Abstract
The C. elegans gonad provides a well-defined model for a stem cell niche and its control of self-renewal and differentiation. The distal tip cell (DTC) forms a mesenchymal niche that controls germline stem cells (GSCs), both to generate the germline tissue during development and to maintain it during adulthood. The DTC uses GLP-1/Notch signaling to regulate GSCs; germ cells respond to Notch signaling with a network of RNA regulators to control the decision between self-renewal and entry into the meiotic cell cycle.
Keywords: C. elegans, Germline stem cells (GSCs), Stem cell niche, Notch signaling, RNA regulation, FBF
1. Introduction
Stem cells are widely used in multicellular organisms, both to generate tissues during development and to maintain them during adulthood. One major control of stem cells is their niche, which is the microenvironment that surrounds and maintains them. This concept was put forward over 30 years ago [1], and the first niche was identified soon thereafter [2]. Since those early days, tremendous progress has been made analyzing a variety of stem cell niches in multiple organisms, including both animals and plants [3-5].
Our review focuses on the stem cell niche for GSCs in C. elegans. This well-defined niche is formed by a mesenchymal cell, the distal tip cell (DTC). We begin by introducing the system and basic concepts critical to this system. We then review molecular controls that are responsible for generating and maintaining the DTC niche itself as well as molecular controls used by the DTC niche to control the decision between germline self-renewal and differentiation, both during development and in adults.
2. The Distal Tip Cell Provides a Niche for Germline Stem Cells
The cellular simplicity and genetic tractability of C. elegans contributed greatly to early identification of the DTC cellular niche and its use of Notch signaling for GSC maintenance. In this section, we provide essential background on the C. elegans germline and briefly review evidence that the DTC and Notch signaling regulate GSCs and germline self-renewal. We also include more recent studies that begin to delineate the DTC and its function in more depth. Importantly, Notch signaling is now recognized as a key regulator of stem cells more broadly [e.g. 6].
2.1 Germ cell background
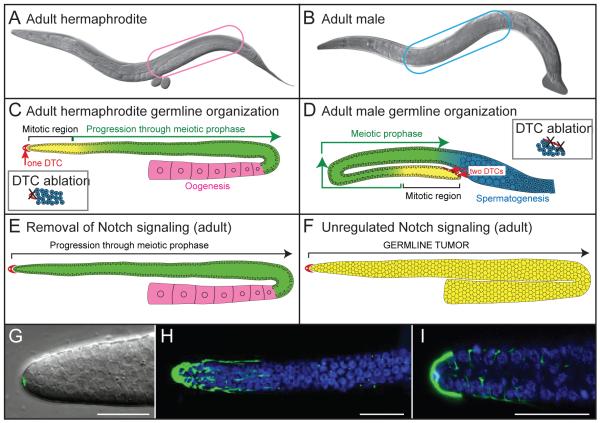
C. elegans can exist as either a hermaphrodite (Fig. 1A) or male (Fig. 1B), with the germline being organized similarly in the two sexes (Fig. 1C-D). All dividing germ cells reside within the “mitotic region” in the distal gonad. As new cells are born, germ cells move proximally out of the mitotic region, enter the meiotic cell cycle and progress through meiotic prophase, ultimately maturing as either sperm or oocytes. Hermaphrodites produce sperm in late stage larvae and oocytes in adults (Fig. 1C); males make sperm continuously from late stage larvae throughout adulthood (Fig. 1D).
Fig. 1.
The C. elegans germline and its DTC niche. (A-B) Nomarski micrographs show gonad arms in situ. (A) Adult hermaphrodite possesses two gonadal arms; the posterior arm is in focus and enclosed by a pink oval (micrograph from M. Gallegos). (B) Adult male has a single gonadal arm (blue oval). (C-D) Cartoons showing DTC niche (red) and germline in the two sexes. Shown are mitotically dividing germ cells (yellow) at the distal end, which include GSCs, germ cells in early meiotic prophase (green) and developing gametes. Insets: DTC ablation (X) abolishes all germline mitotic divisions and causes all germ cells to differentiate. (C) Adult hermaphrodite germline possesses one DTC distally and developing oocytes (pink) proximally. (D) Adult male germline has two DTCs distally and developing sperm (blue) proximally. (E-F) Notch mutant germlines (conventions same as in C and D). (E) When Notch signaling is removed in adults, germline self-renewal is not maintained: germ cells leave the mitotic cell cycle and enter meiosis. (F) When Notch signaling is unregulated, germline differentiation fails: germ cells remain in the mitotic cell cycle and form a tumor. (G-I) DTC position and morphology. (G) Nuclear-localized GFP (green) reveals DTC nucleus at distal end. (H) Cytoplasmic GFP (green) reveals DTC processes that extend proximally along the germline surface (DAPI stained nuclei, blue). (I) Membrane-localized GFP (green) shows intercalation of DTC processes between germ cells (Hoechst stained nuclei, blue) in a single transverse confocal slice through the gonad arm.
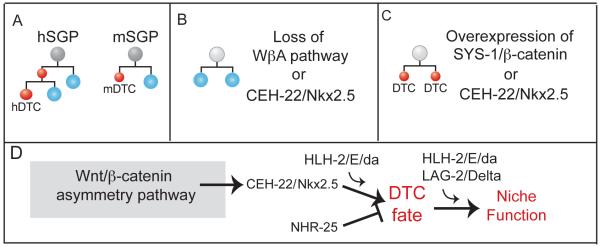
C. elegans GSCs and their controls were first investigated during larval development [2]; more recently, they have been analyzed in adults [reviewed in 3]. Newly hatched larvae possess a four-celled gonadal primordium with two somatic gonadal progenitor cells (SGPs) embracing two primordial germ cells (PGCs). Each SGP divides asymmetrically to produce one DTC (Fig. 2A,B), but the PGCs and their GSC daughters divide symmetrically to expand germ cell number and generate the adult germline tissue. Although larval GSCs generate daughters that are equivalent in developmental potential, those daughters acquire distinct fates based on their proximity to the DTC niche: daughters near the DTC remain in the mitotic cell cycle and continue to generate new germ cells, whereas daughters further from the DTC enter the meiotic cell cycle during the third larval stage (L3) and ultimately differentiate as sperm or oocyte.
