Abstract
Prostate cancer (PCa) continues to represent a burgeoning medical problem in the United States. Recent studies suggest that gossypol, a bioactive phytochemical produced by cotton plants, is a promising agent against prostate cancer. The current studies were undertaken to examine the chemotherapeutic efficacy of gossypol on human prostate cancer cell lines and prostate tumor-initiating cells (pTICs). Gossypol reduced viability of three prostate cancer cell lines (LAPC4, PC3, and DU145) with an IC50 between 3–5 μM. Additionally, gossypol was effective at inhibiting pTIC-driven tumor growth in a NOD/SCID xenograft model. Our integrated molecular profiling approach encompassing proteomics, activated transcription factors and genomics suggests that the decrease in viability was associated with increased DNA damage and the induction of apoptosis. Exposure of DU145 cells to gossypol (1 – 10 μM) resulted in the activation of 13 proteins, 7 transcription factors, and expression of 17 genes involved in the mitochondrial pathway of apoptosis. These studies demonstrate for the first time that gossypol treatment induces DNA damage and activates p53. Collectively, this data supports the use of gossypol as a novel agent for PCa.
Keywords: Gossypol, prostate tumor-initiating cells, p53, apoptosis, prostate cancer
Introduction
Prostate cancer (PCa) continues to be the most common malignancy diagnosed in American men and the second leading cause of male cancer mortality (1). Current estimates indicate that over 2 million American men have been diagnosed with prostate cancer (2). Though localized forms of the disease can often be successfully treated by surgery or radiotherapy, a significant proportion of patients having undergone such interventions are at risk of disease recurrence. Androgen deprivation therapy (ADT) can prolong the life expectancy of these patients; however, ADT is associated with a number of distressing side effects, including loss of libido, bone density, muscle mass along with erectile dysfunction and cardiovascular morbidity (3). Moreover, nearly all the patients undergoing ADT are expected to fail hormone therapy and progress to an androgen-independent phenotype (AIPCa) (4). As currently available treatment options for AIPCa are lacking, there is a growing need for novel therapeutics that are effective against this fatal disease with minimal toxicity.
Gossypol, a yellowish compound produced by cotton plants (Gossypium species), has a long history of use in Chinese folk medicine and has been extensively studied for its use as a male contraceptive agent (5). In recent years, however, this polyphenolic compound has generated interest in the scientific community as a promising agent against cancer. For instance, studies conducted by three independent laboratory have demonstrated that gossypol specifically targets cancer cells and is not toxic to primary normal cells or non-cancerous cells (6,7,8). Clinical trials indicate gossypol is apparently safe in doses up to 70 mg/day (9) and pharmacokinetic studies revealed that this compound can reach peak plasma concentrations in micromolar ranges [0.79 ± 0.4 μg/ml (1.4 μM)] (10). Although cell cultures (11,12), animal studies (13,14), and clinical trials (15,16) have demonstrated that gossypol inhibits cell proliferation and prevents metastases of many types of cancers (16), the ability of this compound to modulate prostate cancer has not been well studied. Accordingly, the primary objective of this study was to access the chemotherapeutic effects of gossypol on human prostate cancer cell lines. Furthermore, in order to decipher the mechanism by which this compound exerts its anticancer effects, we chose DU145 as our model and employed an integrated molecular profiling approach in which we screened 650 proteins (377 pan-specific proteins and 273 phospho-specific proteins), 345 transcription factors and the entire human genome.
Increasing evidence suggests that prostate tumors originate from a subpopulation of stem cell-like cells called prostate tumor-initiating cells (pTIC). These pTICs are thought to be responsible for tumorigenesis, tumor differentiation and tumor maintenance. Patrawala et. al., have previously demonstrated that CD44 +/hi cells, isolated from various human prostatic cancer cells lines, are enriched for pTICs and confirmed that this subpopulation of cells are highly tumorigenic and metastatic (17,18). Similarly, we have demonstrated that as little as 100 CD44 +/hi cells isolated from DU-145 prostate carcinoma cell lines were sufficient for tumor formation when engrafted into the flanks of NOD-SCID mice (19). We and others have documented that the CD44 +/hi subpopulation of cells over-express genes known to play a role in stem cell self-renewal, such as OCT-3/4 and Bmi1 (19,17). The existence of a sinister subset of pTICs suggests that successful elimination of prostate cancer requires anti-cancer therapy targeting this subpopulation of cells as well as the differentiated tumor cells. Therefore, our second objective was to assess the efficacy of gossypol on pTICs.
In this study, we demonstrate for the first time that gossypol is effective at reducing the viability of three prostate cancer cell lines (LAPC4, PC3, DU145) and inhibiting tumor growth in a NOD/SCID xenograft model. Furthermore, the growth of pTICs isolated from DU145 (CD44+/hi) are also inhibited. Our integrated molecular profiling approach suggests that the inhibitory effect of gossypol is attributable to induction of DNA damage, which consequently leads to the stabilization of p53 and the activation of the mitochondrial pathway of apoptosis.
Materials and Methods
Cell Culture and Chemicals
Human prostate cancer cell lines DU145 and PC-3 were purchased from the American Type Culture Collection (Manassas, VA) and LAPC4 cells were a kind gift from Dr. Charles Sawyers from Memorial Sloan-Kettering Cancer Center (MSKCC). The DU145, LAPC4 and PC3 cell lines were cultured in DMEM, Iscove’s DMEM and F-12 Kaighn’s, respectively. All cell lines were supplemented with 10% heat-inactivated FBS, 50 U/ml penicillin/streptomycin and 2 mM L-glutamine. Sorted cells were cultured in a serum free medium as described previously (19). Gossypol (> 98% pure) was purchased from LKT Laboratories, Inc. (St. Paul, MN) and reconstituted in dimethylsufoxide (DMSO; Sigma Chemical Co. St Louis, MO). For analytical purposes, a standard stock solution of gossypol (10 mM) was prepared and stored at −20 °C. The final concentration of DMSO in all cell culture experiments was 0.1%. Control cells were grown in the same concentrations of DMSO.
