Abstract
The left parietal lobe has been proposed as a major language area. However, parietal cortical function is more usually considered in terms of the control of actions, contributing both to attention and cross-modal integration of external and reafferent sensory cues. We used positron emission tomography to study normal subjects while they overtly generated narratives, both spoken and written. The purpose was to identify the parietal contribution to the modality-specific sensorimotor control of communication, separate from amodal linguistic and memory processes involved in generating a narrative. The majority of left and right parietal activity was associated with the execution of writing under visual and somatosensory control irrespective of whether the output was a narrative or repetitive reproduction of a single grapheme. In contrast, action-related parietal activity during speech production was confined to primary somatosensory cortex. The only parietal area with a pattern of activity compatible with an amodal central role in communication was the ventral part of the left angular gyrus (AG). The results of this study indicate that the cognitive processing of language within the parietal lobe is confined to the AG and that the major contribution of parietal cortex to communication is in the sensorimotor control of writing.
Keywords: parietal, PET, somatosensory, speech, writing
Introduction
It is claimed that the left inferior parietal cortex, and the angular gyrus (AG) in particular, is central to amodal (spoken and written) word comprehension (Geschwind 1965; Mesulam 1998). A meta-analysis of functional neuroimaging studies on normal subjects is interpreted as supporting this view (Vigneau et al. 2006). In terms of production as well as comprehension, Catani and ffytche (2005) have proposed that Geschwind's “territory” (in inferior parietal cortex), anatomically connected to the territories of Wernicke (in auditory association cortex) and Broca (in the inferior frontal gyrus), is a core area for speech comprehension and production. In support of this hypothesis, a recent functional neuroimaging study demonstrated that activity in the left AG was common to both speech comprehension and production (Awad et al. 2007). By contrast, a recent model of the anatomy of language excludes the AG altogether from speech comprehension and production and attributes the processing of language to the temporal and frontal lobes alone (Hickok and Poeppel 2007).
Although usually considered in terms of cognitive processing, expressive language, spoken or written, is a complex motor act sustained over time. During development, speech is acquired first without explicit training. One computational model emphasizes the key roles of both auditory and somatosensory feedback during both the acquisition and maintenance of speech (Guenther 2006; Guenther et al. 2006). Although the response of auditory cortex to one's own voice is less than to the voice of another (Creutzfeldt et al. 1989; Houde et al. 2002), processing reafferent feedback is necessary when vocalizations are susceptible to many different forms of error (Garrett 1975; Levelt 1983; Smotherman 2007), such as spoonerisms (e.g., “you have tasted this worm,” instead of “you have wasted this term”). Experimentally, auditory neurons in both nonhuman primates and humans remain sensitive to manipulations of auditory feedback (Eliades and Wang 2008; Tourville et al. 2008). Limited studies on the role of parietal cortex in somatosensory monitoring during speech production suggest that it contributes to compensatory articulatory movements when jaw and lip movements are perturbed (Tremblay et al. 2003).
Writing is acquired later and, unlike speech, requires formal training (Feder and Majnemer 2007); but, akin to speech, models of writing skills postulate a dependence on polysensory (visual and somatosensory) feedback (Grossberg and Paine 2000). Writing is likely to be dependent on parietal function, as the control of upper limb movements places an emphasis on shifts of eye gaze, focused visual attention, and predictive representations of visual movement. These guide reaching, pointing, grasping, and precise placing of hand position in response to external visual cues (Corbetta and Shulman 2002; Castiello 2005). Although somatosensory processes have received rather less attention, they are also recognized as important in the control of actions in general (Dijkerman and de Haan 2007) and specifically in goal-directed hand movements (Gardner et al. 2007).
In this study, we directly contrasted the production of written (WrNa) and spoken narratives (SpNa). The purpose was to compare directly the role of parietal cortex in the control and maintenance of speech and writing and to assess whether inferior parietal cortex, and in particular the left AG, performs an amodal central role in narrative production. Previous studies that have investigated writing have mostly been performed with functional magnetic resonance imaging (fMRI), in a scanning environment that severely hampers upper limb movements and prevents direct visual control of writing. To avoid these major confounds, we used positron emission tomography (PET).
