Abstract
Understanding the biophysical basis of animal magnetoreception has been one of the greatest challenges in sensory biology. Recently, it was discovered that the light-dependent magnetic sense of Drosophila melanogaster is mediated by the ultraviolet (UV)-A/blue light photoreceptor Cryptochrome (Cry)1. We now show using a transgenic approach that the photoreceptive, Drosophila-like Type 1 Cry and the transcriptionally repressive, vertebrate-like Type 2 Cry of the monarch butterfly (Danaus plexippus) can both function in the magnetoreception system of Drosophila and require UV-A/blue light (<420nm) to do so. The lack of magnetic responses for both Cry types under wavelengths >420 nm does not fit the widely held view that tryptophan triad-generated radical pairs mediate Cry’s ability to sense a magnetic field. We bolster this assessment using a mutant form of Drosophila and monarch Type 1 Cry and confirm that the tryptophan triad pathway does not play a critical role in magnetic transduction. Together, these results suggest that animal Crys can mediate light-dependent magnetoreception, but do so through an unconventional photochemical mechanism. This work emphasizes the utility of Drosophila transgenesis for elucidating the precise mechanisms of Cry-mediated magnetosensitivity in insects and also in vertebrates, like migrating birds.
A wide variety of animals are able to detect the Earth’s magnetic field and use it as a source of directional information2,3. In many cases, animal magnetic detection appears to depend on chemical reactions initiated by specific wavelengths of light4,5. The most popular chemical reaction model proposes that light-dependent magnetoreception is mediated by radical pair reactions that are generated in specialized photoreceptors6,7,8.
Because of their photoreceptor function and biochemical properties, the DNA photolyase-related Crys have been postulated to be the key photoreceptor molecules that generate magnetically sensitive radical-pair products7,9. However, all animals Crys are not functionally equivalent. In fact, two phylogenetically and functionally distinct groups of animal Crys have been identified and characterized based largely on their roles in the regulation of circadian clocks10,11. Drosophila-like Type 1 Crys are sensitive to ultraviolet-A/blue wavelengths of light12,13 and function mainly as circadian photoreceptors. Vertebrate-like Type 2 Crys, on the other hand, are thought to function primarily as negative regulators of the clock’s transcriptional feedback loop, the quintessential intracellular gear of the molecular clock. Although both types of Cry appear to be widespread in the animal kingdom, there is considerable variation in their distribution among taxa11; insects can have Type 1 (the only one found in Drosophila), Type 2, or both types of Cry, whereas vertebrates only have Type 2 Crys. Type 2 Cry proteins are thought to mediate light-dependent magnetoreception in many vertebrate groups14 including migratory birds, but direct evidence for such a role has yet to be established for any animal due to the lack of available tools for genetic manipulation.
Recently, we integrated genetic and behavioural approaches to show that light-dependent magnetoreception in Drosophila is mediated by its Type 1 Cry1. The necessity of Drosophilia Cry for magnetosensitivity was shown using two distinct Cry-deficient mutations, which abolished magnetosensitive behavioural responses. This work established Drosophila as a viable genetic system to delineate the molecular underpinnings of Cry-based magnetoreception in animals.
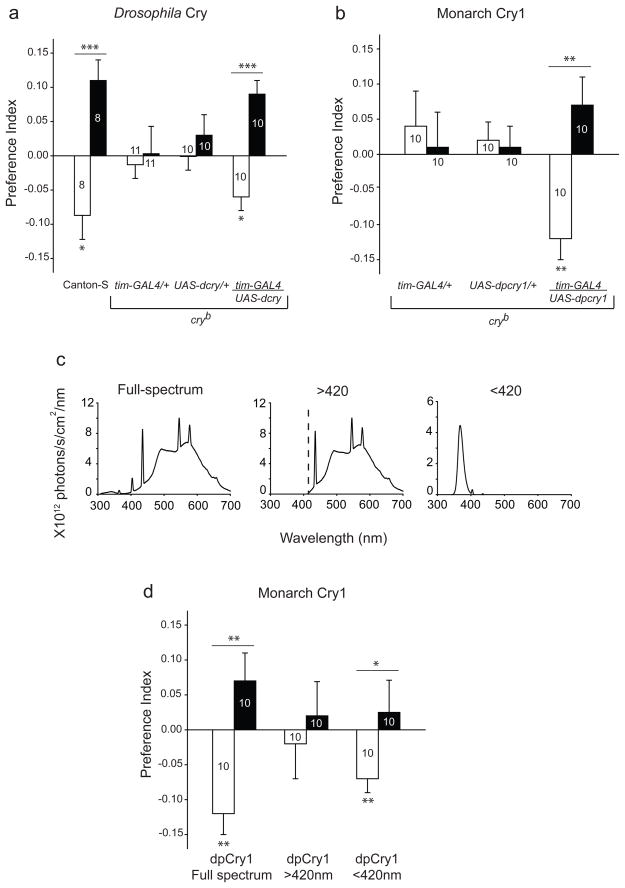
