Abstract
Coping with mild early life stress tends to make subsequent coping efforts more effective and therefore more likely to be used as a means of arousal regulation and resilience. Here we show that this developmental learning-like process of stress inoculation increases ventromedial prefrontal cortical volumes in peripubertal monkeys. Larger volumes do not reflect increased cortical thickness but instead represent surface area expansion of ventromedial prefrontal cortex. Expansion of ventromedial prefrontal cortex coincides with increased white matter myelination inferred from diffusion tensor magnetic resonance imaging. These findings suggest that the process of coping with early life stress increases prefrontal myelination and expands a region of cortex that broadly controls arousal regulation and resilience.
Key Words: Emotion regulation, Cognitive control, Fear, Curiosity, Cortisol
Introduction
Stressful experiences that are challenging but not overwhelming appear to promote the development of subsequent resilience in children [1,2,3,4,5]. Variously described as inoculating, immunizing, steeling, toughening, or thriving [6,7,8], the notion that mild early life stress induces the development of resilience is further supported by longitudinal studies of nonhuman primates. Squirrel monkey mothers and other group members periodically leave newly weaned offspring beginning at 3–6 months of age to forage for food on their own [9, 10]. Initially, brief intermittent separations studied in controlled experimental conditions elicit distress peep calls and increase plasma levels of cortisol with partial habituation of these measures of arousal observed over repeated social separations [11, 12]. Later in life, monkeys exposed to intermittent separations show fewer behavioral indications of anxiety, increased exploration of novel situations, and diminished stress levels of cortisol compared to age-matched monkeys not exposed to prior separations [13,14,15,16,17]. These behavioral and hormonal outcomes reflect a nonspecific form of stress inoculation [15] as exposure to one type of early life stress enhances subsequent arousal regulation and resilience in coping with different stressors encountered later in life.
Prior exposure to separation stress also enhances prefrontal-dependent cognitive control of impulsive behavior [18] and appears to increase ventromedial but not dorsolateral prefrontal volumes determined in vivo by noninvasive neuroimaging of the squirrel monkey brain [19, 20]. These findings are of interest because large ventromedial prefrontal size in humans predicts diminished impulsivity [21], lower harm avoidance [22], and greater retention of learned extinction of fear [23]. Recent neuroimaging studies of humans support results from animal research confirming that learned extinction of fear is mediated by prefrontal downregulation of arousal via inhibitory connections that diminish neural output from the amygdala [24, 25]. Additional evidence likewise suggests that differences in the balance between top-down prefrontal regulation and arousal inducing amygdala activation may account for global trait-like differences in coping with stress [26, 27].
In the following study, we further examine prefrontal plasticity in a new sample of monkeys randomized to intermittent social separation stress or a no-stress condition. Whole brain T1-weighted images acquired at high resolution were processed for predefined measures of prefrontal cortical volumes and an unbiased analysis of cortical thickness unconstrained by predefined regions of interest. Diffusion tensor imaging was used to subsequently investigate prefrontal connections in terms of fractional anisotropy in white matter tissue. Increased fractional anisotropy occurs when tissue microstructure constrains water proton diffusion directionality as exemplified by myelination of axons in white matter [28]. Myelination increases nerve conduction velocities and facilitates synchronous firing of neurons by reducing travel distance effects in distributed networks [29]. Coordination of firing inputs to maximize temporal summation at postsynaptic neurons is the foundation for a key concept in neural plasticity and development-neurons that fire together, wire together. In children, myelination of prefrontal connections determined by fractional anisotropy increases with age [30] and maturation of prefrontal-dependent functions [31]. Results from our studies of monkeys suggest that the learning-like process of coping with stress likewise increases prefrontal myelination and expands a region of cortex that broadly controls arousal regulation and resilience.