Experimental Procedures
Subjects
Functional neuroimaging data were collected from 13 healthy, right-handed volunteers (7 males, age range 40–70 years, mean 51.7). Each gave written informed consent to participate in the study for a total of 16 approved scans per subject in males aged >35 years and females aged >40 years. Prior approval was obtained from the Administration of Radioactive Substances Advisory Committee (Department of Health, UK) and from the research ethics committee of the Hammersmith Hospitals National Health Service Trust (now Imperial Academic Health Sciences Centre).
Behavioral Tasks
Sixteen scans were performed on each subject. Spoken and written responses were recorded for later analysis. Condition order was randomized within and between subjects. The subjects were required to produce written (WrNa) and spoken (SpNa) self-referential narratives, elicited by prompts (e.g., “tell me about the last family gathering you attended”). Three scans each were allocated to speech and writing. During the writing condition, the subjects wrote on a paper fixed to a board that was placed slightly above the subject's head, at a 45-degree angle. The board was clearly visible to the subject while his or her head was positioned in the scanner gantry. The right arm was supported at the elbow by foam rubber to achieve as normal a writing posture as possible while lying supine. A third condition requiring articulation was the production of a sequence of repeated syllables, either /ma/ or /la/ (SpSyl). The 2 spoken syllable conditions were allocated 2 scans each. A fourth condition was the production of a sequence of repeated single graphemes (w) over the course of 3 scans (WrG). A final unmatched condition, allocated 3 scans, was an odd/even number decision (NmTsk). The subjects determined if a heard number, randomly selected from 1 to 10, was odd or even and were instructed to press 1 of 2 computer mouse buttons after each number to indicate their decision. Each button press resulted in the presentation of the next number. The simple button presses were performed under tactile but not visual control with the left hand.
Functional Neuroimaging
The subjects were scanned on a Siemens (Knoxville, TN) HR++ (966) PET camera operated in high-sensitivity 3-dimensional mode, performed in conjunction with Hammersmith Imanet. The field of view (20 cm) covers the whole brain with a resolution of 5.1 mm full width at half maximum in x-, y-, and z-axes. A transmission scan was performed for attenuation correction.
The dependent variable in functional imaging studies is the hemodynamic response: a local increase in synaptic activity is associated with increased local metabolism coupled to an increase in regional cerebral blood flow (rCBF). Water, labeled with a positron-emitting isotope of oxygen (H215O), was supplied by Hammersmith Imanet. The tracer was used to demonstrate changes in rCBF, equivalent to changes in tissue concentration of H215O. During each scan, approximately 5 mCi H215O was infused as a slow bolus over 40 s, resulting in a rise in measurable emitted radioactivity (head counts) that peaked after 30–40 s. The tasks encompassed the incremental phase by commencing 10 s before the rise in head counts and continuing for 10–15 s after the counts began to decline because of washout and radioactive decay. Individual scans were separated by intervals of 6 min.
Image Analyses
Standard image preprocessing (image realignment, anatomical normalization, and smoothing with a 12-mm Gaussian filter) and whole-brain statistical analyses were performed using SPM2 software (Wellcome Department of Cognitive Neurology, Queen Square, London). We used a fixed-effects model to generate statistical parametric maps representing the results of voxelwise t-test comparisons for the contrasts between language and baseline conditions. The voxel-level statistical threshold was set at P < 0.05, with familywise error correction for multiple comparisons and a cluster extent threshold of 10 voxels. Use of a fixed-effects model allowed relative activation for all conditions to be directly visualized and assessed in all contrasts of interest. Describing regional activity during one condition relative to activity across all conditions avoids some of the potential errors of interpretation inherent in cognitive subtractions (Friston et al. 1996).
Image contrasts were performed in a number of stages. Five conditions were employed, and so a balanced design required 3 scans for each condition, out of a total of 16 permitted per subject. The additional scan was used to allow 2 scans each for the utterance of /la/ and /ma/ in the spoken syllable condition. This was done with the supplementary aim of defining motor cortex for the differences between an alveolar/ la/, and a bilabial /ma/, articulated with the tongue tip and lips, respectively. This contrast did not produce a result, probably because of the resolution of PET and/or close overlap of the motor somatotopy. For subsequent analysis, 1 scan of the 4 during the spoken syllable condition was randomly excluded across subjects, resulting in 3 scans, matching the number of scans in the other 4 conditions.