Fig. 2.
Control of DTC specification and function. (A) DTC lineage. Hermaphrodite (left) and male (right) SGPs (grey) generate DTCs (red) by asymmetric divisions. Other daughters (blue) generate other components of the somatic gonad. (B) SGP division is symmetrical with DTC loss and extra other somatic gonadal cells when the Wnt β-catenin asymmetry pathway or ceh-22 is depleted. (C) SGP division is symmetrical with extra DTCs and loss of other gonadal cells when SYS-1/β-catenin or ceh-22 is overexpressed. (D) Genetic pathway for control of DTC fate specification and DTC niche function (see text for explanation).
Adult GSCs are likely to behave like larval GSCs, with daughters in close proximity to the DTC retaining stem cell potential and daughters further from the DTC destined to differentiate [7]. All germ cells in the adult mitotic region actively divide and their division planes are variable [7]. We surmise that germ cells in the mitotic region retain stem cell potential but that most are destined to differentiate as they are continuously pushed away from the DTC. The “actual GSCs”, defined as those cells within the niche that actually accomplish both self-renewal and the generation of differentiated cells, are likely to reside at the distal end of the mitotic region, immediately adjacent to the body of the DTC.
Germ cells begin to enter pre-meiotic S-phase in the proximal-most rows of the mitotic region [7-9]. It is not yet known when and where germ cells adopt a sperm or oocyte fate, but it seems to happen as they enter the meiotic cell cycle [10]. Importantly, the sperm/oocyte decision continues to be made in adults, suggesting that adult GSCs are not committed to either fate. Moreover, even when germ cells have entered the meiotic cell cycle and therefore embarked on one aspect of their differentiation, they remain totipotent: if the GLD-1 and MEX-3 RNA regulators are removed, germ cells in meiotic prophase can adopt any of several somatic fates [11]. Therefore, germ cells are normally totipotent, but their differentiation is constrained to meiotic progression and gametogenesis by RNA regulators, a theme we will come back to.
2.2 The DTC uses Notch signaling to control GSCs in both sexes
The DTC provides the stem cell niche for GSCs in both hermaphrodite and male germlines [2]. In hermaphrodites, a single DTC caps the distal-most germ cells in each of two gonad arms (Fig. 1C), while in males, two DTCs are positioned together at the distal end of the single male gonad arm (Fig. 1D). DTCs are both necessary and sufficient for GSCs to generate germline tissue during larval development. When the DTC is killed in hermaphrodites (or when both are killed in males), germ cells in the niche stop mitotic divisions, enter meiosis, and differentiate (Fig. 1C,D); a single male DTC is sufficient to generate an essentially normal germline. Manipulation of DTC position or number has corresponding effects on GSCs: DTC relocation moves the stem cell pool, and DTC duplication generates additional stem cell pools [2, 12-14].
The DTC governs germline self-renewal by GLP-1/Notch signaling, henceforth referred to simply as Notch signaling [3]. Briefly, the DTC expresses two DSL ligands, LAG-2 and APX-1 [15, 16], while the germline expresses the GLP-1 Notch-like receptor, the LAG-1 CSL DNA binding protein and the LAG-3 transcriptional co-activator (also known as SEL-8). Importantly, Notch signaling is both necessary and sufficient for stem cell maintenance. Loss of Notch signaling eliminates germline self-renewal: germ cells stop dividing mitotically, enter meiosis, and undergo gametogenesis [17]; this effect is seen when Notch signaling is turned off at any time during larval development or adulthood in either sex (Fig. 1E) [18]. Conversely, unregulated Notch signaling drives the germline into a tumorous state (Fig. 1F) [19]. Interestingly, germline tumors can also form if germ cells inappropriately contact non-DTC somatic tissues that express DSL ligands [20-22]. Therefore, while the DTC forms the normal niche for GSC self-renewal, other DSL-expressing tissues can function either as ectopic or latent niches to drive unregulated GSC divisions and to form tumors. Intriguingly, this finding suggests that latent niches could contribute to tumorigenesis and metastasis in humans [22]. Latent niches that become cancer microenvironments may be good targets for therapeutics [23].
2.3 DTC cell biology and Notch signaling
The DTC architecture reveals more about this key regulatory cell. Nuclearly localized GFP highlights the position of the DTC nucleus at the distal end of the gonad (Fig. 1G). By contrast, cytoplasmic GFP reveals DTC processes that extend proximally and either intercalate between the distal-most germ cells or extend along their surface [7, 24, 25, 26; D. Byrd, unpublished] (Fig. 1H-I). The size and shape of the adult DTC make it an intriguing candidate for regulating the size of the mitotic region. However, a correlation between the length of DTC processes and the length of the early adult mitotic region [24, 25] is not seen at other times or in mutants with longer or shorter mitotic regions [7].
Analyses of Notch ligand regulation in the DTC are just beginning. In Drosophila and mammalian cells, both ligand and receptor internalization are necessary for Notch activation [27]. Although it is unclear why internalization of a ligand is important for receptor activation, two models are favored. Endocytosis of the Notch-bound ligand might “activate” the receptor by exertion of a pulling force or “activate” the ligand by modification or localization [27]. In C. elegans, two potential effectors of GLP-1/Notch signaling include EPN-1/epsin and the Bro1-domain containing protein EGO-2 [28, 29]. Epsin binds specific phospholipids, selects cargo, and is important for inducing membrane curvature [reviewed in 30], while Bro1-domain containing proteins are targeted to endosomes [31]. Loss of either epn-1 or ego-2 enhances a weak glp-1 allele. RNAi assays suggest that epn-1 and ego-2 may function in the soma to affect GLP-1/Notch signaling in the germline [28, 29]. One possibility is that EPN-1 and EGO-2 function in the endocytic “activation” of somatically expressed DSL ligands. Consistent with this idea, functional LAG-2 and endocytic markers both localize in puncta near or at the cell surface of the DTC body and along its membranous processes [7, 32; D. Byrd, unpublished]. Ligand processing could regulate the timing and level of DSL signaling. Thus the number of germline stem cells could be expanded or contracted by altering the activity of DSL ligands.