We began our current investigations by showing that a Drosophila cry transgene can rescue magnetosensitivity in Cry-deficient flies. We used the GAL4-UAS system, with timeless (tim)-GAL4 as the driver, which drives transgene expression in tim-expressing and most cry-expressing cells15. We attempted rescue of magnetosensitive behavioural responses in the Cry loss-of-funtion cryb mutant background by expressing a UAS- Drosophila cry transgene. Magnetosensitivity was assessed using our behavioural assay in which flies experience an electric coil-generated magnetic field and display their behavioural responses in a binary-choice T-maze housed in an illuminated black box1. The two-coil system produces a magnetic field on one side of the T-maze, while producing no field on the opposite side. Flies were tested for either their response to the magnetic field in the naïve state or after a training session in which the field was paired with a sucrose reward. Response to the magnetic field was expressed as a Preference Index (PI), with negative values representing an avoidance of the field and positive values a preference for the field. As previously described, wild-type Canton-S flies expressing endogenous Cry show differential behavioural responses to the magnet in the T-maze – a naïve avoidance of the field and a trained preference for the field (white and black bars, respectively; Fig. 1a, left bar set)1. This establishes a standard of behavioural responses for the transgenic studies.
Figure 1. Type 1 Crys rescue light-dependent magnetoreception in Cry-deficient flies.
a, A tim-GAL4 driven Drosophila transgene (tim-GAL4/UAS-dcry) rescues magnetic responses in cryb flies, similar to the responses of wild-type Canton-S flies, while tim-GAL4/+ or UAS-dcry/+ alone do not. Bars show preference index values for naïve (white) and trained (black) groups. Numbers represent groups tested. *, P<0.05; ***, P<0.001. Genotypes in parentheses: tim-GAL4/+ (y w; tim-GAL4/+; cryb), UAS-dcry/+ (y w; UAS-mycdcry/+; cryb), and tim-GAL4/UAS-dcry (y w; tim-GAL4/UAS-mycdcry; cryb).
b, A tim-GAL4 driven monarch (dp)cry1 transgene (tim-GAL4/ UAS-dpcry1) rescues magnetic responses in cryb flies, while tim-GAL4/+ or UAS-dpcry1/+ alone do not. Bars show preference index values for naïve (white) and trained (black) groups. **, P<0.01. Genotypes in parentheses: tim-GAL4/+ (y w; tim-GAL4/+; cryb), UAS-dpcry1/+ (y w; UAS-mycdpCry1#15b/+; cryb), and tim-GAL4/ UAS-dpcry1.
c, Irradiance curves for different light conditions. Light measurements were taken from inside the training and test tube. Full-spectrum and >420 nm irradiance curves were reported before1.
d, Wavelength-dependence of magnetic responses is rescued by monarch (dp)Cry1 (y w; tim-GAL4/UAS-mycdpCry1#15b; cryb). The full-spectrum data are the same as those depicted in b. Bars show preference index values for naïve (white) and trained (black) groups. *, P<0.05, **, P<0.01.
Values from a, b, and d are mean ± s.e.m.
Predictably, Cry-deficient cryb lines expressing either the tim-GAL4 driver alone or the Drosophila cry transgene alone did not respond to the magnetic field (Fig. 1a, middle two bars sets). However, cryb flies expressing the Drosophila cry transgene under control of the tim-GAL4 driver now manifested significant naïve and trained responses to the field (tim-GAL4/UAS-dcry; Fig. 1a, right bar set), similar in both direction and magnitude to those of wild-type flies (Fig. 1a, left bar set). Thus, the tim-GAL4 driver expresses Cry appropriately so that it can rescue the magnetosensitive behavioural responses in the cryb background, making possible study of the biochemical mechanism through which animal Crys mediate magnetosensitivity.
We next used the tim-GAL4 driver in cryb flies for evaluating the functional roles of monarch butterfly Crys in magnetosensitivity. Monarchs have both a Type 1 Cry (designated Cry1) and a Type 2 Cry (designated Cry2). In vitro and in vivo studies have shown that they are functionally distinct16; Cry1 functions as a circadian photoreceptor, while Cry2 functions as a core clock component. Likewise, monarch Cry2 does not exhibit light sensitivity, at least in the assay systems studied so far10,11. Monarch butterfly Crys thus provide a unique opportunity to directly compare the functional equivalency for magnetosensitivity of Type 1 and Type 2 Crys from the same species in vivo, using transgenesis in Drosophila.