The main analysis was based on 4 conditions: SpNa, SpSyl, WrNa, and WrG. This factorial design contained the factors MODE (spoken and written) and CONTENT (narrative and nonnarrative). One main effect investigated the mode of output [i.e., (SpNa + SpSyl) − (WrNa + WrG) and (WrNa + WrG) − (SpNa + SpSyl)]. These contrasts demonstrated the distributed system for overt articulation (a compound of the activity for the muscular control of respiration, the larynx, and the articulators during overt “speech mode,” with accompanying auditory and somatosensory feedback) and that for writing (a compound of the activity controlling the right arm and hand during overt “writing mode,” with accompanying oculomotor control and visual and somatosensory feedback). The second main effect investigated narrative production relative to the meaningless but sustained production of spoken and written elements of language [i.e., (SpNa + WrNa) − (SpSyl + WrG) and (SpSyl + WrG) − (SpNa + WrNa)]. The interactions [i.e., (SpNa − SpSyl) − (WrNa − WrG) and (SpSyl − SpNa) − (WrG − WrNa)] are meaningless in terms of the functional anatomy of language processing. Therefore, these contrasts were not specified in the analysis.
We also investigated additional contrasts using the fifth condition, number task, as a baseline for the narrative conditions. A potential confound was that the monotonous production of a single syllable or grapheme might be accompanied by activity within the “default mode” network, which includes lateral parietal areas. Activity within this network is thought to reflect, at least in part, “self-referential” or “stimulus-independent” thoughts, mental ruminations that rely on declarative (semantic and episodic) memories perhaps expressed internally as covert language (inner speech) (Binder et al. 1999; Gusnard and Raichle 2001; Gusnard et al. 2001; Mazoyer et al. 2001). As in previous studies (Spitsyna et al. 2006; Awad et al. 2007), the number task condition, originally developed for the study of memory-related hippocampal function (Stark and Squire 2001), was used to ascertain whether the intrusion of stimulus-independent thoughts during the monotonous baseline tasks might have masked some activity associated with language processing. The assumption was made that processing of number semantics, executive processes associated with 2-way decision making and error monitoring, had little effect on activity with amodal processes, linguistic or memory related, associated with narrative production.
The overt generation of spoken and written language was associated with widely distributed activity in cortical, subcortical, and cerebellar areas. The purpose of this study was to investigate parietal function during speech and writing, and therefore, the presentation of the results and subsequent discussion focus on dissociations of parietal cortical activity across the different conditions. Activity in other areas is summarized in the Supplementary Table.
Results
Behavioral Performance
The subjects spoke and wrote at their preferred rate, resulting in different rates of output between individuals, tasks, and, in particular, modes of output. In relation to brain activity, there was no equivalence in terms of the rate of production of the responses between modes (grapheme to phoneme and written to spoken syllable). Even within a mode, there was no consistent unit; uttering a 3-syllable word like “holiday” is not equivalent to saying /la/ or /ma/ 3 times, due to differences in coarticulation across syllables. Similarly, letters in cursive script are not directly comparable to the repetitive production of /w/. Nevertheless, we considered the measures of output listed in Table 1 when interpreting the results to exclude the possibility that a difference in focal activity in a particular contrast might simply be the result of a confounding rate effect.
Table 1.
Measures of mean output across the different conditions
| Condition | Mean syllables/minute | Mean graphemes/minute | Mean words/minute |
| Sp/ma/ | 106 (range 68–151) | ||
| Sp/la/ | 118 (range 67–154) | ||
| SpNa | 192 (range 131–256) | 143 (range 101–182) | |
| WrNa | 165 (range 98–255) | 41 (range 25–59) | |
| WrG | 76 (range 50–106) |
Main Effect of MODE: Writing
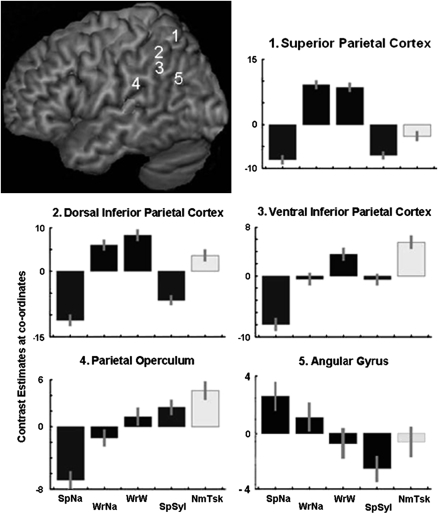