2.4 Hermaphrodite DTCs also control gonadal morphogenesis
In addition to their niche function, hermaphrodite DTCs are also “leader” cells during larval development: they lead the growing gonadal arm as it extends and turns [2]. The leader and niche functions are allocated to distinct cells in males, and they are genetically separable in hermaphrodites. Nonetheless regulators that drive leader function may also influence the hermaphrodite niche. Such regulators include matrix metalloproteinases [33-36], the netrin guidance system [37], TGF signaling [38], Rac-GTPase signaling components [39], and integrin cell adhesion receptors [40]. All of these components could theoretically affect both leader and niche functions.
3. Molecular Controls of Niche Specification and Maintenance
The size and strength of a stem cell niche affects stem cells [41-44]. Therefore, it is critical to know how the stem cell niche itself is specified and maintained. Regulation of the DTC has now been worked out in some detail, and at least some of those controls are likely to be conserved in other animals.
3.1 Wnt pathway and CEH-22/Nkx2.5 control DTC specification
The DTC is generated by asymmetrical cell division in both sexes (Fig. 2A) [45]. Molecularly, the DTCs are specified by the major C. elegans Wnt pathway, called the Wnt/®-catenin asymmetry (WβA) pathway [reviewed in 46]. Details of the WβA pathway are not critical here, but briefly, it activates transcription using the POP-1/TCF DNA-binding protein and the SYS-1/β-catenin transcriptional co-activator [reviewed in 47]. For DTC specification, the WβA pathway directly activates transcription of the ceh-22 gene, which encodes the single C. elegans homolog of a homeodomain transcription factor known as Nkx2.5 in vertebrates or tinman in flies [14, 48]. In addition, NHR-25, an orphan nuclear hormone receptor, antagonizes the WβA control of DTC specification [49], while the HLH-2/E/daughterless transcription factor promotes DTC fate specification or fate maintenance [50, 51]. Importantly, the DTC is lost in mutants lacking the WβA pathway or CEH-22 (Fig. 2B), while extra DTCs are generated with overexpression of either SYS-1/β-catenin or CEH-22 (Fig. 2C) [13, 14, 52]. Therefore, these regulators are both necessary and sufficient for DTC specification.
3.2 HLH-2 controls maintenance of DTC niche function
HLH-2/E/da affects DTC niche function in addition to its role in DTC specification [51]. Recall that LAG-2 is a DTC signaling ligand that activates Notch-dependent events in germ cells. In young larvae, HLH-2 depletion reduces expression of a lag-2 transcriptional reporter in those DTCs successfully made, and when hlh-2 RNAi is performed in later larvae and adults, HLH-2 depletion also reduces lag-2 expression in DTCs. By contrast, a mutant lag-2 reporter that lacks HLH-2 binding sites is not affected by HLH-2 loss. Importantly, hlh-2 RNAi results in a shortened mitotic region in both hermaphrodites and males. By contrast, loss of ceh-22 or other components of the WβA pathway does not reduce lag-2 expression. The simplest explanation is that HLH-2 acts early to control DTC specification and that it also acts continuously after the DTC is specified to promote lag-2 expression and maintain niche function.
3.3 Conservation of niche regulators?
How conserved are the DTC regulators for controlling stem cell niches more broadly? In mice, Wnt signaling controls differentiation of osteoblasts, which are part of the mammalian haematopoietic stem cell niche [41, 42, 53]. [Wnt signaling is also thought to promote self-renewal by acting on stem cells themselves, at least in some cases [54].] In several organisms, the Wnt pathway and nuclear hormone receptors can exhibit cross-regulation [55]. Also in mice, Nkx2.5 is critical for development of the vasculature [56], which can serve as part of the niche for hematopoietic stem cells [57]. Therefore, key controls of the nematode DTC niche have been implicated in regulating niches more broadly.
4. Germ Cell Intrinsic Controls of Self-renewal
Downstream of Notch signaling, a network of RNA regulators functions within germ cells to control their decision between self-renewal and early differentiation. This network has been described in detail elsewhere [3]. Here we focus on stem cell control by two PUF RNA-binding proteins, which are conserved stem cell regulators [58], but best understood in C. elegans.
4.1 FBF RNA-binding proteins control germline self-renewal
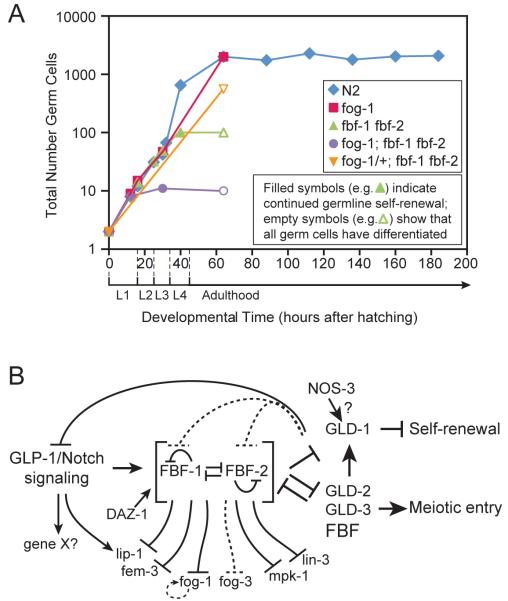
FBF, the collective term for the nearly identical FBF-1 and FBF-2 proteins, is the major germ cell intrinsic regulator of self-renewal [59, 60]. Normally, germ cells proliferate during larval development to generate ~2000 adult germ cells and they continue dividing in adults to maintain that number (Fig. 3A, blue). By contrast, in fbf-1 fbf-2 double mutants, germ cells stop dividing in L4 larvae after ~120 germ cells have been made; the fbf-1 fbf-2 germ cells then all enter the meiotic cell cycle and differentiate (Fig. 3A, green). Therefore, FBF is essential for germline self-renewal in adults.