Materials and Methods
Socially housed squirrel monkeys (Saimiri sciureus) were randomized to either brief intermittent separation stress (n = 4 males and 7 females) or a no-stress control condition (n = 1 male and 8 females) at 17 weeks of age. For each of ten total separation sessions, monkeys were removed from their natal group for a one-hour period once a week [15]. In the no-stress control condition, age-matched monkeys remained undisturbed in their natal groups. After completion of these protocols at 27 weeks of age, all of the monkeys were maintained in identical conditions. Behavioral and neuroendocrine measures of anxiety were assessed in the presence of mothers at 35 and 50 weeks of age [15]. Mothers were then permanently removed and their offspring were housed with peers. Cognitive control of behavior was assessed at 1.5 years of age [18] and exploratory behavior was examined at 2.5 years of age [17]. Puberty occurs at 2–3 years of age and the average maximum life span is ∼21 years. Noninvasive magnetic resonance imaging was performed at 3.3 years of age as described below. All procedures were conducted in accordance with the NIH Guide and were IACUC approved.
Brain Image Acquisition
Brain images were acquired on a General Electric 3T Signa MR scanner (Milwaukee, Wisc., USA) with protocols we developed for squirrel monkeys. All monkeys were scanned under anesthesia induced by subcutaneous injection of 20 mg/kg ketamine hydrochloride, 4 mg/kg xylazine hydrochloride, and 0.04 mg/kg atropine sulfate followed by 0.5–1.5% isoflurane gas. Body temperatures were maintained within the normal range using a cushioned heat pad. Ear plugs provided protection from noises generated by the scanner.
Whole brain T1-weighted images were acquired in the coronal plane with a three-dimensional inversion recovery-prepared fast-spoiled gradient pulse sequence [32]: TR = 12 ms, TE = 3 ms, TI = 300 ms, flip angle = 15, NEX = 4, matrix = 256 × 256, FOV = 8 cm, voxel size = 0.312 × 0.312 × 1.0 mm, slice thickness = 1 mm, gap = 0 mm, total scan time = 17 min. Diffusion tensor imaging data were then acquired in the coronal plane with a diffusion-weighted self-navigated interleaved spiral technique [33]: TR = 2,500 ms, TE = 46.5 ms, NEX = 4, matrix = 128 × 128, FOV = 8 cm, slice thickness = 3 mm, gap = 0 mm, bandwidth = ±125 kHz, number of spiral interleaves = 19, total scan time = 22 minutes. The amplitude of the diffusion-sensitizing gradients was 5 Gauss/cm with a duration and separation of, respectively, 15.8 and 22 ms. This resulted in a b value of 800 s/mm2. Diffusion was measured along six noncollinear directions (x, y, z) = [(1, 1, 0),(0, 1, 1),(1, 0, 1),(−1, 1, 0),(0, −1, 1),(1, 0, −1)], and a reference measurement was made along these directions with a b value of 10 s/mm2.
Brain Image Processing
All image data were analyzed without knowledge of the treatment conditions. Initially, T1-weighted images were processed to measure predefined prefrontal volumes using BrainImage software (http://spnl.stanford.edu/tools/brainimage.htm). Image processing included removal of non-brain tissue, AC-PC positional normalization, and resampling into cubic voxels (0.312 × 0.312 × 0.312 mm). A trained rater then traced ventromedial and dorsolateral prefrontal cortical volumes in the left (fig. 1a) and right cerebral hemispheres on 26–34 contiguous coronal images per monkey from the genu of the corpus callosum to the frontal pole. The specific rules used to identify these predefined regions are described in our earlier research [20]. Inter-rater reliabilities expressed as intra-class correlations from fixed effects models were greater than 0.90.
Fig. 1.
Social separation stress-induced adaptations in prefrontal cortical development. a Representative T1-weighted coronal images of dorsolateral (dlPFC) and ventromedial (vmPFC) prefrontal cortex. b Ventromedial prefrontal cortical volumes are larger in monkeys exposed to intermittent social separation stress compared to the no-stress condition (n = 9–10 monkeys per condition; mean ± SEM). The asterisk signifies a stress main effect (p < 0.05). Stress effects were not discerned in dorsolateral prefrontal regions and none of the stress-by-brain side interactions were statistically significant.
