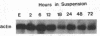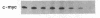Abstract
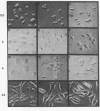
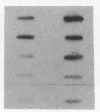
A methylcellulose suspension system that prevents cell-surface contact with the substrate was used to study the role of cell adhesion in the regulation of proliferation. The nonadhesive conditions established by suspension culture cause BALB/c 3T3 (A31) cells to enter a G0 state of growth arrest within 48 hr as defined by an inhibition of DNA synthesis and a suppression of c-myc and histone mRNA expression. The adhesion of these suspension-arrested cells rapidly induces c-fos, c-myc, and actin gene expression. This stimulation did not depend on the presence of serum since the adhesion of suspension-arrested cells, in the absence of serum, also induced the expression of c-fos and c-myc mRNAs. In addition, adhesion onto fibronectin increased the number of cells able to respond to epidermal growth factor and insulin and progress into S phase. These results indicate that adhesion of suspension-arrested cells activates the G0/G1 transition independent of growth factors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada K. M. Fibronectin. Adv Enzymol Relat Areas Mol Biol. 1987;59:1–57. doi: 10.1002/9780470123058.ch1. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell. 1980 Sep;21(2):365–372. doi: 10.1016/0092-8674(80)90473-0. [DOI] [PubMed] [Google Scholar]
- Benecke B. J., Ben-Ze'ev A., Penman S. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell. 1978 Aug;14(4):931–939. doi: 10.1016/0092-8674(78)90347-1. [DOI] [PubMed] [Google Scholar]
- Benecke B. J., Ben-Ze'ev A., Penman S. The regulation of RNA metabolism in suspended and reattached anchorage-dependent 3T6 fibroblasts. J Cell Physiol. 1980 May;103(2):247–254. doi: 10.1002/jcp.1041030209. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bond J. F., Farmer S. R. Regulation of tubulin and actin mRNA production in rat brain: expression of a new beta-tubulin mRNA with development. Mol Cell Biol. 1983 Aug;3(8):1333–1342. doi: 10.1128/mcb.3.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. F., Robinson G. S., Farmer S. R. Differential expression of two neural cell-specific beta-tubulin mRNAs during rat brain development. Mol Cell Biol. 1984 Jul;4(7):1313–1319. doi: 10.1128/mcb.4.7.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Burckhardt J., Curran T., Müller R. Expression of c-fos in NIH3T3 cells is very low but inducible throughout the cell cycle. EMBO J. 1986 Apr;5(4):695–700. doi: 10.1002/j.1460-2075.1986.tb04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Morreo G., Pardee A. B. Kinetics of G1 transit following brief starvation for serum factors. Exp Cell Res. 1984 Jun;152(2):459–466. doi: 10.1016/0014-4827(84)90647-5. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Regulation of cell growth and transformation by tyrosine-specific protein kinases: the search for important cellular substrate proteins. Curr Top Microbiol Immunol. 1983;107:125–161. doi: 10.1007/978-3-642-69075-4_4. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Kent R. B., Sonenshein G. E. Transcriptional activation of immunoglobulin alpha heavy-chain genes by translocation of the c-myc oncogene. 1983 Sep 29-Oct 5Nature. 305(5933):443–446. doi: 10.1038/305443a0. [DOI] [PubMed] [Google Scholar]
- Elder P. K., Schmidt L. J., Ono T., Getz M. J. Specific stimulation of actin gene transcription by epidermal growth factor and cycloheximide. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7476–7480. doi: 10.1073/pnas.81.23.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. R., Ben-Ze'av A., Benecke B. J., Penman S. Altered translatability of messenger RNA from suspended anchorage-dependent fibroblasts: reversal upon cell attachment to a surface. Cell. 1978 Oct;15(2):627–637. doi: 10.1016/0092-8674(78)90031-4. [DOI] [PubMed] [Google Scholar]