Fig. 3.
Control of germ cell self-renewal and proliferation. (A) Graph showing total germ cell number with time. Wild-type and mutants are depicted with distinct symbols: solid symbols show that germline self-renewal is continuing; empty symbols show that germline self-renewal has failed and that all germ cells have differentiated. In wild-type and fog-1 mutants, larval proliferation is equivalent; in wild-type, germline self-renewal maintains germ cell number during adulthood; in fog-1 mutants, germ cell counts have not been done in later adults, but the size of the germline appears to be maintained. In other key mutants, larval proliferation produces 10 germ cells (fog-1; fbf-1 fbf-2), 120 germ cells (fbf-1 fbf-2), or 500 germ cells (fog-1/+; fbf-1 fbf-2), but those divisions stop by the stage indicated as an open symbol. Therefore, germline proliferation is limited and self-renewal fails, either in L2 larvae (fog-1; fbf-1 fbf-2), L4 larvae (fbf-1 fbf-2), or adults (fog-1/+; fbf-1 fbf-2). (B) Network of regulators for germ cell self-renewal and differentiation. See text for explanation. Gene X indicates that Notch signaling likely controls additional genes that are not yet identified.
FBF also controls larval GSC divisions, but that effect is masked in fbf-1 fbf-2 double mutants by the upregulation of another regulator that promotes germline proliferation but not continued self-renewal [61]. That other regulator is FOG-1, a homolog of the cytoplasmic polyadenylation element binding (CPEB) protein [62]. The germ cells in fbf-1 fbf-2; fog-1 triple mutants stop dividing early in larval development with only ~10 total germ cells; these few germ cells also enter meiosis and differentiate (Fig. 3A, purple) [61]. In fbf-1 fbf-2 double mutants, FOG-1 protein abundance increases abnormally and promotes proliferation, but FOG-1 cannot promote divisions indefinitely. In fog-1 null mutants, by contrast, FBF drives GSC divisions without limit. Therefore, FBF is the key regulator of self-renewal while FOG-1 promotes a limited proliferation.
4.2 FBF acts downstream of Notch signaling
The FBF-1 and FBF-2 proteins are enriched in germ cells within the mitotic region [60, 63], and fbf-2 is a direct target of Notch transcriptional activation [63]. Given that FBF is essential for germline self-renewal and that fbf-2 is a Notch target gene, the prediction was that FBF would be required for the germline tumor in a gain-of-function mutant of the GLP-1/Notch receptor [glp-1(gf)]. Surprisingly, glp-1(gf); fbf-1 fbf-2 germlines remain tumorous; however, if fog-1 is also removed, germline tumors are lost [61]. Importantly, FOG-1 removal on its own does not abolish a glp-1(gf) germline tumor. Therefore, the glp-1(gf) tumor requires FOG-1 in the absence of FBF, much as larval germline proliferation does. Although FOG-1 upregulation complicates the genetic epistasis results, the major point is that FBF acts downstream of Notch signaling as expected.
4.3 FBF represses a broad spectrum of mRNAs
FBF controls germline self-renewal by repressing a battery of mRNAs (Fig. 3B). These FBF target mRNAs reveal how FBF controls multiple facets of germline self-renewal and differentiation; some target mRNAs also have implications for how FBF may maintain germline totipotency.
FBF prevents meiotic entry by repressing gld-1 and gld-3 mRNAs [60, 64, 65], which encode key regulators of meiotic entry [3].
FBF prevents differentiation by repressing mpk-1 mRNA [66], which encodes the C. elegans homolog of ERK/MAPK [67]. MPK-1 promotes the sperm fate and affects germline apoptosis in the oogenic germline among other functions [66, 68, 69]. Importantly, the PUF control of ERK/MAPK is conserved: human PUM2 represses ERK/MAPK in human embryonic stem cells [66].
FBF prevents differentiation by repressing lin-3 mRNA [70], which encodes the C. elegans homolog of EGF [71]. While LIN-3 has no apparent role in the germline, it is a potent regulator of somatic development [40]; indeed when LIN-3 is aberrantly made in fbf-1 fbf-2 germlines, vulval development is defective [70]. The lin-3 case suggests that FBF may repress potent developmental regulators more broadly, not just those that control germline development.
FBF maintains the normal balance between self-renewal and differentiation, at least in part by controlling its own mRNAs [63]. The idea is that FBF downregulates its own expression so that regulators promoting differentiation (e.g. the GLDs) can overcome its influence.
FBF promotes the oocyte fate by repressing fem-3 and fog-1 mRNAs [59, 61], which promote the sperm fate [3].
FBF promotes proliferation by maintaining FOG-1 abundance at a low level in young larval germ cells and in the proximal mitotic region of males [61]. FOG-1 effects are dose-dependent: low FOG-1 promotes continued mitotic divisions while high FOG-1 promotes the sperm fate.
All these FBF targets have been identified by a candidate approach. A genomic approach, now in progress, reveals that FBF is associated with many mRNAs (>1000) (A. Kershner and J. Kimble, unpublished). Therefore FBF is likely to be a broad-spectrum regulator of mRNAs. Because the function of FBF within the germline is specialized for self-renewal, understanding its full range of targets is likely to illuminate how it controls stem cells and totipotency.
4.4 FBF also promotes meiotic entry
FBF is a well-documented mRNA repressor [e.g. 58], but two recent reports suggest that FBF can also activate mRNAs [72, 73]. The first clue was genetic. The major role of FBF is to drive germline self-renewal, but it also promotes meiotic entry. Meiotic entry relies on two redundant regulatory branches (Fig. 3B) [3]. The GLD-1 translational repressor defines one branch, while the GLD-2/GLD-3 poly(A) polymerase and translational activator defines the other branch. FBF acts genetically within the GLD-2/GLD-3 branch [60, 72]. Thus, in gld single mutants, germ cells enter meiosis, but in gld-1 gld-2, gld-1 gld-3 or gld-1 fbf-1 fbf-2 mutants, meiotic entry is essentially abolished and a germline tumor results [60, 64, 74, 75]).