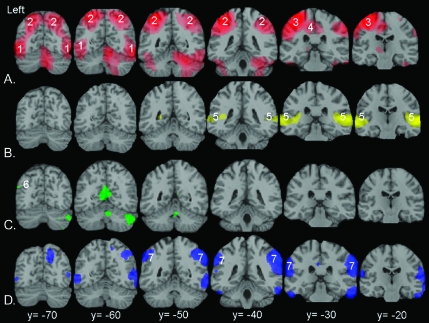
The writing mode (WNa + WrG) contrasted with the speech mode (SpNa + SpSyl) demonstrated distributed posterior cerebral and cerebellar activity (Fig. 2). Writing is executed under both visual and somatosensory guidance and is associated predominantly with forward (left to right) saccades along a line of self-generated writing, with some regressive saccades and an intermittent large right-to-left saccade for the beginning of the next line. The most posterior cerebral activity was in the foveal representation of primary visual cortex, which divided into 2 broad streams, ventral (along the middle occipital and inferior temporal gyri) and dorsal (into left and right posterior parietal cortices). Dorsal activity on the left continued forward into the superior parietal lobe, with peaks dorsal to the intraparietal sulcus (IPS) but extending down to include cortex within the sulcus. Corresponding activity was present on the right, although a greater effect size was evident on the left (Fig. 1). On the left, posterior parietal activity blended with the strong activation of primary sensorimotor and premotor cortices for the right hand in the left pre- and postcentral gyrus and central sulcus. Activity was also observed in medial parietal cortex in the supplementary sensory area within or close to the cingulate sulcus, which was continuous with activity in the ventral part of the medial premotor area. Throughout the extensive parietal system demonstrated by this contrast, activity during written narrative production was no greater than for written graphemes, even with the statistical threshold lowered to P < 0.05, uncorrected, with the exception of the left AG. Activity in the left AG was apparent as a main effect of narrative and is discussed below.
Figure 2.
Plots of contrast estimates, with 90% confidence intervals, for all 5 conditions (normalized around zero) at chosen peak voxels in the left cerebral hemisphere. These were located in the following: 1) the superior parietal cortex (Montreal Neurological Institute coordinates X = −30, Y = −56, Z = +58); 2) dorsal inferior parietal cortex (−42, −40, +44); 3) more ventral and lateral inferior parietal cortices (−60, −42, +36); 4) parietal operculum (−62, −26, +18); and the AG (−50, −68, +26).
Figure 1.
Significant activity overlayed onto coronal slices of a standard magnetic resonance imaging anatomical brain template from 20 to 70 mm caudal to the anterior commissure. (A) MODE: writing (WrNa + WrG) − (SpNa + SpSyl) in red. (1 and 2) Identify activity in the ventral and dorsal processing streams in left and right occipitotemporal and posterior parietal cortices, respectively. (3) Identifies activity in left primary sensorimotor cortex. (4) Identifies activity in the medial supplementary sensory and cingulate motor areas. (B) MODE: speaking (SpNa + SpSyl) − (WrNa + WrG) in yellow. (5) Identifies activity in the left and right superior temporal gyri, including the planum temporale. The only parietal activity was confined to the postcentral gyrus (not shown). (C) CONTENT: narrative (SpNa + WrNa) − (SpSyl + WrG) in green. (6) Identifies the spatially limited activity within the left ventral AG. (D) CONTENT: baseline (SpSyl + WrG) − (SpNa + WrNa) in blue. (7) Identifies activity in left and right inferior parietal cortices. Statistical threshold P < 0.05, familywise error corrected, spatial extent >10 voxels.
Main Effect of MODE: Speaking
The speech mode (SpNa + SpSyl) contrasted with the writing mode (WrNa + WrG) demonstrated bilateral activity within the ventral half of the pre- and postcentral gyri (Fig. 1), which was contiguous with activity along the length of both superior temporal gyri, including the planum temporale. The only parietal activity was confined to the postcentral gyrus (somatosensory cortex).
Main Effect of CONTENT: An Amodal System for Narrative Language
The contrast of narrative (SpNa + WrNa) with meaningless (SpSyl + WrG) conditions demonstrated prominent activity in frontal and left temporal areas. There was only a small volume of activity observed in parietal cortex, confined to the ventral part of the left AG, an area that we have previously termed the left temporo-occipito-parietal junction (Spitsyna et al. 2006; Awad et al. 2007).
