Abstract
Recent studies in various rodent models of pathologic ventricular hypertrophy report the re-expression of deiodinase type 3 (D3) in cardiomyocytes. D3 inactivates thyroid hormone (T3) and is mainly expressed in tissues during development. The stimulation of D3 activity in ventricular hypertrophy and subsequent heart failure is associated with severe impairment of cardiac T3 signaling. Hypoxia-induced signaling appears to drive D3 expression in the hypertrophic cardiomyocyte, but other signaling cascades implicated in hypertrophy are also capable of stimulating transcription of the DIO3 gene. Many cardiac genes are transcriptionally regulated by T3 and impairment of T3 signaling will not only reduce energy turnover, but also lead to changes in gene expression that contribute to contractile dysfunction in pathologic remodeling. Whether stimulation of D3 activity and the ensuing local T3-deficiency is an adaptive response of the stressed heart or part of the pathologic signaling network leading to heart failure, remains to be established.
Keywords: Thyroid hormone, Deiodinase, Hypertrophy, Ischemia
Introduction
Persistent pressure and/or volume overload of the heart triggers a hypertrophic response that is aimed at normalising the increase in ventricular wall stress and the accompanying rise in energy turnover. Depending on the level of hemodynamic load, ventricular remodeling may be successfully compensatory or it may result in progressive contractile dysfunction and ultimately heart failure. This pathologic ventricular hypertrophy is characterized by changes in cardiomyocyte gene expression that affect contractile function and energy metabolism. The complex hypertrophic response is now known to be driven by numerous interacting signal-transduction pathways that may be triggered by mechanical stress of the cardiomyocyte as well as by various neurohumoral factors [1–3]. For the most part, these pathways converge on the promoters of specific genes changing the expression levels of the encoded proteins.
Under normal conditions, the level of circulating thyroid hormone (TH) determines the cardiac phenotype to a considerable extent. This is illustrated by the marked differences in cardiac contractility, electrophysiology, and energy metabolism in the absence of TH (hypothyroidism) and presence of excess levels (hyperthyroidism) [4]. Some of these differences are secondary to the effect of the thyroid status on heart rate and systemic blood pressure, whereas others result from transcriptional regulation of genes by TH [4]. Involvement of impaired TH signaling in pathologic ventricular hypertrophy is suggested by similar changes in expression of a number of key cardiac genes in hypothyroidism and in heart failure [4]. Typical examples are the reduced expression of the sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) and the myosin heavy chain (MHC) α isoform, and the increased expression of the MHCβ isoform, although a hypothyroid-like expression profile does not apply to all genes that are co-regulated by TH [5]. Reduced TH signaling in the hemodynamically overloaded heart could in principle result from changes in the expression of TH receptors or their co-factors; by diminished active TH uptake through its transporters; by the reduction of plasma T3 levels that is seen in severe illness, including advanced heart failure; or by changes in cellular metabolism of TH. In this article, we will focus on the latter possibility and review recent data from different rodent models showing the induction of a TH-inactivating enzyme in the chronically overloaded heart. The multiple mechanisms of induction of this enzyme suggest that reducing TH signaling in the overloaded heart may be either an adaptive or ultimately a maladaptive response.
Thyroid-hormone deiodination: activation and inactivation
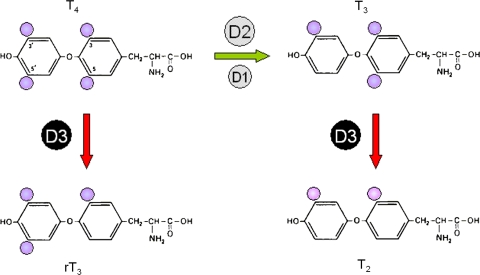
The high-affinity ligand of the nuclear thyroid-hormone receptors is 3,5,3′-triiodothyronine (T3), but the thyroid synthesizes and secretes primarily 3,5,3′,5′-tetraiodothyronine (T4) under conditions of sufficient iodine intake. T4 is considered a pro-hormone because of its much lower biological activity compared to T3. The periferal metabolism of secreted T4 involves the stepwise removal of iodine residues, yielding T3 as well as inactive derivatives of the hormone. These reactions are catalyzed by a group of enzymes called deiodinases. Three types exist, i.e., D1, D2, and D3. These oxido-reductases share the presence of the rare amino acid selenocysteine in the conserved active center of the protein. However, they differ in their catalytic properties, tissue distribution and developmental expression (reviewed in [6]). The principal deiodinative reactions catalyzed by these enzymes are depicted schematically in Fig. 1.
Fig. 1.
Principal enzymatic activities of the deiodinases type 1, 2, and 3. Removal of iodine (purple spheres) from the outer ring of T4 (3,5,3′,5′-tetraiodothyronine) by D1 and D2 converts T4 to the active hormone T3 (3,5,3′-triiodothyronine), with D2 having a higher affinity for T4 than D1. Inner-ring deiodination of T4 and T3 by D3 generates the biologically inactive metabolites reverse T3 (3,3′,5′-triiodothyronine) and T2 (3,3′-diiodothyronine), respectively
Removal of an iodine residue from the outer phenolic ring of T4 yields the active hormone T3. This reaction is catalyzed by both D1 and D2, with D2 having ~1,000-fold higher affinity for T4 than D1. D1 is primarily expressed in liver and kidney, whereas D2 is present in a number of tissues, including brown adipose tissue (BAT), brain, pituitary, and, at low levels, also in human heart and skeletal muscle. D1 activity provides the major part of total plasma T3, but D2 activity in humans also appears to be a substantial source of extra-thyroidal T3 [7]. Primarily, however, D2 provides T3 in those tissues where it is expressed. In brain, D2 activity in tanycytes is also thought to act in a paracrine fashion, providing T3 for surrounding neurons [8, 9].
