Abstract
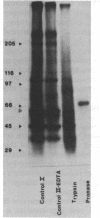
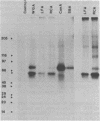
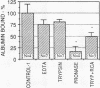
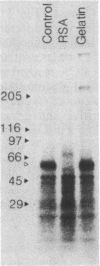
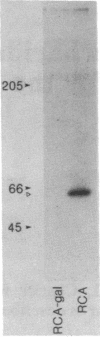
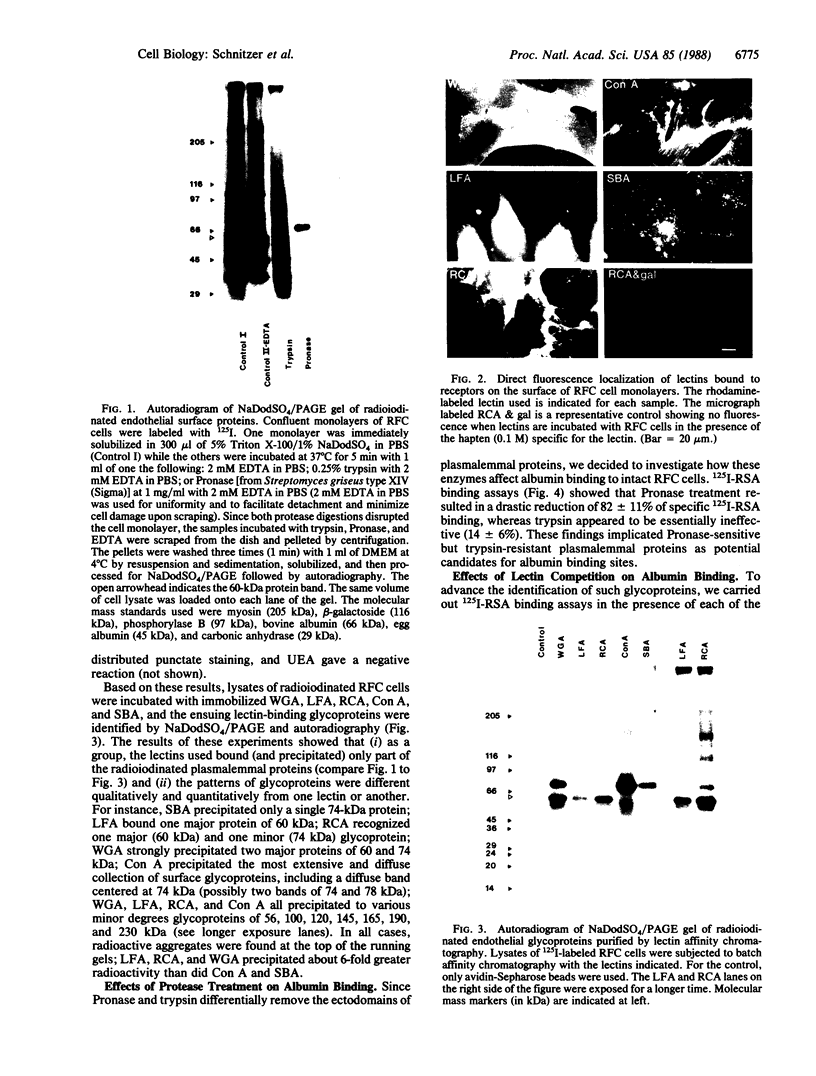
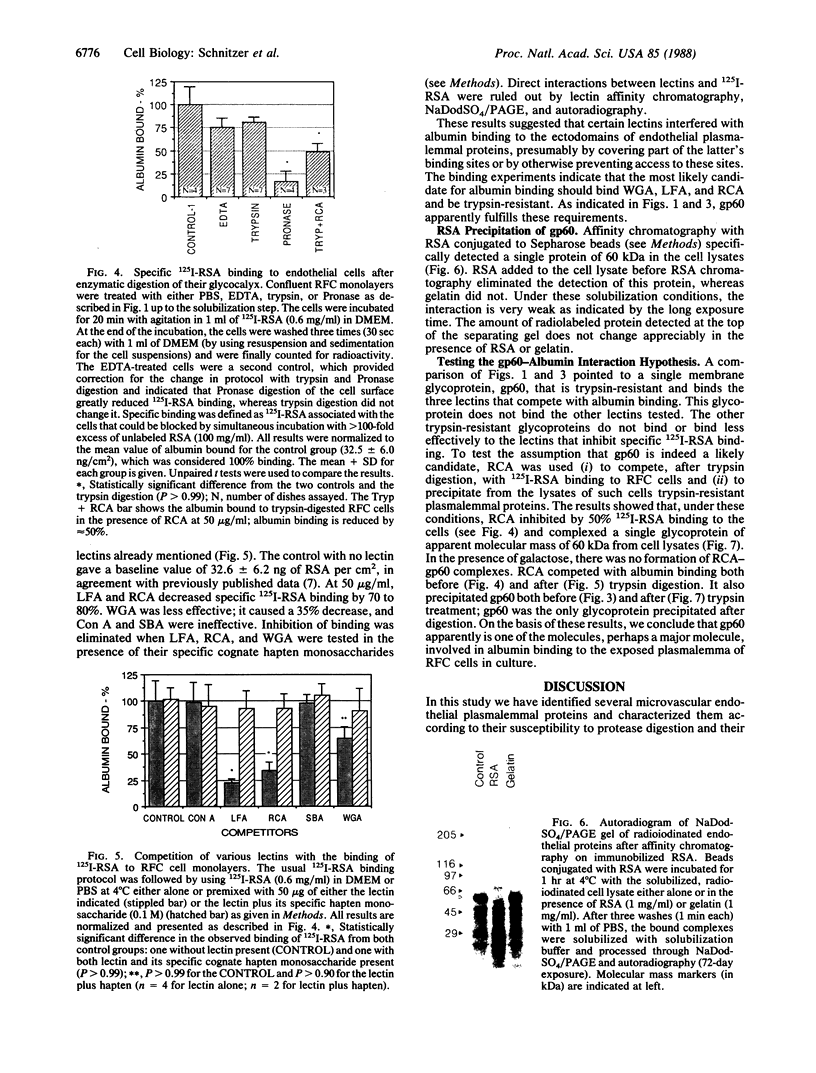
Confluent monolayers of microvascular endothelial cells, derived from the rat epididymal fat pad and grown in culture, were radioiodinated by using the lactoper-oxidase method. Their radioiodinated surface polypeptides were detected by NaDodSO4/PAGE (followed by autoradiography) and were characterized by both lectin affinity chromatography and protease digestion to identify the proteins involved in albumin binding. All detected polypeptides were sensitive to Pronase digestion, whereas several polypeptides were resistant to trypsin. Pronase treatment of the cell monolayer significantly reduced the specific binding of radioiodinated rat serum albumin, but trypsin digestion did not. Limax flavus, Ricinus communis, and Triticum vulgaris agglutinins competed significantly with radioiodinated rat serum albumin binding, whereas other lectins did not. A single 60-kDa glyco-protein was precipitated in common by these three lectins and was trypsin-resistant and Pronase-sensitive. Rat serum albumin affinity chromatography columns weakly but specifically bound a 60-kDa polypeptide from cell lysates derived from radioiodinated cell monolayers. These findings indicate that the 60-kDa glycoprotein is directly involved in a specific interaction of albumin with the cultured microvascular endothelial cells used in these experiments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alroy J., Goyal V., Skutelsky E. Lectin histochemistry of mammalian endothelium. Histochemistry. 1987;86(6):603–607. doi: 10.1007/BF00489554. [DOI] [PubMed] [Google Scholar]
- Feuerstein I. A., Kush J. Platelet adherence and detachment--in what ways is surface-bound albumin different from surface-bound fibrinogen? Thromb Haemost. 1986 Apr 30;55(2):184–188. [PubMed] [Google Scholar]
