Figure 2);the standard deviations for
 were 0.4, 0.8, 0.4 and 0.8 for each
were 0.4, 0.8, 0.4 and 0.8 for each 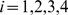 , respectively (Figure 2) – we define Symbiodinium types 1 and 3 as specialists because they have narrow environmental tolerances;
, respectively (Figure 2) – we define Symbiodinium types 1 and 3 as specialists because they have narrow environmental tolerances;the standard deviations for
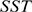 were 0.4, 1, 0.4 and 1 for each
were 0.4, 1, 0.4 and 1 for each 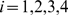 , respectively – again, Symbiodinium types 1 and 3 are defined as specialists because they have narrow environmental tolerances;
, respectively – again, Symbiodinium types 1 and 3 are defined as specialists because they have narrow environmental tolerances;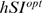 and
and 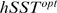 for the holobionts, were 5.5 and 27 for SI and SST respectively;
for the holobionts, were 5.5 and 27 for SI and SST respectively;standard deviations for
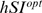 and
and 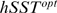 were 2.0 and 3.0, respectively;
were 2.0 and 3.0, respectively;each Symbiodinium type had an initial population density of 1.0×106 cells cm−2;
each month was set at 30 days, and one year was set at 360 days;
Since Equation 1 is a discrete time model, the solutions (i.e., population densities) were approximated in discrete time (10 yr) by numerical iteration. The SIi opt and SSTi opt (values for condition 1 above) and the values for the standard deviations (for conditions 2 and 3 above), were derived from normal distributions for each zooxanthellae type for each iteration step (with mean, μ, and the standard deviation, σ, of the distributions given in conditions 1 and 2, respectively). To introduce real-world thermal stress, Symbiodinium populations were randomly subjected to +1°C above-average temperatures in July, +1.5°C in August, and +1°C in September. The results were compared with a 4-year study, which tagged host corals and regularly monitored Symbiodinium types and their densities from 2000 to 2004, in Key Largo, Florida, and Lee Stocking Island, the Bahamas [41].
Results and Discussion
Seasonal Dynamics
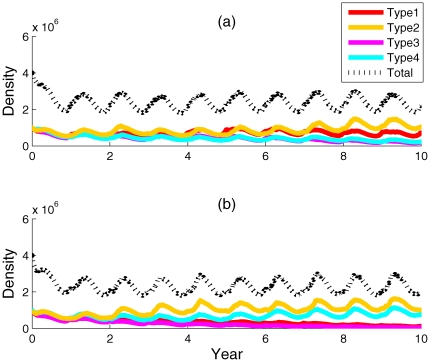
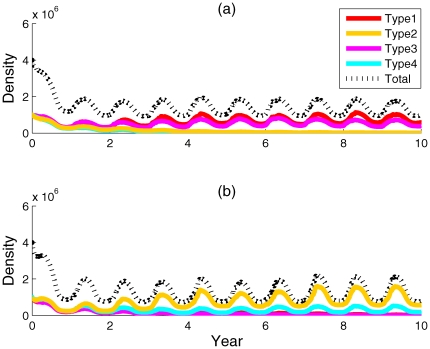
Symbiodinium densities varied seasonally, showing highest densities from December to April; extreme solar insolation and temperature conditions induced high 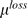 in summer for both localities (Figures 2, 3). Symbiodinium dynamics were more variable in Key Largo, Florida, than at Lee Stocking Island, the Bahamas (Figure 3). Predictions for Lee Stocking Island, the Bahamas, where temperatures were relatively homogenous throughout the year, showed a dominance of Symbiodinium type 2, which had high phenotypic variance, and a type 1 high-temperature and high-insolation specialist (type 1). The densities of Symbiodinium types 3 and 4 remained consistently low (Figure 3). In contrast, predictions for Key Largo, Florida, where environmental conditions were more seasonally variable, showed the co-dominance of two Symbiodinium populations (types 2 and 4), both with high phenotypic variance. The specialist symbionts, types 1 and 3, with low phenotypic variance, were present but in very low densities (Figure 3). At elevated temperatures, population densities showed endogeneous ‘shuffling’ at both sites and an emergence of types 1 and 3, the high-temperature specialists, with low phenotypic variance, at Lee Stocking Island (Figure 4). In contrast, the elevated temperatures allowed types 2 and, somewhat less of, type 4 to remain dominate in Key Largo corals, with extremely low densities of types 1 and 3 specialists (Figure 4).