Using the number task as the baseline condition for the 2 narrative conditions did not reveal any additional activity in higher order heteromodal or amodal cortical areas, including parietal cortex. Therefore, any intrusion of stimulus-independent thoughts during the monotonous baseline conditions of repetitively producing single syllables or graphemes was insufficient to reduce activity associated with the linguistic and memory processes that support narrative production.
Main Effect of CONTENT: An Amodal System for Repeated Meaningless Articulatory and Hand Movements
The repetitive, “meaningless” gestures (SpSyl + WrG) contrasted with the narrative mode (SpNa + WrNa) revealed activation in bilateral inferior parietal cortex, which was more extensive and had a greater effect size on the right (Fig. 1). There was additional activity in cortical areas outside the parietal lobes, in particular in prefrontal cortex (Supplementary Table).
Dorsal-to-Ventral Profiles of Activity within the Left Parietal Lobe
Following the statistical analyses, summary descriptive profiles of activity across all 5 conditions were constructed for 5 peak voxels within the left parietal lobe (Fig. 2). For the peak in the left superior parietal lobe, activity was present in the 2 writing conditions relative to the 3 others. A more ventral peak in the dorsal inferior parietal cortex close to the anterior part of the lateral bank of the IPS, an area associated with the control of grasping (Castiello 2005), showed activity for the 2 writing conditions, but with activity also during the number task (a condition that required grasping a computer mouse with the left hand and signaling decisions with finger presses) relative to the speech conditions. Activity during the written grapheme, number task, and, to a lesser extent, written narrative conditions persisted in more ventral inferior parietal cortex, including second-order somatosensory cortex (SII) in the parietal operculum, but here activity was also present for spoken syllables, that is, in SII activity was least during spoken narrative production. The fifth peak, located in the AG, confirmed that activity was increased during the narrative conditions relative to all other conditions. Activity during spoken narratives was greater than during written narratives, which may relate to the much greater narrative content produced during speaking (Table 1).
Discussion
By using PET, this is the first functional imaging study that has investigated normal narrative writing under both visual and somatosensory control. There is general agreement that the contralateral superior parietal cortex (including cortex within the IPS) is involved in writing (Nakamura et al. 2000; Menon and Desmond 2001; Beeson et al. 2003; Sugihara et al. 2006). This is in accord with focal lesion studies of patients with agraphia due predominantly to a deficit in the motor execution of writing rather than a central linguistic deficit (Basso et al. 1978; Alexander et al. 1992; Otsuki et al. 1999). However, this study has demonstrated more widely distributed parietal activity. Whether writing involved creating lines of text or repeated /w/s, there was bilateral activity in superior parietal cortex and the IPSs. Activity was also present in primary somatosensory cortex for the right upper limb, weakly present in bilateral SII and in the medial supplementary sensory area. This bilateral parietal activity would have encompassed a number of parallel processes. Foremost was the execution of writing under polysensory control, including pen grasp and self-directed upper limb movements to achieve letter shapes retrieved from procedural memory. We have assumed that directed saccades, with gaze predominantly focused on the tip of the pen, would have made additional contribution to the activity, although there is a paucity of studies investigating eye movements during writing (Gowen and Miall 2006.). If there was differential activity involved in retrieving multiple different letters to form words as opposed to repeatedly retrieving a single letter shape, the study was not sufficiently sensitive to reveal this distinction. Additional processes would have included the attentional and visuospatial resources required to control the layout and spacing of the text.
The very extensive parietal activity associated with writing was not matched by that observed during narrative speech production. Therefore, this study has demonstrated very limited involvement of the parietal lobes in the organization, execution, and monitoring of the complex movements that the many muscles that control respiration, the larynx, and the articulators make during normal speech production. This null result extended to the absence, or suppression, of activity within secondary somatosensory cortex, a region that nevertheless responded to meaningless articulatory movements—an observation also made in a recent study of speech production using fMRI (Dhanjal et al. 2008). One interpretation of this dissociation is that both sensorimotor integration and attention are directed predominantly toward auditory feedback during normal speech, whereas attention is divided between the 2 sensory modalities during the unusual task of syllable repetition. An alternative interpretation is that, once speech has been acquired, the online control of speech output shifts entirely to the superior temporal gyrus (STG) but without the implication that processing of reafferent sensory feedback is confined to the auditory domain. There is evidence that neurons in caudal auditory association cortex respond to somatosensory cues (Smiley et al. 2007). Thus, the response in the STG during speech may combine reafferent auditory and somatosensory signals, with little or no contribution from SII during the processing of reafferent sensory signal generated during speech production.