The T1-weighted images were subsequently processed for whole brain cortical thickness analysis unconstrained by predefinedregions of interest using FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/). Images were segmented into gray matter, white matter, and cerebrospinal fluid according to voxel intensity values and geometrical constraints [34]. A surface deformation algorithm was applied which first fits the white matter surface and then expands outward to find the gray matter surface [34, 35]. White and gray matter surfaces were carefully inspected and irregularities were manually corrected. For each monkey's left and right hemispheres, the algorithm produced separate cortical mesh models comprised of ∼138,500 vertices connected with triangular faces having an average face area of ∼0.05 mm2. Cortical thickness was computed as the distance between each linked vertex in the mesh models [36]. To allow for statistical comparisons (see below), all mesh models were spatially realigned to a group-average spherical surface representation that optimally co-registered corresponding sulcal and gyral brain features [35, 37]. Cortical thickness values were re-sampled into the common average spherical coordinate system and smoothed using an iterative nearest-neighbor averaging procedure with full width at half maximum of 10 mm.
The diffusion tensor imaging data were reconstructed prior to averaging on a Linux workstation. Motion-induced phase variation from interleaf to interleaf was measured using the center portion of k-space data and corrected with a conjugate gradient method. Fractional anisotropy was computed for each voxel as previously described [33]. Prefrontal white matter regions traced on T1-weighted images were spatially realigned to the fractional anisotropy images using SPM software (http://www.fil.ion.ucl.ac.uk/spm/). Voxel values of fractional anisotropy were extracted with a Matlab script and used to calculate a mean value per monkey for each white matter region of interest.
Data Analysis
Mixed factor analysis of variance was used to test the hypothesis that intermittent separation stress increases ventromedial and not dorsolateral prefrontal cortical volumes. The stress versus no-stress comparison was considered a between-subjects factor and brain side was included as a within-subjects factor. The study was not adequately powered to detect gender differences and gender was therefore excluded from analysis.
Anatomical mesh modeling was used to subsequently examine cortical thickness unconstrained by predefined cortical regions of interest. The effects of intermittent separation stress were analyzed using least squares regression with statistically significant differences visualized on a group-average brain template. Group differences were verified on individual brains by inverting the spherical surface transformations for each monkey subject. One male and one female from the separation stress condition were excluded from the cortical thickness analysis because their anatomical mesh models could not be resolved.
Lastly, analysis of variance was used to examine stress effects on prefrontal white matter fractional anisotropy. Pearson correlations were determined to assess relationships between white matter anisotropy and prefrontal cortical volumes. The descriptive statistics presented below are mean ± SEM and all test statistics are evaluated with two-tailed probabilities (p < 0.05).
Results
In keeping with previous studies we found that intermittent separation stress increased ventromedial (F(1,17) = 6.49, p = 0.021) and not dorsolateral (p = 0.11) prefrontal cortical volumes compared to the no-stress control condition with total brain volume variation controlled as a statistical covariate (fig. 1b). The stress-by-brain side interactions were not significant for either volumetric measure of prefrontal cortex.
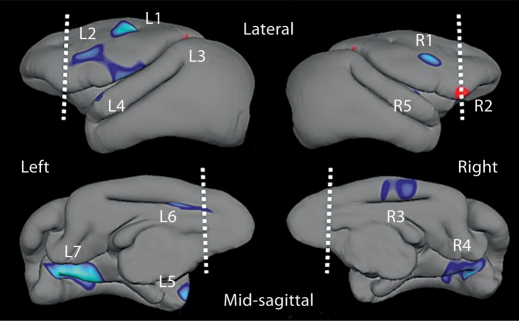
Intermittent separation stress also increased cortical thickness in several clusters that together encompassed 6% of all cortical tissue (table 1). None of these clusters were found within the pre-defined prefrontal regions (fig. 2). Clusters of increased cortical thickness induced by intermittent separation stress were located in dorsolateral premotor cortex (clusters R1 and L2), medial cingulate cortex (clusters R3 and L6), and the posterior parahippocampal gyrus (clusters R4 and L7) on both sides of the brain (fig. 2). On average, cortical thickness was only 1% greater in prefrontal regions of monkeys exposed to separation stress compared to the no-stress condition (p = 0.610). In contrast, the surface area of prefrontal cortex was 8% greater (F(1,16) = 4.92, p = 0.041) in monkeys exposed to intermittent separation stress (2,226 ± 49 mm2) compared to the no-stress condition (2,066 ± 53 mm2).