Cell proliferation and cytotoxicity assay
PCa cell lines (DU145, LAPC4, and PC3) were seeded at a density of 5 × 104/well in 96-well tissueculture dishes and allowed to attach overnight. Triplicate samples of growing cells were treated with gossypol for time and concentrations as indicated. Cell proliferation was accessed by CellTiter-Glo assay (Promega, Madison, WI). To evaluate the cytotoxicity of gossypol on PCa cell lines, CytoTox-ONE™ membrane integrity assay was employed. For this assay, PCa cells were seeded at a density of 10,000 cells per 100μl per well in 96-well plates. After 24h, cells were treated with either gossypol or 0.1% DMSO as vehicle control for 48hrs. Cytotoxicity was determined according to the manufacturer’s recommendations. Briefly, lactate dehydrogenase (LDH) leakage was measured as the ratio of treatment-induced LDH to maximum LDH release. Samples were measured in triplicate, and repeated in at least 3 independent experiments.
Fluorescence-activated cell sorting (FACS) of CD44−/lo and CD44+/hi Prostate cancer cells
Trypsinized cells were washed twice with FACs buffer (PBS containing 1% BSA) and stained with 5ul of PE conjugated anti-CD44 (Invitrogen, Carlsbad, CA) per million cells at 4 C for 15 min. After staining, cells were washed twice with FACs buffer, re-suspended at 2×107 cells/ml, and were sorted using a MoFlo high-speed cell sorter (Dako Cytomation, Carpinteria, CA, USA). Live cells were gated on the basis of forward and side scatter and only the top 5% (CD44 hi/+) and the bottom 5% (CD44 lo/−) of stained cells were used for experiments.
Mouse xenograft studies
Eighteen male NOD/SCID mice were purchased at 4 weeks of age from NCI’s Animal Genetics and Production Facility and housed in the AAALAC-accredited animal facility at NCI. Animal care was provided in accordance with the procedures outlined in the “Guide for Care and Use of Laboratory Animals” (National Research Council, 1996). The mice were injected subcutaneously, at 6 weeks of age, with 100 CD44hi DU145 cells as described previously (19) and were randomly divided into 3 groups (6 mice per group). Group 1 was the positive control (no test reagent), whereas, groups 2 and group 3 received low dose (5mg/kg) and high dose (15mg/kg) of gossypol by oral gavage once daily, three times a week. Mice were monitored weekly for palpable tumor formation for 106 days.
Quantative apoptotic cell death assay
Cell death was measured by fluorescein-conjugated annexin V (Annexin V-FITC) and propidium iodide (PI) staining. DU145 cells in log phase growth were plated into 6-well plates at a density of 1 ×106 cells/well. Twenty-four hours later, cells were treated with gossypol (1, 5, and 10 μM) for the indicated periods of time. After the treatment, the attached cells were trypsinized, washed twice with cold PBS, and resuspended in binding buffer (1 ×105 cells/ml). Five microliters of Annexin V-FITC and PI were added to the suspended cells, incubated for 15 min at room temperature in the dark, cells were washed twice with PBS and analyzed by flow cytometry.
Caspase activity assay
DU145 cells were seeded at a density of 2.5 × 103 cells per well in a 96-well plate and cultured for 24 hours. After incubation with 10 μM gossypol for the indicated times, caspase catalytic activity of caspase 3/7, caspase 8 and caspase 9 were determined by means of Caspase-Glo 3/7, Caspase-Glo 8, and Caspase-Glo 9 Assay kit, (Promega, Madison, WI) according to manufactures instructions. Briefly, after treatment with gossypol, 100μL culture supernatant was transferred into an white walled 96-well plate and equal volume of caspase substrate was added and samples were incubated at room temperature for 1 hr. Culture medium was used as a blank and luminescence was measured using an Infinite M200 luminometer (Tecan US; Research Triangle Park, NC).
Comet assay for detecting DNA damage
An alkali comet assay, used for detecting DNA damage, was performed according to instructions provided by the manufacturer (Trevigen, Gaithersburg, MD). A suspension of 10,000 unsorted and sorted (CD44 +/hi and CD 44−/lo) cells either treated with or untreated with 10 uM gossypol for 48 hr were gently mixed with 100 ul of low melting point agrose. Seventy five microliters of the cells/agarose mixture was directly added to the comet slides and was allowed to gel on ice for 10 min. After solidification of agarose, the slide was immersed in lysis solution at 4 °C for 1h. Following lysis, electrophoresis (1 V/cm) was performed in alkali solution (0.3 M NaOH, 1 mM EDTA, pH > 13) for 20 min at 4 °C. The slides were immersed in ethanol (5 min), air-dried, and stained with SYBR Green and analyzed immediately at 200X in a fluorescence microscope (Olympus 70) under green light. An image analysis system (Image J), provided by the National Institute of Health (http://rsb.info.nih.gov/ij/) was used to quantify DNA damage. The DNA damage in non-treated (N = 32 for CD44 +/hi, N = 38 for CD 44−/lo and N = 62 for unsorted cells) and gossypol-treated cells (N = 22 for CD44 +/hi, N = 56 for CD 44−/lo and N = 95 for unsorted cells) was quantified as tail moment (the mean migration distance in the tail). One-way analysis of variance (ANOVA’s) was used to analyze normally distributed tail migration and head diameter. Overall differences were considered significant when p < 0.05.
Statistical Analysis
Data are presented as means ± standard deviation (SD). Statistical analysis was done using one-way ANOVA for multiple samples. P-value less than 0.05 were considered statically significant. Cell viability, DNA damage and caspase activity assay were presented graphically in the form of histograms, using Microsoft Excel computer program.
Protein Microarray, Transcription Factor/DNA binding Array, and Gene Array
See the Supplemental Experimental Procedures.