- Ghinea N., Fixman A., Alexandru D., Popov D., Hasu M., Ghitescu L., Eskenasy M., Simionescu M., Simionescu N. Identification of albumin-binding proteins in capillary endothelial cells. J Cell Biol. 1988 Jul;107(1):231–239. doi: 10.1083/jcb.107.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitescu L., Fixman A., Simionescu M., Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. J Cell Biol. 1986 Apr;102(4):1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley V. H., Curry F. E. Albumin modulation of capillary permeability: test of an adsorption mechanism. Am J Physiol. 1985 Feb;248(2 Pt 2):H264–H273. doi: 10.1152/ajpheart.1985.248.2.H264. [DOI] [PubMed] [Google Scholar]
- Hütter J. F., Piper H. M., Spieckermann P. G. Myocardial fatty acid oxidation: evidence for an albumin-receptor-mediated membrane transfer of fatty acids. Basic Res Cardiol. 1984 May-Jun;79(3):274–282. doi: 10.1007/BF01908027. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Brandon M. R., Hunt S. V., Williams A. F., Gatter K. C., Mason D. Y. Transferrin receptor on endothelium of brain capillaries. Nature. 1984 Nov 8;312(5990):162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- King G. L., Johnson S. M. Receptor-mediated transport of insulin across endothelial cells. Science. 1985 Mar 29;227(4694):1583–1586. doi: 10.1126/science.3883490. [DOI] [PubMed] [Google Scholar]
- Kragh-Hansen U. Molecular aspects of ligand binding to serum albumin. Pharmacol Rev. 1981 Mar;33(1):17–53. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann G. E. Alterations of myocardial capillary permeability by albumin in the isolated, perfused rabbit heart. J Physiol. 1981;319:311–323. doi: 10.1113/jphysiol.1981.sp013910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C., Phillips M. E., Turner M. R. The effects of native and modified bovine serum albumin on the permeability of frog mesenteric capillaries. J Physiol. 1985 Mar;360:333–346. doi: 10.1113/jphysiol.1985.sp015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milici A. J., Watrous N. E., Stukenbrok H., Palade G. E. Transcytosis of albumin in capillary endothelium. J Cell Biol. 1987 Dec;105(6 Pt 1):2603–2612. doi: 10.1083/jcb.105.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin. Adv Clin Chem. 1970;13:37–111. doi: 10.1016/s0065-2423(08)60385-6. [DOI] [PubMed] [Google Scholar]