A monarch cry1 transgene driven by tim-GAL4 successfully restored both naïve and trained responses to the magnetic field in the cryb background (tim-GAL4/UAS-dpcry1; Fig. 1b). Furthermore, we showed that monarch Cry1 rescues magnetosensitivity in a wavelength-dependent manner (Fig. 1c and Fig. 1d). Similar to Drosophila Cry1, monarch Cry1-rescued flies exhibited significant naïve and trained responses to a magnetic field under full-spectrum light (~300–700 nm) and under UV-A/blue light (<420 nm), but the behavioural responses were abolished when only long wavelength light (>420 nm) was available. Thus, at the intensities used, light in the UV-A/blue range is both necessary and sufficient for monarch Cry1 to function in the Drosophila magnetoreception system. Together, these results indicate that Type 1 Crys play a key role in the magnetic transduction pathway of Drosophila and provide evidence for the functional equivalency of Type 1 Crys in magnetoreception across insect species.
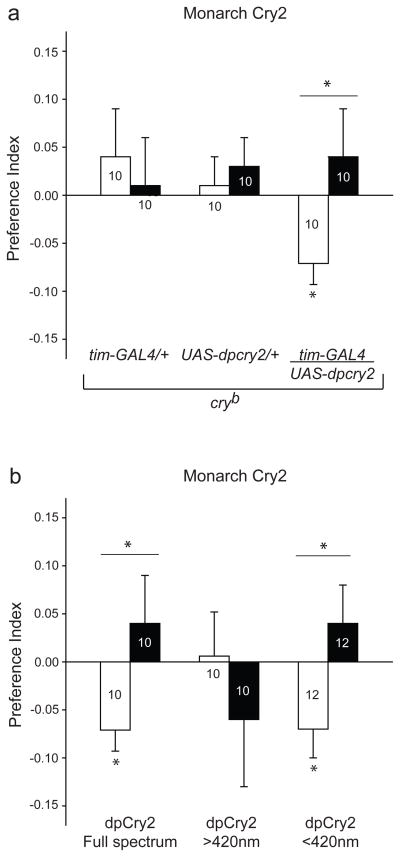
Remarkably, a monarch cry2 transgene driven by tim-GAL4 also successfully restored both naïve and trained responses to the magnet in the cryb background (tim-GAL4/UAS-dpcry2; Fig. 2a) and did so in a wavelength-dependent manner similar to that of the monarch cry1 transgene (Fig. 2b). The reliance of monarch Cry2-induced magnetosensitivity on UV-A/blue light is consistent with the predicted spectral sensitivities of chicken Type 2 Crys, as measured from the embryonic iris ex vivo17. To our knowledge, these are the first genetic data to show that a Type 2 Cry can function in a light-dependent behavioural response; another transgenic study has suggested a light-induced proteolytic response for a Type 2 protein18. The light-dependence of magnetosensitivity in flies expressing monarch cry1 or cry2 transgenes suggests that both proteins undergo the photochemical reactions necessary for magnetic sensing.
Figure 2. Monarch Type 2 Cry rescues light-dependent magnetosensivitiy in Cry-deficient flies.
a, A tim-GAL4 driven monarch (dp)cry2 transgene (tim-GAL4/ UAS-dpcry2) rescues magnetic responses in cryb flies, while UAS-dpcry2/+ alone does not. The tim-GAL4/+ data are re-plotted from Fig. 1b. Bars show preference index values for naïve (white) and trained (black) groups. Numbers represent groups tested. *, P<0.05. Genotypes in parentheses: tim-GAL4/+ (y w; tim-GAL4/+; cryb), UAS-dpcry2/+ (y w; UAS- mycdpCry2#125a/+; cryb), and tim-GAL4/ UAS-dpcry2 (y w; tim-GAL4/UAS- mycdpCry2#125a; cryb).
b, Wavelength-dependence of magnetic responses is rescued by monarch (dp)Cry2 (y w; tim-GAL4/UAS- mycdpCry2#125a; cryb). The full spectrum data are the same that those depicted in a. Bars show preference index values for naïve (white) and trained (black) groups. *, P<0.05. Values from a and b are mean ± s.e.m.