Results
Growth-inhibitory effect of gossypol on human PCa cell lines
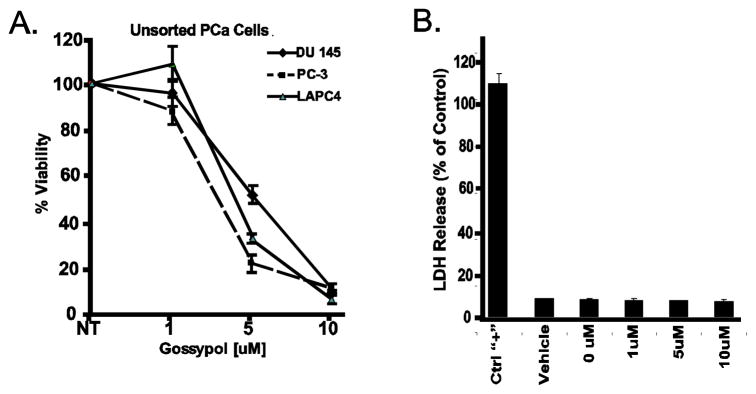
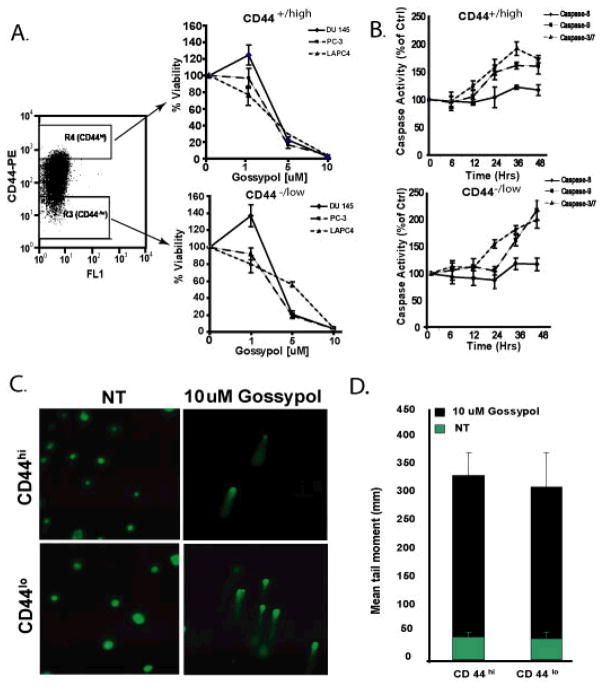
Gossypol inhibits proliferation and prevents metastases of Dunning prostate cells in xenograft Copenhagen rats (20). However, the oncostatic effects of gossypol have not been thoroughly investigated in other human prostate cancer cell lines and the molecular basis underlying these anticancer properties is still poorly understood. Hence, we first sought to access the effect of gossypol on the viability of three prostate cancer cell lines, DU145, PC3, and LAPC4. As shown in Figure 1A, treatment with gossypol for 72 hours induced a dose-dependent decrease in cell viability compared with untreated cells. Although the IC50 of gossypol on these three cell lines ranged from 3–5 μM (3 μM for PC3, 4 μM for LAPC4 and 5 μM for DU145), the 90 inhibitory concentration % (IC90) of gossypol was 10 μM on all three cells lines. These results show that gossypol can dramatically inhibit growth of PCa cells.
Figure 1. Effects of gossypol on viability and cytotoxicity of human prostate cancer cell lines.
(A) Three PCa cell lines (LAPC4, PC3, DU145) were cultured in the presence of various concentrations of gossypol (1 – 10 μM) for 72 hours and assayed for viability using Promega’s CellTiter-Glo assay. Results are expressed as the percentage decrease in viability in comparison with the control (DMSO treated) cells. All data shown are the mean ± SD of three separate experiments. (B) Depicts the effects of gossypol on LDH release. All data shown are the mean ± SD of three separate experiments.
A reduction in cell number can either be the consequence of gross injury to the cells, cytotoxicity, or the consequence of an actively driven biochemical process such as cell cycle arrest or apoptosis. To ensure that the growth inhibitory effect of gossypol on PCa cells was not a consequence of cytotoxicity, LDH leakage in response to 1, 5, and 10 μM of gossypol was performed. As depicted by Figure 1B, even at high concentrations (10 μM), gossypol exerted no cytotoxicity on DU145 cells. Taken together, these experiments suggest that gossypol can inhibit growth of prostate cancer cells and that this inhibition is not due to cytotoxicity.
Proteins modulated by gossypol
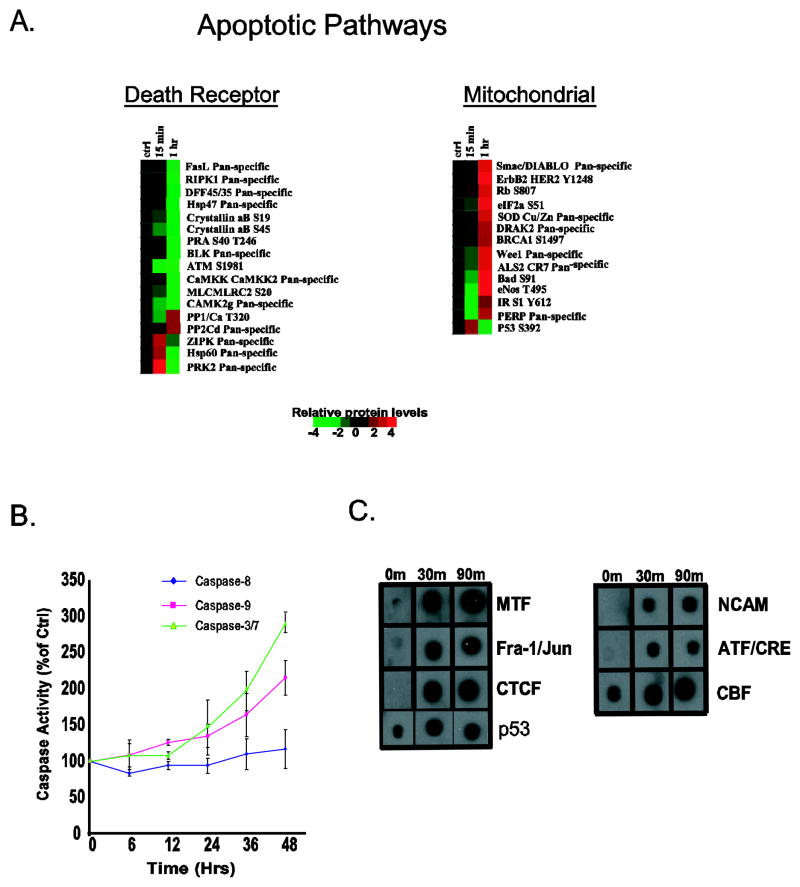
The effects of biological disruption are usually observed within the networks of biochemical intracellular systems. Using an integrated molecular profiling platform (proteomics, activated transcription factors and genomics), we examined the effects of gossypol on three systems important to the biological functions of cells. Since gossypol was equally potent across all three cell lines (Figure 1A) examined, we selected DU145 as a model to determine gossypol’s growth inhibitory effects. Cells exposed to 10 μM gossypol for 15 or 60 min were subjected to kinexus antibody microarray which screens 273 phospho-specific proteins and 377 pan specific proteins. Out of the 650 proteins, we found a 2-fold or greater down regulation in phosphorylation or expression of 96 proteins at either 15 or 60 min time point. As shown in Supplemental Figure 1, the majority of the proteins involved with ERK/MAPK signaling pathway, JAK/Stat signaling pathway, and cell structure were inactivated. Gossypol activated proteins that are involved in the mitochondrial apoptotic pathway (Figure 2A) and increased the phosphorylation of p53 at serine-392, which is phosphorylated in response to DNA damage (21). In order to validate these findings, the activity of initiator caspases (Caspase 8 and 9) and the effector caspases (Caspase 3/7) were accessed. As shown in Figure 2B, addition of gossypol to DU145 cells lead to a time-dependent increase in Caspase-9 and Caspase-3/7 but had no effect on Caspase-8. These results suggest that gossypol induces DNA damage and triggers apoptosis by utilizing the mitochondrial pathway.
