Abstract
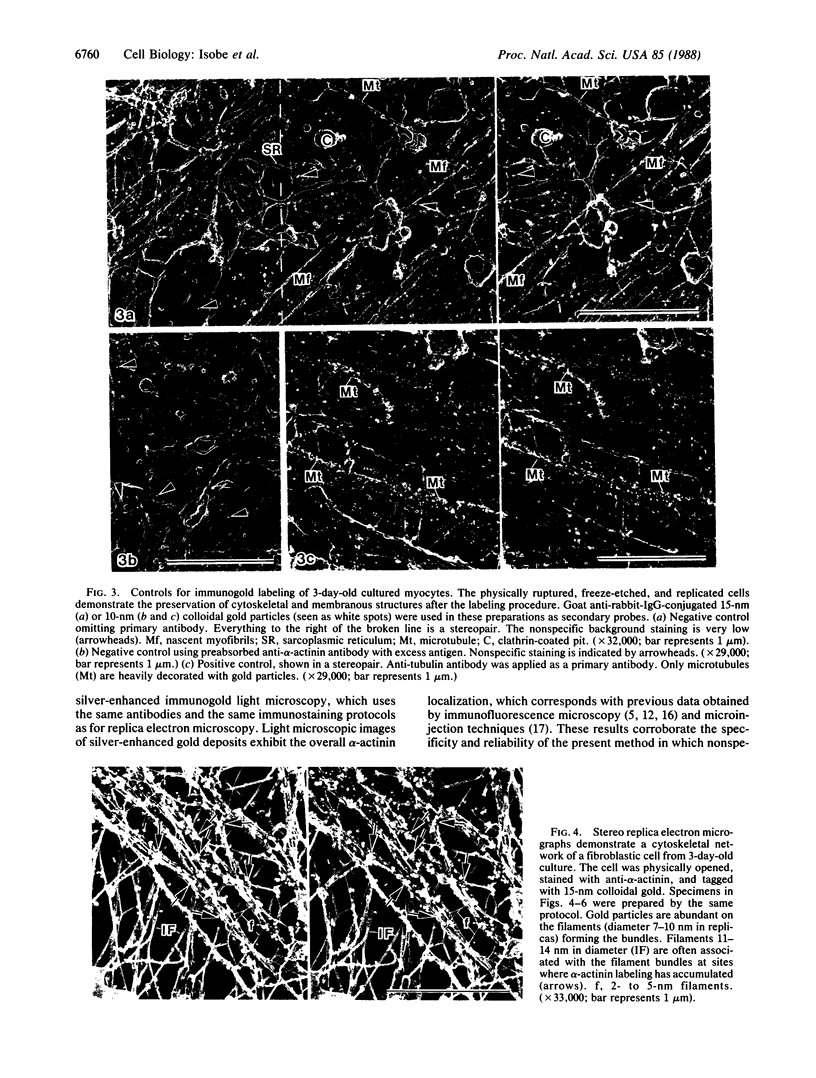
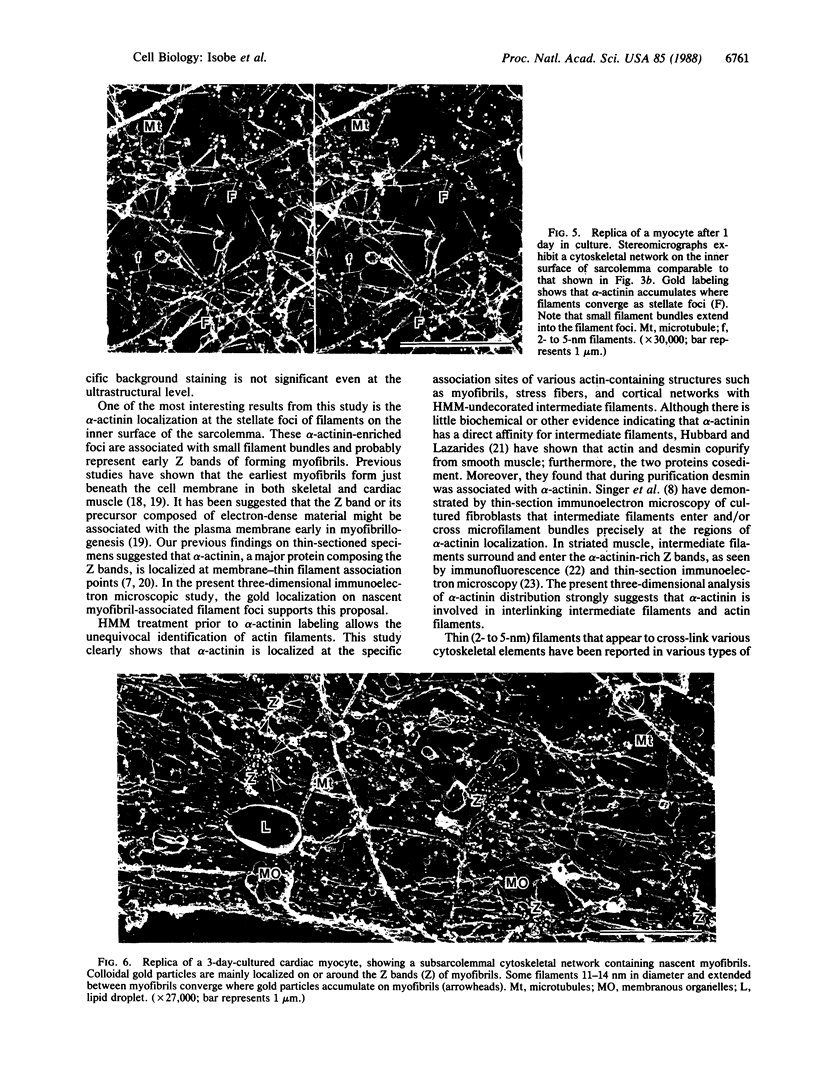
The ultrastructural distribution of alpha-actinin was studied in cultured hamster heart cells by immunogold replica electron microscopy. This technique enabled us to localize alpha-actinin within the cytoskeletal networks at high resolution and in three dimensions. Colloidal gold, indicating the presence of alpha-actinin, was localized on the Z bands of nascent myofibrils in myocytes and on stress fiber bundles in nonmuscle cells. alpha-Actinin staining was also seen on stellate foci, where cytoskeletal filaments converged along the inner myocyte cell membranes. Intermediate filaments were associated with Z bands of myofibrils, stress fibers, and subplasmalemmal actin networks at the specific points where alpha-actinin was localized on these structures. Heavy meromyosin treatment prior to immunostaining confirmed that the thin filaments contained actin. These results suggest that alpha-actinin serves to interlink these various cytoskeletal elements. In addition, this protein may be involved in the initial phases of filament organization during myofibrillogenesis along the inner surface of the myocyte plasma membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dlugosz A. A., Antin P. B., Nachmias V. T., Holtzer H. The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J Cell Biol. 1984 Dec;99(6):2268–2278. doi: 10.1083/jcb.99.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Dutton A. H., Tokuyasu K. T., Singer S. J. Immunoelectron microscope studies of membrane-microfilament interactions: distributions of alpha-actinin, tropomyosin, and vinculin in intestinal epithelial brush border and chicken gizzard smooth muscle cells. J Cell Biol. 1981 Dec;91(3 Pt 1):614–628. doi: 10.1083/jcb.91.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. Membrane-cytoskeleton interaction. Biochim Biophys Acta. 1983 Aug 11;737(3-4):305–341. doi: 10.1016/0304-4157(83)90005-9. [DOI] [PubMed] [Google Scholar]