The widely held radical pair model for Cry-mediated magnetoreception is derived from the biochemical reactions proposed for the photocycle of plant Crys6. In this model, magnetically sensitive radical pair products are generated when the fully oxidized flavin adenine dinucleotide (FAD) chromophore is photoreduced through a sequence of intraprotein electron transfers along a chain of three tryptophan residues; these three residues are conserved in photolyases and Crys (the so-called ‘trp triad’; Supplementary Fig. S1)6. It is well established that blue light in the 450 nm range is sufficient for the photoreduction of oxidized flavin and the generation of trp triad-mediated radicals 6,12,18,19,20. The lack of a magnetic response for both Type 1 and Type 2 Crys under wavelengths >420 nm suggests that neither type signals through the photoreduction of oxidized flavin. The results further imply that the photoactivation of either Cry type for magneto-sensing does not depend on the presence of trp triad-mediated radical pairs.
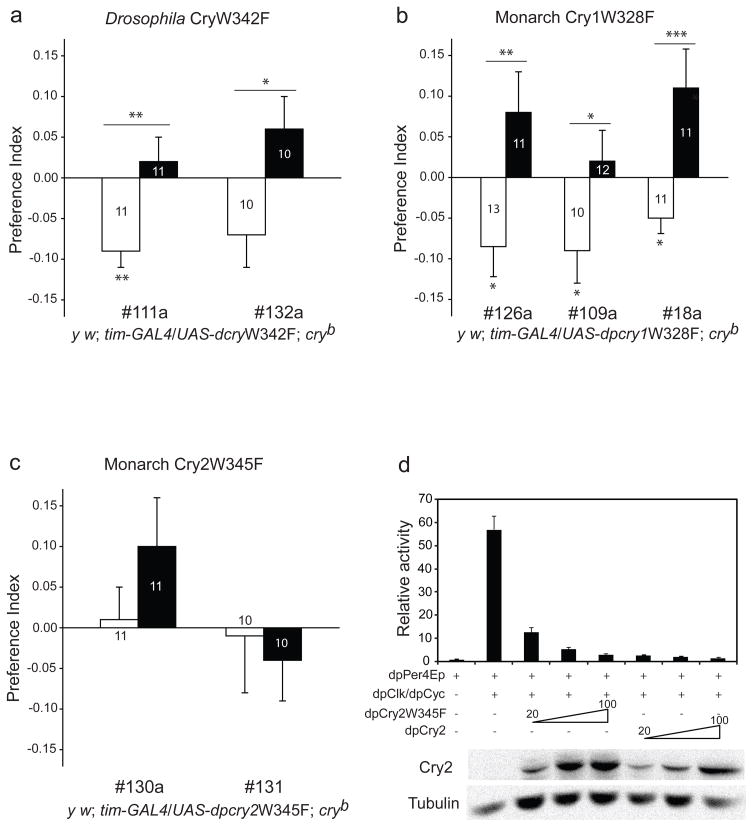
We directly tested whether the trp triad pathway is necessary for Cry-mediated magnetoreception by mutating the terminal tryptophan (W) to phenylalanine (F) in Drosophila Cry (W342F, two independent UAS lines) as well as in monarch Cry1 (W328F, three independent lines) and in monarch Cry2 (W345F, two independent lines). Magnetosensitivity was then assessed in cryb flies expressing each of the trp-mutated Crys as UAS transgenes under control of the tim-GAL4 driver. These mutations were chosen because previous biochemical studies have shown that mutating this tryptophan in an identical way in Drosophila Cry and in monarch Cry1 blocks photoreduction of oxidized FAD in vitro12,20, thus preventing the generation of trp triad-mediated radical pair products. No such biochemical data exist for any Type 2 Cry protein.
Mutating the terminal tryptophan in Drosophila Cry and monarch Cry1 did not affect the ability of transgenic flies to respond to the magnetic field, as lines of both tim-GAL4/UAS-dcryW342F and tim-GAL4/UAS-dpcry1W328F flies exhibited appropriate, significant naïve and trained responses in the cryb background (Fig. 3a and Fig. 3b), as expected based on the lack of magnetic sensing under >420 nm light. Notably, the trp triad is important but not essential for Drosophila Cry to function in the circadian system, because the trp mutant trangenes modestly rescued circadian photoreceptive responses in cryb flies (Supplementary Fig. S2). Although other ways of generating radical pairs in Cry have been proposed, for example through superoxides, all described radical pair mechanisms depend on a functional trp triad6,21,22. Therefore, radical pairs generated through those mechanisms are not necessary for magnetoreception through Type 1 Crys.