Figure 2. Apoptotic proteins modulated by gossypol.
(A) Protein array of DU145 treated with gossypol. Relative protein level changes are indicated by the color bar. Red indicates an increase, green indicates a decrease and black indicates no change. Time points (0, 15 and 60 min) are shown at the top of each protein cluster: regulatory proteins of the death receptor pathway (top left panel), and the mitochondrial apoptotic pathway (top right panel). (B) Caspase activity following treatment with 10 μM was assessed. Error bars indicate mean ± standard deviations and the graph is representative of at least three independent experiments. (C) Regulation of several apoptotic transcription factors binding in DU145 cells treated with gossypol at 0, 30 and 90 min.
Transcription factors (Protein/DNA) altered by gossypol
The next phase of our integrated molecular profiling was to identify transcription factors (TFs) that were modulated by gossypol; for this, we used a Panomics TransSignal protein/DNA combo array. Exposing DU145 cells to 10 μM gossypol for either 30 or 90 min showed that gossypol altered the binding of 17 out of 345 TFs (5%); three decreased and 14 increased DNA binding (Supplemental Figure 2 and Figure 2C). Interestingly, fifty percent (7/14) of the TFs which show increased DNA binding are known to transcribe genes which participate in apoptosis (Figure 2C); the most noteworthy being p53. The ability of gossypol to increase DNA binding of p53 correlates well with our protein array data which showed an increase in activation (phosphorylation) of this protein (Figure 2B).
Gene Expression changes induced by gossypol
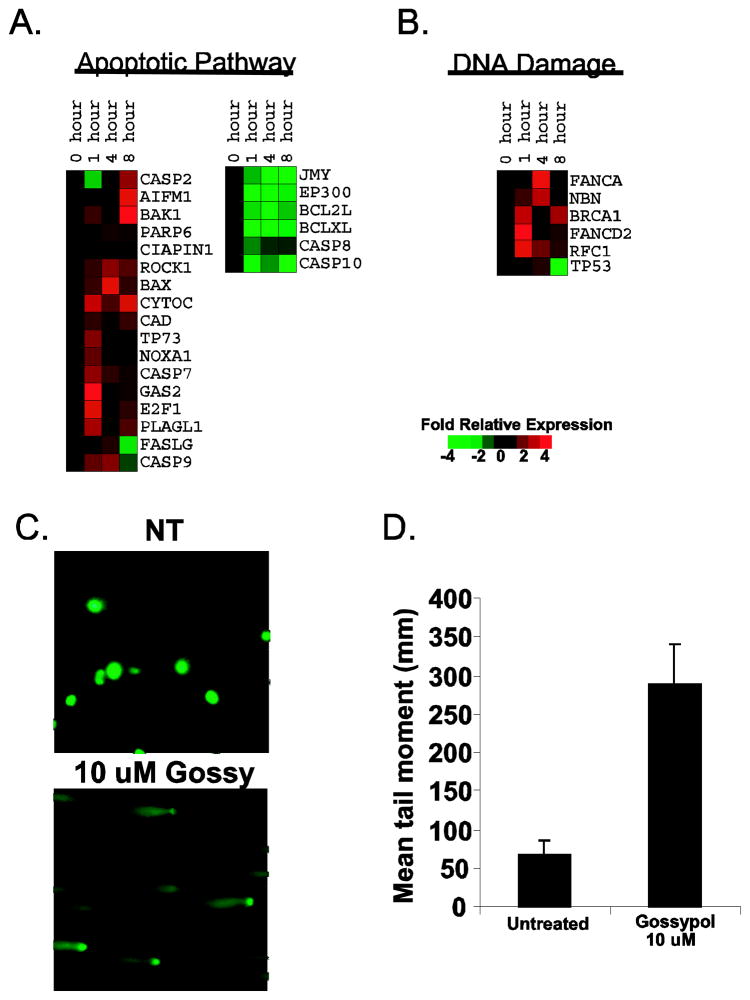
To finalize our integrated molecular profiling approach, gene expression changes were monitored after exposure to 10 μM gossypol using DU145 cells. By using Ingenuity System’s Pathways Analysis software, we focused our analysis to the transcripts involved in apoptotic pathways. Figure 3A reveals that treating DU145 cells with gossypol for 0, 1, 4, and 8 hrs increased the expression of pro-apoptotic genes such as Bax, Bak, AIF, NOXA1, CAD, PARP, and cytochrome C and decreased the expression of anti-apoptotic genes such as Bcl-2 and Bcl-xL. Additionally, analysis of the caspases involved in apoptosis showed an increase in transcripts of caspase-9 and caspase-7 and a decrease in transcripts of caspase-8 and caspase-10, thus, confirming the involvement of the mitochondrial pathway. Also, consistent with our protein and TF array data, we noticed an increase expression of p53 occurred within 1 hr after gossypol treatment, indicating that DNA damage might be responsible for gossypol-induced apoptosis. Therefore, we enriched our data for genes involved in DNA damage and found 6 genes (BRCA-1, FANCA, NHN, RFC1, FANCD2, and p53) up-regulated (Figure 3B). To verify these results, we performed a comet assay to detect DNA damage. As shown in Figure 3C and 3D, exposing DU145 cells to gossypol for 48 hrs lead to a marked increase in the mean tail moment; thus, confirming DNA damage.