in summer for both localities (Figures 2, 3). Symbiodinium dynamics were more variable in Key Largo, Florida, than at Lee Stocking Island, the Bahamas (Figure 3). Predictions for Lee Stocking Island, the Bahamas, where temperatures were relatively homogenous throughout the year, showed a dominance of Symbiodinium type 2, which had high phenotypic variance, and a type 1 high-temperature and high-insolation specialist (type 1). The densities of Symbiodinium types 3 and 4 remained consistently low (Figure 3). In contrast, predictions for Key Largo, Florida, where environmental conditions were more seasonally variable, showed the co-dominance of two Symbiodinium populations (types 2 and 4), both with high phenotypic variance. The specialist symbionts, types 1 and 3, with low phenotypic variance, were present but in very low densities (Figure 3). At elevated temperatures, population densities showed endogeneous ‘shuffling’ at both sites and an emergence of types 1 and 3, the high-temperature specialists, with low phenotypic variance, at Lee Stocking Island (Figure 4). In contrast, the elevated temperatures allowed types 2 and, somewhat less of, type 4 to remain dominate in Key Largo corals, with extremely low densities of types 1 and 3 specialists (Figure 4).
Figure 3. Ten-year iterations of four Symbiodinium population densities in corals modeled at two Caribbean localities.
The models were run with C1 = 0.01, and R1 = R2 = R3 = R4 = 1. Panel (a) shows the results for Lee Stocking Island, the Bahamas and panel (b) shows the results for Key Largo, Florida.
Figure 4. Ten-year iterations of four Symbiodinium population densities in corals that have been subjected to above-average water temperature increases.
Where panel (a) represents predictions for Lee Stocking Island, the Bahamas, subjected to above-average temperatures of +1°C July, +1.5°C August, +1°C September; and panel (b) represents predictions for Key Largo, Florida, subjected to the same above average temperatures.
In 2006, Thornhill et al. [41] noted that Symbiodinium in Montastrea annularis and Montastrea faveolata varied in accordance with locality and depth. They also showed a 2–3 year changeover from one symbiont to another in certain shallow colonies from Florida, and that M. annularis and M. faveolata supported more Symbiodinium types in Key Largo than the same hosts at Lee Stocking Island. Therefore the Thornhill et al. [41] study and the present (modeling) study agree; Key Largo corals support more Symbiodinium types than Lee Stocking Island. Thornhill et al. [41] attributed these differences to six potential factors, including environmental variation and human impacts. We suggest insolation and temperature differences between the sites may have the same effect. More interesting, however, was that both studies showed high symbiont diversity directly following extreme thermal stress, followed by stability and reduced diversity. In 2009, Thornhill and colleagues [42] showed that while the 2005 bleaching event caused compositional changes in Montastrea annularis and M. faveolata symbiont populations, they noted that the recovered genotypes were consistent with the population prior to the thermal stress. Furthermore, they demonstrated remarkable endemism and specificity within host corals. Clearly thermal stress events trigger within-host instability, which may equilibrate through time under more optimal conditions. Still, an increase in the frequency and intensity of disturbance may cause a more ‘permanent’ state of instability.
Limits
The present model uses a Gaussian distribution to represent environmental optimality. There is no information in the literature confirming or denying such a distribution, although it seems reasonable based on numerous plant-physiology studies [53]. Empirical studies may be best directed at examining physiological variance of Symbiodinium in relation to temperature, irradiance and nutrient concentrations. Yet, phenotypic variance may be best expressed as log-normal distributions (i.e., geometric normal) [Gingerich 54]. Similarly, the model assumed random, non-selective Symbiodinium loss; selective loss may also follow a Gaussian distribution, but more studies are needed to test this premise. Loss and recovery rates may even follow different distribution functions. For example, loss may follow a continuous exponential or a Weibull distribution, with loss decreasing over time after a threshold is exceeded, while recovery may follow a normal distribution that incorporates a lag-phase. Such adjustments are highly dependent on the outcomes of much needed physiological studies examining in hospite responses of Symbiodinium to environmental conditions and extremes.