Figure 3. Effects of terminal tryptophan mutations on Type 1 and Type 2 Cry-mediated magnetosensitivity.
a, The Drosophila CryW342F mutation rescues magnetosensitive responses. Two mutant lines (111a and 132a) of y w; tim-GAL4/UAS-dcryW342F; cryb were tested. Bars show preference index values for naïve (white) and trained (black) groups. Numbers represent groups tested. *, P<0.05, **, P<0.01.
b, The monarch Cry1W328F mutation rescues magnetosensitive responses. Three mutant lines (126a, 109a, and 18a) of y w; tim-GAL4/UAS-dpcry1W328F; cryb were tested. Bars show preference index values for naïve (white) and trained (black) groups. Numbers represent groups tested. *, P<0.05, **, P<0.01; ***, P<0.001.
c, The monarch Cry2W345F mutation does not rescue magnetosensitive responses. Two mutant lines (130a and 131) of y w; tim-GAL4/ UAS-dpcry2W345F; cryb were tested. Bars show preference index of the naïve (white) and trained (black) groups. Numbers represent groups tested. For a–c, none of the UAS-trp mutant/+ lines (without driver) restored magnetosensitive behavioural responses (data not shown). Values from a–c are mean ± s.e.m. All Cry mutations were sequenced and confirmed from all of the different transgenic lines used.
d, Cry2W345F still inhibits Clock:Cycle-mediated transcription in Schneider 2 cells. The dpPerEp reporter (20 ng) was tested in presence (+) or absence (−) of dpClk/dpCyc expression plasmids (10 ng each); wild-type dpCry2 (20, 50 and 100 ng) or dpCry2W345F (20, 50 and 100 ng) were used10,11. Luciferase activity relative to β-galactosidase activity was computed. Lower, representative western blot of Cry2 (dpCry2 or dpCry2W345F) and tubulin expression. Values are mean ± s.e.m for three independent experiments.
Another possibility is that there are light-sensitive biochemical reactions for magnetosensing that involve Type 1 Crys but do not require a functional trp triad, such as those that may occur in a photolyase-like photocycle12,20. In this photocycle, UV-A/blue light (<420 nm) induces reduced flavin to initiate electron transfer to an unknown substrate that generates a radical and initiates the signal; back transfer turns off the signal23. Whether this photocycle could generate radical pairs appropriate for magnetoreception warrants consideration.
Surprisingly, magnetosensitivity was not restored in either of the two transgenic lines expressing the monarch Cry2 trp mutation (tim-GAL4/UAS-dpcry2W345F) in the cryb background (Fig. 3c), even though protein expression was stable (Supplemental Fig. S3). The Cry2 trp mutation did alter the ability of the protein to function as a transcriptional repressor, although the mutant protein could still clearly repress Clock:Cycle-mediated transcription in a dose-dependent manner in cell culture (Fig. 3d). Because monarch Cry2 cannot rescue magnetic sensing under >420 nm light (Fig. 2b), the lack of a magnetic response in Cry2 trp mutant flies under full-spectrum light is unlikely caused by the absence of radical pairs generated via the trp-triad pathway. Rather, the Cry2 trp mutation may have caused a nonspecific effect on magnetosensing function due to protein misfolding. Thus, Cry2 may use a photochemical mechanism common to both Type 1 and Type 2 Cry, such as the photolyase-like photocycle mentioned above. Alternatively, Cry2 could use a photochemical mechanism that is dependent on the terminal trp, but independent of trp triad-generated radical pairs.
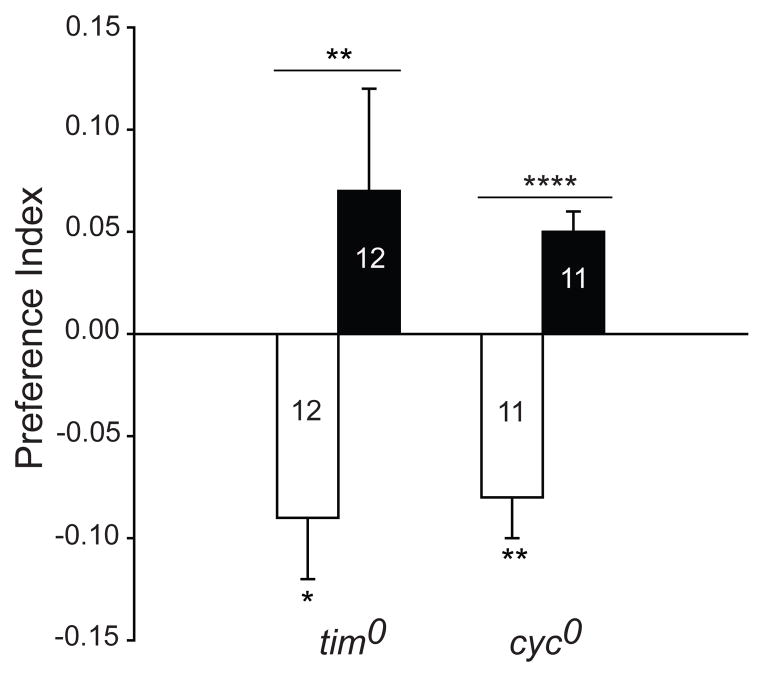
A recent report described a Cry-mediated effect of magnetic fields on circadian period in Drosophila studied under constant light24. In the circadian system of Drosophila, Cry interacts with the key clock protein Timeless (Tim) in a light-dependent manner, leading to its degradation and subsequent effects on circadian period25. Thus, this reported light-induced magnetosensitive effect of Cry on circadian period is entirely dependent on its interaction with Tim. It is important to note that the behavioural responses to a magnetic field in the present study do not depend on Tim; Tim-deficient tim0 mutant flies showed robust naïve and trained responses to the magnetic field in the T-maze (Fig. 4).
