Abstract
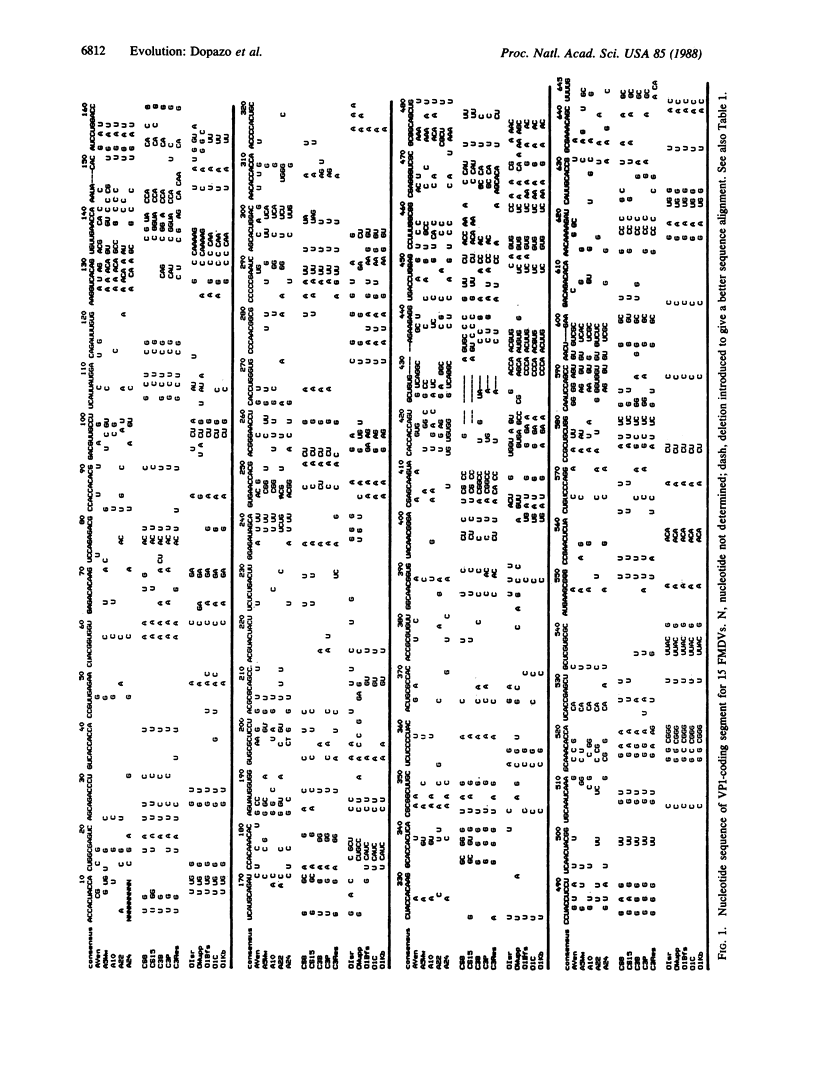
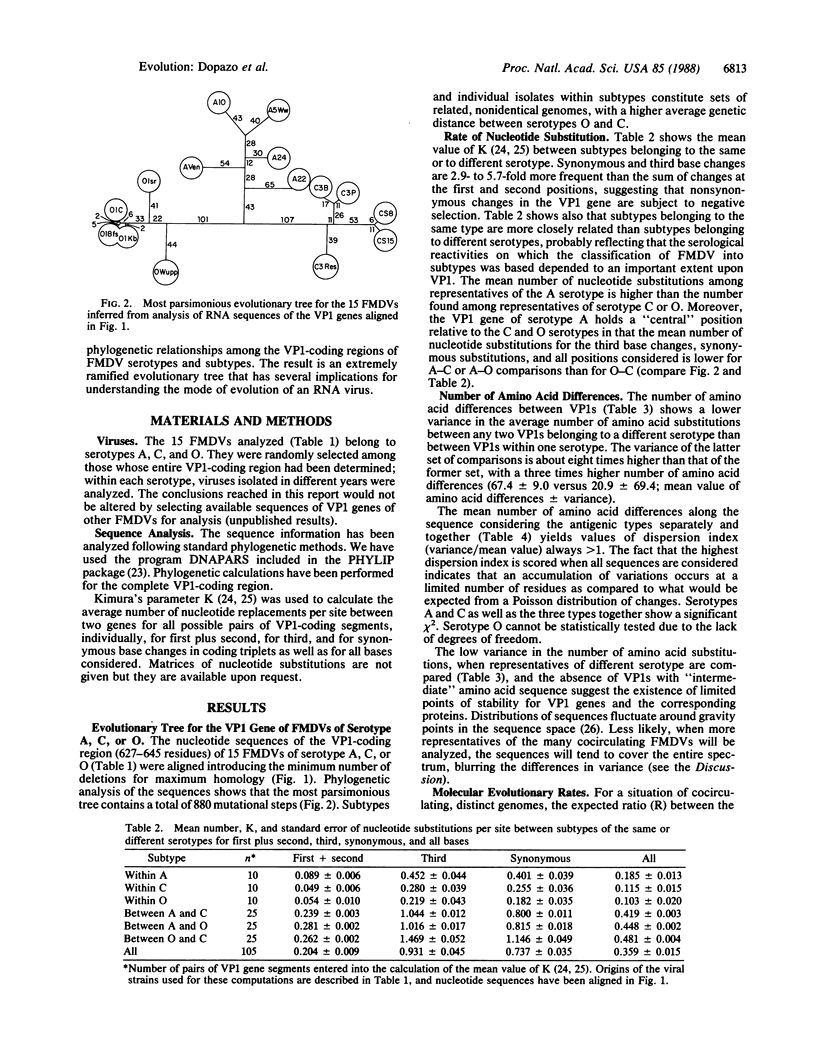
A phylogenetic tree relating the VP1 gene of 15 isolates of foot-and-mouth disease virus (FMDV) of serotypes A, C, and O has been constructed. The most parsimonious tree shows that FMDV subtypes and isolates within subtypes constitute sets of related, nonidentical genomes, in agreement with a quasispecies mode of evolution of this virus. The average number of nucleotide replacements per site for all possible pairs of VP1 coding segments is higher among representatives of serotype A than serotype C or O. In comparing amino acid sequences, the values of dispersion index (variance/mean value) are greater than 1, with the highest values scored when all sequences are considered. This indicates an accumulation of mutations at a limited number of residues, suggesting that distributions of sequences fluctuate around points of high stability. Evolution of FMDV follows a path very distant from that of a star phylogeny, and it has not been possible to derive conclusions on constancy of evolutionary rates with the test applied to the analysis. FMDVs, as other RNA viruses, are of limited genetic complexity and their population sizes are extremely large. Their evolution concerns complex, indeterminate mixtures of genomes rather than a single, determinate species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. C., Underwood B. O., Brown F., Ngichabe C. K. Variation in foot-and-mouth disease virus isolates in Kenya: an examination of field isolates by T1 oligonucleotide fingerprinting. Vet Microbiol. 1985 Aug;10(5):409–423. doi: 10.1016/0378-1135(85)90023-9. [DOI] [PubMed] [Google Scholar]
- Batschelet E., Domingo E., Weissmann C. The proportion of revertant and mutant phage in a growing population, as a function of mutation and growth rate. Gene. 1976;1(1):27–32. doi: 10.1016/0378-1119(76)90004-4. [DOI] [PubMed] [Google Scholar]
- Beck E., Forss S., Strebel K., Cattaneo R., Feil G. Structure of the FMDV translation initiation site and of the structural proteins. Nucleic Acids Res. 1983 Nov 25;11(22):7873–7885. doi: 10.1093/nar/11.22.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Strohmaier K. Subtyping of European foot-and-mouth disease virus strains by nucleotide sequence determination. J Virol. 1987 May;61(5):1621–1629. doi: 10.1128/jvi.61.5.1621-1629.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittle J. L., Houghten R. A., Alexander H., Shinnick T. M., Sutcliffe J. G., Lerner R. A., Rowlands D. J., Brown F. Protection against foot-and-mouth disease by immunization with a chemically synthesized peptide predicted from the viral nucleotide sequence. Nature. 1982 Jul 1;298(5869):30–33. doi: 10.1038/298030a0. [DOI] [PubMed] [Google Scholar]
