Abstract
Functional neuroimaging and lesion studies have frequently reported thalamic and putamen activation during reading and speech production. However, it is currently unknown how activity in these structures interacts with that in other reading and speech production areas. This study investigates how reading aloud modulates the neuronal interactions between visual recognition and articulatory areas, when both the putamen and thalamus are explicitly included. Using dynamic causal modeling in skilled readers who were reading regularly spelled English words, we compared 27 possible pathways that might connect the ventral anterior occipito-temporal sulcus (aOT) to articulatory areas in the precentral cortex (PrC). We focused on whether the neuronal interactions within these pathways were increased by reading relative to picture naming and other visual and articulatory control conditions. The results provide strong evidence that reading boosts the aOT–PrC pathway via the putamen but not the thalamus. However, the putamen pathway was not exclusive because there was also evidence for another reading pathway that did not involve either the putamen or the thalamus. We conclude that the putamen plays a special role in reading but this is likely to vary with individual reading preferences and strategies.
Keywords: dynamic causal modeling, effective connectivity, functional MRI, subcortical structures, word reading
Introduction
In this dynamic causal modeling (DCM) study, we were interested in how the putamen and thalamus contribute to reading. Previous functional imaging and lesion studies have already shown that the putamen and thalamus are involved in reading but little is known about how they participate in the conversion of orthographic input to articulatory output. Our questions focused on how reading aloud modulated the neuronal interactions between visual recognition and articulatory areas, specifically, whether these interactions were mediated by activity in the putamen, the thalamus, both, or neither. In addition, by including data from 28 healthy subjects reading regularly spelled English words, we were also able to consider intersubject variability in the neuronal pathways that support reading because both cognitive and anatomical models invariably include 2 or more possible mechanisms for reading regularly spelled words (Seidenberg and McClelland 1989; Coltheart et al. 1993; Plaut et al. 1996; Coltheart et al. 2001). Below, we discuss the previous studies that observed putamen and thalamic activation in reading and we then present the motivation for our experimental design and the rationale for our DCM analysis.
Our review of both the lesion and imaging literature provides some evidence that the putamen and thalamus are involved in reading at the level of speech production (e.g., Sakurai et al. 1993; Rosen et al. 2000; Riecker et al. 2002; Kuljic-Obradovic 2003; Binder et al. 2005; Bohland and Guenther 2006). For example, both regions were more activated when subjects read meaningless written syllables (Bohland and Guenther 2006) or completed stem words (Rosen et al. 2000) when performed aloud compared with silently. Activation in the left putamen and thalamus also increased when subjects monitored verbal output during syllable production (Riecker et al. 2002). Nevertheless, putamen and thalamic activation do not always co-occur as illustrated by observations that increased speech production rate increases thalamus activation but not putamen activation (Price et al. 1996; Palmer et al. 2001; Riecker et al. 2005, 2006). Likewise, several studies have reported increased activation in the thalamus for reading unfamiliar relative to familiar words but with no corresponding effect in the putamen (Fiebach et al. 2002; Binder et al. 2005; Borowsky et al. 2006). The left thalamus, but not the putamen, also plays a consistent role in name retrieval, irrespective of whether the stimuli are pictures of objects, written words, letters, or colors (Price and Friston 1997). Moreover, in a metanalysis of reading studies, Turkeltaub et al. (2002) found that the left thalamus was consistently activated but putamen activation was not mentioned.
One explanation for why putamen activation is inconsistent across reading studies is that its involvement depends on the reading strategy used. We recently, presented evidence to support this hypothesis in a study that segregated 2 different networks that were differentially activated across subjects who were all reading aloud familiar words with regular spellings (Seghier, Lee, et al. 2008). The left putamen was associated with one of these networks and was significantly more correlated with activation in anterior occipito-temporal sulcus (aOT) than posterior occipito-temporal cortex (pOT) (Seghier, Lee, et al. 2008). Intersubject variability in putamen activation has also been put forward in other contexts, including for instance counting (Hinton et al. 2004; Gandini et al. 2008), consistent with putamen activity varying with the cognitive strategy used by different subjects. Alternatively, instead of depicting the involvement of the putamen as a localized focus in an activation map that compared reading with baseline conditions, its role can be pictured as part of a processing network. This perspective fits with recent studies that advocated the importance of considering what is specific to reading not only as a set of localized regions but also in terms of specific interactions and networks (see discussion in Reinke et al. 2008). For instance, although pictures and words have been found to activate the same regions in the left occipito-temporal cortex (e.g., Moore and Price 1999; Price et al. 2006; Starrfelt and Gerlach 2007; Wright et al. 2008), modality specific processing becomes more evident at the system/network level (e.g., see Joseph et al. 2003; Reinke et al. 2008). It is in this perspective that assessing the interactions of both the putamen and thalamus during reading aloud takes on its importance. Specifically, we were interested here in the interactions between word recognition areas in occipito-temporal cortex (Cohen et al. 2002; Jobard et al. 2003; Price and Mechelli 2005; Vinckier et al. 2007) and articulation areas in the left precentral cortex (PrC) and whether these interactions are mediated via activity in the putamen, the thalamus or both.