How do we explain the role of FBF in the GLD-2/GLD-3 branch? One idea is that FBF acts directly with GLD-2 and GLD-3 to activate mRNAs and promote meiotic entry. This model is attractive because it can provide a molecular mechanism to drive the transition from self-renewal to differentiation. FBF target mRNAs that had been repressed in GSCs could be subsequently activated by changing the FBF molecular complex as germ cells move proximally and enter meiosis. Consistent with this model, FBF does in fact bind directly to both GLD-2 and GLD-3 both in vitro and in vivo [72, 76], and FBF can promote GLD-2-dependent polyadenylation in vitro [72]. An alternative model is that FBF acts indirectly to promote meiotic entry, but no good candidates exist to date for that mechanism.
4.5 FBF is a major hub of the network controlling self-renewal versus differentiation
The network controlling germline self-renewal and early differentiation was assembled regulator by regulator and regulatory relationship by regulatory relationship — all from detailed genetic, biochemical and cellular data. This process is not complete, but the assembly now emerges as a network. One emergent network property is that FBF is a major hub, albeit a complex hub composed of FBF-1 and FBF-2. Thus, FBF is connected to all other nodes in the network by either regulation or protein interaction, and FBF removal is catastrophic.
A second emergent property of the RNA network is its robustness, a property deriving from multiple redundancies in the network. For example, either FBF-1 or FBF-2 is sufficient for self-renewal, and either the GLD-1 branch or the GLD-2/GLD-3 branch is sufficient for meiotic entry. Therefore, removal of any single component in the network leaves both the mitotic region and the switch into meiosis intact. These redundancies mean that the network is remarkably robust and provide a mechanism for fine-tuning and network modulation in response to different regulatory inputs. Importantly, the size of the mitotic region changes subtly when any one regulator is removed (e.g. fbf-1 mutants have a smaller than normal mitotic region), making mitotic region size a sensitive measure of network state.
The prevalence of RNA regulation in the germline network is extraordinary. FBF is one example, but in addition, NOS-3 (Nanos), GLD-1 (quaking), GLD-2 (poly(A) polymerase), GLD-3 (BicC) and FOG-1 (CPEB) are all RNA regulators [3]. Therefore, Notch signaling controls the decision between self-renewing mitotic divisions and meiotic entry by a network of RNA regulators. RNA regulation is also critical for maintaining germline totipotency [11]. We suggest that RNA regulation is particularly well suited for the plasticity required to adjust the balance of self-renewal and differentiation in response to changes in reproduction, aging, nutrition, or other environmental cues.
5. Conclusions and Future Directions
Stem cells are controlled by both extrinsic and intrinsic factors. In the C. elegans germline, the DTC provides a cellular stem cell niche that maintains germline self-renewal via the GLP-1/Notch signaling pathway. The Wnt pathway and CEH-22/Nkx2.5 specify the DTC fate and HLH-2/E/da maintains DTC niche function. Many conserved RNA regulators act intrinsically within the germline tissue to control self-renewal and differentiation, including meiotic entry and the sperm/oocyte fate decision. In particular, FBF is a conserved stem cell regulator that functions in the C. elegans germline as a hub in the network controlling self-renewal and differentiation. Future analyses will reveal target mRNAs of all the key RNA regulators at a genomic scale and also probe the dynamics of RNA regulatory complexes as germ cells transition from a stem cell state through early steps in the path toward differentiation.
While much is known about the C. elegans GSC niche, much remains to be discovered. The DTC niche provides the essential microenvironment for continued germline self-renewal, but additional cells and factors from the “macroenvironment” may also impinge on GSC number or activity. For the C. elegans germline, that “macroenvironment” is likely to include gonadal sheath cells [77, 78], the extracellular matrix [79], and the animal’s metabolism [80, 81]. The presence of gonadal sheath cells affects the number of germ cells in the mitotic region and the total number of germ cells made, but germline self-renewal still occurs in their absence [77, 78]. The extracellular matrix affects the integrity of the germline tissue [79], and cholesterol starvation lowers brood size and total germ cell number [80, 81]. Important goals for future work include understanding how the niche and macroenvironment work together to control the equilibrium achieved between self-renewal and differentiation and exploring how GSCs are regulated under different physiological conditions (i.e. nutrition, stress, and aging).
Acknowledgements
We thank A. Helsley and L. Vanderploeg for help preparing the manuscript and figures. We also thank members of the Kimble lab, especially S. Crittenden, for helpful discussions, and S. Crittenden, K. Knobel, C. Stumpf and D. Greenstein for providing comments on the manuscript. D.T.B. was supported by NIH postdoctoral fellowship F32 GM072126. J.K. is an investigator of the Howard Hughes Medical Institute.
