Abstract
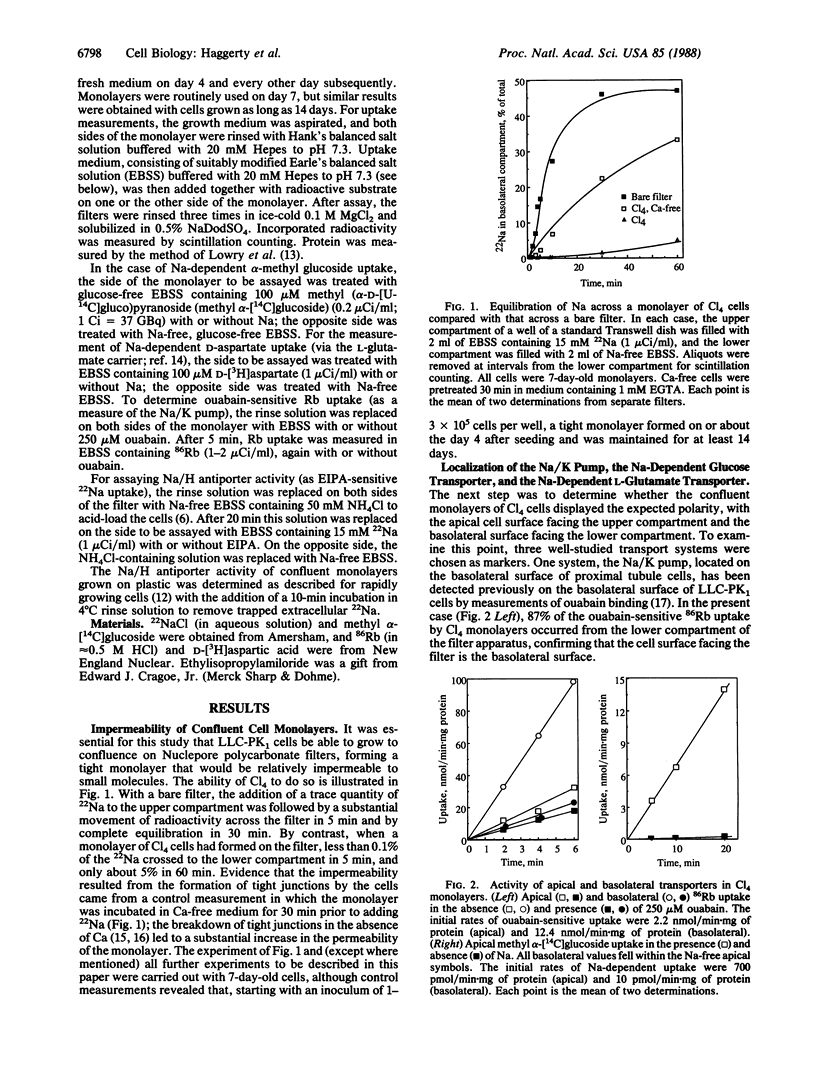
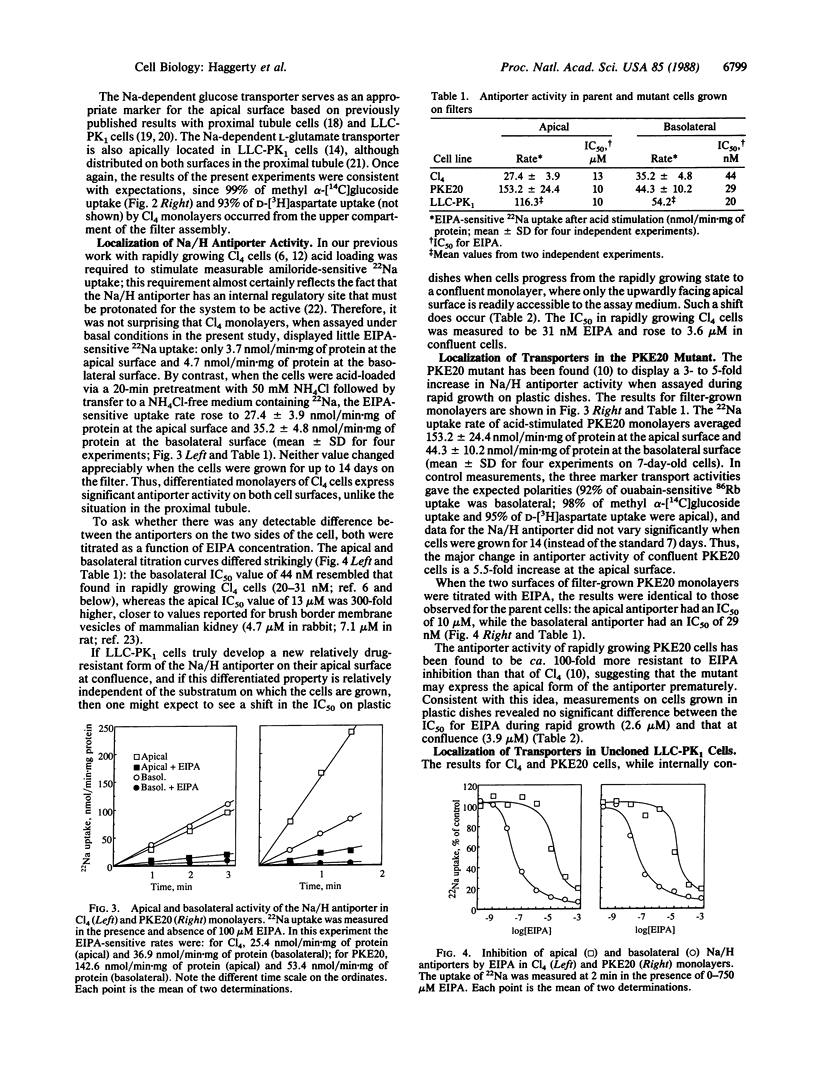
Proximal tubule cells of the kidney contain, on their apical surface, an amiloride-sensitive Na/H antiporter that functions in Na reabsorption and proton secretion. We have investigated the localization of the antiporter in a cloned cell line of porcine renal origin, LLC-PK1/Cl4, which is often considered to be a useful model of the proximal tubule. Transport measurements were performed with differentiated monolayers grown on Nuclepore filters, permitting independent access to the apical and basolateral cell surfaces. In control experiments with LLC-PK1/Cl4 monolayers, three marker transport systems showed the expected polarity: 87% of ouabain-sensitive Rb uptake was at the basolateral surface, and 99% of Na-dependent alpha-methylglucoside transport and 93% of Na-dependent D-aspartate (L-glutamate) transport were at the apical surface. By contrast, the monolayers displayed significant Na/H antiporter activity (assayed as ethylisopropylamiloride-sensitive 22Na uptake) at both cell surfaces, with an apical uptake rate amounting to 44% and a basolateral rate amounting to 56% of the total. Significantly, the apical and basolateral antiporters could readily be distinguished from one another on the basis of ethylispropylamiloride sensitivity. The apical system had an IC50 of 13 microM, close to that reported for kidney brush border vesicle preparations, whereas the basolateral system had an IC50 of 44 nM, similar to values seen in undifferentiated LLC-PK1 cells and other cultured cell lines. The PKE20 mutant, previously selected from LLC-PK1/Cl4 on the basis of resistance to ethylisopropylamiloride, was found to overexpress the more resistant antiporter both during rapid growth and on its apical cell surface at confluence; normal amounts of the more sensitive antiporter were seen on the basolateral surface of confluent PKE20 cells. Taken together, these results suggest that there are two distinct forms of the Na/H antiporter, which are under separate genetic control.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal N., Haggerty J. G., Adelberg E. A., Slayman C. W. Isolation and characterization of a Na-H antiporter-deficient mutant of LLC-PK1 cells. Am J Physiol. 