Deiodination of the inner tyrosyl ring of T4 and T3 generates the inactive iodothyronines reverse T3 (3,3′,5′-triiodothyronine, rT3) and T2 (3,3′-diiodothyronine), respectively (see Fig. 1). This reaction is also catalyzed by D1, albeit at low rates. However, sulfation of the phenolic hydroxyl group of T4 and T3 greatly increases these rates, while blocking outer-ring deiodination [10]. Iodothyronine sulfation is at least present in liver and D1 activity is considered important in the clearance of T4 and T3 [10]. rT3 is also a substrate for D2 to generate T2. The third deiodinase, D3, has high affinity for both T4 and T3, but it has exclusively inner-ring deiodinative activity producing rT3 and T2. This TH-inactivating activity is virtually absent in adult tissues, with the exception of skin and different areas and cell types in the brain. It is also high in placenta and in most fetal tissues, including the heart. The D3 protein is primarily located in the plasma membrane and although extracellular catalytic activity of D3 has been proposed [11], intracellular TH appears to be the principal substrate for D3 and this is in line with the reducing environment required for activity [12].
It is now becoming clear that the different deiodinase activities are not static, but that they are highly regulated, both during development and in adult life [13, 14]. A well-studied example of this is the induction of D2 activity in rodent BAT during cold exposure, which results in a local increase in T3 levels that enables the thermogenic response of this tissue [15]. Tight regulation of tissue T3 levels is particularly critical during development. Adult plasma levels of T3 are not compatible with normal fetal development and in mammals high D3 activity in the pregnant uterus and placenta protects the fetus from too much maternal TH. In addition, most fetal tissues express D3. Spatial and temporal patterns of expression of D2 and D3 appear to precisely regulate the T3-dependent aspects of cell proliferation and, particularly, cell differentiation. For example, development and maturation of the cochlea in mice is dependent on induction of D2 activity [16], whereas repression of D2 activity plays a role during bone development [17]. Additionally, cell-specific expression of D3 in the retina during the T3-driven metamorphic climax in tadpoles orchestrates the development of the adult frog eye [18]. D3 expression is also essential for the development of the thyroid axis in mice [19] and regulated regional expression of D2 and D3 in the developing human brain is associated with concomitant up or down regulation of local T3 levels [20]. Taken together, these data show that regulated expression of the different deiodinases, either as part of a developmental program or in response to environmental cues, allows for active modulation of tissue-specific TH signaling irrespective of systemic hormone levels.
Cardiac deiodinase expression in pathologic ventricular remodeling
Current studies suggest that activation of TH in the healthy heart is only marginal. D1 and D2 activity are low in the rodent myocardium and conversion of T4 accounts for <7% of cardiac T3 [21, 22]. Equally low levels of deiodinase activity have been reported for human cardiac tissue [23], although D2 mRNA levels are considerably higher than in rat heart [24]. Overexpression of D2 in mouse myocardium results in only a mild increase in cardiac T3 levels [25, 26], suggesting that active uptake of T4 by cardiomyocytes through the recently identified TH transporters [27] may be limited. T3 levels in the healthy heart are therefore primarily determined by the level of plasma T3. Nevertheless, the heart does appear to have some capacity for regulating its T3 levels. Normal cardiac T3 levels were observed in rats subjected to severe iodine deficiency, which reduces plasma T3 and T4 levels by 50 and 90%, respectively [28]. These low levels of plasma T4 make increased local conversion an unlikely source for the extra tissue T3, eventhough the cardiac D2 activity increases under conditions of reduced circulating TH [29]. Alternative options include increased active uptake of T3 or reduced clearance, but these aspects have not yet been studied.
Myocardial activity of the three deiodinases in cardiac pathology was determined for the first time in a rat model of right-ventricular (RV) hypertrophy and failure [22]. In this model, chronic pulmonary arterial hypertension (PAH) is induced by a single dose of the pyrrolizidine alkaloid monocrotaline (MCT). The bioactive metabolite of MCT selectively injures the vascular endothelium of the lung vessels and progressive pulmonary vasculitis leads to an increase in vascular resistance and a gradual rise in arterial pressure, which in turn induces ventricular hypertrophy. This hypertrophy progresses to a stable compensated state, designated HYP, or to congestive heart failure (CHF) and death within 4–5 weeks, depending on the level of PAH [22, 30]. A greater degree of RV hypertrophy in the CHF group compared to the HYP group was associated with more pronounced changes in gene expression that characterize pathologic remodeling, such as the reduction of mRNA levels of SERCA2a and the shift in mRNA expression from the MHCα to the MHCβ isoform [22, 30]. The partial shift in MHC isoform expression seen in the RV of the HYP group was strictly related to hypertrophy of this ventricle since no change occurred in the left ventricle (LV). On the other hand, the almost complete shift in the RV of the CHF group is partly related to hypertrophy and partly to the reduction of plasma T3 levels in these critically ill animals, because a significant shift was seen in the LV. Analysis of deiodinase enzyme activities in the myocardium showed a low level of D1 activity in both ventricles of control animals, which was reduced in the HYP and CHF groups [22]. However, the maximal D1 activity was <1% of that found in the livers of these animals and is consequently thought to be irrelevant for cardiac TH metabolism [22]. D2 activity could not be detected in any of the groups, but a low level of D3 activity was found in LV and RV of control animals. Unexpectedly, this activity increased in the chronically overloaded RV, with no change of activity in the LV’s of the same hearts. The five fold increase in D3 activity in the RV of the CHF group was furthermore significantly higher than that in the RV of the HYP group (three fold) [22]. A more extensive recent analysis of this model showed an average 10-fold stimulation of RV D3 activity in the CHF group, amounting to ~20% of the level of D3 activity found in brains of these rats [31]. Moderate induction of D3 activity is therefore associated with the development of compensatory RV hypertrophy, whereas high levels of D3 activity are associated with overt RV failure and death in these animals. In a mouse model of chronic pressure overload of the LV due to aortic constriction, Trivieri et al. [26] also reported a five fold increase in cardiac D3 activity in the hypertrophic LV, however, absolute activity levels were not presented in that study.
Induction of D3 activity was also found recently in the LV following myocardial infarction in rats (MI) [32]. Loss of viable LV tissue due to MI results in a mixture of pressure and volume overload, which drives hypertrophic remodeling of the non-infarcted tissue. A 12-week analysis of post-MI LV remodeling showed chronic cardiac dysfunction with reduced ejection fraction and increased LV end-diastolic diameters. High activity levels of D3 were observed in the infarcted LV at the first week following MI, identical to the levels shown previously for the overloaded RV in the study by Wassen et al. [22]. The authors suggest that this activity is responsible for the transient decrease in plasma T3 levels that is typically seen during the first 3 weeks following MI [32]. Confirmation of this idea awaits analysis of D3 activity at later time points.