To visualize the dynamics and the directions of the interaction in both putamen and thalamus, we went beyond the functional connectivity concept (e.g., Bokde et al. 2001; Hampson et al. 2006; Prat et al. 2007) and used DCM to estimate the neuronal dynamics of the modeled processes by means of a hemodynamic forward model (Friston et al. 2003). Several other studies have used DCM to model neuronal interactions with occipito-temporal (OT) regions during reading (Bitan et al. 2005; Mechelli et al. 2005; Booth et al. 2007; Nakamura et al. 2007; Cao et al. 2008; Chow et al. 2008; Heim et al. 2009); however, only one study (Booth et al. 2007) explored the interaction between the putamen and OT (with OT referred to as fusiform). Contrary to the expectations of the current study, Booth et al. (2007) did not find reading modulated inputs from the OT (fusiform) to the putamen. However, there are several key differences between the Booth et al. (2007) study and ours. First, the Booth et al. (2007) study used a very large OT region that included data from both pOT and aOT; therefore, it did not accommodate the known functional subdivisions within OT along the posterior-to-anterior axis (Moore and Price 1999; Jobard et al. 2003; Mechelli et al. 2005; Price and Mechelli 2005; Vinckier et al. 2007; Levy et al. 2008; Seghier, Lee, et al. 2008). Second, the interaction between OT and putamen was estimated in the context of a silent rhyme detection task that would have required a different strategy to the reading aloud task that we used. Third, the Booth et al. (2007) model included frontal, temporal and cerebellar regions associated with phonological processing, whereas our model included PrC and the thalamus. The context of the OT to putamen interactions are therefore not the same.
Last but not least, to determine whether regional interactions in our model were selective to reading, we need to include relatively matched baselines. Our experimental design therefore included 4 different conditions: Reading aloud, picture naming; saying “1,2,3” to meaningless symbols matched in size and complexity to the visual words; and saying 1,2,3 to pictures of meaningless nonobjects matched in size and complexity to the pictures of real objects. The 1,2,3 conditions partially controlled for visual input and articulatory output but not for the links between visual and articulatory regions. The picture naming condition fully controlled for speech output because the written words were the names of the objects in the pictures. It also controlled for some of the processes involved in retrieving phonology from visual inputs (Bookheimer and Zeffiro 1995; Moore and Price 1999; Price et al. 2006). However, unlike reading, picture naming cannot proceed without semantics (Glaser and Glaser 1989). Therefore, the DCM analysis will be able to identify the interactions that increased selectively during reading aloud when visual processing and articulation are fully controlled.
In summary, we aimed to characterize the interactions between OT and PrC regions that are mediated by the putamen, the thalamus, or both during reading aloud. Our discussion above leads us to the following hypotheses: 1) the interactions between left putamen and aOT will increase even when articulation is fully controlled; 2) the regional interactions with the putamen will be independent of the regional interactions with the thalamus; 3) there will be intersubject variability in the most effective reading pathway; and therefore 4) the pathway from aOT to PrC that involved the putamen will not be exclusive. We therefore set up a DCM model that included the following 5 left hemisphere regions: the posterior OT (pOT), the anterior OT (aOT), the putamen, thalamus, and the left PrC. Our aim was to identify the pathway between the (aOT) and output (PrC) regions that best explained the data. Our analyses compared regional interactions between the different conditions of our design and focused on pathways that were selective to reading.
Materials and Methods
Subjects
We started with a cohort of 58 right-handed healthy subjects. From this cohort, we selected 28 subjects who had robust and consistent activation in all 5 of our regions of interest (ROIs, see below for details). Our 28 selected subjects (12 females, 16 males, aged 27 ± 18 years) gave written informed consent to participate in this study. Subjects were native English speakers, had normal or corrected-to-normal vision, and had no history of neurological or psychiatric disorders. The study was approved by the National Hospital for Neurology and Institute of Neurology Joint Ethic's Committee.
Experimental Design
All stimuli were derived from a set of 192 objects with 3–6 letter common names with regular spelling-to-sound relationships: Thirty-three objects had 3-letter names (cat, bus, and hat), 65 had 4-letter names (ship, bell, frog, and hand), 58 had 5-letter names (teeth, camel, and snake), and 36 had 6-letter names (spider, dagger, and button). During 2 separate scanning sessions, subjects were asked to 1) read aloud 96 3- to 6-letter object names with consistent spelling-to-sound relationships (e.g., hat, tent, horse, and carrot); 2) name presented pictures of familiar objects; and 3) say 1,2,3 to meaningless pictures of symbols or nonobjects (unfamiliar stimuli). In each session, there were 4 different word-reading blocks that each lasted 18 s, with 12 words per block presented in triads (3 words together) every 4.5 s. By presenting triads of words (3 on the screen at a time), subjects were able to read the 3 high-frequency words very rapidly. This maximizes the efficiency of our experimental design by blocking events of interest together. There were also 4 blocks of object naming and 4 blocks of saying “123” to unfamiliar (meaningless) pictures of symbols or nonobjects, presented in exactly the same way as the reading blocks. In addition there were 6 blocks of fixation (14.4 s per block). For the reading and object naming conditions, triads of stimuli were constructed where there was no obvious semantic relationship between the 3 different items in the triad (e.g., slide, axe, and cup). Accuracy of vocal responses during all conditions was recorded with a MRI-compatible microphone. To minimize artefacts from head motion and airflow caused by the mouth opening and closing, subjects were instructed to whisper their response with minimal mouth movement. Although a sound cancellation system allowed us to identify the accuracy of vocal response, it was not possible to extract the response times. Stimulus presentation was via a video projector, a front-projection screen and a system of mirrors fastened to a head coil. Additional details about the paradigm and stimuli can be found in our previous work (cf. Seghier, Lee, et al. 2008; Kherif et al. 2009).