There was unexpected bilateral inferior parietal activity, right more than left, extending into ventral right dorsolateral prefrontal cortex, associated with the repetitive production of syllables and repeated writing of /w/s. This distribution of activity corresponds well with the cortical regions involved in sustained attention (Husain and Nachev 2007). Although normally considered in terms of sustained monitoring of sensory stimuli, based on the data from this study, the same system may be engaged during the sustained production of repeated meaningless gestures. Although greatest for repetitive syllable and grapheme production, activity in this system was also greater during narrative writing than narrative speech. This is in accord with the common perception that it is more “effortful” to write, whereas natural speech flows effortlessly.
The only parietal area that demonstrated amodal activity during narrative production was the ventral part of the left AG. Separate analyses of written narrative with written graphemes and spoken narrative with spoken syllables did not reveal activity in additional parietal areas that could be attributed to modality-specific linguistic or mnemonic processing. The spatial extent of this area was small relative to the other parietal areas revealed across the range of contrasts and therefore easy to dismiss as of little functional significance. However, the left AG is associated with lexical semantic processing according to the meta-analysis of Vigneau et al. (2006) and with analyzing meaning, over and above lexical semantics, that is conveyed by combinations of words in phrases and sentences (combinatorial semantics) (Humphries et al. 2007; Lau et al. 2008). A recent meta-analysis by Binder et al. (2009) proposes that the AG is at the top of a processing hierarchy “underlying concept retrieval and conceptual integration” and perhaps playing a particular role in “behaviors requiring fluent conceptual combination such as sentence comprehension.” Hickok and Poeppel (2007) proposed that the “combinatorial network” of speech processing involves the anterior middle temporal gyrus and the anterior inferior temporal sulcus, and they excluded the AG in their proposed network. However, it has been included in a more recent model proposed by Poeppel's group (Lau et al. 2008). Our group has repeatedly observed activation of this area in amodal narrative language comprehension and production (Blank et al. 2002; Spitsyna et al. 2006; Awad et al. 2007). Overall, the evidence strongly supports a role for the left AG as a component of a distributed language network, operating at high-order amodal level. An alternative suggestion is that the left AG forms part of a distributed working memory system (Buchsbaum and D'Esposito 2008), a system recruited during speech comprehension and production (Jacquemot and Scott 2006) and also required during written narrative production.
Defining the role of this spatially restricted cortical area will be assisted by behavioral observations following an isolated lesion of the AG, but these are very rare. The AG is most often destroyed after a stroke that also encompasses more anterior inferior parietal and posterior temporal cortices, when lost function may relate to a number of cortical areas or to anatomical disconnection secondary to damage to short and long white matter tracts (Catani and ffytche 2005). The occasional reports of a lesion confined to the AG report disorders of reading, spelling, or both. However, there may be a greater and more diverse loss of function soon after the lesion, with compensatory recovery intervening within days or weeks, and so detailed behavioral observations in the early phase after the ictus may be required (Hillis and Rapp 2004). Dejerine (1892) observed patients with chronic lesions in the AG who presented with disorders of reading, both with and without agraphia. However, more recent lesion studies and functional imaging data suggest that the critical region for processing orthographic stimuli when reading is more ventrally located in the left fusiform gyrus (Dehaene et al. 2002; Leff et al. 2006), and it no longer seems plausible that the function of the left AG is confined to processing written word forms. Therefore, it remains to be seen whether a widely accepted “neophrenological” interpretation of the AG's function emerges with further studies or whether its function is best understood as a core component of a distributed language network.
In conclusion, the results presented indicate extensive parietal activity associated with the planning, execution, and monitoring of writing, even if this involves only repeated formation of a single letter. The parietal lobes make little, if any, contribution to the planning, execution, and monitoring of articulation during normal spoken language production. Parietal activity for amodal linguistic or mnemonic processing was confined to the left AG. This posterior region was spatially insignificant compared with that associated with the execution of writing, but it is an area repeatedly observed in functional imaging studies of language (Blank et al. 2002; Spitsyna et al. 2006; Vigneau et al. 2006; Awad et al. 2007). It may be premature to exclude the AG from models of the functional anatomy of language (Hickok and Poeppel 2007; Saur et al. 2008), but it remains to be established whether its role is related more to lexical semantics, as proposed by Geschwind (1965), or alternative roles, such as the temporary storage of short sequences of the elements of language during production or comprehension (Jacquemot and Scott 2006), particularly when this involves executive (frontal) control of posterior cortical systems (Jefferies and Lambon Ralph 2006).