Abbreviations
- DTC
distal tip cell
- GSCs
germline stem cells
- SGPs
somatic gonadal progenitor cells
- PGCs
primordial germ cells
- DSL
Delta/Serrate/LAG-2
- CSL
CBF-1/Su(H)/LAG-1
- WβA
Wnt/β-catenin asymmetry
- PUF
Pumilio and FBF
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- [2].Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans. Dev Biol. 1981;81:208–19. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- [3].Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2007;23:405–33. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- [4].Xie T, Li L. Stem cells and their niche: an inseparable relationship. Development. 2007;134:2001–6. doi: 10.1242/dev.002022. [DOI] [PubMed] [Google Scholar]
- [5].Dinneny JR, Benfey PN. Plant stem cell niches: standing the test of time. Cell. 2008;132:553–7. doi: 10.1016/j.cell.2008.02.001. [DOI] [PubMed] [Google Scholar]
- [6].Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–47. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- [7].Crittenden SL, Leonhard KA, Byrd DT, Kimble J. Cellular analyses of the mitotic region in the Caenorhabditis elegans adult germ line. Mol Biol Cell. 2006;17:3051–61. doi: 10.1091/mbc.E06-03-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hansen D, Wilson-Berry L, Dang T, Schedl T. Control of the proliferation versus meiotic development decision in the C. elegans germline through regulation of GLD-1 protein accumulation. Development. 2004;131:93–104. doi: 10.1242/dev.00916. [DOI] [PubMed] [Google Scholar]
- [9].Jaramillo-Lambert A, Ellefson M, Villeneuve AM, Engebrecht J. Differential timing of S phases, X chromosome replication, and meiotic prophase in the C. elegans germ line. Dev Biol. 2007;308:206–21. doi: 10.1016/j.ydbio.2007.05.019. [DOI] [PubMed] [Google Scholar]
- [10].Barton MK, Kimble J. fog-1, a regulatory gene required for specification of spermatogenesis in the germ line of Caenorhabditis elegans. Genetics. 1990;125:29–39. doi: 10.1093/genetics/125.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ciosk R, DePalma M, Priess JR. Translational regulators maintain totipotency in the Caenorhabditis elegans germline. Science. 2006;311:851–3. doi: 10.1126/science.1122491. [DOI] [PubMed] [Google Scholar]
- [12].Kipreos ET, Gohel SP, Hedgecock EM. The C. elegans F-box/WD-repeat protein LIN-23 functions to limit cell division during development. Development. 2000;127:5071–82. doi: 10.1242/dev.127.23.5071. [DOI] [PubMed] [Google Scholar]
- [13].Kidd AR, 3rd, Miskowski JA, Siegfried KR, Sawa H, Kimble J. A β-catenin identified by functional rather than sequence criteria and its role in Wnt/MAPK signaling. Cell. 2005;121:761–72. doi: 10.1016/j.cell.2005.03.029. [DOI] [PubMed] [Google Scholar]
- [14].Lam N, Chesney MA, Kimble J. Wnt signaling and CEH-22/tinman/Nkx2.5 specify a stem cell niche in C. elegans. Curr Biol. 2006;16:287–95. doi: 10.1016/j.cub.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Henderson ST, Gao D, Lambie EJ, Kimble J. lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C. elegans. Development. 1994;120:2913–24. doi: 10.1242/dev.120.10.2913. [DOI] [PubMed] [Google Scholar]
- [16].Nadarajan S, Govindan JA, McGovern M, Hubbard EJ, Greenstein D. MSP and GLP-1/Notch signaling coordinately regulate actomyosin-dependent cytoplasmic streaming and oocyte growth in C. elegans. Development. 2009;136:2223–34. doi: 10.1242/dev.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kimble J, Simpson P. The LIN-12/Notch signaling pathway and its regulation. Annu Rev Cell Dev Biol. 1997;13:333–61. doi: 10.1146/annurev.cellbio.13.1.333. [DOI] [PubMed] [Google Scholar]
- [18].Austin J, Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987;51:589–99. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- [19].Berry LW, Westlund B, Schedl T. Germ-line tumor formation caused by activation of glp-1, a Caenorhabditis elegans member of the Notch family of receptors. Development. 1997;124:925–36. doi: 10.1242/dev.124.4.925. [DOI] [PubMed] [Google Scholar]
- [20].Seydoux G, Schedl T, Greenwald I. Cell-cell interactions prevent a potential inductive interaction between soma and germline in C. elegans. Cell. 1990;61:939–51. doi: 10.1016/0092-8674(90)90060-r. [DOI] [PubMed] [Google Scholar]
- [21].Pepper AS, Lo TW, Killian DJ, Hall DH, Hubbard EJ. The establishment of Caenorhabditis elegans germline pattern is controlled by overlapping proximal and distal somatic gonad signals. Dev Biol. 2003;259:336–50. doi: 10.1016/s0012-1606(03)00203-3. [DOI] [PubMed] [Google Scholar]
- [22].McGovern M, Voutev R, Maciejowski J, Corsi AK, Hubbard EJ. A “latent niche” mechanism for tumor initiation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11617–22. doi: 10.1073/pnas.0903768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kenny PA, Lee GY, Bissell MJ. Targeting the tumor microenvironment. Front Biosci. 2007;12:3468–74. doi: 10.2741/2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fitzgerald K, Greenwald I. Interchangeability of Caenorhabditis elegans DSL proteins and intrinsic signalling activity of their extracellular domains in vivo. Development. 1995;121:4275–82. doi: 10.1242/dev.121.12.4275. [DOI] [PubMed] [Google Scholar]
- [25].Hall DH, Winfrey VP, Blaeuer G, Hoffman LH, Furuta T, Rose KL, et al. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev Biol. 1999;212:101–23. doi: 10.1006/dbio.1999.9356. [DOI] [PubMed] [Google Scholar]
- [26].Finger FP, Kopish KR, White JG. A role for septins in cellular and axonal migration in C. elegans. Dev Biol. 2003;261:220–34. doi: 10.1016/s0012-1606(03)00296-3. [DOI] [PubMed] [Google Scholar]