MRI Acquisition
Experiments were performed on a 1.5-T Siemens system (Siemens Medical Systems, Erlangen, Germany). In the functional MRI (fMRI) experiment, imaging consisted of a single shot gradient Echo Planar Imaging (EPI) sequence (repetition time/echo time/Flip = 3600 ms/50 ms/90°, field of view = 192 mm, matrix = 64 × 64, 40 axial slices, 2 mm thick with a 1-mm gap). Functional scanning was always preceded by 14.4 s of dummy scans to ensure tissue steady-state magnetization. To avoid ghost-EPI artefacts, a generalized reconstruction algorithm was used for data preprocessing.
fMRI Data Analysis
Data processing and statistical analyses were performed with the Statistical Parametric Mapping SPM5 software package (Wellcome Trust Centre for Neuroimaging, London, United Kingdom, http://www.fil.ion.ucl.ac.uk/spm/). All functional volumes were spatially realigned, un-warped, normalized to the Montreal Neurological Institute (MNI) space using the unified normalization–segmentation procedure of SPM5, and smoothed with an isotropic 6-mm full width at half maximum Gaussian kernel, with resulting voxels size of 2 × 2 × 2 mm3. Time series from each voxel were high-pass filtered (1/128-Hz cutoff) to remove low-frequency noise and signal drift. The preprocessed functional volumes of each subject were then submitted to a fixed-effects analysis, using the general linear model at each voxel. Each stimulus onset was modeled as an event in condition-specific “stick-functions” with a duration of 4.32 s per trial and a stimulus onset interval of 4.5 s. The resulting stimulus functions were convolved with a canonical hemodynamic response function that provided regressors for the linear model. The appropriate summary or contrast image was then entered into a second-level analysis (i.e., random-effects analysis) to enable inferences at the group level. From this second-level analysis, we generated statistical parametric maps of the t statistic at each voxel SPM{t}, which characterized differences in activation for any condition (i.e., word reading, object naming, and saying 123 to unfamiliar symbols and nonobjects) relative to fixation.
ROI Selection
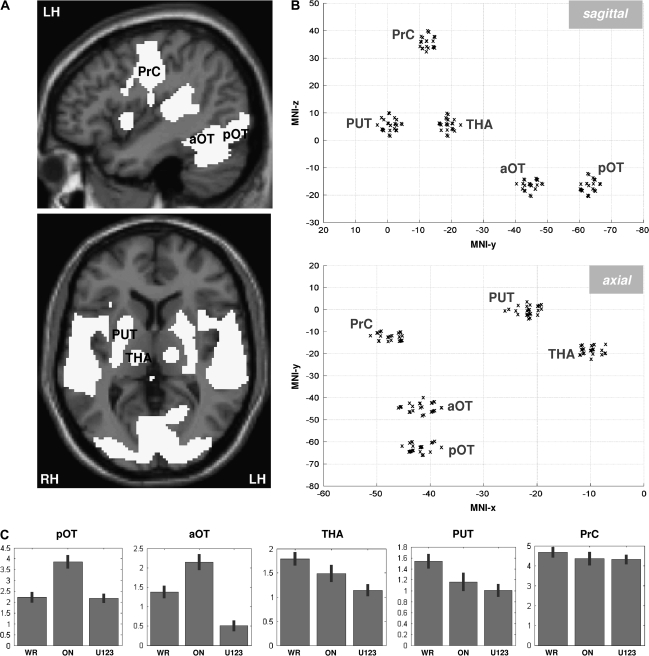
As described in the Introduction, our model included 5 regions. We hypothesized that visual information entered at the level of pOT and exited our model at PrC; we could then explore the pathways between these 2 regions and the intermediate roles played by aOT, thalamus, and putamen. Activation in pOT and PrC was consistent across all 3 conditions (reading, picture naming and saying 1,2,3) relative to fixation. In contrast, activation in aOT, putamen, and thalamus was higher during reading and naming than saying 1,2,3, which suggests that these areas were playing a role in linking visual inputs to motor outputs (Fig. 1). The coordinates (in MNI space) for our 5 ROIs were identified from the group analysis for the contrast “reading versus fixation” (P < 0.05 corrected): pOT = {x = −42, y = −62, z = −16}, aOT = {x = −42, y = −44, z = −16}, thalamus = {x = −10, y = −18, z = 6}, putamen = {x = −22, y = 0, z = 6}, and (v) PrC = {x = −48, y = −12, z = 36}, see Figure 1. Note that pOT and aOT here are very close to the 2 most consistent subdivisions of OT identified in a previous metanalysis of 35 neuroimaging studies of reading at {−44, −58, −15} and {−48, −41, −16}, respectively (see Jobard et al. 2003). In concordance with previous studies (Huang et al. 2001; Sakurai et al. 2001), the articulatory region PrC in the precentral gyrus is close to the “MLT–PMC” region (i.e., the “mouth, lips, and tongue” region of the primary motor cortex) that has been shown to be involved in motor processing of the mouth (e.g., see Fig. 1 of Huang et al. 2001).
Figure 1.
(A) Activation pattern for reading relative to fixation (group analysis, P < 0.05 corrected). The localization of the 3 cortical regions is illustrated on the sagittal view (x-MNI = −44 mm, top). The 2 subcortical regions are illustrated on the axial slice (z-MNI = +6 mm, bottom). pOT = posterior occipito-temporal sulcus, aOT = anterior occipito-temporal sulcus, THA = thalamus, PUT = putamen, and PrC = precentral gyrus. (B) Schematic projection of the individual coordinates of the 5 regions on a sagittal (top, Y–Z plan) and axial (bottom, X–Y plan) view. (C) Bar plot of the parameter estimates (±standard deviation) of each ROI at the group peak during word reading (WR), object naming (ON), and saying 123 to unfamiliar stimuli (U123).