Some studies have clearly shown that Symbiodinium population dynamics are influenced by nutrient concentrations [21]. Incorporating nutrient dynamics (in the water column) into the model will require a different approach, especially considering the volatility of many nutrient species and their unpredictability in the environment [50]. A more threshold-based response model may be required to reasonably estimate Symbiodinium populations with respect to nutrient dynamics. For example, seasonal extremes (i.e., wet and dry seasons), and event-driven nutrient concentrations may be best input as functions of time (in Equation 3). We input optimality at slightly different parameters; however, theoretically, multiple types of symbionts can also coexist in the same niche space, especially in benign environments where there are no differences between intra- and inter-specific competition [55]–[57]. Although Hutchinson [58] and Huston [59] argued for enhanced diversity at environmentally dynamic localities, because competitive displacement is prevented, the present model predicts that several Symbiodinium populations are likely to be present in locations where the physical environment is benign. We add that diversity depends on the phenotypic variance of the populations and highly dynamic localities are less likely to support specialist Symbiodinium types.
Adjustment Capacity
None of the Symbiodinium types 1–4 reached zero densities after 10 years, although some densities were extremely low (<1 cell cm−2), well below in situ levels of detectability (∼5%, which was the state-of-the-art in 2005) [15], [42]. The model showed possibilities of potentially endogenous shifts in the relative abundance of Symbiodinium populations, especially under thermal stress. This hidden, vestigial component may be non-adaptive but could become useful when conditions change, especially on reefs away from large land masses. Field studies show that survival through a thermal-stress event, of the multi-claded Stylophora pistillata on the Great Barrier Reef, is directly related to whether hosts harbor resistant symbionts [63]. Therefore, sustaining these ‘hidden’ specialists becomes advantageous under thermal stress conditions because the coral holobiont is pre-adapted to thermal stress. In other words, corals harboring multiple symbionts may a have a greater capacity to acclimate to environmental change, but only if those symbionts include thermally tolerant types. Corals harboring thermally sensitive symbionts are rapidly selected out of the gene pool through elevated temperature anomalies [63]. This contrasts with the suggested need to derive novel symbionts from the environment, implied by the adaptive-bleaching hypothesis [60]–[62].
But acclimation reaches a ‘dead end’ under extreme environmental stress; populations can only adjust by evolving – or adapting to the new environment. In principle, a population can adapt to gradual environmental change depending on the amount of genetic variation within a population. But because evolution is the outcome of the interaction between (i) genetic variation, and (ii) natural selection, the capacity to adapt is often limited by the first step – the capacity of a population to produce enough variation upon which selection can act [63]. The second step, in a rapidly changing environment, is ubiquitous and a natural consequence of selective pressure by the environment [64]. Is it then reasonable to assume that corals supporting multiple-species symbionts would have the genetic material to potentially become more thermally tolerant, conceivably adjusting to rapid climate change scenarios, compared with more extinction prone reef corals that strictly support only one specialist Symbiodinium type? No. Certainly the multi-symbiont hosts may have a greater capacity to acclimate, but only if they harbor temperature resistant symbionts [65]. There is no evidence that these multi-symbiont hosts have an advantage in their capacity to adapt. Adaptation requires new material, generated through recombination and mutation. Furthermore, a series of independent molecular studies have shown clear evidence of symbiont endemicity [7], [41], [42], suggesting (i) that new symbiont-coral relationships are unlikely in the short term, and (ii) shuffling symbionts is not a mechanism by which corals can adapt to rapidly warming oceans, but it is a useful acclimation mechanism.