Figure 3. Gene expression analysis of gossypol.
(A) Apoptotic genes modulated by gossypol. DU145 cells treated with 10μM gossypol for 0, 1, 4, and 8 hrs lead to increased expression of pro-apoptotic genes and decreased expression of anti-apoptotic genes. Relative gene expression changes are indicated by the color bar. (B) Gossypol modulates genes involved in DNA damage. Red represents an increase, green represents a decrease and black represents no change. Time points (0, 1, 4 or 8 hr) are shown at the top. (C) Example of “comet” produced by no treatment (top) and treatment with 10 μM gossypol (bottom) for 48 hrs in DU145 human prostate cancer cells. (D) Histogram summarizing the results of the comet assay. Results are represented as the mean tail moment (mm) + SD.
Gossypol induces apoptosis of DU145 cells
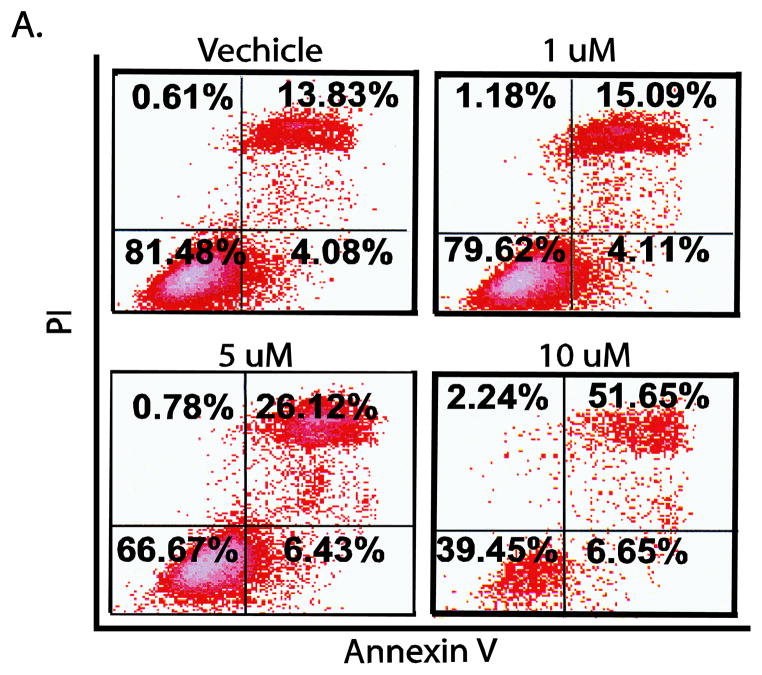
Because apoptosis appeared to be the major mechanism by which gossypol reduced viability DU145 cells were examined using Annexin V and PI staining. As shown in Figure 4, gossypol treatment of different doses (1, 5, and 10 μM) for 72 hours resulted in 15%, 26% and 52% apoptosis compared with the vehicle (14%). Similarly, exposing DU145 cells to 10 μM of gossypol for 24, 48, and 72 hrs resulted in 5%, 12%, and 52% apoptosis, respectively (data not shown).
Figure 4. Gossypol induces apoptosis in DU145 cells.
Flow cytometric analysis of annexin V-FITC and PI show the induction of apoptosis in DU145 cells after a 72 hr exposure to gossypol (1, 5, and 10 μM).
Gossypol inhibits growth and induces apoptosis of both pTICs (CD44+/hi) and bulk tumor (CD44−/lo) cells
Several studies suggest that pTICs are responsible for tumor growth and maintenance (19,17). We have previously shown that CD44+/hi cells isolated for DU145 cells are enriched for pTICs. Indeed, injecting as little as 100 CD44+/hi cells into NOD/SCID mice was sufficient to induce tumors (22). In order to assess whether gossypol induces growth inhibitory effects on this subpopulation of cells, we isolated the top 5% (CD44+/hi ) and the bottom 5% (CD 44−/lo ) of cells fractions from cultured parental prostate cancer cell lines by FACs. As shown in Figure 5A, gossypol inhibited growth of both CD44+/hi and CD 44−/lo cells. Gossypol at 5 μM reduced cellular viability to 10 – 28% for CD44+/hi cells in all cell lines. At the same concentration, for the CD44−/lo cells, gossypol reduced cellular viability to 18% and 20% for PC3 and DU145, respectively. However, increasing the concentration to 10 μM reduced viability of all three cells lines to 1 – 3% in both CD44+/hi and CD 44−/lo cells. To determine whether the decrease in cell number of CD44+/hi and CD 44−/lo cells was due to the ability of gossypol to induce apoptosis, the ability of gossypol to modulate the activity of initiator caspases (caspase 8 and 9) and effector caspases (caspase 3/7) was evaluated. Similar to our observation in unsorted DU145 cells, CD44+/hi and CD 44−/lo cells exposed to10μM gossypol resulted in a time-dependent increase in caspase 3/7 activity and caspase 9 activity, but had no effect of the caspase 8 activity (Figure 5B).
Figure 5. Effects of gossypol on PTICs (CD44+/hi) and bulk tumor (CD44−/lo) cells isolated from DU145.