Table 1.
Social separation stress-induced differences in cortical thickness determined by unbiased mesh modeling analysis
| Cluster | Area mm2 | Mean (SEM) cortical thickness mm |
p | |
|---|---|---|---|---|
| intermittent separations | no separations | |||
| L1 | 132.74 | 2.80 (0.02) | 2.64 (0.03) | 0.001 |
| L2 | 358.79 | 2.23 (0.03) | 2.07 (0.03) | 0.001 |
| L3 | 29.07 | 1.82 (0.04) | 1.97 (0.05) | 0.039 |
| L4 | 83.72 | 1.55 (0.04) | 1.34 (0.04) | 0.003 |
| L5 | 50.84 | 1.19 (0.04) | 0.96 (0.07) | 0.010 |
| L6 | 65.32 | 2.23 (0.045) | 2.03 (0.05) | 0.008 |
| L7 | 370.92 | 1.84 (0.03) | 1.68 (0.03) | 0.000 |
| R1 | 61.29 | 2.73 (0.04) | 2.55 (0.05) | 0.009 |
| R2 | 97.42 | 1.90 (0.03) | 2.08 (0.04) | 0.003 |
| R3 | 154.71 | 2.17 (0.03) | 2.06 (0.03) | 0.015 |
| R4 | 286.32 | 1.79 (0.04) | 1.65 (0.03) | 0.004 |
| R5 | 8.08 | 1.61 (0.03) | 1.47 (0.05) | 0.029 |
The area and thickness of all clusters identified as significantly different in monkeys exposed to intermittent separation stress compared to the no-stress condition are presented for each cluster location depicted in figure 2 (n = 9 per condition). Total cortical area of the group-average brain template is 25,200 mm2. Clusterwise cortical thickness group differences were confirmed by MANOVA for the left (clusters L1–L7, Wilks' lambda = 0.125, F(7,10) = 9.973, p = 0.001) and right (clusters R1–R5, Wilks' lambda = 0.335, F(5,12) = 4.757, p = 0.013) hemispheres.
Fig. 2.
Social separation stress-induced differences in cortical thickness. Blue clusters on brain templates depict regions where cortex is significantly thicker (p < 0.05) after exposure to intermittent social separation stress compared to the no-stress condition (n = 9 monkeys per condition). Red clusters signify vice versa. Dashed lines demarcate the posterior boundary of prefrontal regions, and measures of cortical thickness for each labeled cluster are provided in table 1.
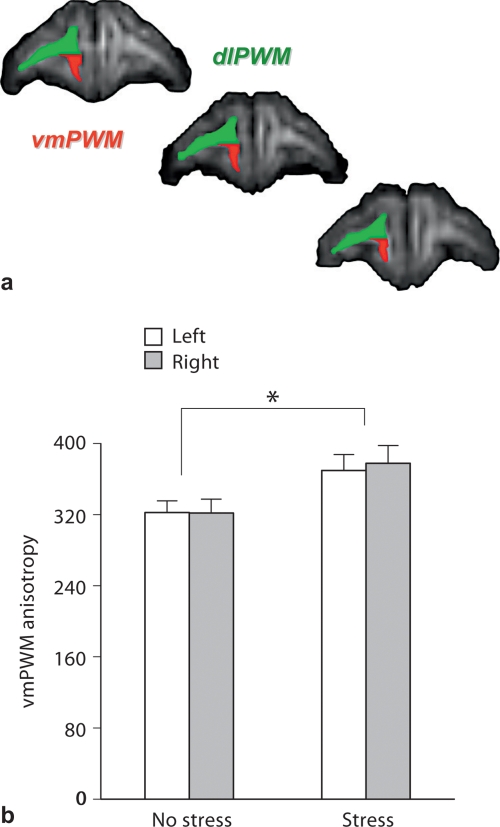
Previously, we found that intermittent separation stress increased prefrontal cortical and underlying white matter volumes in a different sample of monkeys [20]. Here, we used diffusion tensor imaging to examine prefrontal white matter in terms of fractional anisotropy (fig. 3a). Intermittent separation stress increased ventromedial (F(1,18) = 4.64, p = 0.045) and not dorsolateral (p = 0.418) prefrontal white matter fractional anisotropy compared to the no-stress condition (fig. 3b). This region-specific difference coincides with separation stress-induced differences in ventromedial and not dorsolateral prefrontal cortical volumes. White matter measures of fractional anisotropy in ventromedial (r = 0.53, d.f. 18, p = 0.016) and not dorsolateral (p = 0.618) regions correlated with their respective volumes of prefrontal cortex. No other separation stress effects were identified by an unbiased voxel-based exploratory analysis of white matter fractional anisotropy.
