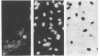
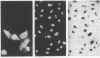
Abstract
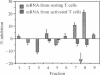
Naturally quiescent human lymphocytes, consisting predominantly of T cells, contain mRNA(s) that can inhibit DNA synthesis when injected into either human diploid fibroblasts (IMR-90) or transformed recipient cells (HeLa). By using an automated capillary microinjection system and a fluorescent coinjection marker (fluorescein isothiocyanate-dextran), individually injected cells can be retrieved and analyzed for DNA synthesis. mRNA isolated from resting T cells is able to block the cells from entering the S phase. The block is reversible and leads to a delay in DNA synthesis. The inhibitory effect is not observed if the injected mRNA is isolated from growth-activated T cells. The disappearance of the inhibition coincides with the approach of the G1/S boundary in both the donor T cells and the recipient human fibroblasts. The mRNA of resting T cells was size-fractionated and the peak inhibitory activity was recovered in a fraction approximately equal to 1.5 kilobases long.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansorge W. Improved system for capillary microinjection into living cells. Exp Cell Res. 1982 Jul;140(1):31–37. doi: 10.1016/0014-4827(82)90152-5. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Brooks R. F. The kinetics of serum-induced initiation of DNA synthesis in BHK 21/C13 cells, and the influence of exogenous adenosine. J Cell Physiol. 1975 Oct;86(2 Pt 2 Suppl 1):369–377. doi: 10.1002/jcp.1040860409. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Delia D., Greaves M., Villa S., DeBraud F. Characterization of the response of human thymocytes and blood lymphocytes to the synergistic mitogenicity of 12-O-tetradecanoylphorbol-13-acetate (TPA)-ionomycin. Eur J Immunol. 1984 Aug;14(8):720–724. doi: 10.1002/eji.1830140809. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Drescher-Lincoln C. K., Smith J. R. Inhibition of DNA synthesis in senescent-proliferating human cybrids is mediated by endogenous proteins. Exp Cell Res. 1984 Jul;153(1):208–217. doi: 10.1016/0014-4827(84)90462-2. [DOI] [PubMed] [Google Scholar]
- Lumpkin C. K., Jr, McClung J. K., Pereira-Smith O. M., Smith J. R. Existence of high abundance antiproliferative mRNA's in senescent human diploid fibroblasts. Science. 1986 Apr 18;232(4748):393–395. doi: 10.1126/science.2421407. [DOI] [PubMed] [Google Scholar]
- Lumpkin C. K., Jr, McClung J. K., Smith J. R. Entry into S phase is inhibited in human fibroblasts by rat liver poly(A)+RNA. Exp Cell Res. 1985 Oct;160(2):544–549. doi: 10.1016/0014-4827(85)90201-0. [DOI] [PubMed] [Google Scholar]
- Massagué J. The TGF-beta family of growth and differentiation factors. Cell. 1987 May 22;49(4):437–438. doi: 10.1016/0092-8674(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Rabinovitch P. S., Norwood T. H. Comparative heterokaryon study of cellular senescence and the serum-deprived state. Exp Cell Res. 1980 Nov;130(1):101–109. doi: 10.1016/0014-4827(80)90046-4. [DOI] [PubMed] [Google Scholar]
- Sager R. Genetic suppression of tumor formation: a new frontier in cancer research. Cancer Res. 1986 Apr;46(4 Pt 1):1573–1580. [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. M. Quiescent human diploid cells can inhibit entry into S phase in replicative nuclei in heterodikaryons. Proc Natl Acad Sci U S A. 1981 May;78(5):3025–3029. doi: 10.1073/pnas.78.5.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Werner D., Chemla Y., Herzberg M. Isolation of poly(A)+ RNA by paper affinity chromatography. Anal Biochem. 1984 Sep;141(2):329–336. doi: 10.1016/0003-2697(84)90050-2. [DOI] [PubMed] [Google Scholar]
- Zetterberg A., Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5365–5369. doi: 10.1073/pnas.82.16.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]