PCa cells were FACS sorted into two populations; CD44+/hi and CD 44−/lo. R3 and R4 represent the lowest and highest 5% of CD44-expressing cells, respectively. Cell viability was measured using Promega’s Cell-Titer Glo assay. Y-axis represents percent viability normalized to untreated (0.1% DMSO) control. (B) Activity of initiator caspase (caspase-8 and -9) and effector caspase (caspase-3/7) in response to 10μM gossypol accessed at various time points (6 – 48 hrs) in CD44+/hi (right top) and CD 44−/lo (right bottom)cells isolated from DU145. After treatment, the cells were lysed and caspase-3/7, -8, and -9 activity was measured using Caspase-Glo assay kit. All experiments were done in triplicate and were performed at least three times. (C) A representative comet assay image showing DNA damage induced by gossypol on CD44+/hi and CD 44−/lo cells. (D) A histogram summarizing the Mean Tail Moment of DNA.
Gossypol induces DNA damage in both pTICs (CD44+/hi) and bulk tumor (CD44−/lo) cells
Our results on unsorted DU145 cells suggest that the apoptotic effect of gossypol was mainly due to induction of DNA damage, in order to investigate whether gossypol also induced DNA damage in CD44+/hi and CD 44−/lo cells, we performed an alkaline comet assay. Cells treated with gossypol show statically significant longer tails (Figure 5C; right panel) than control (untreated) cells (Figure 5C, left panel). Also, the tail length observed for both CD44+/hi and CD 44−/lo cells were approximately the same (Figure 5D), indicating that gossypol is equally potent on both cell types.
Gossypol inhibits tumor incidence in a NOD/SCID xenograft model
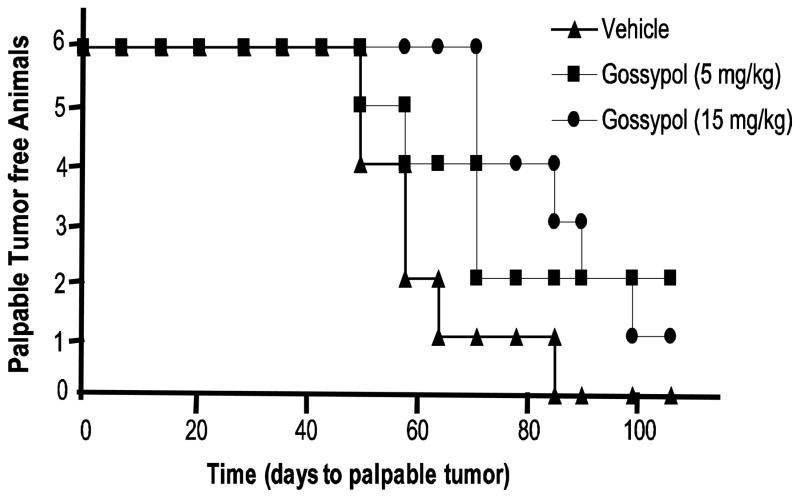
The in vitro data prompted us to investigate the efficacy of DU145 in a pTIC-driven tumor-initiation study. NOD/SCID mice were injected subcutaneously with 100 CD44+/hi cells and gossypol was administered by oral gavage three times a week over the course of the entire experiment (up to 106 days) at two concentrations (5 mg/kg and 15 mg/kg). As shown in Figure 6, gossypol reduced both tumor incidence and tumor latency. At 82 days, 100% of vehicle treated mice had tumors, whereas only 67% (4/6) of 5 mg/kg gossypol treated mice had tumors and 50% (3/6) of 15 mg/kg gossypol treated mice had tumors. The average latency period for vehicle treated was 61 days, whereas the average latency period was 78 and 90 days 5 mg/kg and 15 mg/kg, respectively.
Figure 6. Gossypol inhibits tumor incidence in a NOD/SCID xenograft model.
Kaplan-Meier plot for time to palpable tumor formation. CD44+/hi and CD 44−/lo (100 sorted cells) were injected subcutaneously into the left and right flanks, respectively, of six-week old NOD/SCID mice. Injections were supplemented with an equal volume of matrigel. Each group contained six animals and was monitored for palpable tumor formation weekly.
Discussion
Prostate cancer causes significant morbidity and mortality and is a major public problem in the United States. Although ADT can prolong the life expectancy of these patients, acquired drug resistance as well as induction of side effects remains a major obstacle in clinical settings. Therefore, attention has been focused on natural products as potential sources of novel anticancer drugs over the last few decades (23,24,25,26). Accordingly, in this study we evaluated the effect of gossypol on the growth of human prostate cancer cell lines. These results indicate that gossypol acts as an inhibitor of prostate cancer cell growth as demonstrated by its ability to reduce viability of three prostate cancer cell lines (LAPC4, PC3, DU145) and the putative prostate cancer stem cells isolated from DU145 (CD44+/high) in culture and to inhibit tumor growth in a NOD/SCID xenograft model. Furthermore, these investigations provide a plausible molecular basis for the development of naturally occurring anticancer agents for better management of androgen-independent prostate cancer.
Humans have consumed gossypol and gossypol-containing drugs and food for a long period of time with few adverse consequences. Chinese contraceptive studies conducted by Qian et. al., found that gossypol given orally at a dose of 60 – 70 mg per day for 35 – 42 days caused a gradual increase in the percentage of nonmotile spermatozoa in the ejaculate in 25 (100%) volunteers suggesting that gossypol may act on epididymal or testicular spermatozoa (5). The side effects of this dosage were reversible and generally of mild degree, mainly including decrease or increase in appetite, fatigue, dryness of mouth, and a seemingly decreased libido. A preliminary clinical study conducted by Stein et. al., reported that oral gossypol at lower doses (20 mg daily) can safely be given to patients with advance cancer and the main subjective toxicity appeared to be emesis (27). In our study, mice fed gossypol by oral gavage at 5 and 15 mg/kg displayed no signs of toxicity during the 106 days of study. Furthermore our in vitro studies on prostate cancer cell lines showed that gossypol can inhibit growth of prostate cancer cells without inducing cytotoxicity as measured by the membrane integrity assay.
Many chemotherapeutic agents have been found to retain the activity of apoptosis (28). The integrated molecular profiling approach utilized in our study reveals that the growth inhibiting effects of gossypol appeared to be mostly associated with induction of apoptosis. We observed that exposure of DU145 cells to 10 μM gossypol resulted in activation of 13 proteins (Fig. 2A), 7 transcription factors (Fig. 2C), and expression of 17 genes (Fig. 3) involved in apoptosis. Furthermore, our observation that gossypol treatment lead to an increase in PI and Annexin V staining and increased activity of caspase 3/7 confirms that gossypol induces apoptosis of prostate cancer cells.