Fig. 3.
Social separation stress-induced adaptations in prefrontal white matter development. a Representative diffusion tensor images of fractional anisotropy in dorsolateral (dlPWM) and ventromedial (vmPWM) prefrontal white matter. b Ventromedial prefrontal white matter fractional anisotropy is greater in monkeys exposed to intermittent social separation stress compared to the no-stress condition (n = 9–10 monkeys per condition; mean ± SEM). The asterisk signifies a stress main effect (p < 0.05). Stress effects were not discerned in dorsolateral prefrontal regions and none of the stress-by-brain side interactions were statistically significant.
Discussion
In neuroscience, plasticity has traditionally been studied at the synaptic level in the context of learning and memory. Results from our studies of monkeys suggest that the learning-like process of coping with stress has broader effects on plasticity in prefrontal development. Exposure to intermittent social separations that simulate a naturally occurring but stressful transition in development increases ventromedial and not dorsolateral prefrontal cortical volumes. Increased ventromedial prefrontal cortical volumes reflect surface area expansion and coincide with increased white matter myelination inferred from neuroimaging. In published studies of the same monkeys, we previously reported that intermittent separations diminish subsequent stress-levels of cortisol, increase exploration of novel situations, and enhance prefrontal-dependent cognitive control of behavior [15, 17, 18]. Taken together, these findings suggest a role for prefrontal neuroadaptations in arousal regulation and resilience.
The cellular basis of stress-induced prefrontal neuroadaptations is unknown. Prefrontal cortical cell proliferation, dendritic elaboration, and synapse formation are largely complete soon after parturition in human and nonhuman primates [38, 39]. Around puberty, prefrontal cortical volumes then undergo a significant decline that corresponds with synaptic pruning [40] and possibly neuronal cell loss [41]. Diminished usage-dependent cell loss and selective retention of synaptic connections in ventromedial prefrontal cortex may accompany the learning-like process of coping with early life stress. This possibility is consistent with evidence of experience-dependent prefrontal plasticity in adolescent rats [42].
Unlike the pattern of prefrontal gray matter growth and regression, prefrontal white matter increases linearly throughout childhood and adolescence in primates [30, 43]. White matter is composed of axons sheathed in myelin produced by oligodendrocytes, and increased myelination induced by early experiences appears to affect information processing in distributed neural networks [29]. Despite evidence that coping with stress depends on myelinated prefrontal cortical and subcortical interconnections [24, 25, 44], myelination is seldom considered in discussions of neural plasticity as a mechanism for experience-dependent resilience [45,46,47].
The observation that stress affects ventromedial and not dorsolateral prefrontal white matter interconnections corresponds with structural and functional differences between these regions. The dorsolateral region is primarily comprised of granular cortex [48, 49] and its connections are consistent with executive functions, i.e. attention, planning, and working memory [50, 51]. The ventromedial region largely consists of agranular cortex [48, 49] and its connections indicate a role in visceral and sensory information integration, autonomic and neuroendocrine systems regulation, and the control of adaptive emotional behavior [44, 52, 53].