Acknowledgments
We sincerely thank Sandra van Woesik and Todd Lajeunesse for comments on the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Funding for study to KS was provided by the Okinawa International Exchange & Human Resources Development Foundation. This research was supported in part by the World Bank and the Global Environmental Facility through the Coral Reef Targeted Research and Capacity Building for Management program, Coral Bleaching and Local Environmental Responses working group. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stanley GD, Fautin DG. Paleontology and evolution: The origin of modern corals. Science. 2001;291:1913–1914. doi: 10.1126/science.1056632. [DOI] [PubMed] [Google Scholar]
- 2.Muscatine L, Cernichiari E. Assimilation of photosynthetic products of Symbiodinium by a reef coral. Biol Bull. 1969;137:506–523. doi: 10.2307/1540172. [DOI] [PubMed] [Google Scholar]
- 3.Gates RD, Baghdasarian G, Muscatine L. Temperature stress causes host cell detachment in symbiotic cnidarians: Implications for coral bleaching. Biol Bull. 1992;182:324–332. doi: 10.2307/1542252. [DOI] [PubMed] [Google Scholar]
- 4.Taylor DL. Symbiotic marine algae: taxonomy and biological fitness. In: Vernberg WB, editor. Symbiosis in the Sea. Colombia: University of South Carolina Press; 1974. pp. 245–262. [Google Scholar]
- 5.Rowan R, Powers DA. A molecular genetic classification of Symbiodinium and the evolution of animal-algal symbioses. Science. 1991;251:1348–1351. doi: 10.1126/science.251.4999.1348. [DOI] [PubMed] [Google Scholar]
- 6.Rowan R, Powers DA. Ribosomal NA sequences and the diversity of symbiotic dinoflagellates (Symbiodinium). Proc Natl Acad Sci USA. 1992;89:3639–3643. doi: 10.1073/pnas.89.8.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaJeunesse TC. Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol. 2002;141:387–400. [Google Scholar]
- 8.LaJeunesse TC. “Species” radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol Biol Evol. 2005;22:570–581. doi: 10.1093/molbev/msi042. [DOI] [PubMed] [Google Scholar]
- 9.Baker AC. Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography. Annu Rev Ecol Evol Syst. 2003;34:661–68. [Google Scholar]
- 10.Iglesias-Prieto R, Beltran VH, LaJeunesse TC, Reyes-Bonilla H, Thome PE. Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc R Soc Lond B. 2004;271:1757–1763. doi: 10.1098/rspb.2004.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Little AF, van Oppen MJH, Willis BL. Flexibility in algal endosymbioses shapes growth in reef corals. Science. 2004;304:1492–1494. doi: 10.1126/science.1095733. [DOI] [PubMed] [Google Scholar]
- 12.Rowan R. Thermal adaptation in reef coral symbionts. Nature. 2004;430:742. doi: 10.1038/430742a. [DOI] [PubMed] [Google Scholar]
- 13.Warner ME, LaJeunesse TC, Robison JD, Thur RM. The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: Potential implications for coral bleaching. Limnol Oceanogr. 2006;51:1887–1897. [Google Scholar]
- 14.Fitt WK, Cook CB. The effects of feeding or addition of dissolved inorganic nutrients in maintaining the symbiosis between dinoflagellates and a tropical marine cnidarian. Mar Biol. 2001;139:507–517. [Google Scholar]
- 15.LaJeunesse TC, Loh WKW, van Woesik R, Hoegh-Guldberg O, Schmidt GW, et al. Low symbiont diversity in southern Great Barrier Reef corals relative to those of the Caribbean. Limnol Oceanogr. 2003;48:2046–2054. [Google Scholar]
- 16.Goulet TL. Most corals may not change their symbionts. Mar Ecol Prog Ser. 2006;321:1–7. [Google Scholar]
- 17.Rowan R, Knowlton N, Baker A, Jara J. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature. 1997;388:265–269. doi: 10.1038/40843. [DOI] [PubMed] [Google Scholar]
- 18.Baker AC. Reef corals bleach to survive change. Nature. 2001;411:765–766. doi: 10.1038/35081151. [DOI] [PubMed] [Google Scholar]
- 19.Stimson J. The annual cycle of density of Symbiodinium in the tissues of field and laboratory-held Pocillopora damicornis (Linnaeus). J Exp Mar Biol Ecol. 1997;214:35–48. [Google Scholar]
- 20.Brown BE, Ambarsari I, Warner ME, Fitt WK, Dunne RP, et al. Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: evidence for photoinhibition and photoprotection. Coral Reefs. 1999;18:99–105. [Google Scholar]