There are two pathways currently proposed to play major rolesin regulating apoptosis in mammalian cells: caspase-8 mediated extrinsic pathway and mitochondria-related intrinsic (29). Although both pathways share a common downstream caspase-3/7 protease signaling step, the intrinsic pathway involves the permeabilization of the outer mitochondrial membrane by proapoptotic proteins such as Bax and Bad, resulting in the release of Smac/DIABLO and cytochrome c, which in turn leads to the activation of caspase-9 (30). These studies demonstrate that gossypol activated proteins involved in the mitochondrial pathway such as Bad and Smac/Diablo as early as 15 min post treatment. After 1 hour of gossypol treatment, we noticed increased transcript levels of proapoptotic genes such as Bak1, PARP6, AIF, Cyto C, and Noxa and a decrease in transcript levels of Bcl-2 and Bcl-XL. Moreover, an increase in the activation of caspase-9, and not caspase-8, strongly suggests that the intrinsic mitochondrial pathway is engaged in gossypol-induced apoptosis.
Activation of the p53 pathway is required for apoptosis induction by growth factor withdrawal, hypoxia, and DNA damage (31). It is well established that p53 mediates apoptosis by transcribing genes that encode the mitochondrial pathway of apoptosis, such as Bax (32,31). The current studies reveal that the gossypol-induced apoptotic response in prostate cancer cells is, in part, achieved through DNA damage and p53 activation. Treating DU145 cells with gossypol lead to an increase in length of the mean tail moment and transcripts of genes involved in DNA damage. Furthermore, we observed that within 15 min of exposure to gossypol, p53 was stabilized, as evident by increased phosphorylation at S392 (Figure 2A). After 30 min, we noticed an increase in its ability to bind DNA (Figure 2C) and in as little as 1 hrs time, we detected an increase of p53 transcripts (Figure 3B) for up to 4 hrs. These results, taken together, demonstrate that gossypol-induced apoptosis in prostate cancer cells is associated with DNA damage and activation of p53. While gossypol has been reported to induce apoptosis by inhibiting antiapoptotic Bcl-2 family members and interaction with the mitochondrial caspase pathway (33), to our knowledge, this is the first report to demonstrate that gossypol induces DNA damage leading to activation of p53 and apoptosis.
There is a growing literature supporting that prostate cancer is the result of the hierarchical expansion of “prostate tumor-initiating cells” (pTIC), which function as stem-like cells to maintain malignant growth and contribute to drug resistance (19,17). Although most cancer cells are apparently killed during chemotherapy, a few pTICs can survive and lead to relapse of disease. The current studies indicate that gossypol is a potential anti-prostate cancer agent that can target both the tumor-initiating cells (pTICs) and their differentiated progeny (Figure 5A). Additionally, we showed that mice engrafted with pTIC and treated with gossypol exhibited a marked depression in tumor incidence and latency (Figure 6). Furthermore, gossypol increased the activation of caspase 3/7 and caspase 9 activity, but had no effect on the activity of caspase 8, suggesting that the mechanism by which gossypol reduced viability of pTICs is similar to that of the unsorted cells (Figure 5B).
In conclusion, we have demonstrated, for the first time that gossypol inhibits tumor growth in a NOD/SCID xenograft model and reduces viability of three prostate cancer cell lines (LAPC4, PC3, DU145). The inhibitory effect can be attributed to the ability of gossypol to induce apoptosis. Gossypol leads to DNA damage with subsequent activation of p53, which in turn activates apoptosis via the mitochondrial pathway. These results provide molecular information for further investigations on gossypol’s clinical application for prostate cancer prevention/therapy. Our findings that gossypol also inhibits growth of pTICs could be important in devising a target-based therapeutic strategies that have effects on tumor initiating cells which may be resistant to conventional therapies. Moreover, gossypol may be an appropriate post conventional treatment as a relatively non-toxic means of suppressing residual pTICs.
Supplementary Material
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. In addition, this work was funded in part by the Office of Dietary Supplements, National Institutes of Health. This work has been funded in part with Federal funds fro the National Cancer Institute, under contract no. N01-CO-12400. The content of this paper does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Abbreviations List
- Pca
Prostate cancer
- ADT
Androgen deprivation therapy
- AIPCa
Androgen independent prostate cancer
- pTIC
Prostate tumor-initiating cells
- LDH
Lactate dehydrogenase
- FACs
Fluorescence-activated cell sorting
- TFs
Transcription factors
- PI
Propidium iodide
- FITC
Fluorescein isothiocyanate
- MSKCC
Memorial Sloan-Kettering Cancer Center
- NCI
National Cancer Institute
- AAALAC
Association for Assessment and Accrediatation of Laboratory Animal Care
Footnotes
The corresponding authors have no conflict of interest to report.