Transient expression of D3 following MI was in fact not found in a recent study of post-MI LV remodeling in the mouse [33, 34]. Also in this model, strong induction of D3 was found in the hypertrophic, non-infarcted area of the LV 1 week following MI, but D3 activity remained high at 4 and 8 weeks. Using immunohistochemistry and validated D3 antibodies, it was also shown for the first time that D3 protein localizes to cardiomyocytes in the hypertrophic LV [33]. As in the rat model, LV function was severely reduced from the first week post-MI onward, with increased LV end-diastolic and end-systolic diameters and reduced fractional shortening. The D3 activity levels were again similar to those found in failing RV [22], but although LV function was compromised in these mice, they did not succumb to heart failure.
Consequences of cardiac D3 expression in pathologic ventricular remodeling
The stimulation of TH-degrading activity in hypertrophic myocardium was suggested to lead to a reduction of T3 levels in cardiomyocytes [22]. Tissue TH content has so far only been determined in the PAH model of RV failure [31]. The high D3 activity in the RV in CHF correlated with a 35% lower total T3 content compared to LV of the same heart. Furthermore, T3-dependent transcription in the cardiomyocyte was determined to assess whether T3 signaling was indeed reduced in the hypertrophic RV. An in vivo T3-transcription probe was used consisting of a reporter plasmid in which the Firefly luciferase gene is placed under control of a T3-responsive minimal promotor that has no cardiac-specific regulatory sequences. Direct injection of the plasmid, together with a normalisation plasmid expressing Renilla luciferase, into the free wall of the RV and LV leads to transfection of only cardiomyocytes. Measurement of tissue luciferase activities after several days gives a measure of T3-dependent transcriptional activity. Analysis of CHF animals showed that normalized Firefly luciferase activity remained at control levels in the LV, but that it was reduced by 45% in the hypertrophic RV. By comparison, normalized Firefly luciferase activity showed a 50% reduction in hearts of animals that were severely hypothyroid (plasma T3: 0.03 nM) compared to euthyroid controls (plasma T3: 0.94 nM). This RV-restricted reduction of T3-dependent transcriptional activity, together with the results mentioned above, suggests that D3 expression in the remodeling cardiomyocyte reduces cellular T3 content to a level equal or close to that seen in severe systemic hypothyroidism. However, at this point it cannot be ruled out that other changes in the hypertrophic cardiomyocyte contribute to, or cause the observed reduction of T3-dependent transcription. Indeed, changes in TR expression levels, including differential effects on the α1, α2 and β1 isoforms, have been reported in models of LV remodeling induced by MI [35] and pressure overload [36, 37]. The role of TRs is still a matter of debate with both increased and decreased expression of TR being suggested to suppress T3 signaling. Nevertheless, in a recent study cardiac function in a mouse model of LV pressure overload improved by increasing TR expression through viral transfection of the myocardium [38].
On the other hand, the view that a reduction of cellular T3 levels is a principal cause of the hypothyroid condition of hypertrophic cardiomyocytes is supported by the study of Trivieri et al. [26]. Cardiac-specific overexpression of D2 was used in a model of LV hypertrophy and dysfunction due to aortic constriction. In control mice, overexpression of D2 increased total cardiac T3 levels by ~25%, resulting in enhancement of contractile function. When these animals were then subjected to aortic constriction, LV hypertrophy developed, but without the characteristic decrease in SERCA2a and increase in MHCβ expression that is seen in wild-type mice. Preservation of function in the D2-overexpressing hearts was confirmed in isolated cardiomyocytes by measurement of Ca2+-transients and contractility. As mentioned earlier, D3 expression was increased five fold in the hypertrophic LV in this study, suggesting that the overexpression of D2 maintains T3-responsive gene expression by effectively balancing the TH-degrading activity of D3. Detailed analyses of the tissue T3 levels in the hypertrophic LV of control and transgenic mice are required to show that this is indeed the case.
Regulation of cardiac D3 expression in pathologic ventricular remodeling
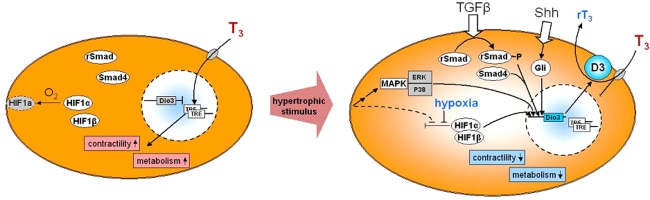
A large number of signal-transduction pathways are known to be involved in ventricular remodeling [1–3]. The outcome of the process depends on the particular mix of pathways which results from the type and level of stimulation of the cardiomyocyte, as well as from processes secondary to remodeling. Surprisingly, stimulation of D3 expression may be accounted for by at least three of these pathways and the synergistic interactions between them suggests that expression is potentially regulated over a wide range. The factors and pathways discussed below are depicted schematically in Fig. 2.
Fig. 2.