After defining our 5 ROIs from the group analysis, eigenvectors (i.e., time series) were extracted in each subject (individual map thresholded at P < 0.05 uncorrected) at the closest maxima within a distance of 4 mm. Critically, this limit of 4-mm distance ensured that DCM models were comparable across subjects by incorporating consistent functional regions (for a similar rationale see Stephan, Marshall, et al. 2007). Previous DCM studies with reading that have included OT regions have used very liberal distances between regions across subjects (e.g., more than 20 mm in Bitan et al. 2005; Booth et al. 2007; Cao et al. 2008). This may yield inconsistent effects across subjects because data will come from different functional regions/subdivisions in different subjects. Therefore, we used much stricter criteria for region selection at the individual level to ensure robust and consistent effects across subjects and the most optimal implementation of the DCM. This was possible because we started with a large cohort of 58 right-handed subjects. We then selected those that had robust activation (P < 0.05 uncorrected) at a 4-mm distance from the group peaks of our 5 ROIs. The choice of ROIs within 4 mm from the group peaks maximized the spatial resolution of our data and ensured that there was no overlap in the voxels included in different ROIs. An examination of activity in our 58 subjects within 4 mm from the group peaks indicated that pOT was identified in 53 subjects, aOT in 45 subjects, thalamus in 40 subjects, putamen in 41 subjects, and PrC in 57 subjects. Although 42 subjects (72%) activated pOT, aOT, and PrC, only 28 (48%) also activated the thalamus and putamen. The remaining subjects did not satisfy our strict criteria even though activation was evident if we increased the distance from the group peaks (>8 mm) or lowered the statistical threshold (P > 0.1 uncorrected). Figure 1B illustrates the consistency of the ROI locations across our 28 subjects. Data (principal eigenvariates) were extracted for each session separately within each ROI (4-mm-radius sphere) and adjusted to the F-contrast (i.e., effects of interest) of each subject.
DCM Parameters
More details about DCM can be found elsewhere (e.g., Friston et al. 2003; Penny et al. 2004b; Stephan, Harrison, et al. 2007). Briefly, DCM can be considered as a hypothesis-driven neurodynamics model that uses a bilinear state equation to characterize an experimentally perturbed cognitive system. Basically, after defining a model with a set of regions and connections, DCM estimates the different parameters of this model at the neuronal level, using a hemodynamic forward model. This model then compares the generated/modeled functional responses to the measured ones (i.e., the extracted time series).
For a given model, DCM estimates 3 different sets of parameters: 1) input parameters that quantify how brain regions respond to external stimuli, 2) intrinsic parameters reflecting the effective or the latent connectivity that characterizes the coupling between regions, and 3) modulatory parameters that measure changes in effective connectivity induced by some experimental conditions. These different parameters are expressed in Hz within the DCM framework. It is important to keep in mind that 1) DCM is not an exploratory method because it is a generic approach designed to estimate and test explicit models, 2) parameters (intrinsic and modulatory) are estimated at the neuronal level (they are not directly accessible from the hemodynamic measures in fMRI), 3) the coupling between ROIs is not necessarily constrained by anatomical connections, and 4) the estimated model is context dependent, which means that interactions and coupling among regions are constrained by the user-specified driving and modulatory inputs.
DCM Model
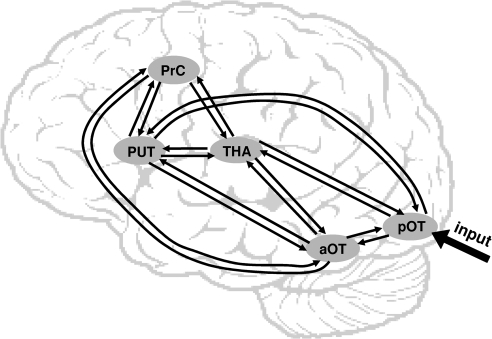
Practically, the extracted ROI time series were concatenated over the 2 sessions and incorporated in the DCM model. For each subject, we specified the model as follows (see Fig. 2): 1) the driving input (i.e., words, objects, unfamiliar symbols, and nonobjects grouped as one regressor) was connected to pOT, 2) pOT was connected to all regions except PrC, 3) PrC was considered as the system-output region that received inputs from all regions except pOT, 4) the connections between aOT, thalamus, and putamen were both forward and backward, and 5) word reading was used as a modulatory input to estimate the change in connection strength as a function of the reading task relative to all other conditions. Although we know that there are many other regions that participate in reading, the inclusion of these regions is not needed to estimate our questions of interest. For example, the direct connections between aOT and PrC would model any effects induced by other brain regions not included in our DCM.
Figure 2.
Schematic view of the DCM model (5 regions, input to pOT, 18 connections, modulated by reading).
Critically, the rationale for specifying this model is to ensure equivalence between the positions of the 3 regions that showed differential activations in reading versus saying 123 (e.g., Fig. 1C). Indeed, aOT, thalamus, and putamen occupied equivalent positions (i.e., level in the processing hierarchy) in the model as they all 1) received the same driving input propagation from pOT, 2) converged to the same motor output region PrC, and 3) their position in the DCM model was hence interchangeable. Importantly, by including object naming in the driving input, any significant modulation of effective connectivity by reading would imply that these modulations are highly specific to reading (i.e., object naming was used as a matched baseline). All parameters (intrinsic and modulatory) of the DCM model and their posterior probabilities were then assessed with Bayesian inversion by means of expectation-maximization algorithm (Friston et al. 2003).