Reference List
- 1.Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–94. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith MR. Androgen deprivation therapy for prostate cancer: new concepts and concerns. Curr Opin Endocrinol Diabetes Obes. 2007;14:247–54. doi: 10.1097/MED.0b013e32814db88c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isbarn H, Boccon-Gibod L, Carroll PR, Montorsi F, Schulman C, Smith MR, et al. Androgen Deprivation Therapy for the Treatment of Prostate Cancer: Consider Both Benefits and Risks. Eur Urol. 2008 doi: 10.1016/j.eururo.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer EA, Golijanin DJ, Messing EM. Androgen deprivation therapy for advanced prostate cancer: why does it fail and can its effects be prolonged? Can J Urol. 2008;15:4381–7. [PubMed] [Google Scholar]
- 5.Qian SZ, Wang ZG. Gossypol: a potential antifertility agent for males. Annu Rev Pharmacol Toxicol. 1984;24:329–60. doi: 10.1146/annurev.pa.24.040184.001553. [DOI] [PubMed] [Google Scholar]
- 6.Hu F, Mah K, Teramura DJ. Gossypol effects on cultured normal and malignant melanocytes. In Vitro Cell Dev Biol. 1986;22:583–8. doi: 10.1007/BF02623517. [DOI] [PubMed] [Google Scholar]
- 7.Tuszynski GP, Cossu G. Differential cytotoxic effect of gossypol on human melanoma, colon carcinoma, and other tissue culture cell lines. Cancer Res. 1984;44:768–71. [PubMed] [Google Scholar]
- 8.Yeow WS, Baras A, Chua A, Nguyen DM, Sehgal SS, Schrump DS, et al. Gossypol, a phytochemical with BH3-mimetic property, sensitizes cultured thoracic cancer cells to Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J Thorac Cardiovasc Surg. 2006;132:1356–62. doi: 10.1016/j.jtcvs.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Van PC, Seidman AD, Reidenberg MM, Moasser MM, Sklarin N, Van ZK, et al. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res Treat. 2001;66:239–48. doi: 10.1023/a:1010686204736. [DOI] [PubMed] [Google Scholar]
- 10.Qian SZ, Wang ZG. Gossypol: a potential antifertility agent for males. Annu Rev Pharmacol Toxicol. 1984;24:329–60. doi: 10.1146/annurev.pa.24.040184.001553. [DOI] [PubMed] [Google Scholar]
- 11.Karaca B, Kucukzeybek Y, Gorumlu G, Erten C, Gul MK, Cengiz E, et al. Profiling of angiogenic cytokines produced by hormone- and drug-refractory prostate cancer cell lines, PC-3 and DU-145 before and after treatment with gossypol. Eur Cytokine Netw. 2008;19:176–84. doi: 10.1684/ecn.2008.0139. [DOI] [PubMed] [Google Scholar]
- 12.Macoska JA, Adsule S, Tantivejkul K, Wang S, Pienta KJ, Lee CT. -(−)Gossypol promotes the apoptosis of bladder cancer cells in vitro. Pharmacol Res. 2008;58:323–31. doi: 10.1016/j.phrs.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Meng Y, Tang W, Dai Y, Wu X, Liu M, Ji Q, et al. Natural BH3 mimetic (−)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol Cancer Ther. 2008;7:2192–202. doi: 10.1158/1535-7163.MCT-08-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paoluzzi L, Gonen M, Gardner JR, Mastrella J, Yang D, Holmlund J, et al. Targeting Bcl-2 family members with the BH3 mimetic AT-101 markedly enhances the therapeutic effects of chemotherapeutic agents in in vitro and in vivo models of B-cell lymphoma. Blood. 2008;111:5350–8. doi: 10.1182/blood-2007-12-129833. [DOI] [PubMed] [Google Scholar]
- 15.Dodou K, Anderson RJ, Small DA, Groundwater PW. Investigations on gossypol: past and present developments. Expert Opin Investig Drugs. 2005;14:1419–34. doi: 10.1517/13543784.14.11.1419. [DOI] [PubMed] [Google Scholar]
- 16.Stein RC, Joseph AE, Matlin SA, Cunningham DC, Ford HT, Coombes RC. A preliminary clinical study of gossypol in advanced human cancer. Cancer Chemother Pharmacol. 1992;30:480–2. doi: 10.1007/BF00685601. [DOI] [PubMed] [Google Scholar]
- 17.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 18.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 19.Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98:756–65. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CJ, Ghosh PK, Hu YF, Brueggemeier RW, Lin YC. Antiproliferative and antimetastatic effects of gossypol on Dunning prostate cell-bearing Copenhagen rats. Res Commun Chem Pathol Pharmacol. 1993;79:293–312. [PubMed] [Google Scholar]
- 21.Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–7. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki BT, Hurt EM, Kalathur M, Duhagon MA, Milner JA, Kim YS, et al. Effects of the sesquiterpene lactone parthenolide on prostate tumor-initiating cells: An integrated molecular profiling approach. Prostate. 2009;69:827–37. doi: 10.1002/pros.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartsmann G, Ratain MJ, Cragg GM, Wong JE, Saijo N, Parkinson DR, et al. Anticancer drug discovery and development throughout the world. J Clin Oncol. 2002;20:47S–59S. [PubMed] [Google Scholar]
- 24.Goswami SK, Das DK. Resveratrol and chemoprevention. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 25.Perabo FG, von Low EC, Siener R, Ellinger J, Muller SC, Bastian PJ. A critical assessment of phytotherapy for prostate cancer. Urologe A. 2009;48:270–83. doi: 10.1007/s00120-008-1929-5. [DOI] [PubMed] [Google Scholar]
- 26.Volate SR, Davenport DM, Muga SJ, Wargovich MJ. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin) Carcinogenesis. 2005;26:1450–6. doi: 10.1093/carcin/bgi089. [DOI] [PubMed] [Google Scholar]
- 27.Stein RC, Joseph AE, Matlin SA, Cunningham DC, Ford HT, Coombes RC. A preliminary clinical study of gossypol in advanced human cancer. Cancer Chemother Pharmacol. 1992;30:480–2. doi: 10.1007/BF00685601. [DOI] [PubMed] [Google Scholar]
- 28.Smets LA. Programmed cell death (apoptosis) and response to anti-cancer drugs. Anticancer Drugs. 1994;5:3–9. doi: 10.1097/00001813-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 30.Rustin P, Kroemer G. Mitochondria and cancer. Ernst Schering Found Symp Proc. 2007:1–21. doi: 10.1007/2789_2008_086. [DOI] [PubMed] [Google Scholar]
- 31.Colman MS, Afshari CA, Barrett JC. Regulation of p53 stability and activity in response to genotoxic stress. Mutat Res. 2000;462:179–88. doi: 10.1016/s1383-5742(00)00035-1. [DOI] [PubMed] [Google Scholar]
- 32.Amundson SA, Myers TG, Fornace AJ., Jr Roles for p53 in growth arrest and apoptosis: putting on the brakes after genotoxic stress. Oncogene. 1998;17:3287–99. doi: 10.1038/sj.onc.1202576. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M, Liu H, Tian Z, Griffith BN, Ji M, Li QQ. Gossypol induces apoptosis in human PC-3 prostate cancer cells by modulating caspase-dependent and caspase-independent cell death pathways. Life Sci. 2007;80:767–74. doi: 10.1016/j.lfs.2006.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.