1986 Nov;251(5 Pt 1):C825–C830. doi: 10.1152/ajpcell.1986.251.5.C825. [DOI] [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Cantiello H. F., Scott J. A., Rabito C. A. Polarized distribution of the Na+/H+ exchange system in a renal cell line (LLC-PK1) with characteristics of proximal tubular cells. J Biol Chem. 1986 Mar 5;261(7):3252–3258. [PubMed] [Google Scholar]
- Friedrich T., Sablotni J., Burckhardt G. Species differences between rat and rabbit renal Na+/H+ exchangers. Biochem Biophys Res Commun. 1987 Apr 29;144(2):869–875. doi: 10.1016/s0006-291x(87)80045-1. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Gstraunthaler G., Pfaller W., Kotanko P. Biochemical characterization of renal epithelial cell cultures (LLC-PK1 and MDCK). Am J Physiol. 1985 Apr;248(4 Pt 2):F536–F544. doi: 10.1152/ajprenal.1985.248.4.F536. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986 Feb;102(2):457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty J. G., Cragoe E. J., Jr, Slayman C. W., Adelberg E. A. Na+/H+ exchanger activity in the pig kidney epithelial cell line, LLC-PK1: inhibition by amiloride and its derivatives. Biochem Biophys Res Commun. 1985 Mar 29;127(3):759–767. doi: 10.1016/s0006-291x(85)80008-5. [DOI] [PubMed] [Google Scholar]
- Hull R. N., Cherry W. R., Weaver G. W. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro. 1976 Oct;12(10):670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- Ives H. E., Yee V. J., Warnock D. G. Asymmetric distribution of the Na+/H+ antiporter in the renal proximal tubule epithelial cell. J Biol Chem. 1983 Nov 25;258(22):13513–13516. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahnensmith R. L., Aronson P. S. The plasma membrane sodium-hydrogen exchanger and its role in physiological and pathophysiological processes. Circ Res. 1985 Jun;56(6):773–788. doi: 10.1161/01.res.56.6.773. [DOI] [PubMed] [Google Scholar]
- Martinez-Palomo A., Meza I., Beaty G., Cereijido M. Experimental modulation of occluding junctions in a cultured transporting epithelium. J Cell Biol. 1980 Dec;87(3 Pt 1):736–745. doi: 10.1083/jcb.87.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. W., Macknight A. D., Dayer J. M., Ausiello D. A. Localization of [3H]ouabain-sensitive Na+ pump sites in cultured pig kidney cells. Am J Physiol. 1979 Mar;236(3):C157–C162. doi: 10.1152/ajpcell.1979.236.3.C157. [DOI] [PubMed] [Google Scholar]