Schematic representation of some of the pathways that may contribute to the expression of D3 in pathologic ventricular remodeling. A normal cardiomyocyte is depicted on the left. T3 is taken up by specific transporters and genes that are transcriptionally regulated by T3 are characterized by the presence in their promoters of thyroid hormone response elements (TRE) to which the T3 receptor binds. HIF-1α is degraded under normoxic conditions, whereas HIF-1β is stable. Transition to the hypertrophic cardiomyocyte may be triggered by various stimuli (see text for details). Several signaling pathways converge on the mitogen activated protein kinases (MAPK), of which ERK and p38 activate DIO3 gene transcription. Mismatch of oxygen delivery and consumption, caused by ischemia and/or enlargement of the cardiomyocyte, results in hypoxia and stabilization of HIF-1α. Dimerization with HIF-1β forms the HIF-1 complex. HIF-1α may also be stabilized directly as a result of hemodynamic overload and mechanical stress. TGFβ stimulates the Smad signaling pathway by phosphorylation of R-Smad2 and -3, which form a complex with Smad4. Together with HIF-1, phosphorylated ERK, and p38 this results in the synergistic stimulation of transcription of the DIO3 gene. D3 expression is further stimulated by the secreted morphogen Sonic hedgehog (Shh) which signals through the Gli family of transcription factors. D3 activity converts T3 to the inactive metabolite reverse T3, resulting in reduced T3-dependent gene expression and a concomitant reduction of contractile activity and energy turnover
Hypoxia-inducible factor 1
The DIO3 gene has recently been shown to be a direct target of hypoxia-inducible factor 1 (HIF-1) [31]. A reduction of oxygen availability triggers HIF-1 signaling and its down-stream effects are aimed at reducing cellular oxygen consumption and stimulating oxygen delivery [39]. HIF-1 is a heterodimer and its activity is determined by the oxygen-dependent level of the HIF-1α subunit. Under normoxic conditions HIF-1α is ubiquinated and rapidly degraded, but as cellular oxygen tension drops, it accumulates and associates with the stably expressed HIF-1β to form HIF-1. HIF-1 then translocates to the nucleus and activates a number of genes, many of them involved in glucose metabolism and angiogenesis. In vitro analysis of the effect of hypoxia on D3 activity showed strong induction in human neurons (SK-N-AS cells) and choriocarcinoma cells (JEG-3), rhesus monkey hepatocytes (NCLP6E cells), as well as in rat neonatal cardiomyocytes, while human endometrial cells and fibroblasts were unresponsive [31]. Induction of D3 activity was dynamic with transient exposure to hypoxia resulting in a transient increase in D3 mRNA and protein expression. Furthermore, this induction correlated with increased HIF-1α levels and ChiP analysis confirmed the direct interaction of HIF-1 with the DIO3 promoter. The latter most likely involving a conserved HIF-1-binding site present in this region. The induction of D3 activity in the hypoxic cells fits the adaptive response orchestrated by HIF-1, as it markedly reduces T3-dependent metabolic rate in these cells [31].
Involvement of HIF-1 signaling in cardiac D3 expression in vivo is suggested by data from the model of PAH-induced RV hypertrophy and failure. RV-specific stimulation of D3 mRNA expression and enzyme activity was associated with a similarly specific stimulation of HIF-1α levels [31]. This is in line with the earlier reported increased nuclear HIF-1α content in RV cardiomyocytes [40] and increased expression of HIF-1 regulated genes [30]. Similarly, in the mouse model of LV pressure-overload in which D3 was induced [26], Sano et al. reported increased HIF-1 activity to be required for adaptive LV hypertrophy and angiogenesis [41].
Cardiomyocyte hypoxia can occur in hypertrophy as a result of a mismatch between oxygen supply and consumption. Capillary density and oxygen diffusion distances may become limiting factors for the enlarged cardiomyocytes [40], particularly given the higher energy turnover as a result of the increase in wall tension. HIF signaling therefore constitutes a secondary pathway affecting gene expression in hypertrophic remodeling. However, rapid HIF-1α accumulation was also observed following an increase in ventricular wall tension under normoxic conditions [42]. The HIF-1 response to this hypertrophy stimulus appeared to be triggered by stretch-activated channels signaling through the phosphatidylinositol-3-kinase pathway. Consequently, HIF-1 may also be a factor in early hypertrophic signaling irrespective of changes in oxygen tension.
Hypoxia-triggered HIF-1 signaling may obviously account for the early induction of D3 expression seen in cardiac ischemia. HIF-1α levels are increased following ischemia or MI in rat [43–45], hamster [43], mouse [46], and human myocardium [47–49]. Studies using transgenics [46], gene transfer [50] or pharmacological intervention [51] to increase HIF-1α have clearly shown the adaptive nature of the HIF-1 response in cardiac ischemia. HIF-1 activation in rodent cardiomyocytes occurred in multiple areas of the ventricle but the expression persisted particularly in the peri-infarct area. Progressively increasing expression of HIF-2α was also found in remote areas of the infarcted rat ventricle for at least 4 weeks following MI [45]. HIF-2α is structurally related to HIF-1α and similarly regulated, but its expression has so far been considered to be mainly involved in the endothelial response to hypoxia. Persistent HIF-1 signaling in the post-MI ventricle may also be related to the hypertrophic remodeling of surviving tissue (see above) and is in line with the observed stable expression of D3 for up to 8 weeks [33, 34]. Immunohistochemical localization of HIF-1 activation and D3 expression in individual cardiomyocytes is needed to further support this suggested route for D3 activation.
Transforming growth factor β
Cellular signaling induced by TGF-β leads to transcriptional activation of the DIO3 gene. This effect was analysed in detail by Huang et al. using various non-transformed human cell types [52]. TGF-β-activated cell surface receptor kinases phosphorylate receptor-associated members of the Smad family of trans-activating factors (R-Smad), which then migrate to the nucleus to activate target genes in conjunction with other transcription factors (reviewed in [53]). Combinations of the common Smad4 isoform with either the R-Smad2 or -3 isoform strongly activated the promoter of the DIO3 gene in cell types that expressed D3 endogenously, such as hepatocarcinoma cells, fibroblasts and skeletal muscle myocytes [52]. TGF-β1, -2 and -3 were equally potent and this signaling pathway is a potential candidate for cardiac D3 regulation, since TGF-β is known to play a role in the development of ventricular hypertrophy and adverse remodeling (reviewed in [54, 55]). TGF-β1 mRNA levels increased rapidly in LV pressure overload (aortic constriction) in the rat, and either remained elevated at 28 days [56] or returned to basal values after 14 days [57]. Similarly, in volume overload (aortacaval fistula), TGF-β1 mRNA levels increased and returned to basal levels after 21 days [58]. Following MI in rat and mouse, TGF-β expression increased throughout the ventricle, particularly in infarct and border zones, persisting for up to 82 days [59–61].
TGF-β signaling in hypertrophy and heart failure is best known for its role in activating fibrosis, but more recent data have shown that it is also involved in cardiomyocyte hypertrophy. For instance, cardiac hypertrophy induced by Angiotensin II (Ang II) is in part dependent on the upregulation of cardiomyocyte TGF-β expression, which then acts in an autocrine loop to stimulate cell growth (reviewed in [54]). Ang II stimulation of TGF-β expression involved the stress-activated branch of the Mitogen Activated Protein Kinase (MAPK) system, i.e., p38 MAPK. This factor itself is a down-stream target of TGF-β through the action of TGF-β-activated kinase 1 (TAK1), which was shown to be upregulated in cardiomyocytes in non-infarcted myocardium following MI [62]. TAK1 signaling is a second mode of TGF-β action next to the Smad route. Involvement of the latter route in TGF-β signaling in cardiomyocytes is nevertheless also likely, since Smads were shown to be critical in myocyte proliferation and growth during development [63]. Additionally, Smads play a role in hypertrophic remodeling (reviewed in [55]), including Ang II-induced apoptosis [64].