Figure 4. The clock proteins Tim and Cyc are not required for Drosophila magnetoreception.
Naïve and trained responses to a magnetic field are not impaired in either Tim-deficient tim0 mutant flies or Cyc-deficient cyc0 flies. Bars show preference index values for naïve (white) and trained (black) groups. Numbers represent groups tested. Values are mean ± s.e.m. *, P<0.05; **, P<0.01; ****, P<0.0001.
Our tim0 results also show that light-dependent magnetoreception through Type 1 Crys is not mediated through photochemical interactions between Cry and Tim. In addition, other core clock proteins are not likely involved in magnetoreception. Indeed, flies deficient in the essential clockwork transcription factor Cycle (homozygous cyc0 mutant flies26) also showed a strong response to the magnetic field (Fig. 4), and we have shown previously that disrupting the molecular clock under constant light conditions does not affect magnetosensitivity in wild-type Canton-S flies1.
Our results advance the field of magnetoreception in animals by strongly suggesting that both Type 1 and Type 2 Crys can function in magnetosensitive responses without generating radical pairs via the trp triad, which is widely thought to be the critical element of a Cry-based magnetoreception mechanism6,7,9. The future challenge will be understanding more precisely how magnetosensitive chemical reactions are actually generated, and determining how, once generated, they are transduced into neural signals that lead to behavioural responses. It is noteworthy that a recent study suggests that light-activated Drosophila Cry can directly modulate ion channel activity in fly brain, independent of its light effects on the molecular clock mechanism27. Although more work is needed to address the mechanistic details of this possibility, it is an exciting way in which magnetosensitive Cry of either type could transduce directional information to the brain for behavioural responses.
Our foray into animal magnetoreception was initiated on the idea that monarch butterflies, like other long-distance migrants, used several navigational strategies, including magnetoreception28. Indeed, our transgenic results now show that both types of monarch Cry proteins have the molecular capacity to transduce magnetic information, and the hunt is now on for a light-sensitive behavioural correlate of magnetosensitivity in migrating monarchs. This study highlights how the unique biology of monarch butterflies continues to advance our understanding of general biological principles.
METHODS SUMMARY
Fly stocks were raised on standard cornmeal/agar medium at 25°C and 60% relative humidity under a 12 h light:12 h dark lighting cycle. The cryb, UAS-dcry (UAS-mycdcry), tim0, and cyc0 lines were a gift from Patrick Emery. The generation of UAS-dpcry1 (UAS-mycdpCry1#15b) and UAS-dpcry2 (UAS-mycdpCry2#125a) lines were previously described16. The trp mutant lines were generated as described in METHODS. The tim-GAL4 (tim-GAL4/CyO29) driver line was used for all Drosophila and monarch transgene expression in cryb flies. The apparatus and behavioural assay used to test for magnetosensitivity in flies has been described1. The apparatus consisted of a choice chamber and an illuminated black box containing the two-coil system connected to an adjustable DC power supply. The two-coil system allows for the production of a magnetic field on one side while producing no field on the opposite side and alternation of the field between sides. Coils were positioned at 45° to the horizontal. We adjusted the power supply so that the magnetic field intensity ranged from 0.1G at the tube entrance to 5G at the end of the tube. For each population of flies tested (100–150 individuals), we calculated a Preference Index (PI) value based on the following equation: (PM−0.5)/[(PM +0.5) −(PM)], where PM is the proportion of flies on the magnetic field side of the T-maze. To test if flies responded to the experimental magnetic field, we either used a Student’s t-test to compare PI values between trained and naïve groups or a one-sample t-test to compare PI values to zero (i.e., PI value expected with no response to the magnetic field).