- Goli D. E., Suzuki A., Temple J., Holmes G. R. Studies on purified -actinin. I. Effect of temperature and tropomyosin on the -actinin-F-actin interaction. J Mol Biol. 1972 Jun 28;67(3):469–488. doi: 10.1016/0022-2836(72)90464-0. [DOI] [PubMed] [Google Scholar]
- Hill C. S., Lemanski L. F. Immunoelectron microscopic localization of alpha-actinin and actin in embryonic hamster heart cells. Eur J Cell Biol. 1986 Jan;39(2):300–312. [PubMed] [Google Scholar]
- Hubbard B. D., Lazarides E. Copurification of actin and desmin from chicken smooth muscle and their copolymerization in vitro to intermediate filaments. J Cell Biol. 1979 Jan;80(1):166–182. doi: 10.1083/jcb.80.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe Y., Shimada Y. Organization of filaments underneath the plasma membrane of developing chicken skeletal muscle cells in vitro revealed by the freeze-dry and rotary replica method. Cell Tissue Res. 1986;244(1):47–56. doi: 10.1007/BF00218380. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langanger G., de Mey J., Moeremans M., Daneels G., de Brabander M., Small J. V. Ultrastructural localization of alpha-actinin and filamin in cultured cells with the immunogold staining (IGS) method. J Cell Biol. 1984 Oct;99(4 Pt 1):1324–1334. doi: 10.1083/jcb.99.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Burridge K. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975 Nov;6(3):289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lemanski L. F., Fuldner R. A., Paulson D. J. Immunofluorescence studies for myosin, alpha-actinin and tropomyosin in developing hearts of normal and cardiac lethal mutant Mexican axolotls, Ambystoma mexicanum. J Embryol Exp Morphol. 1980 Feb;55:1–15. [PubMed] [Google Scholar]
