Abstract
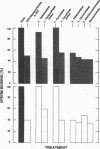
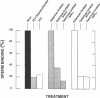
During fertilization in mice, zona pellucida glycoprotein ZP3 mediates initial sperm-egg interactions by serving as receptor for sperm. Purified egg ZP3, as well as ZP3-derived O-linked oligosaccharides, exhibit sperm receptor activity in vitro. We report that treatment of purified egg ZP3 and ZP3-derived O-linked oligosaccharides with either alpha-galactosidase or galactose oxidase results in loss of sperm receptor activity. In the latter case, sperm receptor activity can be restored to the oxidized glycoprotein and O-linked oligosaccharides by treatment with sodium borohydride. We conclude that galactose, located in alpha-linkage at the nonreducing terminus of O-linked oligosaccharides, is at least one of the sugar determinants on ZP3 responsible for binding of sperm to the zona pellucida.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleil J. D., Wassarman P. M. Autoradiographic visualization of the mouse egg's sperm receptor bound to sperm. J Cell Biol. 1986 Apr;102(4):1363–1371. doi: 10.1083/jcb.102.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell. 1980 Jul;20(3):873–882. doi: 10.1016/0092-8674(80)90334-7. [DOI] [PubMed] [Google Scholar]
- Dodd J., Jessell T. M. Lactoseries carbohydrates specify subsets of dorsal root ganglion neurons projecting to the superficial dorsal horn of rat spinal cord. J Neurosci. 1985 Dec;5(12):3278–3294. doi: 10.1523/JNEUROSCI.05-12-03278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florman H. M., Bechtol K. B., Wassarman P. M. Enzymatic dissection of the functions of the mouse egg's receptor for sperm. Dev Biol. 1984 Nov;106(1):243–255. doi: 10.1016/0012-1606(84)90079-4. [DOI] [PubMed] [Google Scholar]
- Florman H. M., Wassarman P. M. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell. 1985 May;41(1):313–324. doi: 10.1016/0092-8674(85)90084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Klein L. E., Britten R. J., Davidson E. H. Sequence of mRNA coding for bindin, a species-specific sea urchin sperm protein required for fertilization. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8634–8638. doi: 10.1073/pnas.83.22.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Dodd J. Structure and expression of differentiation antigens on functional subclasses of primary sensory neurons. Philos Trans R Soc Lond B Biol Sci. 1985 Feb 19;308(1136):271–281. doi: 10.1098/rstb.1985.0027. [DOI] [PubMed] [Google Scholar]
- Kao K. J., Pizzo S. V., McKee P. A. Factor VIII/von Willebrand protein. Modification of its carbohydrate causes reduced binding to platelets. J Biol Chem. 1980 Nov 10;255(21):10134–10139. [PubMed] [Google Scholar]
- Lambert H. Role of sperm-surface glycoproteins in gamete recognition in two mouse species. J Reprod Fertil. 1984 Jan;70(1):281–284. doi: 10.1530/jrf.0.0700281. [DOI] [PubMed] [Google Scholar]
- Lis H., Jaffe C. L., Sharon N. Variations in the susceptibility of erythrocyte membrane glycolipids to galactose oxidase. FEBS Lett. 1982 Oct 4;147(1):59–63. doi: 10.1016/0014-5793(82)81011-9. [DOI] [PubMed] [Google Scholar]
- Lopez L. C., Bayna E. M., Litoff D., Shaper N. L., Shaper J. H., Shur B. D. Receptor function of mouse sperm surface galactosyltransferase during fertilization. J Cell Biol. 1985 Oct;101(4):1501–1510. doi: 10.1083/jcb.101.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa T., Yanagimachi R., Nicolson G. L. Wheat germ agglutinin blocks mammalian fertilization. Nature. 1973 Jan 26;241(5387):256–259. doi: 10.1038/241256a0. [DOI] [PubMed] [Google Scholar]
- Pink J. R. Changes in T-lymphocyte glycoprotein structures associated with differentiation. Contemp Top Mol Immunol. 1983;9:89–113. doi: 10.1007/978-1-4684-4517-6_3. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A. Carbohydrate-binding proteins: tertiary structures and protein-sugar interactions. Annu Rev Biochem. 1986;55:287–315. doi: 10.1146/annurev.bi.55.070186.001443. [DOI] [PubMed] [Google Scholar]
- Salzmann G. S., Greve J. M., Roller R. J., Wassarman P. M. Biosynthesis of the sperm receptor during oogenesis in the mouse. EMBO J. 1983;2(9):1451–1456. doi: 10.1002/j.1460-2075.1983.tb01607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmell E. D., Gulyas B. J. Mammalian sperm-egg recognition and binding in vitro. I. Specificity of sperm interactions with live and fixed eggs in homologous and heterologous inseminations of hamster, mouse, and guinea pig oocytes. Biol Reprod. 1980 Dec;23(5):1075–1085. doi: 10.1095/biolreprod23.5.1075. [DOI] [PubMed] [Google Scholar]
- Shalgi R., Matityahu A., Nebel L. The role of carbohydrates in sperm-egg interaction in rats. Biol Reprod. 1986 Apr;34(3):446–452. doi: 10.1095/biolreprod34.3.446. [DOI] [PubMed] [Google Scholar]
- Shur B. D., Hall N. G. A role for mouse sperm surface galactosyltransferase in sperm binding to the egg zona pellucida. J Cell Biol. 1982 Nov;95(2 Pt 1):574–579. doi: 10.1083/jcb.95.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacquier V. D., Moy G. W. Isolation of bindin: the protein responsible for adhesion of sperm to sea urchin eggs. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2456–2460. doi: 10.1073/pnas.74.6.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman P. M., Bleil J. D., Florman H. M., Greve J. M., Roller R. J., Salzmann G. S., Samuels F. G. The mouse egg's receptor for sperm: what is it and how does it work? Cold Spring Harb Symp Quant Biol. 1985;50:11–19. doi: 10.1101/sqb.1985.050.01.004. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Early events in mammalian fertilization. Annu Rev Cell Biol. 1987;3:109–142. doi: 10.1146/annurev.cb.03.110187.000545. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. The biology and chemistry of fertilization. Science. 1987 Jan 30;235(4788):553–560. doi: 10.1126/science.3027891. [DOI] [PubMed] [Google Scholar]
- Yurewicz E. C., Sacco A. G., Subramanian M. G. Structural characterization of the Mr = 55,000 antigen (ZP3) of porcine oocyte zona pellucida. Purification and characterization of alpha- and beta-glycoproteins following digestion of lactosaminoglycan with endo-beta-galactosidase. J Biol Chem. 1987 Jan 15;262(2):564–571. [PubMed] [Google Scholar]