- [27].Furthauer M, Gonzalez-Gaitan M. Endocytic regulation of Notch signalling during development. Traffic. 2009;10:792–802. doi: 10.1111/j.1600-0854.2009.00914.x. [DOI] [PubMed] [Google Scholar]
- [28].Tian X, Hansen D, Schedl T, Skeath JB. Epsin potentiates Notch pathway activity in Drosophila and C. elegans. Development. 2004;131:5807–15. doi: 10.1242/dev.01459. [DOI] [PubMed] [Google Scholar]
- [29].Liu Y, Maine EM. The Bro1-domain protein, EGO-2, promotes Notch signaling in Caenorhabditis elegans. Genetics. 2007;176:2265–77. doi: 10.1534/genetics.107.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Horvath CA, Vanden Broeck D, Boulet GA, Bogers J, De Wolf MJ. Epsin: inducing membrane curvature. Int J Biochem Cell Biol. 2007;39:1765–70. doi: 10.1016/j.biocel.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [31].Kim J, Sitaraman S, Hierro A, Beach BM, Odorizzi G, Hurley JH. Structural basis for endosomal targeting by the Bro1 domain. Developmental Cell. 2005;8:937–47. doi: 10.1016/j.devcel.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Henderson ST, Gao D, Christensen S, Kimble J. Functional domains of LAG-2, a putative signaling ligand for LIN-12 and GLP-1 receptors in Caenorhabditis elegans. Mol Biol Cell. 1997;8:1751–62. doi: 10.1091/mbc.8.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Blelloch R, Kimble J. Control of organ shape by a secreted metalloprotease in the nematode Caenorhabditis elegans. Nature. 1999;399:586–90. doi: 10.1038/21196. [DOI] [PubMed] [Google Scholar]
- [34].Hesselson D, Newman C, Kim KW, Kimble J. GON-1 and fibulin have antagonistic roles in control of organ shape. Curr Biol. 2004;14:2005–10. doi: 10.1016/j.cub.2004.11.006. [DOI] [PubMed] [Google Scholar]
- [35].Kubota Y, Kuroki R, Nishiwaki K. A fibulin-1 homolog interacts with an ADAM protease that controls cell migration in C. elegans. Curr Biol. 2004;14:2011–8. doi: 10.1016/j.cub.2004.10.047. [DOI] [PubMed] [Google Scholar]
- [36].Nishiwaki K, Kubota Y, Chigira Y, Roy SK, Suzuki M, Schvarzstein M, et al. An NDPase links ADAM protease glycosylation with organ morphogenesis in C. elegans. Nat Cell Biol. 2004;6:31–7. doi: 10.1038/ncb1079. [DOI] [PubMed] [Google Scholar]
- [37].Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- [38].Nash B, Colavita A, Zheng H, Roy PJ, Culotti JG. The forkhead transcription factor UNC-130 is required for the graded spatial expression of the UNC-129 TGF-β guidance factor in C. elegans. Genes Dev. 2000;14:2486–500. doi: 10.1101/gad.831500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Reddien PW, Horvitz HR. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat Cell Biol. 2000;2:131–6. doi: 10.1038/35004000. [DOI] [PubMed] [Google Scholar]
- [40].Sternberg PW, Lesa G, Lee J, Katz WS, Yoon C, Clandinin TR, et al. LET-23-mediated signal transduction during Caenorhabditis elegans development. Mol Reprod Dev. 1995;42:523–8. doi: 10.1002/mrd.1080420422. [DOI] [PubMed] [Google Scholar]
- [41].Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- [42].Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- [43].Ward EJ, Shcherbata HR, Reynolds SH, Fischer KA, Hatfield SD, Ruohola-Baker H. Stem cells signal to the niche through the Notch pathway in the Drosophila ovary. Curr Biol. 2006;16:2352–8. doi: 10.1016/j.cub.2006.10.022. [DOI] [PubMed] [Google Scholar]
- [44].Song X, Call G, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–80. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- [45].Kimble J, Hirsh D. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev Biol. 1979;70:396–417. doi: 10.1016/0012-1606(79)90035-6. [DOI] [PubMed] [Google Scholar]
- [46].Mizumoto K, Sawa H. Two βs or not two βs: regulation of asymmetric division by β-catenin. Trends Cell Biol. 2007;17:465–73. doi: 10.1016/j.tcb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- [47].Phillips BT, Kimble J. A new look at TCF and beta-catenin through the lens of a divergent C. elegans Wnt pathway. Developmental Cell. 2009;17:27–34. doi: 10.1016/j.devcel.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Okkema PG, Fire A. The Caenorhabditis elegans NK-2 class homeoprotein CEH-22 is involved in combinatorial activation of gene expression in pharyngeal muscle. Development. 1994;120:2175–86. doi: 10.1242/dev.120.8.2175. [DOI] [PubMed] [Google Scholar]
- [49].Asahina M, Valenta T, Silhankova M, Korinek V, Jindra M. Crosstalk between a nuclear receptor and β-catenin signaling decides cell fates in the C. elegans somatic gonad. Dev Cell. 2006;11:203–11. doi: 10.1016/j.devcel.2006.06.003. [DOI] [PubMed] [Google Scholar]
- [50].Karp X, Greenwald I. Multiple roles for the E/Daughterless ortholog HLH-2 during C. elegans gonadogenesis. Dev Biol. 2004;272:460–9. doi: 10.1016/j.ydbio.2004.05.015. [DOI] [PubMed] [Google Scholar]
- [51].Chesney MA, Lam N, Morgan DE, Phillips BT, Kimble J. C. elegans HLH-2/E/Daughterless controls key regulatory cells during gonadogenesis. Dev Biol. 2009;331:14–25. doi: 10.1016/j.ydbio.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Siegfried KR, Kimble J. POP-1 controls axis formation during early gonadogenesis in C. elegans. Development. 2002;129:443–53. doi: 10.1242/dev.129.2.443. [DOI] [PubMed] [Google Scholar]
- [53].Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- [54].Nusse R. Wnt signaling and stem cell control. Cell research. 2008;18:523–7. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- [55].Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/β-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev. 2005;26:898–915. doi: 10.1210/er.2003-0034. [DOI] [PubMed] [Google Scholar]
- [56].Terami H, Hidaka K, Katsumata T, Iio A, Morisaki T. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Commun. 2004;325:968–75. doi: 10.1016/j.bbrc.2004.10.103. [DOI] [PubMed] [Google Scholar]