Funding
The Wellcome Trust (S.L.E.B.).
Supplementary Material
Supplementary material can be found at http://www.cercor.oxfordjournals.org/.
Supplementary Material
Acknowledgments
Conflict of Interest: None declared.
References
- Alexander MP, Fischer RS, Friedman R. Lesion localization in apractic agraphia. Arch Neurol. 1992;49:246–251. doi: 10.1001/archneur.1992.00530270060019. [DOI] [PubMed] [Google Scholar]
- Awad M, Warren JE, Scott SK, Turkheimer FE, Wise RJ. A common system for the comprehension and production of narrative speech. J Neurosci. 2007;27:1455–1464. doi: 10.1523/JNEUROSCI.5257-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso A, Taborelli A, Vignolo LA. Dissociated disorders of speaking and writing in aphasia. J Neurol Neurosurg Psychiatry. 1978;41:556–563. doi: 10.1136/jnnp.41.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson P, Rapcsak S, Plante E, Chargualaf J, Chung A, Johnson S, Trouard T. The neural substrates of writing: a functional magnetic resonance imaging study. Aphasiology. 2003;17:647–665. [Google Scholar]
- Binder J, Desai R, Graves W, Conant L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp055. Advance Access published March 27, doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J, Frost J, Hammeke T, Bellgowan P, Rao S, Cox R. Conceptual processing during the conscious resting state: a functional MRI study. J Cogn Neurosci. 1999;11:80–93. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Blank S, Scott S, Murphy K, Warburton E, Wise RJ. Speech production: Wernicke, Broca and beyond. Brain. 2002;125:1829–1838. doi: 10.1093/brain/awf191. [DOI] [PubMed] [Google Scholar]
- Buchsbaum B, D'Esposito M. The search for the phonological store: from loop to convolution. J Cogn Neurosci. 2008;20:762–778. doi: 10.1162/jocn.2008.20501. [DOI] [PubMed] [Google Scholar]
- Castiello U. The neuroscience of grasping. Nat Rev Neurosci. 2005;6:726–736. doi: 10.1038/nrn1744. [DOI] [PubMed] [Google Scholar]
- Catani M, ffytche D. The rises and falls of disconnection syndromes. Brain. 2005;10:2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman G. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O, Ojemann G, Lettich E. Neuronal activity in the human lateral temporal lobe. II. Responses to the subject's own voice. Exp Brain Res. 1989;77:476–489. doi: 10.1007/BF00249601. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec’ HG, Poline JB, Le Bihan D, Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport. 2002;13:321–325. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Contribution à l'étude anatomo-pathologique et clinique des différentes variétés de cécité verbale. Mém Soc Biol. 1892;4:61–90. [Google Scholar]
- Dhanjal N, Handunnetthi L, Patel M, Wise RJS. Perceptual systems controlling speech production. J Neurosci. 2008;28(40):9969–9975. doi: 10.1523/JNEUROSCI.2607-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkerman H, de Haan E. Somatosensory processes subserving perception and action. Behav Brain Sci. 2007;30:189–239. doi: 10.1017/S0140525X07001392. [DOI] [PubMed] [Google Scholar]
- Eliades S, Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008;453:1102–1106. doi: 10.1038/nature06910. [DOI] [PubMed] [Google Scholar]
- Feder KP, Majnemer A. Handwriting development, competency, and intervention. Dev Med Child Neurol. 2007;49:312–317. doi: 10.1111/j.1469-8749.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Price CJ, Fletcher P, Moore C, Frackowiak RS, Dolan RJ. The trouble with cognitive subtraction. Neuroimage. 1996;4:97–104. doi: 10.1006/nimg.1996.0033. [DOI] [PubMed] [Google Scholar]
- Gardner E, Babu K, Reitzen S, Ghosh S, Brown A, Chen J, Hall A, Herzlinger M, Kohlenstein J, Ro J. Neurophysiology of prehension. I. Posterior parietal cortex and object-oriented hand behaviors. J Neurophysiol. 2007;97:387–406. doi: 10.1152/jn.00558.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett MF. The analysis of speech production. In: Bower G, editor. The psychology of learning and motivation. London: Academic Press; 1975. pp. 133–177. [Google Scholar]