DCM Group Analysis: The Local Approach
This approach aimed to identify the consistent effects across subjects for each parameter separately (intrinsic and modulatory) of the DCM. The significance of the parameters was thus assessed at the local (connection) level. Practically, in the absence of an a priori hypothesis about the exact modulatory effects, we modeled all the connections in Figure 2 by word reading (for a similar rationale see Bitan et al. 2005; Booth et al. 2007). Then, all intrinsic and modulatory effects (i.e., parameters) were assessed for each subject. Because of the known interindividual variability in effective connectivity (e.g., Mechelli et al. 2002), these estimated effects in each subject were subsequently submitted to t-tests (i.e., random-effect analyses) to identify the most consistent parameters across our 28 subjects. Significant effects were reported at P < 0.05 corrected (correction based on the number of tested connections: equivalent to P < 0.0028 for 18 tested connections; for a similar rationale see Booth et al. 2007; Sonty et al. 2007). The results from this local approach were then used to build further hypotheses for the global approach (see below) by limiting the plausible modulations to the connections that were consistent across our 28 subjects.
DCM Group Analysis: The Global Approach
This approach aimed to identify the set of modulatory connections that was most plausible in the context of word reading. Whereas the local approach (described above) guarantees that the connections considered for model selection are consistent across subjects, the global approach finds the optimal combination of these consistent local effects (connections) at the system level. By limiting our global models to those that are validated at the local level, we were also able to reduce the total number of models to 27 (as opposed to the 262,143 possible ways of adding 18 modulations!).
Practically, for each subject, we generated and estimated 27 models that had the same intrinsic connections (as in Fig. 2) but differed in where modulatory effects were specified (see Results section for more details). Then, to select the most plausible models, we used the Bayesian model selection (BMS) procedure as implemented in the most recent SPM8b version (Wellcome Trust Centre for Neuroimaging, London, United Kingdom, http://www.fil.ion.ucl.ac.uk/spm/). Although all previous DCM studies have used the Bayesian information criterion (BIC) and Akaike's information criterion (AIC) as measures of model evidence (for more details see Penny et al. 2004a), we preferred here to use the more robust and sensitive criterion based on the negative Free energy (F), see Stephan et al. (2009). Basically, the 3 criteria (AIC, BIC, and F) point to the optimal compromise between the accuracy and the complexity of a given model (i.e., “accuracy minus complexity”). However, unlike the AIC and BIC, the free energy F provides a better Laplace approximation for the complexity term (for more details see eq. 19 in Penny et al. 2004a) because it takes into account the interdependency between the estimated parameters. This is an important issue that may explain why some previous DCM studies have frequently observed high evidence in favor of the simplest model when using AIC/BIC criteria (e.g., a bias for high cost in penalty for complex models). In fact, as demonstrated recently, AIC and BIC are blind to how much the estimated parameters are dependent on each other, both a priori and a posteriori (Stephan et al. 2009), whereas the F criterion can take into account such interdependency and thus does not penalize models on the number of parameters alone. In other words, by using this optimal criterion F, we ensured here that 1) model complexity will not increase if additional parameters are “redundant” to existing parameters and 2) the parameter estimates of a good model are as precise and uncorrelated as possible (for a detailed discussion see Stephan et al. 2009).
After estimating all models and their evidence (the negative free energy F expressed here as a log evidence), we then computed the group evidence (of 27 models over 28 subjects) using the BMS procedure. To ensure that our BMS at the group level is robust (e.g., not affected by outliers, e.g., Ethofer et al. 2006; Allen et al. 2008), we used the hierarchical Bayesian approach developed recently by Stephan et al. (2009) that is considerably more robust in dealing with outliers (Stephan et al. 2009). Fundamentally, using a hierarchical model and variational Bayes, this “robust” BMS approach treats each model as a random variable and estimates the parameters of a Dirichlet distribution, which describes the probabilities for all models considered. It quantifies, in the context of a group of subjects, how likely it is that a specific model generated the data of a subject chosen at random. Here, we computed 2 measures for the group evidence of a given model (Stephan et al. 2009): 1) the Dirichlet parameter estimates (“alpha”) as a representative measure of the effective number of subjects in which a given model generated the observed data (sum of all alphas is equal to the number of subjects plus the number of compared models), and 2) the “exceedance” probability (xp) that describes the belief that a particular model is more likely than any other model given the group data (sum of all xp is equal to 1). Note, however, that these measures (i.e., alpha and xp) are not “absolute” for a particular model as their values depend on the relative preference/occurrence within the selected models. Although both measures are comparable with rank models at the group level, we preferred to use exceedance probability xp because it is particularly intuitive (i.e., all exceedance probabilities sum to one over all tested models). Throughout the results section, a winning model should therefore have an exceedance probability xp > 90%.
Finally, it is worth noting that it is perfectly valid to reverse the order of the local and global approaches if someone already has an a priori knowledge about the plausible models to be tested with DCM (see examples in Stephan, Marshall, et al. 2007; Allen et al. 2008; Heim et al. 2009). After comparing the plausible models with BMS, the local approach can then be used to identify the consistent parameters across subjects of the winning models.
Results
Behavioral Results
Accuracy across sessions was on average 99 ± 1% for word reading and 90 ± 9% for object naming. An accurate response was 1 when all 3 stimuli in a triad were read/named correctly.