Effect sizes for stress-induced prefrontal neuroadaptations are small but similar in magnitude to brain changes induced by environmental enrichment in marmoset monkeys [54] and rats [55, 56]. Enrichment entails exposure to novel inanimate and/or social stimulation [57] and elicits neuroendocrine indications of mild stress in rats [58]. After exposure to enrichment, however, rats show diminished anxiety [57] and enhanced prefrontal-dependent learning [59] compared to nonenriched controls. These findings combined with our studies suggest that enrichment effects may be mediated, in part, by the process of coping with stress.
Controlled exposure to stress-related cues is also a feature of resiliency training for people that work in conditions where performance in the face of adversity is required, e.g. medical and military personnel, aviators, police, firefighters, and rescue workers [7, 60]. A similar process likewise occurs during cognitive behavior exposure therapy for stress-induced psychopathology. Patients are taught to confront their stress-related memories in imagination and then to interact with stress-inducing objects or situations in vivo. Repeated exposure to stress-related cues is thought to activate cognitive and emotional processing within and between exposure sessions, and thereby modify erroneous conditions that underlie the disorder [61, 62]. Although exposure therapy for patients and resiliency training for healthy humans are administered by professional psychologists and psychiatrists, our findings support Epstein's [63] suggestion that these interventions build on a basic stress coping process that tends to occur spontaneously without formal instruction or guidance.
All studies have their limitations and ours is no exception. The ratio of males to females is skewed toward females and our findings may or may not hold true for males. The surface features of cortex we used to differentiate prefrontal boundaries on T1-weighted images only roughly coincide with conventional maps based on cytoarchitectural borders. Increased fractional anisotropy determined by diffusion tensor imaging may reflect microstructural changes other than increased myelination, e.g. increased cell packing densities or larger nerve fiber diameters. The limitations of noninvasive neuroimaging are offset by opportunities for longitudinal follow-up studies of these adolescent monkeys. Such studies are needed to determine whether stress inoculation-induced prefrontal structural and functional adaptations persist into adulthood.
Acknowledgements
Supported by Public Health Service grants MH47573, MH66537, MH77884, DA16902, and Swiss National Research Funds 323500-111165, 3200-063135, 3232-063134, and PP00B-102864.
References
- 1.Khoshaba DM, Maddi SR. Early experiences in hardiness development. Consult Psychol J. 1999;51:106–116. [Google Scholar]
- 2.Mortimer JT, Staff J. Early work as a source of developmental discontinuity during the transition to adulthood. Dev Psychopathol. 2004;16:1047–1070. doi: 10.1017/s0954579404040131. [DOI] [PubMed] [Google Scholar]
- 3.Boyce WT, Ellis BJ. Biological sensitivity to context. I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 4.Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garmezy N, Masten AS, Tellegen A. The study of stress and competence in children: a building block for developmental psychopathology. Child Dev. 1984;55:97–111. [PubMed] [Google Scholar]
- 6.Rutter M. Implications of resilience concepts for scientific understanding. Ann NY Acad Sci. 2006;1094:1–12. doi: 10.1196/annals.1376.002. [DOI] [PubMed] [Google Scholar]
- 7.Meichenbaum D. Stress inoculation training: a preventative and treatment approach. In: Lehrer PM, Woolfolk RL, Sime WE, editors. Principles and Practice of Stress Management. New York: Guildford Press; 2007. pp. 497–518. [Google Scholar]
- 8.Fergus S, Zimmerman MA. Adolescent resilience: a framework for understanding healthy development in the face of risk. Annu Rev Publ Health. 2005;26:399–419. doi: 10.1146/annurev.publhealth.26.021304.144357. [DOI] [PubMed] [Google Scholar]
- 9.Boinski S, Fragaszy DM. The ontogeny of foraging in squirrel monkeys, Saimiri oerstedi. Anim Behav. 1989;37:415–428. [Google Scholar]