METHODS
Fly lines
For generating UAS-dcryW342F transgenic lines, the dcry open reading frame containing a W342F mutation was PCR amplified from pAC5.1V5/His-dcryW342F and subcloned into the XhoI and XbaI sites of the pUAST vector. To generate the UAS-dpcry1W328F and UAS-dpcry2W345F transgenic lines, the tryptophan to phenylalanine mutations were introduced into the pUAST-dpcry1 and pUAST-dpcry2 constructs, respectively, using the Stratagene QuikChange II XL Site-Directed Mutagenesis kit. The mutated open reading frames were PCR amplified and subcloned into the KpnI and XbaI sites of new pUAST vectors to ensure that there were no vector mutations. All constructs contain a full Kozak sequence. All constructs were sequenced prior to injection into w1118 embryos by Genetic Services. During balancing, the w1118 X chromosome was replaced with the y w-containing chromosome.
Behavioural apparatus
The choice chamber was comprised of a one-tube training section, a two-tube choice section (T-maze), and an elevator section to transfer flies between training and T-maze sections. The housing box was constructed such that the choice chamber could be placed between the two coils in either an upright position (used during training) or a horizontal position (used during testing). The box containing the coil system was open on the top so that the chamber, regardless of position, could be illuminated by one ZooMed Reptisun 10.0 UVB fluorescent tube (F20T12) and one Agrobrite full spectrum fluorescent grow tube. Wavelength-dependence of magnetosensitivity was examined by either covering the top of the box with a long-wavelength filter that transmitted wavelengths of light > 420 nm (E400 and E420 from Gentex, Carbondale PA) or using a General Electric Black Light 20 to transmit wavelengths < 420nm.
Behavioural procedure
Flies were starved for 18 hours prior to testing their magnetic response. All experiments were performed between 8:00 and 12:00 EST. Flies in the Trained Group were moved into the training section facing one of two adjustable coils with no field for 2 minutes and then were reloaded into the training tube containing sucrose reinforcement and a magnetic field for an additional 2 minutes. After being held for 1 minute in the elevator section, flies were tested for their magnetic preference by transferring them to the T-maze section and allowing them to choose between the sides with or without a magnetic field for 2 minutes. Flies in the Naïve Group were loaded into the elevator section and immediately transferred to the T-maze and allowed to choose between the sides with or without a magnetic field for 2 minutes. Trained and Naïve Groups were tested consecutively and with the magnetic field on the same side. As an additional control for side preferences independent of magnetic stimuli, we alternated the side of the T-maze containing the field after each consecutive set of trained and naïve flies.
Supplementary Material
Acknowledgments
We thank Quan Yuan for performing the assays in Figure 3d and the cryptochrome alignments in Figure S1. We thank Patrick Emery, Christine Merlin and David R. Weaver for discussions. This work was supported by a grant from the NIH.
Footnotes
Supplemental Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions. All authors contributed to experimental design, execution, data analysis and writing the paper.
References
- 1.Gegear RJ, Casselman A, Waddell S, Reppert SM. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature. 2008;454:1014–U1061. doi: 10.1038/nature07183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiltschko W, Wiltschko R. Magnetic orientation and magnetoreception in birds and other animals. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 2005;191:675–693. doi: 10.1007/s00359-005-0627-7. [DOI] [PubMed] [Google Scholar]
- 3.Lohmann KJ, Lohmann CMF, Putman NF. Magnetic maps in animals: nature’s GPS. Journal of Experimental Biology. 2007;210:3697–3705. doi: 10.1242/jeb.001313. [DOI] [PubMed] [Google Scholar]
- 4.Wiltschko R, Ritz T, Stapput K, Thalau P, Wiltschko W. Two different types of light-dependent responses to magnetic fields in birds. Current Biology. 2005;15:1518–1523. doi: 10.1016/j.cub.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 5.Phillips JB, Borland SC. Wavelength Specific Effects of Light on Magnetic Compass Orientation of the Eastern Red-Spotted Newt Notophthalmus-Viridescens. Ethology Ecology & Evolution. 1992;4:33–42. [Google Scholar]