FMRI Univariate Voxel-Based Analysis
Over our 28 subjects, word reading relative to fixation activated a large set of regions associated with visual, occulo-motor, attention, semantic access and articulatory processing (all 5 of our ROIs were activated in this contrast, see Fig. 1A). The comparison of reading versus saying 123 to symbols showed increased activation in aOT, thalamus, and putamen (e.g., Fig. 1C). In addition, this contrast included superior and middle temporal cortices bilaterally and the cerebellum but these regions were not included in our DCM analyses. More details about these activated patterns can be found elsewhere (e.g., see Seghier, Lee, et al. 2008; Kherif et al. 2009). For the current paper, the purpose of this analysis was only to select the coordinates for our 5 ROIs.
DCM Results
The Local Approach
Intrinsic connections.
All intrinsic connections were significant (i.e., consistent across all subjects) at P < 0.05 corrected. They were all positive, varying from 0.08 to 0.41 Hz (see Table 1). These intrinsic connections correspond to the latent (i.e., fixed) effective connectivity in our DCM model. In other words, these intrinsic connections represent the effective connectivity that is context independent (i.e., irrespective of input).
Table 1.
Consistent intrinsic connections in Hz at the group level (all significant at P < 0.05 corrected)
| To (in) | From (out) |
||||
| pOT | aOT | THA | PUT | PrC | |
| pOT | — | 0.15 | 0.09 | 0.08 | — |
| aOT | 0.33 | — | 0.12 | 0.11 | 0.14 |
| THA | 0.24 | 0.11 | — | 0.08 | 0.10 |
| PUT | 0.18 | 0.09 | 0.08 | — | 0.09 |
| PrC | — | 0.41 | 0.33 | 0.28 | — |
Note: pOT = posterior occipito-temporal sulcus, aOT = anterior occipito-temporal sulcus, THA = thalamus, PUT = putamen, and PrC = precentral gyrus.
Modulatory effects for word reading.
All forward connections were significantly and positively modulated by word reading (at P < 0.05 corrected, Table 2), except the connection from pOT to aOT, with one significant backward connection from PrC to putamen and reciprocal connections between putamen and thalamus. None of the modulations on the backward connections to pOT and aOT were significant (Table 2). Positive significant modulatory effects represent increased effective connectivity that is induced by the reading task relative to all other tasks.
Table 2.
Consistent modulatory effects in Hz during reading aloud over all subjects (all significant at P < 0.05 corrected)
| To (in) | Modulatory factor = reading |
||||
| From (out) | |||||
| pOT | aOT | THA | PUT | PrC | |
| pOT | — | ns | ns | ns | — |
| aOT | ns | — | ns | ns | ns |
| THA | 0.06 | 0.02 | — | 0.02 | ns |
| PUT | 0.08 | 0.03 | 0.03 | — | 0.02 |
| PrC | — | 0.06 | 0.05 | 0.05 | — |
Note: Positive values indicate an increase in interactions between regions during reading as compared with object naming and saying 123 to unfamiliar stimuli.
Consistent modulations across all 28 subjects.
There were 3 pathways (routes) between aOT and PrC that were positively and consistently modulated by reading across all 28 of our subjects. These were 1) via the putamen; 2) via the thalamus; and 3) a “direct” route that did not involve putamen or thalamus but may include other regions that are not explicitly modeled in our DCM. There were also 3 other connections that were positively modulated by reading. These were 4) pOT to thalamus, 5) pOT to putamen, 6) PrC to putamen, and 7) reciprocal connections between putamen and thalamus (see Table 2).
Critically, a consistent pathway (e.g., connection) at the local (parameter) level does not mean that the same pathway will be plausible at the global (systems) level across subjects. This is because the local approach does not test how evidence for one pathway changes with the presence of another pathway. The next analysis (the global approach below) aimed to test if all the positive local modulations were plausible at the system level. This identified the consistent effects that combined into the most efficient (i.e., plausible) systems (see Materials and Methods section). As mentioned above, previous studies have used the global level approach first and then conducted post hoc tests at the local level to check that all the local pathways are consistent across subjects. In our study, we reversed this procedure because knowing which pathways are consistent at a local level enabled us to limit the number of models tested at the global level to those that would be plausible following post hoc tests at the local level.
The Global Approach
Building the plausible models.
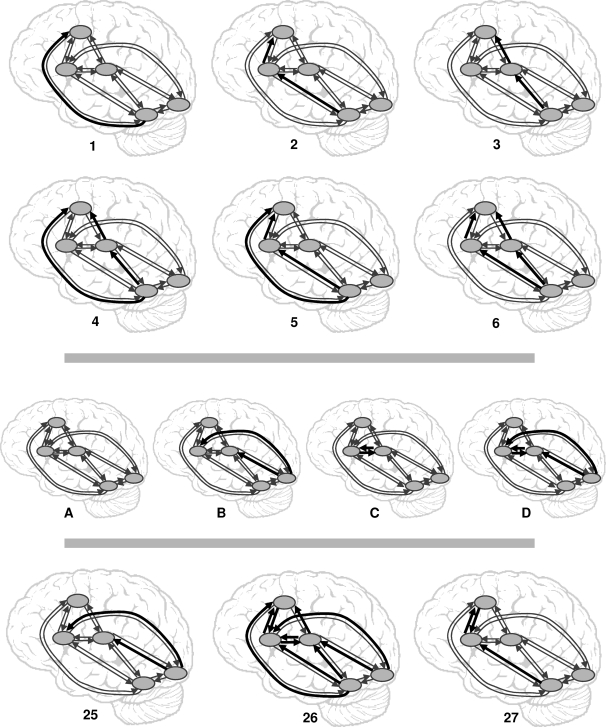
On the basis of the findings from the local analysis, we first generated 24 possible combinations of the 3 consistent routes between aOT to PrC. These models are illustrated in Figure 3. They all had the same intrinsic connections (as in Fig. 2) but differed in where word reading was included as a modulatory factor between aOT and PrC. They were categorized at 2 levels. At the first level, there were 6 ways that aOT can connect to PrC: 1) via a direct connection (not via putamen or thalamus), 2) via putamen, 3) via thalamus, 4) directly and via thalamus, 5) directly and via putamen, and 6) via thalamus and via putamen. At the second level, these 6 models were repeated in 4 different configurations: A) in the absence of any additional modulation; B) with modulations on the forward connections from pOT to thalamus and putamen; C) with modulations on the reciprocal connections between putamen and thalamus; and D) with modulations on both forward connections from pOT to thalamus and putamen and reciprocal connections between putamen and thalamus (see Fig. 3).