- Geschwind N. Disconnection syndromes in animals and man. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Gowen E, Miall RC. Eye-hand interactions in tracing and drawing tasks. Hum Mov Sci. 2006;25:568–585. doi: 10.1016/j.humov.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Grossberg S, Paine RW. A neural model of cortico-cerebellar interactions during attentive imitation and predictive learning of sequential handwriting movements. Neural Netw. 2000;13:999–1046. doi: 10.1016/s0893-6080(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Guenther F, Ghosh S, Tourville J. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang. 2006;96:280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH. Cortical interactions underlying the production of speech sounds. J Commun Disord. 2006;39:350–365. doi: 10.1016/j.jcomdis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Gusnard D, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hillis A, Rapp B. Cognitive and neural substrates of written language comprehension and production. In: Gazzaniga M, editor. The new cognitive neurosciences. 3rd ed. 2004. Cambridge (MA): MIT Press. p. 775–787. [Google Scholar]
- Houde J, Nagarajan SS, Sekihara K, Merzenich M. Modulation of the auditory cortex during speech: an MEG study. J Cogn Neurosci. 2002;14:1125–1138. doi: 10.1162/089892902760807140. [DOI] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Time course of semantic processes during sentence comprehension: an fMRI study. Neuroimage. 2007;36:924–932. doi: 10.1016/j.neuroimage.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci. 2007;11(1):30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemot C, Scott SK. What is the relationship between phonological short-term memory and speech processing? Trends Cogn Sci. 2006;10:480–486. doi: 10.1016/j.tics.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Lau EF, Phillips C, Poeppel D. A cortical network for semantics: (de)constructing the N400. Nat Rev Neurosci. 2008;9:920–933. doi: 10.1038/nrn2532. [DOI] [PubMed] [Google Scholar]
- Leff AP, Spitsyna G, Plant GT, Wise RJ. Structural anatomy of pure and hemianopic alexia. J Neurol Neurosurg Psychiatry. 2006;77:1004–1007. doi: 10.1136/jnnp.2005.086983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJ. Monitoring and self-repair in speech. Cognition. 1983;14:41–104. doi: 10.1016/0010-0277(83)90026-4. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdem O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Menon V, Desmond JE. Left superior parietal cortex involvement in writing: integrating fMRI with lesion evidence. Cogn Brain Res. 2001;12:337–340. doi: 10.1016/s0926-6410(01)00063-5. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Honda M, Okada T, Hanakawa T, Toma K, Fukuyama H, Konishi J, Shibasaki H. Participation of the left posterior inferior temporal cortex in writing and mental recall of kanji orthography: a functional MRI study. Brain. 2000;123:954–967. doi: 10.1093/brain/123.5.954. [DOI] [PubMed] [Google Scholar]
- Otsuki M, Soma Y, Arai T, Otsuka A, Tsuji S. Pure apraxic agraphia with abnormal writing stroke sequences: a report of a Japanese patient with a left superior parietal haemorrhage. J Neurol Neurosurg Psychiatry. 1999;66:556–563. doi: 10.1136/jnnp.66.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Kreher B, Schnell S, Kummerer D, Kellmeyer P, Vry M, Umarova R, Musso M, Glauche V, Abel S, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Hackett TA, Ulbert I, Karmas G, Lakatos P, Javitt DC, Schroeder CE. Multisensory convergence in auditory cortex, I. Cortical connections of the caudal superior temporal plane in macaque monkeys. J Comp Neurol. 2007;20:894–923. doi: 10.1002/cne.21325. [DOI] [PubMed] [Google Scholar]
- Smotherman MS. Sensory feedback control of mammalian vocalizations. Behav Brain Res. 2007;182:315–326. doi: 10.1016/j.bbr.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJ. Converging language streams in the human temporal lobe. J Neurosci. 2006;26:7328–7336. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark C, Squire L. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci USA. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara G, Kaminaga T, Sugishita M. Interindividual uniformity and variety of the “Writing center”: a functional MRI study. Neuroimage. 2006;32:1837–1849. doi: 10.1016/j.neuroimage.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Tourville JA, Reilly KJ, Guenther FH. Neural mechanisms underlying auditory feedback control of speech. Neuroimage. 2008;39:1429–1443. doi: 10.1016/j.neuroimage.2007.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Shiller DM, Ostry DJ. Somatosensory basis of speech production. Nature. 2003;423:866–869. doi: 10.1038/nature01710. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.