- Farmer S. R., Wan K. M., Ben-Ze'ev A., Penman S. Regulation of actin mRNA levels and translation responds to changes in cell configuration. Mol Cell Biol. 1983 Feb;3(2):182–189. doi: 10.1128/mcb.3.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Folkman J., Greenspan H. P. Influence of geometry on control of cell growth. Biochim Biophys Acta. 1975 Dec 31;417(3-4):211–236. doi: 10.1016/0304-419x(75)90011-6. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Herman B., Harrington M. A., Olashaw N. E., Pledger W. J. Identification of the cellular mechanisms responsible for platelet-derived growth factor induced alterations in cytoplasmic vinculin distribution. J Cell Physiol. 1986 Jan;126(1):115–125. doi: 10.1002/jcp.1041260116. [DOI] [PubMed] [Google Scholar]
- Herman B., Pledger W. J. Platelet-derived growth factor-induced alterations in vinculin and actin distribution in BALB/c-3T3 cells. J Cell Biol. 1985 Apr;100(4):1031–1040. doi: 10.1083/jcb.100.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst R., Horwitz A., Buck C., Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Ingber D. E., Madri J. A., Folkman J. Endothelial growth factors and extracellular matrix regulate DNA synthesis through modulation of cell and nuclear expansion. In Vitro Cell Dev Biol. 1987 May;23(5):387–394. doi: 10.1007/BF02620997. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey P., Ran W., Campisi J., Rosner M. R. Two independent growth factor-generated signals regulate c-fos and c-myc mRNA levels in Swiss 3T3 cells. J Biol Chem. 1987 Feb 5;262(4):1442–1445. [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Neill C. H., Riddle P. N., Jordan P. W. The relation between surface area and anchorage dependence of growth in hamster and mouse fibroblasts. Cell. 1979 Apr;16(4):909–918. doi: 10.1016/0092-8674(79)90106-5. [DOI] [PubMed] [Google Scholar]
- O'Neill C., Jordan P., Ireland G. Evidence for two distinct mechanisms of anchorage stimulation in freshly explanted and 3T3 Swiss mouse fibroblasts. Cell. 1986 Feb 14;44(3):489–496. doi: 10.1016/0092-8674(86)90470-8. [DOI] [PubMed] [Google Scholar]
- Otsuka H., Moskowitz M. Arrest of 3T3 cells in G1 phase in suspension culture. J Cell Physiol. 1975 Dec;87(2):213–219. doi: 10.1002/jcp.1040870209. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Schümperli D. Cell-cycle regulation of histone gene expression. Cell. 1986 May 23;45(4):471–472. doi: 10.1016/0092-8674(86)90277-1. [DOI] [PubMed] [Google Scholar]
- Stoker M., O'Neill C., Berryman S., Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968 Sep 15;3(5):683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Hamamori Y., Yamashita T., Fukumoto Y., Takai Y. Involvement of three intracellular messenger systems, protein kinase C, calcium ion and cyclic AMP, in the regulation of c-fos gene expression in Swiss 3T3 cells. FEBS Lett. 1986 Nov 10;208(1):39–42. doi: 10.1016/0014-5793(86)81527-7. [DOI] [PubMed] [Google Scholar]
- Tucker R. W., Butterfield C. E., Folkman J. Interaction of serum and cell spreading affects the growth of neoplastic and non-neoplastic fibroblasts. J Supramol Struct Cell Biochem. 1981;15(1):29–40. doi: 10.1002/jsscb.1981.380150104. [DOI] [PubMed] [Google Scholar]
- Verrier B., Müller D., Bravo R., Müller R. Wounding a fibroblast monolayer results in the rapid induction of the c-fos proto-oncogene. EMBO J. 1986 May;5(5):913–917. doi: 10.1002/j.1460-2075.1986.tb04303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittelsberger S. C., Kleene K., Penman S. Progressive loss of shape-responsive metabolic controls in cells with increasingly transformed phenotype. Cell. 1981 Jun;24(3):859–866. doi: 10.1016/0092-8674(81)90111-2. [DOI] [PubMed] [Google Scholar]