Figure 3.
Illustration of the 27 different models estimated and compared here (models 1–6, configurations A–D, and 3 additional models 25–27). These models are based on the most consistent effects across our 28 subjects from the fully connected model. Modulations with reading are shown with black and thick arrows.
In addition, we also considered 3 other models. Model (25) was the same as configuration B) (i.e., modulations on the forward connections between pOT to putamen and thalamus) but without any modulation on connections between aOT to PrC. This model was used to test the evidence when none of the 3 routes from aOT to PrC were modulated by reading. Model (26) represented a complex model that included all the modulatory effects that were consistent across subjects (Table 2). This model was specified to ensure that we were not losing evidence when using simpler models (i.e., 1–6 in Fig. 3) and also to test model evidence when all the 3 routes aOT-PrC were included. Finally, Model 27 modeled connections between aOT and PrC via putamen (i.e., Model 2 in configuration A) with a backward modulatory connection from PrC to putamen. This model was used to explicitly test the effect of the only significant backward modulation from PrC. We therefore had a total of 27 models for each of our 28 subjects. After estimation, these models were compared over subjects using the BMS procedure (as detailed in the Materials and Methods section).
The Best Configuration
The first step of the model comparison was to establish the best configuration (A vs. B vs. C vs. D) for the modulatory effects between aOT and PrC. To do this, we compared each type of configuration while keeping the modulations on aOT–PrC connections constant (e.g., 1A vs. 1B vs. 1C vs. 1D; 2A vs. 2B vs. 2C vs. 2D, etc). The BMS indicated clear evidence (i.e., very high exceedance probability xp > 90%) for configuration “A” for each Model 1-6 (see Table 3). This suggests that the evidence for Models 1–6 did not improve when increasing complexity (i.e., B, C, or D vs. A).
Table 3.
BMS for the group, based on the free energy “F”, of each model (1–6 in their different configurations A–D) and the 2 additional Models 25 and 26
| Model | 1A | 1B | 1C | 1D | 25 | 26 |
| BMS | (14.5; 0.91) | (2.8; 0.0) | (8.2; 0.09) | (2.7; 0.0) | (3.0; 0.0) | (2.9; 0.0) |
| 2A | 2B | 2C | 2D | 25 | 26 | |
| BMS | (17.1; 0.99) | (2.7; 0.0) | (6.2; 0.01) | (2.5; 0.0) | (2.7; 0.0) | (2.7; 0.0) |
| 3A | 3B | 3C | 3D | 25 | 26 | |
| BMS | (15.5; 0.96) | (2.8; 0.0) | (7.1; 0.04) | (2.7; 0.0) | (3.1; 0.0) | (2.8; 0.0) |
| 4A | 4B | 4C | 4D | 25 | 26 | |
| BMS | (15.4; 0.95) | (2.7; 0.0) | (7.5; 0.05) | (2.6; 0.0) | (3.3; 0.0) | (2.5; 0.0) |
| 5A | 5B | 5C | 5D | 25 | 26 | |
| BMS | (16.7; 0.98) | (2.6; 0.0) | (6.7; 0.02) | (2.5; 0.0) | (2.9; 0.0) | (2.4; 0.0) |
| 6A | 6B | 6C | 6D | 25 | 26 | |
| BMS | (16.4; 0.98) | (2.7; 0.0) | (6.8; 0.02) | (2.5; 0.0) | (3.1; 0.0) | (2.4; 0.0) |
Note: The BMS was performed between the models of each row (i.e., BMS analysis on 6 selected models). For each model, the values of alpha and the exceedance probability xp are provided (alpha; xp). Models with xp > 0.9 (shown in bold) are considered as the winning models (i.e., high group evidence).
The 3 Additional Models
As shown in Table 3, there was stronger evidence for Models 1–6 in configuration A (noted 1A–6A) even when the comparison included Models 25 and 26. Likewise, there was strong evidence (xp = 99.2%) for Model 2A compared with Model 27. Thus, BMS found no evidence for our 3 additional models. This suggests that the models were less plausible when they did not include modulations on the connections from aOT to PrC (Model 25) or when the additional modulation on the backward connection was included (Model 27) or when all possible (i.e., consistent) modulations were included (Model 26). Moreover, it illustrates that there was no overall preference for simpler or more complex models, for example, Model 25 is simpler but less plausible than Models 1–6C, whereas Model 26 is more complex but less plausible than Models 1–6A.
The Most Plausible Modulatory Connections between aOT and PrC
Having established that the most plausible configuration was A, the next step involved the pairwise comparison of models 1A–6A. The results are listed in Table 4 with strong evidence (>90%) for one model over another highlighted in bold and weak (trend) evidence (85% < xp < 90%) highlighted in bold italics.
Table 4.