- [57].Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- [58].Wickens M, Bernstein DS, Kimble J, Parker R. A PUF family portrait: 3′UTR regulation as a way of life. Trends Genet. 2002;18:150–7. doi: 10.1016/s0168-9525(01)02616-6. [DOI] [PubMed] [Google Scholar]
- [59].Zhang B, Gallegos M, Puoti A, Durkin E, Fields S, Kimble J, et al. A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature. 1997;390:477–84. doi: 10.1038/37297. [DOI] [PubMed] [Google Scholar]
- [60].Crittenden SL, Bernstein DS, Bachorik JL, Thompson BE, Gallegos M, Petcherski AG, et al. A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature. 2002;417:660–3. doi: 10.1038/nature754. [DOI] [PubMed] [Google Scholar]
- [61].Thompson BE, Bernstein DS, Bachorik JL, Petcherski AG, Wickens M, Kimble J. Dose-dependent control of proliferation and sperm specification by FOG-1/CPEB. Development. 2005;132:3471–81. doi: 10.1242/dev.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jin SW, Kimble J, Ellis RE. Regulation of cell fate in Caenorhabditis elegans by a novel cytoplasmic polyadenylation element binding protein. Dev Biol. 2001;229:537–53. doi: 10.1006/dbio.2000.9993. [DOI] [PubMed] [Google Scholar]
- [63].Lamont LB, Crittenden SL, Bernstein D, Wickens M, Kimble J. FBF-1 and FBF-2 regulate the size of the mitotic region in the C. elegans germline. Dev Cell. 2004;7:697–707. doi: 10.1016/j.devcel.2004.09.013. [DOI] [PubMed] [Google Scholar]
- [64].Eckmann CR, Crittenden SL, Suh N, Kimble J. GLD-3 and control of the mitosis/meiosis decision in the germline of Caenorhabditis elegans. Genetics. 2004;168:147–60. doi: 10.1534/genetics.104.029264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Merritt C, Rasoloson D, Ko D, Seydoux G. 3′ UTRs are the primary regulators of gene expression in the C. elegans germline. Curr Biol. 2008;18:1476–82. doi: 10.1016/j.cub.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lee MH, Hook B, Pan G, Kershner AM, Merritt C, Seydoux G, et al. Conserved regulation of MAP kinase expression by PUF RNA-binding proteins. PLoS Genet. 2007;3:e233. doi: 10.1371/journal.pgen.0030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lackner MR, Kornfeld K, Miller LM, Horvitz HR, Kim SK. A MAP kinase homolog, mpk-1, is involved in ras-mediated induction of vulval cell fates in Caenorhabditis elegans. Genes Dev. 1994;8:160–73. doi: 10.1101/gad.8.2.160. [DOI] [PubMed] [Google Scholar]
- [68].Lee MH, Ohmachi M, Arur S, Nayak S, Francis R, Church D, et al. Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germline development. Genetics. 2007;177:2039–62. doi: 10.1534/genetics.107.081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Arur S, Ohmachi M, Nayak S, Hayes M, Miranda A, Hay A, et al. Multiple ERK substrates execute single biological processes in Caenorhabditis elegans germ-line development. Proc Natl Acad Sci U S A. 2009;106:4776–81. doi: 10.1073/pnas.0812285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Thompson BE, Lamont LB, Kimble J. Germ-line induction of the Caenorhabditis elegans vulva. Proc Natl Acad Sci U S A. 2006;103:620–5. doi: 10.1073/pnas.0510264103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hill RJ, Sternberg PW. The gene lin-3 encodes an inductive signal for vulval development in C. elegans. Nature. 1992;358:470–6. doi: 10.1038/358470a0. [DOI] [PubMed] [Google Scholar]
- [72].Suh N, Crittenden SL, Goldstrohm A, Hook B, Thompson B, Wickens M, et al. FBF and its dual control of gld-1 expression in the Caenorhabditis elegans germline. Genetics. 2009;181:1249–60. doi: 10.1534/genetics.108.099440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kaye JA, Rose NC, Goldsworthy B, Goga A, L’Etoile ND. A 3′UTR pumilio-binding element directs translational activation in olfactory sensory neurons. Neuron. 2009;61:57–70. doi: 10.1016/j.neuron.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Francis R, Barton MK, Kimble J, Schedl T. gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics. 1995;139:579–606. doi: 10.1093/genetics/139.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kadyk LC, Kimble J. Genetic regulation of entry into meiosis in Caenorhabditis elegans. Development. 1998;125:1803–13. doi: 10.1242/dev.125.10.1803. [DOI] [PubMed] [Google Scholar]
- [76].Eckmann CR, Kraemer B, Wickens M, Kimble J. GLD-3, a Bicaudal-C homolog that inhibits FBF to control germline sex determination in C. elegans. Dev Cell. 2002;3:697–710. doi: 10.1016/s1534-5807(02)00322-2. [DOI] [PubMed] [Google Scholar]
- [77].McCarter J, Bartlett B, Dang T, Schedl T. Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev Biol. 1997;181:121–43. doi: 10.1006/dbio.1996.8429. [DOI] [PubMed] [Google Scholar]
- [78].Killian DJ, Hubbard EJ. Caenorhabditis elegans germline patterning requires coordinated development of the somatic gonadal sheath and the germ line. Dev Biol. 2005;279:322–35. doi: 10.1016/j.ydbio.2004.12.021. [DOI] [PubMed] [Google Scholar]
- [79].Kao G, Huang CC, Hedgecock EM, Hall DH, Wadsworth WG. The role of the laminin β subunit in laminin heterotrimer assembly and basement membrane function and development in C. elegans. Dev Biol. 2006;290:211–9. doi: 10.1016/j.ydbio.2005.11.026. [DOI] [PubMed] [Google Scholar]
- [80].Merris M, Wadsworth WG, Khamrai U, Bittman R, Chitwood DJ, Lenard J. Sterol effects and sites of sterol accumulation in Caenorhabditis elegans: developmental requirement for 4α-methyl sterols. J Lipid Res. 2003;44:172–81. doi: 10.1194/jlr.m200323-jlr200. [DOI] [PubMed] [Google Scholar]
- [81].Shim YH, Chun JH, Lee EY, Paik YK. Role of cholesterol in germ-line development of Caenorhabditis elegans. Mol Reprod Dev. 2002;61:358–66. doi: 10.1002/mrd.10099. [DOI] [PubMed] [Google Scholar]