- Lemanski L. F. Heart development in the Mexican salamander, Ambystoma Mexicanum. II. Ultrastructure. Am J Anat. 1973 Apr;136(4):487–525. doi: 10.1002/aja.1001360408. [DOI] [PubMed] [Google Scholar]
- Lemanski L. F., Paulson D. J., Hill C. S., Davis L. A., Riles L. C., Lim S. S. Immunoelectron microscopic localization of alpha-actinin on Lowicryl-embedded thin-sectioned tissues. J Histochem Cytochem. 1985 Jun;33(6):515–522. doi: 10.1177/33.6.3889138. [DOI] [PubMed] [Google Scholar]
- Lemanski L. F., Tu Z. H. Immunofluorescent studies for myosin, actin, tropomyosin and alpha-actinin in cultured cardiomyopathic hamster heart cells. Dev Biol. 1983 Jun;97(2):338–348. doi: 10.1016/0012-1606(83)90091-x. [DOI] [PubMed] [Google Scholar]
- Sanger J. M., Mittal B., Pochapin M. B., Sanger J. W. Myofibrillogenesis in living cells microinjected with fluorescently labeled alpha-actinin. J Cell Biol. 1986 Jun;102(6):2053–2066. doi: 10.1083/jcb.102.6.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Sanger J. M., Jockusch B. M. Differences in the stress fibers between fibroblasts and epithelial cells. J Cell Biol. 1983 Apr;96(4):961–969. doi: 10.1083/jcb.96.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Ball E. H., Geiger B., Chen W. T. Immunolabeling studies of cytoskeletal association in cultured cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):303–316. doi: 10.1101/sqb.1982.046.01.032. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Goll D. E., Singh I., Allen R. E., Robson R. M., Stromer M. H. Some properties of purified skeletal muscle alpha-actinin. J Biol Chem. 1976 Nov 10;251(21):6860–6870. [PubMed] [Google Scholar]
- Tokuyasu K. T., Maher P. A., Singer S. J. Distributions of vimentin and desmin in developing chick myotubes in vivo. II. Immunoelectron microscopic study. J Cell Biol. 1985 Apr;100(4):1157–1166. doi: 10.1083/jcb.100.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]