Involvement of the MAPK system is particularly relevant for a possible mechanism of stimulation of D3 expression. Many stimuli driving hypertrophy converge on this system, which also includes Extracellular Responsive Kinase (ERK) MAPK and c-Jun N-terminal kinases (JNKs) [1–3]. Activation of p38 as well as ERK stimulated D3 expression in a range of human cell types that express D3 [52, 65]. More importantly, D3 induction was greatly enhanced in these cells when MAPK activation was combined with TGF-β stimulation. Further analysis indicated synergistic action of Smads and MAPK signaling driving D3 transcription, possibly involving Sp1 [52]. The relevance of this for the in vivo situation is suggested by the observation of RV-specific activation of p38 MAPK [30] as well as increased RV Ang II signaling and TGF-β mRNA expression [66] in the rat model of PAH-induced RV hypertrophy with high D3 expression [31].
Sonic hedgehog
Sonic hedgehog (Shh) is a secreted signaling protein acting through the Gli-family of transcription factors with numerous effects in vertebrate development. It determines patterns of cell proliferation and differentiation, including cardiomyogenesis and development of the heart [17, 67, 68] (and references therein). The proliferation-promoting aspect of Shh/Gli signaling has recently been shown to be linked to induction of D3 activity [68]. Using malignant keratinocytes, this study indicated that cell proliferation depended on the reduction of cellular T3 levels due to D3 activity, which effectively blocks both T3-stimulated differentiation and inhibition of progression of the cell cycle. High D3 activity was also found in vascular tumors in humans, as well as in a solitary fibrous tumor [69–71]. In these cases D3 activity reached such high levels that a systemic hypothyroid condition ensued. Taken together, these data suggest that D3 is induced as part of the re-induction of the program of cell proliferation that is responsible for D3 expression in fetal tissues. This mechanism is not restricted to tumor growth, as a strong induction of hepatic D3 expression was recently shown in a rat model of liver regeneration following partial hepatectomy [72].
Several studies indicate that Shh also plays a role in the adult heart. Shh signaling in cardiomyocytes and perivascular smooth muscle cells is critical for maintaining normal cardiac function in mice [73] and stimulation of Shh signaling by gene transfer in cardiomyocytes and fibroblasts preserved LV function in acute and chronic ischemia [74]. Endogenous Shh signaling in the LV was also markedly upregulated following MI [74]. Thus far, cardiac Shh signaling has not been analyzed for the models of LV or RV pressure overload in which D3 expression was upregulated. However, increased proliferation signaling is indicated for RV hypertrophy, where expression of several factors involved in cell cycle progression was increased [30], including cyclin D1, a target of Shh [75].
Cross talk
Results from a number of different research lines suggest that the signaling pathways discussed above are interconnected. HIF-1α and TGF-β signaling are potentiated by the strong synergistic action on responsive promoters of HIF-1 and complexes of R-Smad2/3 and Smad 4 (reviewed in [53]). Furthermore, HIF-1α is stabilized by TGF-β-induced inhibition of 1α-associated prolyl hydroxylase, the enzyme responsible for degradation of HIF-1α [76]. Cross talk between hypoxia- and Shh-signaling has also been described recently. Normobaric hypoxia in mice was found to induce Shh signaling in a number of tissues and detailed analysis using the cardiomyocyte cell line H9C2 showed direct activation of the Shh pathway by HIF-1α [77]. These data corroborate the earlier reported HIF-1α-induced re-entry of the cell cycle in rat cardiomyocytes following MI, as well as in cultured adult cardiomycytes [44]. HIF-1 signaling, therefore, appears to play a central role in the induction of D3 expression in adult tissues, as it likely does during fetal development when tissue-oxygen tension is low. Irrespective of the pathway stimulating D3 expression, its fetal characteristic fits the partial re-expression of the fetal gene profile that characterizes pathologic cardiac hypertrophy [78].
Concluding remarks and future directions
Reducing T3-dependent energy turnover in the stressed cardiomyocyte by induction of D3 is consistent with the adaptive nature of HIF-1 signaling. In contrast to extracellularly activated signaling cascades or increases in wall stress, HIF-1 signaling would restrict the induction of D3 to those cells that would benefit from a reduction in energy turnover. So far, D3-immunohistochemistry has only been shown in post-MI LV and the mixed pattern of D3-positive and -negative cardiomyocytes appears to support cell-specific rather than global induction of D3 expression [33].
When stimulation of D3 activity is compounded by other factors involved in pathologic remodeling, e.g., TGF-β signaling, the decrease in cellular T3 levels may further impact on the expression of T3-dependent genes that are implicated in the development of contractile dysfunction, and the adaptive response may become part of the problem. A maladaptive effect of reduced T3-signaling in heart failure is still the prevailing view, but the stable induction of D3 in the post-MI LV [33] and the original study by Wassen et al. [22], suggest that timing and extent of D3 induction may be critical factors in turning an adaptive into a maldaptive response. In the latter study, ventricles that developed stable compensatory hypertrophy showed significantly less induction of D3 activity compared to failing ventricles. The increase in TGF-β signaling in the progression to failure may play a role here [55]. Given the stimulation of DIO3 gene transcription by multiple signaling cascades implicated in hypertrophy, additional studies are needed to deliniate which factors are driving D3 expression over the course of pathologic ventricular remodeling.