- Buonagurio D. A., Nakada S., Desselberger U., Krystal M., Palese P. Noncumulative sequence changes in the hemagglutinin genes of influenza C virus isolates. Virology. 1985 Oct 30;146(2):221–232. doi: 10.1016/0042-6822(85)90006-6. [DOI] [PubMed] [Google Scholar]
- Buonagurio D. A., Nakada S., Parvin J. D., Krystal M., Palese P., Fitch W. M. Evolution of human influenza A viruses over 50 years: rapid, uniform rate of change in NS gene. Science. 1986 May 23;232(4753):980–982. doi: 10.1126/science.2939560. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Rebmann G., Baczko K., Ter Meulen V., Bellini W. J., Rozenblatt S., Billeter M. A. Accumulated measles virus mutations in a case of subacute sclerosing panencephalitis: interrupted matrix protein reading frame and transcription alteration. Virology. 1986 Oct 15;154(1):97–107. doi: 10.1016/0042-6822(86)90433-2. [DOI] [PubMed] [Google Scholar]
- Cheung A., DeLamarter J., Weiss S., Küpper H. Comparison of the major antigenic determinants of different serotypes of foot-and-mouth disease virus. J Virol. 1983 Nov;48(2):451–459. doi: 10.1128/jvi.48.2.451-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo E., Dávila M., Ortín J. Nucleotide sequence heterogeneity of the RNA from a natural population of foot-and-mouth-disease virus. Gene. 1980 Nov;11(3-4):333–346. doi: 10.1016/0378-1119(80)90073-6. [DOI] [PubMed] [Google Scholar]
- Domingo E., Martínez-Salas E., Sobrino F., de la Torre J. C., Portela A., Ortín J., López-Galindez C., Pérez-Breña P., Villanueva N., Nájera R. The quasispecies (extremely heterogeneous) nature of viral RNA genome populations: biological relevance--a review. Gene. 1985;40(1):1–8. doi: 10.1016/0378-1119(85)90017-4. [DOI] [PubMed] [Google Scholar]
- Domingo E., Sabo D., Taniguchi T., Weissmann C. Nucleotide sequence heterogeneity of an RNA phage population. Cell. 1978 Apr;13(4):735–744. doi: 10.1016/0092-8674(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Eigen M. Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften. 1971 Oct;58(10):465–523. doi: 10.1007/BF00623322. [DOI] [PubMed] [Google Scholar]
- Gillespie J. H. Natural selection and the molecular clock. Mol Biol Evol. 1986 Mar;3(2):138–155. doi: 10.1093/oxfordjournals.molbev.a040382. [DOI] [PubMed] [Google Scholar]
- Hayashida H., Toh H., Kikuno R., Miyata T. Evolution of influenza virus genes. Mol Biol Evol. 1985 Jul;2(4):289–303. doi: 10.1093/oxfordjournals.molbev.a040352. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Kimura M. Estimation of evolutionary distances between homologous nucleotide sequences. Proc Natl Acad Sci U S A. 1981 Jan;78(1):454–458. doi: 10.1073/pnas.78.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M. A., Carrillo C., Plana J., Mascarella R., Bergada J., Palma E. L., Domingo E., Sobrino F. Genetic and immunogenic variations among closely related isolates of foot-and-mouth disease virus. Gene. 1988;62(1):75–84. doi: 10.1016/0378-1119(88)90581-1. [DOI] [PubMed] [Google Scholar]