- 21.Fagoonee I, Wilson HB, Hassell MP, Turner JR. The dynamics of Symbiodinium populations: a long term study in the field. Science. 1999;283:843–845. doi: 10.1126/science.283.5403.843. [DOI] [PubMed] [Google Scholar]
- 22.Fitt WK, McFarland FK, Warner ME, Chilcoat GC. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol Oceanogr. 2000;45:677–685. [Google Scholar]
- 23.Chen CA, Wang JT, Fang LS, Yang YW. Fluctuating algal symbiont communities in Acropora palifera (Scleractinia: Acroporidae) from Taiwan. Mar Ecol Prog Ser. 2005;295:113–121. [Google Scholar]
- 24.Pillay RM, Willis B, Terashita H. Trends in the density of Symbiodinium in Acropora millepora (Ehrenberg, 1834) at the Palm Island Group, Great Barrier Reef, Australia. Symbiosis. 2005;38:209–226. [Google Scholar]
- 25.Warner ME, Fitt WK, Schmidt GW. Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc Natl Acad Sci USA. 1999;96:8007–8012. doi: 10.1073/pnas.96.14.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones RJ, Hoegh-Guldberg O. Diurnal changes in the photochemical efficiency of the symbiotic dinoflagellates (Dinophyceae) of corals: photoprotection, photoinactivation and the relationship to coral bleaching. Plant Cell Environ. 2001;24:89–99. [Google Scholar]
- 27.Warner ME, Chilcoat G, McFarland F, Fitt W. Seasonal fluctuations in the photosynthetic capacity of photosystem II in symbiotic dinoflagellates in the Caribbean reef-building coral Montastraea. Mar Biol. 2002;141:31–38. [Google Scholar]
- 28.Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, et al. Coral bleaching: the winners and the losers. Ecol Lett. 2001;4:122–131. [Google Scholar]
- 29.Glynn PW. Coral reef bleaching in the 1980s and possible connections with global warming. Trends Ecol Evol. 1991;6:175–179. doi: 10.1016/0169-5347(91)90208-F. [DOI] [PubMed] [Google Scholar]
- 30.Glynn PW. Coral reef bleaching ecological perspectives. Coral Reefs. 1993;12:1–17. [Google Scholar]
- 31.Brown BE. Coral bleaching: causes and consequences. Coral Reefs. 1997;16(S):129–138. [Google Scholar]
- 32.Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Mar Freshw Res. 1999;50:839–866. [Google Scholar]
- 33.Fitt WK, Brown BE, Warner ME, Dunne RP. Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs. 2001;20:51–65. [Google Scholar]
- 34.Toller WW, Rowan R, Knowlton N. Symbiodinium of the Montastraea annularis species complex: patterns of distribution of four taxa of Symbiodinium on different reefs and across depths. Biol Bull. 2001;201:348–359. doi: 10.2307/1543613. [DOI] [PubMed] [Google Scholar]
- 35.Berkelmans R. Time-integrated thermal bleaching thresholds of reefs and their variation on the Great Barrier Reef. Mar Ecol Prog Ser. 2002;229:73–82. [Google Scholar]
- 36.Takahashi S, Nakamura T, Sakimizu M, van Woesik R, Yamasaki H. Repair Machinery of Symbiotic Photosynthesis as the Primary Target of Heat Stress for Reef-Building Corals. Plant Cell Physiol. 2004;45(2):251–255. doi: 10.1093/pcp/pch028. [DOI] [PubMed] [Google Scholar]
- 37.Dunn SR, Bythell JC, Le Tissier MDA, Burnett WJ, Thomason JC. Programmed cell death and cell necrosis activity during hyperthermic stress-induced bleaching of the symbiotic sea anemone Aiptasia sp. J Exp Mar Biol Ecol. 2002;272:29–53. [Google Scholar]
- 38.McClanahan T, Maina J, Pet-Soede L. Effects of the 1998 coral morality event on Kenyan coral reefs and fisheries. Ambio. 2002;31:543–550. [PubMed] [Google Scholar]
- 39.Sheppard CR. Predicted recurrences of mass coral mortality in the Indian Ocean. Nature. 2005;425:294–297. doi: 10.1038/nature01987. [DOI] [PubMed] [Google Scholar]
- 40.Santos SR. Phylogenetic analysis of a free-living strain of Symbiodinium isolated from Jiaozhou Bay, P.R. China. J Phycol. 2004;40:395–397. [Google Scholar]
- 41.Thornhill DJ, LaJeunesse TC, Kemp DW, Fitt WK, Schmidt GW. Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar Biol. 2006;148:711–722. [Google Scholar]
- 42.Thornhill DJ, Xiang Y, Fitt WK, Santos SR. Reef endemism, host specificity and temporal stability in populations of symbiotic dinoflagellates from two ecologically dominant Caribbean corals. PLoS One. 2009;4(7):e6262. doi: 10.1371/journal.pone.0006262. DOI: 10.1371/journal.pone.0006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baskett ML, Gaines SD, Nisbet RM. Symbiont diversity may help coral reefs survive moderate climate change. Ecol Appl. 2009;19(1):3–17. doi: 10.1890/08-0139.1. [DOI] [PubMed] [Google Scholar]