Although it is tempting to conclude a causal relationship between the induction of D3 activity on the one hand, and adaptive or maladaptive aspects of pathologic hypertrophy on the other, there are no data as yet to confirm this. There are no selective inhibitors of D3 activity and a transgenic approach, using cardiac-specific, conditional knock-out of D3 expression appears to be the optimal way to test the relevance of D3 activity for the development of adaptive or maladaptive ventricular remodeling.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Oka T, Xu J, Molkentin JD. Re-employment of developmental transcription factors in adult heart disease. Semin Cell Dev Biol. 2007;18:117–131. doi: 10.1016/j.semcdb.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Card Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Selvetella G, Hirsch E, Notte A, et al. Adaptive and maladaptive hypertrophic pathways: points of convergence and divergence. Card Res. 2004;63:373–380. doi: 10.1016/j.cardiores.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 5.Athea Y, Garnier A, Fortin D. Mitochondrial and energetic cardiac phenotype in hypothyroid rat. Relevance for heart failure. Eur J Phys. 2007;455:431–442. doi: 10.1007/s00424-007-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gereben B, Zavacki AM, Ribich S et al (2008) Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29(7):898–938 doi:10.1210/er.2008-0019 [DOI] [PMC free article] [PubMed]
- 7.Maia AL, Kim BW, Huang SA, et al. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Investig. 2005;115:2524–2533. doi: 10.1172/JCI25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechan RM, Fekete C. Role of thyroid hormone deiodination in the hypothalamus. Thyroid. 2005;15:883–897. doi: 10.1089/thy.2005.15.883. [DOI] [PubMed] [Google Scholar]
- 9.Bernal J, Guadano-Ferraz A, Morte B. Perspectives in the study of thyroid hormone action on brain development and function. Thyroid. 2003;13:1005–1012. doi: 10.1089/105072503770867174. [DOI] [PubMed] [Google Scholar]
- 10.Visser TJ, Kaptein E, Glatt H, et al. Characterization of thyroid hormone sulfotransferases. Chem Biol Interact. 1998;109:279–291. doi: 10.1016/S0009-2797(97)00139-7. [DOI] [PubMed] [Google Scholar]
- 11.Baqui M, Botero D, Gereben B, et al. Human type 3 iodothyronine selenodeiodinase is located in the plasma membrane and undergoes rapid internalization to endosomes. J Biol Chem. 2003;278:1206–1211. doi: 10.1074/jbc.M210266200. [DOI] [PubMed] [Google Scholar]
- 12.Friesema EC, Kuiper GG, Jansen J et al (2006) Thyroid hormone transport by the human monocarboxylate transporter 8 and its rate-limiting role in intracellular metabolism. Mol Endocrinol 20:2761–2772 [DOI] [PubMed]
- 13.Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Investig. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gereben B, Zeold A, Dentice M, et al. Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cell Mol Life Sci. 2008;65:570–590. doi: 10.1007/s00018-007-7396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jesus LA, Carvalho SD, Ribeiro MO, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Investig. 2001;108:1379–1385. doi: 10.1172/JCI13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng L, Goodyear RJ, Woods CA, et al. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci USA. 2004;101:3474–3479. doi: 10.1073/pnas.0307402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dentice M, Bandyopadhyay A, Gereben B, et al. The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol. 2005;7:698–705. doi: 10.1038/ncb1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsh-Armstrong N, Huang H, Remo BF, et al. Asymmetric growth and development of the Xenopus laevis retina during metamorphosis is controlled by type III deiodinase. Neuron. 1999;24:871–878. doi: 10.1016/S0896-6273(00)81034-X. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez A, Martinez ME, Fiering S, et al. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Investig. 2006;116:476–484. doi: 10.1172/JCI26240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kester MH, Martinez de Mena R, Obregon MJ, et al. Iodothyronine levels in the human developing brain: major regulatory roles of iodothyronine deiodinases in different areas. J Clin Endocrinol Metab. 2004;89:3117–3128. doi: 10.1210/jc.2003-031832. [DOI] [PubMed] [Google Scholar]
- 21.Escobar-Morreale HF, Obregon MJ, Escobar del Rey F, et al. Tissue-specific patterns of changes in 3, 5, 3′-triiodo-L-thyronine concentrations in thyroidectomized rats infused with increasing doses of the hormone. Which are the regulatory mechanisms? Biochimie. 1999;81:453–462. doi: 10.1016/S0300-9084(99)80095-9. [DOI] [PubMed] [Google Scholar]
- 22.Wassen FW, Schiel AE, Kuiper GG, et al. Induction of thyroid hormone-degrading deiodinase in cardiac hypertrophy and failure. Endocrinology. 2002;143:2812–2815. doi: 10.1210/en.143.7.2812. [DOI] [PubMed] [Google Scholar]
- 23.Sabatino L, Iervasi G, Ferrazzi P, et al. A study of iodothyronine 5′-monodeiodinase activities in normal and pathological tissues in man and their comparison with activities in rat tissues. Life Sci. 2000;68:191–202. doi: 10.1016/S0024-3205(00)00929-2. [DOI] [PubMed] [Google Scholar]
- 24.Dentice M, Morisco C, Vitale M, et al. The different cardiac expression of the type 2 iodothyronine deiodinase gene between human and rat is related to the differential response of the Dio2 genes to Nkx-2.5 and GATA-4 transcription factors. Mol Endocrinol (Baltimore Md.) 2003;17:1508–1521. doi: 10.1210/me.2002-0348. [DOI] [PubMed] [Google Scholar]
- 25.Pachucki J, Hopkins J, Peeters R, et al. Type 2 iodothyronine deiodinase transgene expression in the mouse heart causes cardiac-specific thyrotoxicosis. Endocrinology. 2001;142:13–20. doi: 10.1210/en.142.1.13. [DOI] [PubMed] [Google Scholar]