- 6.Rodgers CT, Hore PJ. Chemical magnetoreception in birds: The radical pair mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:353–360. doi: 10.1073/pnas.0711968106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophysical Journal. 2000;78:707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda K, et al. Chemical compass model of avian magnetoreception. Nature. 2008;453:387–390. doi: 10.1038/nature06834. [DOI] [PubMed] [Google Scholar]
- 9.Mouritsen H, Ritz T. Magnetoreception and its use in bird navigation. Current Opinion in Neurobiology. 2005;15:406–414. doi: 10.1016/j.conb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Zhu HS, et al. The two CRYs of the butterfly. Current Biology. 2005;15:R953–R954. doi: 10.1016/j.cub.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Yuan Q, Metterville D, Briscoe AD, Reppert SM. Insect cryptochromes: Gene duplication and loss define diverse ways to construct insect circadian clocks. Molecular Biology and Evolution. 2007;24:948–955. doi: 10.1093/molbev/msm011. [DOI] [PubMed] [Google Scholar]
- 12.Oeztuerk N, Song SH, Selby CP, Sancar A. Animal type 1 cryptochromes - Analysis of the redox state of the flavin cofactor by site-directed mutagenesis. Journal of Biological Chemistry. 2008;283:3256–3263. doi: 10.1074/jbc.M708612200. [DOI] [PubMed] [Google Scholar]
- 13.vanVickle-Chavez SJ, van Gelder RN. Action spectrum of Drosophila cryptochrome. Journal of Biological Chemistry. 2007;282:10561–10566. doi: 10.1074/jbc.M609314200. [DOI] [PubMed] [Google Scholar]
- 14.Ritz T, Dommer DH, Phillips JB. Shedding light on vertebrate magnetoreception. Neuron. 2002;34:503–506. doi: 10.1016/s0896-6273(02)00707-9. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko M, Hall JC. Neuroanatomy of cells expressing clock genes in Drosophila: Transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. Journal of Comparative Neurology. 2000;422:66–94. doi: 10.1002/(sici)1096-9861(20000619)422:1<66::aid-cne5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhu HS, et al. Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. Plos Biology. 2008;6:138–155. doi: 10.1371/journal.pbio.0060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu DC, Batten ML, Palczewski K, Van Gelder RN. Nonvisual photoreception in the chick iris. Science. 2004;306:129–131. doi: 10.1126/science.1101484. [DOI] [PubMed] [Google Scholar]
- 18.Hoang N, et al. Human and Drosophila cryptochromes are light activated by flavin photoreduction in living cells (vol 6, pg e160, 2008) Plos Biology. 2008;6:1811–1811. doi: 10.1371/journal.pbio.0060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berndt A, et al. A novel photoreaction mechanism for the circadian blue light photoreceptor Drosophila cryptochrome. Journal of Biological Chemistry. 2007;282:13011–13021. doi: 10.1074/jbc.M608872200. [DOI] [PubMed] [Google Scholar]
- 20.Song SH, et al. Formation and function of flavin anion radical in cryptochrome 1 blue-light photoreceptor of monarch butterfly. Journal of Biological Chemistry. 2007;282:17608–17612. doi: 10.1074/jbc.M702874200. [DOI] [PubMed] [Google Scholar]
- 21.Solov’yov IA, Schulten K. Magnetoreception through Cryptochrome May Involve Superoxide. Biophysical Journal. 2009;96:4804–4813. doi: 10.1016/j.bpj.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogben HJ, Efimova O, Wagner-Rundell N, Timmel CR, Hore PJ. Possible involvement of superoxide and dioxygen with cryptochrome in avian magnetoreception: Origin of Zeeman resonances observed by in vivo EPR spectroscopy. Chem Phys Lett. 2009;480:118–122. [Google Scholar]
- 23.Ozturk N, et al. Cold Spring Harbor Lab Press. Publications Dept; pp. 119–131. [Google Scholar]
- 24.Yoshii T, Ahmad M, Helfrich-Forster C. Cryptochrome Mediates Light-Dependent Magnetosensitivity of Drosophila’s Circadian Clock. Plos Biology. 2009;7:813–819. doi: 10.1371/journal.pbio.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanewsky R. Genetic analysis of the circadian system in Drosophila melanogaster and mammals. Journal of Neurobiology. 2003;54:111–147. doi: 10.1002/neu.10164. [DOI] [PubMed] [Google Scholar]
- 26.Rutila JE, et al. CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell. 1998;93:805–814. doi: 10.1016/s0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- 27.Sheeba V, Gu H, Sharma VK, O’Dowd DK, Holmes TC. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of drosophila circadian pacemaker neurons. J Neurophysiol. 2008;99:976–988. doi: 10.1152/jn.00930.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reppert SM. A colorful model of the circadian clock. Cell. 2006;124:233–236. doi: 10.1016/j.cell.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.