- Pino R. M., Thouron C. L. Identification of lectin-receptor monosaccharides on the endothelium of retinal capillaries. Curr Eye Res. 1986 Aug;5(8):625–628. doi: 10.3109/02713688609015128. [DOI] [PubMed] [Google Scholar]
- Rasio E. A., Bendayan M., Goresky C. A. The effect of hyperosmolality on the permeability and structure of the capillaries of the isolated rete mirabile of the eel. Circ Res. 1981 Sep;49(3):661–676. doi: 10.1161/01.res.49.3.661. [DOI] [PubMed] [Google Scholar]
- Schneeberger E. E., Hamelin M. Interaction of serum proteins with lung endothelial glycocalyx: its effect on endothelial permeability. Am J Physiol. 1984 Aug;247(2 Pt 2):H206–H217. doi: 10.1152/ajpheart.1984.247.2.H206. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. E., Carley W. W., Palade G. E. Specific albumin binding to microvascular endothelium in culture. Am J Physiol. 1988 Mar;254(3 Pt 2):H425–H437. doi: 10.1152/ajpheart.1988.254.3.H425. [DOI] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Differentiated microdomains on the luminal surface of capillary endothelium: distribution of lectin receptors. J Cell Biol. 1982 Aug;94(2):406–413. doi: 10.1083/jcb.94.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorstensen K., Romslo I. Albumin prevents nonspecific transferrin binding and iron uptake by isolated hepatocytes. Biochim Biophys Acta. 1984 Aug 17;804(4):393–397. doi: 10.1016/0167-4889(84)90065-x. [DOI] [PubMed] [Google Scholar]
- Vasile E., Simionescu M., Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low-density lipoprotein in the arterial endothelium in situ. J Cell Biol. 1983 Jun;96(6):1677–1689. doi: 10.1083/jcb.96.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. C., Robinson C. S., Cross P. J., Devenny J. J. Endocytosis and exocytosis of transferrin by isolated capillary endothelium. Microvasc Res. 1983 May;25(3):387–396. doi: 10.1016/0026-2862(83)90028-6. [DOI] [PubMed] [Google Scholar]