- 44.Ware JR, Fautin DG, Buddemeier RW. Patterns of coral bleaching: Modeling the adaptive bleaching hypothesis. Ecol Model. 1996;84:199–214. [Google Scholar]
- 45.Norberg J, Swaney DP, Dushoff J, Lin J, Casagrandi R, et al. Phenotypic diversity and ecosystem functioning in changing enveironments: A theoretical framework. PNAS. 2001;98:11376–11381. doi: 10.1073/pnas.171315998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hellman JJ, Pineda-Krch M. Constraints and reinforcement on adaptation under climate change: Selection of genetically correlated traits. Biol Conser. 2007;137:599–609. [Google Scholar]
- 47.Day T, Nagel L, van Oppen MJH, Caley MJ. Factors affecting the evolution of bleaching resistance in corals. The American Naturalist. 2008;171:E72–E87. doi: 10.1086/524956. [DOI] [PubMed] [Google Scholar]
- 48.Jones RJ, Yellowlees D. Regulation and control of intracellular algae ( = Symbiodinium) in hard corals. Philos Trans R Soc Lond B Biol Sci. 1997;352:457–468. [Google Scholar]
- 49.Falkowski PG, Dubinsky Z, Muscatine L, Porter JW. Light and the bioenergetics of a symbiotic coral. Bioscience. 1984;34:705–709. [Google Scholar]
- 50.Wagner D, Mielbrecht E, van Woesik R. Application of landscape ecology to spatial variance of water-quality parameters along the Florida Keys reef tract. Bull Marine Science. 2008;83(3):553–569. [Google Scholar]
- 51.van Woesik R, Lacharmoise F, Koksal S. Annual cycles of solar insolation predict spawning times of Caribbean corals. Ecol Lett. 2006;9:390–398. doi: 10.1111/j.1461-0248.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 52.Tilman D, Lehman CL, Thomson KT. Plant diversity and ecosystem productivity: Theoretical considerations. Proc Natl Acad Sci USA. 1997;94:1857–1861. doi: 10.1073/pnas.94.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pulliam HR. On the relationship between niche and distribution. Ecol Lett. 2000;3:349–361. [Google Scholar]
- 54.Gingerich PD. Arithmetic or geometric normality of biological variation: an empirical test of theory. J Theoretical Biology. 2000;204:201–221. doi: 10.1006/jtbi.2000.2008. [DOI] [PubMed] [Google Scholar]
- 55.Hubbell SP. Vol. 375. Princeton: Princeton University Press; 2001. The unified neutral theory of biodiversity and biography. [Google Scholar]
- 56.van Woesik R. Processes regulating coral communities. Comments Theor Biol. 2002;7:201–214. [Google Scholar]
- 57.Scheffer M, van Nes EH. Self-organized similarity, the evolutionary emergence of groups of similar species. Proc Natl Acad Sci USA. 2006;103:6230–6235. doi: 10.1073/pnas.0508024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutchinson GE. Ecological aspects of succession in natural populations. Amer Nat. 1941;75:406–418. [Google Scholar]
- 59.Huston M. A general hypothesis of species diversity. Am Nat. 1979;113:81–101. [Google Scholar]
- 60.Buddemeier RW, Fautin DG. Coral bleaching as an adaptive mechanism. Bioscience. 1993;43:320–326. [Google Scholar]
- 61.Kinzie RT, Takayama M, Santos SR, Coffroth MA. The Adaptive Bleaching Hypothesis: experimental tests of critical assumptions. Biol Bull. 2001;200:51–58. doi: 10.2307/1543084. [DOI] [PubMed] [Google Scholar]
- 62.Baker AC, Starger CJ, McClanahan TR, Glynn PW. Coral's adaptive response to climate change. Nature. 2004;430:741. doi: 10.1038/430741a. [DOI] [PubMed] [Google Scholar]
- 63.Bradshaw AD. Genostasis and the limits to evolution. Phil Tran Royal Soc B. 1991;333:289–305. doi: 10.1098/rstb.1991.0079. [DOI] [PubMed] [Google Scholar]
- 64.Thompson D, van Woesik R. Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proc Royal Society B. 2009;276(1669):2893–2901. doi: 10.1098/rspb.2009.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sampayo EM, Ridgway T, Bongaerts P, Hoegh-Guldberg O. Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. PNAS. 2008;105(30):10444–10449. doi: 10.1073/pnas.0708049105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Woesik R, Koksal S. Washington D.C.: American Geophysical Union; 2006. A coral population response (CPR) model for thermal stress, Coastal and Estuarine Studies 61: Coral reefs and climate change: science and management. pp. 129–144. [Google Scholar]