- Misfeldt D. S., Sanders M. J. Transepithelial transport in cell culture: D-glucose transport by a pig kidney cell line (LLC-PK1). J Membr Biol. 1981 Mar 15;59(1):13–18. doi: 10.1007/BF01870816. [DOI] [PubMed] [Google Scholar]
- Montrose M. H., Friedrich T., Murer H. Measurements of intracellular pH in single LLC-PK1 cells: recovery from an acid load via basolateral Na+/H+ exchange. J Membr Biol. 1987;97(1):63–78. doi: 10.1007/BF01869615. [DOI] [PubMed] [Google Scholar]
- Moran A. Sodium-hydrogen exchange system in LLC-PK1 epithelium. Am J Physiol. 1987 Jan;252(1 Pt 1):C63–C67. doi: 10.1152/ajpcell.1987.252.1.C63. [DOI] [PubMed] [Google Scholar]
- Rabito C. A., Karish M. V. Polarized amino acid transport by an epithelial cell line of renal origin (LLC-PK1). The apical systems. J Biol Chem. 1983 Feb 25;258(4):2543–2547. [PubMed] [Google Scholar]
- Rabito C. A. Localization of the Na+-sugar cotransport system in a kidney epithelial cell line (LLC PK1). Biochim Biophys Acta. 1981 Dec 7;649(2):286–296. doi: 10.1016/0005-2736(81)90417-x. [DOI] [PubMed] [Google Scholar]
- Sacktor B., Rosenbloom I. L., Liang C. T., Cheng L. Sodium gradient- and sodium plus potassium gradient-dependent L-glutamate uptake in renal basolateral membrane vesicles. J Membr Biol. 1981 May 15;60(1):63–71. doi: 10.1007/BF01870833. [DOI] [PubMed] [Google Scholar]
- Silverman M., Black J. High affinity phlorizin receptor sites and their relation to the glucose transport mechanism in the proximal tubule of dog kidney. Biochim Biophys Acta. 1975 Jun 11;394(1):10–30. doi: 10.1016/0005-2736(75)90201-1. [DOI] [PubMed] [Google Scholar]
- Vigne P., Frelin C., Cragoe E. J., Jr, Lazdunski M. Ethylisopropyl-amiloride: a new and highly potent derivative of amiloride for the inhibition of the Na+/H+ exchange system in various cell types. Biochem Biophys Res Commun. 1983 Oct 14;116(1):86–90. doi: 10.1016/0006-291x(83)90384-4. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Cragoe E. J., Jr, Shaikewitz T., Glaser L., Cassel D. Characterization of potent Na+/H+ exchange inhibitors from the amiloride series in A431 cells. Biochemistry. 1984 Sep 11;23(19):4481–4488. doi: 10.1021/bi00314a038. [DOI] [PubMed] [Google Scholar]