- 10.Lyons DM, Kim S, Schatzberg AF, Levine S. Postnatal foraging demands alter adrenocortical activity and psychosocial development. Dev Psychobiol. 1998;32:285–291. [PubMed] [Google Scholar]
- 11.Hennessy MB. Multiple, brief maternal separations in the squirrel monkey: changes in hormonal and behavioral responsiveness. Physiol Behav. 1986;36:245–250. doi: 10.1016/0031-9384(86)90011-9. [DOI] [PubMed] [Google Scholar]
- 12.Coe CL, Glass JC, Wiener SG, Levine S. Behavioral, but not physiological, adaptation to repeated separation in mother and infant primates. Psychoneuroendocrinology. 1983;8:401–409. doi: 10.1016/0306-4530(83)90019-7. [DOI] [PubMed] [Google Scholar]
- 13.Lyons DM, Martel FL, Levine S, Risch NJ, Schatzberg AF. Postnatal experiences and genetic effects on squirrel monkey social affinities and emotional distress. Horm Behav. 1999;36:266–275. doi: 10.1006/hbeh.1999.1547. [DOI] [PubMed] [Google Scholar]
- 14.Levine S, Mody T. The long-term psychobiological consequences of intermittent postnatal separation in the squirrel monkey. Neurosci Biobehav Rev. 2003;27:83–89. doi: 10.1016/s0149-7634(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 15.Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Arch Gen Psychiatry. 2004;61:933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- 16.Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. J Trauma Stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- 17.Parker KJ, Rainwater KL, Buckmaster CL, Schatzberg AF, Lindley SE, Lyons DM. Early life stress and novelty seeking behavior in adolescent monkeys. Psychoneuroendocrinology. 2007;32:785–792. doi: 10.1016/j.psyneuen.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biol Psychiatry. 2005;57:848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Lyons DM. Stress, depression, and inherited variation in primate hippocampal and prefrontal brain development. Psychopharmacol Bull. 2002;36:27–43. [PubMed] [Google Scholar]
- 20.Lyons DM, Afarian H, Schatzberg AF, Sawyer-Glover A, Moseley ME. Experience-dependent asymmetric variation in primate prefrontal morphology. Behav Brain Res. 2002;136:51–59. doi: 10.1016/s0166-4328(02)00100-6. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp. 2009;30:1188–1195. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamasue H, Abe O, Suga M, Yamada H, Inoue H, Tochigi M, Rogers M, Aoki S, Kato N, Kasai K. Gender-common and -specific neuroanatomical basis of human anxiety-related personality traits. Cereb Cortex. 2008;18:46–52. doi: 10.1093/cercor/bhm030. [DOI] [PubMed] [Google Scholar]
- 23.Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci USA. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biol Psychiatry. 2009;65:367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moseley M. Diffusion tensor imaging and aging: a review. NMR Biomed. 2002;15:553–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- 29.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 31.Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, Spicer J, Niogi S, Millner AJ, Reiss A, Garrett A, Hinshaw SP, Greenhill LL, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Glover G. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am J Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- 32.Lyons DM, Yang C, Eliez S, Reiss AL, Schatzberg AF. Cognitive correlates of white matter growth and stress hormones in female squirrel monkey adults. J Neurosci. 2004;24:3655–3662. doi: 10.1523/JNEUROSCI.0324-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C, Bammer R, Kim DH, Moseley ME. Self-navigated interleaved spiral (SNAILS): application to high-resolution diffusion tensor imaging. Magn Reson Med. 2004;52:1388–1396. doi: 10.1002/mrm.20288. [DOI] [PubMed] [Google Scholar]
- 34.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 35.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 36.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rakic P. The development of the frontal lobe: a view from the rear of the brain. Adv Neurol. 1995;66:1–6. [PubMed] [Google Scholar]