- Parvin J. D., Moscona A., Pan W. T., Leider J. M., Palese P. Measurement of the mutation rates of animal viruses: influenza A virus and poliovirus type 1. J Virol. 1986 Aug;59(2):377–383. doi: 10.1128/jvi.59.2.377-383.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff E., Mussgay M., Böhm H. O., Schulz G. E., Schaller H. Antibodies against a preselected peptide recognize and neutralize foot and mouth disease virus. EMBO J. 1982;1(7):869–874. doi: 10.1002/j.1460-2075.1982.tb01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccone M. E., Kaplan G., Giavedoni L., Domingo E., Palma E. L. VP1 of serotype C foot-and-mouth disease viruses: long-term conservation of sequences. J Virol. 1988 Apr;62(4):1469–1473. doi: 10.1128/jvi.62.4.1469-1473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. Polymorphism and evolution of influenza A virus genes. Mol Biol Evol. 1986 Jan;3(1):57–74. doi: 10.1093/oxfordjournals.molbev.a040381. [DOI] [PubMed] [Google Scholar]
- Sangar D. V. The replication of picornaviruses. J Gen Virol. 1979 Oct;45(1):1–13. doi: 10.1099/0022-1317-45-1-1. [DOI] [PubMed] [Google Scholar]
- Sobrino F., Dávila M., Ortín J., Domingo E. Multiple genetic variants arise in the course of replication of foot-and-mouth disease virus in cell culture. Virology. 1983 Jul 30;128(2):310–318. doi: 10.1016/0042-6822(83)90258-1. [DOI] [PubMed] [Google Scholar]
- Sobrino F., Palma E. L., Beck E., Dávila M., de la Torre J. C., Negro P., Villanueva N., Ortín J., Domingo E. Fixation of mutations in the viral genome during an outbreak of foot-and-mouth disease: heterogeneity and rate variations. Gene. 1986;50(1-3):149–159. doi: 10.1016/0378-1119(86)90320-3. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Direct method for quantitation of extreme polymerase error frequencies at selected single base sites in viral RNA. J Virol. 1986 Jan;57(1):219–228. doi: 10.1128/jvi.57.1.219-228.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmaier K., Franze R., Adam K. H. Location and characterization of the antigenic portion of the FMDV immunizing protein. J Gen Virol. 1982 Apr;59(Pt 2):295–306. doi: 10.1099/0022-1317-59-2-295. [DOI] [PubMed] [Google Scholar]
- Weddell G. N., Yansura D. G., Dowbenko D. J., Hoatlin M. E., Grubman M. J., Moore D. M., Kleid D. G. Sequence variation in the gene for the immunogenic capsid protein VP1 of foot-and-mouth disease virus type A. Proc Natl Acad Sci U S A. 1985 May;82(9):2618–2622. doi: 10.1073/pnas.82.9.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C., Martínez-Salas E., Diez J., Villaverde A., Gebauer F., Rocha E., Dávila M., Domingo E. Coevolution of cells and viruses in a persistent infection of foot-and-mouth disease virus in cell culture. J Virol. 1988 Jun;62(6):2050–2058. doi: 10.1128/jvi.62.6.2050-2058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