- 26.Trivieri MG, Oudit GY, Sah R, et al. Cardiac-specific elevations in thyroid hormone enhance contractility and prevent pressure overload-induced cardiac dysfunction. Proc Natl Acad Sci USA. 2006;103:6043–6048. doi: 10.1073/pnas.0601072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friesema EC, Jansen J, Milici C, et al. Thyroid hormone transporters. Vitam Horm. 2005;70:137–167. doi: 10.1016/S0083-6729(05)70005-4. [DOI] [PubMed] [Google Scholar]
- 28.Pedraza PE, Obregon MJ, Escobar-Morreale HF, et al. Mechanisms of adaptation to iodine deficiency in rats: thyroid status is tissue specific. Its relevance for man. Endocrinology. 2006;147:2098–2108. doi: 10.1210/en.2005-1325. [DOI] [PubMed] [Google Scholar]
- 29.Wagner MS, Morimoto RJ, Dora JM, et al. Hypothyroidism induces type 2 iodothyronine deiodinase expression in mouse heart and testis. J Mol Endocrinol. 2003;31:541–550. doi: 10.1677/jme.0.0310541. [DOI] [PubMed] [Google Scholar]
- 30.Buermans HPJ, Redout EM, Schiel AE, et al. Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics. 2005;21:314–323. doi: 10.1152/physiolgenomics.00185.2004. [DOI] [PubMed] [Google Scholar]
- 31.Simonides WS, Mulcahey MA, Redout EM, et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Investig. 2008;118:975–983. doi: 10.1172/JCI32824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olivares EL, Marassi MP, Fortunato RS, et al. Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in rats a time course study. Endocrinology. 2007;148:4786–4792. doi: 10.1210/en.2007-0043. [DOI] [PubMed] [Google Scholar]
- 33.Pol C, Zuidwijk M, Deel E et al (2008) Left ventricular myocardial infarction in mice induces sustained cardiac deiodinase type III activity. In: Proceedings of the XXVIII European Section Meeting of the International Society for Heart Research, Mediamond International Proceedings, Bologna, Italy, pp 57–60
- 34.Pol CJ, Zuidwijk MJ, Deel E, et al. Left ventricular myocardial infarction in mice induces sustained cardiac deiodinase type III activity. J Mol Cell Cardiol. 2008;44:722–723. doi: 10.1016/j.yjmcc.2008.02.029. [DOI] [Google Scholar]
- 35.Pantos C, Mourouzis I, Xinaris C, et al. Time-dependent changes in the expression of thyroid hormone receptor alpha 1 in the myocardium after acute myocardial infarction: possible implications in cardiac remodeling. Eur J Endocrinol. 2007;156:415–424. doi: 10.1530/EJE-06-0707. [DOI] [PubMed] [Google Scholar]
- 36.Kinugawa K, Yonekura K, Ribeiro RC, et al. Regulation of thyroid hormone receptor isoforms in physiological and pathological cardiac hypertrophy. Circ Res. 2001;89:591–598. doi: 10.1161/hh1901.096706. [DOI] [PubMed] [Google Scholar]
- 37.Kinugawa K, Minobe WA, Wood WM, et al. Signaling pathways responsible for fetal gene induction in the failing human heart: evidence for altered thyroid hormone receptor gene expression. Circulation. 2001;103:1089–1094. doi: 10.1161/01.cir.103.8.1089. [DOI] [PubMed] [Google Scholar]
- 38.Belke DD, Gloss B, Swanson EA, et al. Adeno-associated virus-mediated expression of thyroid hormone receptor isoforms-alpha1 and -beta1 improves contractile function in pressure overload-induced cardiac hypertrophy. Endocrinology. 2007;148:2870–2877. doi: 10.1210/en.2007-0009. [DOI] [PubMed] [Google Scholar]
- 39.Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol. 2004;96:1173–1177. doi: 10.1152/japplphysiol.00770.2003. [DOI] [PubMed] [Google Scholar]
- 40.Des Tombes AL, van Beek-Harmsen BJ, Lee-de Groot MBE, et al. Calibrated histochemistry applied to oxygen supply and demand in hypertrophied myocardium. Microsc Res Tech. 2002;58:412–420. doi: 10.1002/jemt.10153. [DOI] [PubMed] [Google Scholar]
- 41.Sano M, Minamino T, Toko H, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 42.Kim CH, Cho YS, Chun YS, et al. Early expression of myocardial HIF-1alpha in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ Res. 2002;90:E25–E33. doi: 10.1161/hh0202.104923. [DOI] [PubMed] [Google Scholar]
- 43.Kakinuma Y, Miyauchi T, Yuki K, et al. Novel molecular mechanism of increased myocardial endothelin-1 expression in the failing heart involving the transcriptional factor hypoxia-inducible factor-1alpha induced for impaired myocardial energy metabolism. Circulation. 2001;103:2387–2394. doi: 10.1161/01.cir.103.19.2387. [DOI] [PubMed] [Google Scholar]
- 44.Bai CG, Liu XH, Liu WQ, et al. Regional expression of the hypoxia-inducible factor (HIF) system and association with cardiomyocyte cell cycle re-entry after myocardial infarction in rats. Heart Vessel. 2008;23:193–200. doi: 10.1007/s00380-007-1029-2. [DOI] [PubMed] [Google Scholar]
- 45.Jürgensen JS, Rosenberger C, Wiesener MS, et al. Persistent induction of HIF-1alpha and -2alpha in cardiomyocytes and stromal cells of ischemic myocardium. FASEB J. 2004;18:1415–1417. doi: 10.1096/fj.04-1605fje. [DOI] [PubMed] [Google Scholar]
- 46.Kido M, Du L, Sullivan CC, et al. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol. 2005;46:2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 47.Parisi Q, Biondi-Zoccai GG, Abbate A. Hypoxia inducible factor-1 expression mediates myocardial response to ischemia late after acute myocardial infarction. Int J Cardiol. 2005;99:337–339. doi: 10.1016/j.ijcard.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 48.Zhu BL, Tanaka S, Ishikawa T, et al. Forensic pathological investigation of myocardial hypoxia-inducible factor-1 alpha, erythropoietin and vascular endothelial growth factor in cardiac death. Leg Med. 2008;10:11–19. doi: 10.1016/j.legalmed.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Lee SH, Wolf PL, Escudero R, et al. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–633. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 50.Shyu KG, Wang MT, Wang BW. Intramyocardial injection of naked DNA encoding HIF–1alpha/VP16 hybrid to enhance angiogenesis in an acute myocardial infarction model in the rat. Cardiovasc Res. 2002;54:576–583. doi: 10.1016/S0008-6363(02)00259-6. [DOI] [PubMed] [Google Scholar]