- 39.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 40.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 41.Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Bock J, Murmu RP, Ferdman N, Leshem M, Braun K. Refinement of dendritic and synaptic networks in the rodent anterior cingulate and orbitofrontal cortex: critical impact of early and late social experience. Dev Neurobiol. 2008;68:685–695. doi: 10.1002/dneu.20622. [DOI] [PubMed] [Google Scholar]
- 43.Malkova L, Heuer E, Saunders RC. Longitudinal magnetic resonance imaging study of rhesus monkey brain development. Eur J Neurosci. 2006;24:3204–3212. doi: 10.1111/j.1460-9568.2006.05175.x. [DOI] [PubMed] [Google Scholar]
- 44.Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- 46.Cicchetti D, Blender JA. A multiple-levels-of-analysis perspective on resilience: implications for the developing brain, neural plasticity, and preventive interventions. Ann NY Acad Sci. 2006;1094:248–258. doi: 10.1196/annals.1376.029. [DOI] [PubMed] [Google Scholar]
- 47.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 48.Rosabal F. Cytoarchitecture of the frontal lobe of the squirrel monkey. J Comp Neurol. 1967;130:87–108. doi: 10.1002/cne.901300202. [DOI] [PubMed] [Google Scholar]
- 49.Sanides F. The architecture of the cortical taste nerve areas in squirrel monkey (Saimiri sciureus) and their relationships to insular, sensorimotor and prefrontal regions. Brain Res. 1968;8:97–124. doi: 10.1016/0006-8993(68)90174-1. [DOI] [PubMed] [Google Scholar]
- 50.Goldman-Rakic PS. The ‘psychic cell’ of Ramon y Cajal. Prog Brain Res. 2002;136:427–434. doi: 10.1016/s0079-6123(02)36035-7. [DOI] [PubMed] [Google Scholar]
- 51.Fuster JM. The prefrontal cortex – an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 52.Price JL. Free will versus survival: brain systems that underlie intrinsic constraints on behavior. J Comp Neurol. 2005;493:132–139. doi: 10.1002/cne.20750. [DOI] [PubMed] [Google Scholar]
- 53.Barbas H, Zikopoulos B. The prefrontal cortex and flexible behavior. Neuroscientist. 2007;13:532–545. doi: 10.1177/1073858407301369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, Stranahan AM, Gould E. Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci USA. 2005;102:17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenzweig MR. Effects of differential experience on the brain and behavior. Dev Neuropsychol. 2003;24:523–540. doi: 10.1080/87565641.2003.9651909. [DOI] [PubMed] [Google Scholar]
- 56.Dong WK, Greenough WT. Plasticity of nonneuronal brain tissue: roles in developmental disorders. Ment Retard Dev Disabil Res Rev. 2004;10:85–90. doi: 10.1002/mrdd.20016. [DOI] [PubMed] [Google Scholar]
- 57.Fox C, Merali Z, Harrison C. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behav Brain Res. 2006;175:1–8. doi: 10.1016/j.bbr.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Moncek F, Duncko R, Johansson BB, Jezova D. Effect of environmental enrichment on stress related systems in rats. J Neuroendocrinol. 2004;16:423–431. doi: 10.1111/j.1365-2826.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- 59.Schrijver NC, Pallier PN, Brown VJ, Wurbel H. Double dissociation of social and environmental stimulation on spatial learning and reversal learning in rats. Behav Brain Res. 2004;152:307–314. doi: 10.1016/j.bbr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 60.Stetz MC, Thomas ML, Russo MB, Stetz TA, Wildzunas RM, McDonald JJ, Wiederhold BK, Romano JA., Jr Stress, mental health, and cognition: a brief review of relationships and countermeasures. Aviat Space Environ Med. 2007;78:B252–B260. [PubMed] [Google Scholar]
- 61.Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychol Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- 62.De Raedt R. Does neuroscience hold promise for the further development of behavior therapy? The case of emotional change after exposure in anxiety and depression. Scand J Psychol. 2006;47:225–236. doi: 10.1111/j.1467-9450.2006.00511.x. [DOI] [PubMed] [Google Scholar]
- 63.Epstein S. Natural healing processes of the mind: graded stress inoculation as an inherent coping mechanism. In: Meichenbaum D, Jaremko ME, editors. Stress Reduction and Prevention. New York: Plenum Press; 1983. pp. 39–66. [Google Scholar]