- 51.Philipp S, Jürgensen JS, Fielitz J. Stabilization of hypoxia inducible factor rather than modulation of collagen metabolism improves cardiac function after acute myocardial infarction in rats. Eur J Heart Fail. 2006;8:347–354. doi: 10.1016/j.ejheart.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Huang SA, Mulcahey MA, Crescenzi A, et al. TGF-{beta} promotes inactivation of extracellular thyroid hormones via transcriptional stimulation of type 3 iodothyronine deiodinase. Mol Endocrinol (Baltimore, Md.) 2005;19:3126–3136. doi: 10.1210/me.2005-0173. [DOI] [PubMed] [Google Scholar]
- 53.Euler-Taimor G, Heger J. The complex pattern of SMAD signaling in the cardiovascular system. Card Res. 2006;69:15–25. doi: 10.1016/j.cardiores.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Rosenkranz S. TGF-β1 and angiotensin networking in cardiac remodeling. Card Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 55.Lim H, Zhu YZ. Role of transforming growth factor-β in the progression of heart failure. Cell Mol Life Sci. 2006;63:2584–2596. doi: 10.1007/s00018-006-6085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuwahara F, Kai H, Tokuda K, et al. Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension. 2004;43:739–745. doi: 10.1161/01.HYP.0000118584.33350.7d. [DOI] [PubMed] [Google Scholar]
- 57.Villarreal FJ, Dillmann WH. Cardiac hypertrophy-induced changes in mRNA levels for TGF-beta 1, fibronectin, and collagen. Am J Physiol. 1992;262:H1861–H1866. doi: 10.1152/ajpheart.1992.262.6.H1861. [DOI] [PubMed] [Google Scholar]
- 58.Dai RP, Dheen ST, He BP, et al. Differential expression of cytokines in the rat heart in response to sustained volume overload. Eur J Heart Fail. 2004;6:693–703. doi: 10.1016/j.ejheart.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 59.Deten A, Hölzl A, Leicht M, et al. Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J Mol Cell Cardiol. 2001;33:1191–1207. doi: 10.1006/jmcc.2001.1383. [DOI] [PubMed] [Google Scholar]
- 60.Hao J, Wang B, Jones SC, et al. Interaction between angiotensin II and Smad proteins in fibroblasts in failing heart and in vitro. Am J Physiol. Heart Circ Physiol. 2000;279:H3020–H3030. doi: 10.1152/ajpheart.2000.279.6.H3020. [DOI] [PubMed] [Google Scholar]
- 61.Ikeuchi M, Tsutsui H, Shiomi T, et al. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc Res. 2004;64:526–535. doi: 10.1016/j.cardiores.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 62.Matsumoto-Ida M, Takimoto Y, Aoyama T, et al. Activation of TGF-beta1-TAK1–p38 MAPK pathway in spared cardiomyocytes is involved in left ventricular remodeling after myocardial infarction in rats. Am J Physiol. Heart Circ Physiol. 2006;290:H709–H715. doi: 10.1152/ajpheart.00186.2005. [DOI] [PubMed] [Google Scholar]
- 63.Song L, Yan W, Chen X, et al. Myocardial smad4 is essential for cardiogenesis in mouse embryos. Circ Res. 2007;101:277–285. doi: 10.1161/CIRCRESAHA.107.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schröder D, Heger J, Piper HM, et al. Angiotensin II stimulates apoptosis via TGF-beta1 signaling in ventricular cardiomyocytes of rat. J Mol Med (Berlin, Germany) 2006;84:975–983. doi: 10.1007/s00109-006-0090-0. [DOI] [PubMed] [Google Scholar]
- 65.Kester MH, Kuiper GG, Versteeg R, et al. Regulation of type III iodothyronine deiodinase expression in human cell lines. Endocrinology. 2006;147:5845–5854. doi: 10.1210/en.2006-0590. [DOI] [PubMed] [Google Scholar]
- 66.Park HK, Park SJ, Kim CS, et al. Enhanced gene expression of renin-angiotensin system, TGF-beta1, endothelin-1 and nitric oxide synthase in right-ventricular hypertrophy. Pharmacol Res. 2001;43:265–273. doi: 10.1006/phrs.2000.0777. [DOI] [PubMed] [Google Scholar]
- 67.Gianakopoulos PJ, Skerjanc IS. Hedgehog signaling induces cardiomyogenesis in P19 cells. J Biol Chem. 2005;280:21022–21028. doi: 10.1074/jbc.M502977200. [DOI] [PubMed] [Google Scholar]
- 68.Dentice M, Luongo C, Huang S, et al. Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc Natl Acad Sci USA. 2007;104:14466–14471. doi: 10.1073/pnas.0706754104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185–189. doi: 10.1056/NEJM200007203430305. [DOI] [PubMed] [Google Scholar]
- 70.Huang SA, Fish SA, Dorfman DM, et al. A 21-year-old woman with consumptive hypothyroidism due to a vascular tumor expressing type 3 iodothyronine deiodinase. J Clin Endocrinol Metab. 2002;87:4457–4461. doi: 10.1210/jc.2002-020627. [DOI] [PubMed] [Google Scholar]
- 71.Ruppe MD, Huang SA, Jan de Beur SM. Consumptive hypothyroidism caused by paraneoplastic production of type 3 iodothyronine deiodinase. Thyroid. 2005;15:1369–1372. doi: 10.1089/thy.2005.15.1369. [DOI] [PubMed] [Google Scholar]
- 72.Kester MH, Toussaint MJ, Punt CA et al (2008) Large induction of type III deiodinase (D3) expression after partial hepatectomy in the regenerating mouse and rat liver. Endocrinology doi:10.1210/en.2008-0344 [DOI] [PubMed]
- 73.Lavine KJ, Kovacs A, Ornitz DM. Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J Clin Investig. 2008;118:2404–2414. doi: 10.1172/JCI34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kusano KF, Pola R, Murayama T, et al. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005;11:1197–1204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- 75.Duman-Scheel M, Weng L, Xin S, et al. Hedgehog regulates cell growth and proliferation by inducing Cyclin D and Cyclin E. Nature. 2002;417:299–304. doi: 10.1038/417299a. [DOI] [PubMed] [Google Scholar]
- 76.McMahon S, Charbonneau M, Grandmont S, et al. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281:24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 77.Bijlsma MF, Groot AP, Oduro JP et al (2008) Hypoxia induces a hedgehog response mediated by HIF-1α. J Cell Mol Med [Epub ahead of print]. doi:10.1111/j.1582-4934.2008.00491.x [DOI] [PMC free article] [PubMed]
- 78.Rajabi M, Kassiotis C, Razeghi P, et al. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]