BMS for the group, based on the free energy F, of each model (1A–6A)
| Versus | Model |
|||||
| 1A | 2A | 3A | 4A | 5A | 6A | |
| 1A | — | (17.8; 0.85) | (9.6; 0.02) | (11.4; 0.09) | (18.2; 0.88) | (12.1; 0.14) |
| 2A | (12.2; 0.15) | — | (6.6; 0.0) | (9.3; 0.02) | (15.5; 0.57) | (7.8; 0.0) |
| 3A | (20.4; 0.98) | (23.4; 1.0) | — | (19.0; 0.93) | (23.5; 1.0) | (16.6; 0.72) |
| 4A | (18.6; 0.91) | (20.7; 0.98) | (11.0; 0.07) | — | (22.3; 1.0) | (13.4; 0.27) |
| 5A | (11.8; 0.12) | (14.5; 0.43) | (6.5; 0.0) | (7.7; 0.0) | — | (6.9; 0.0) |
| 6A | (17.9; 0.86) | (22.2; 1.0) | (13.4; 0.28) | (16.6; 0.73) | (23.1; 1.0) | — |
Note: The BMS was performed between each pair of models (i.e., 15 BMS analyses) by comparing a model in a column to a model in a row. For each model, the values of alpha and the exceedance probability xp are provided (alpha; xp). Models with xp > 0.9 (shown in bold) are considered as the winning models (i.e., high group evidence). Weak (trend) evidences (85% < xp < 90%) are shown in bold italics.
The first thing to note is that reading modulated the connections from aOT to PrC via the putamen (Models 2A and 5A) rather than via the thalamus (Models 3A and 4A). There was also strong evidence that modulatory connections via the putamen were more plausible without the thalamus (Models 2A and 5A) than with the thalamus (Model 6A). From this we conclude that reading boosts the transfer of information from aOT to PrC via the putamen but not via the thalamus. The second thing to note is that the direct route alone (Model 1A) was more plausible than via the thalamus (Models 3A and 6A) or the direct route with the thalamus route (Model 4A). This again suggests that modulatory connections via the thalamus were not a plausible explanation of the data (i.e., thalamic interactions were not selective to reading). Finally, there was weak evidence (85% < xp < 90%) that reading modulations were stronger via the putamen (Models 2A and 5A) than the direct route (i.e., Model 1). However, putamen alone (Model 2A) was equally plausible (Table 4) to putamen combined with the direct route (Model 5A). This suggests that there may be more than one route from aOT to PrC, and thus the route via the putamen was not exclusive.
In summary, the comparison of 27 models suggests that the best fitting model is one where reading modulates connections from aOT to PrC via the putamen without the thalamus. However, the route via the putamen is unlikely to be exclusive.
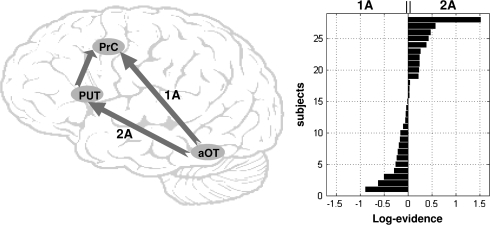
Intersubject Variability in the Best Global Model
The group comparisons suggest that, in addition to the putamen route, the direct route (i.e., not via the putamen or thalamus) may also be a plausible explanation of our data. To investigate this further, we compared Model 1A (direct route) with Model 2A (via putamen) in each subject. The results are illustrated in Figure 4. Some subjects showed a preference (i.e., weak evidence) for Model 1A, whereas other subjects showed a preference for Model 2A (see Fig. 4). These results provide further evidence that, in addition to the modulatory connections via the putamen, there may also be another pathway from aOT to PrC that does not include either the putamen or thalamus. None of the demographic (gender and age) or behavioral (the in-scanner accuracy) variables explained these individual differences (i.e., correlations between the Bayesian factor and these variables were not significant at P < 0.05).
Figure 4.
(Left) schematic view of the most plausible routes from aOT to PrC (direct in 1A, via the putamen in 2A or both in 5A). (Right) bar graph of the log evidence of each subject when comparing the direct route (Model 1A) with the route via the putamen (Model 2A).
Discussion
Although previous functional imaging studies have shown that the putamen and thalamus are activated during reading (e.g., Sakurai et al. 1992, 1993; Price et al. 1994, 1996; Rumsey and Horwitz 1997; Fiez et al. 1999; Kerr et al. 2004; Binder et al. 2005; Dietz et al. 2005; Borowsky et al. 2006; Hernandez and Fiebach 2006), activity in these regions is typically reported in “static” functional maps that ignore the numerous regional interactions that support the reading process. Thus, there is currently no account of how information is passed through these regions or how they interact with one another. In this paper, we investigated whether information flow from word recognition regions in the left ventral anterior occipito-temporal sulcus (aOT) to articulatory regions in the PrC was mediated by activity in the putamen, the thalamus, both, or neither. On the basis of prior studies, we hypothesized that the putamen would interact with aOT; however, we did not know whether this pathway would also include the thalamus, whether there would be an independent pathway via the thalamus or other regions, or how reading pathways might vary over subjects.
Our experimental design focused on regional interactions that were stronger for reading relative to picture naming or saying 1,2,3 to meaningless visual stimuli. Thus, we were looking for what is special about reading rather than looking for regional interactions that are the same for reading and picture naming. Our models were also constrained by the selection of 5 ROIs. Thus, we focused on the most anterior part of the region surrounding the ventral occipito-temporal sulcus rather than including more dorsal occipital or parietal regions. In part, our choice of regions was limited to those activated by reading relative to the 1,2,3, baseline condition (i.e., ventral OT but not parietal regions). We also had prior knowledge from Seghier, Lee, et al. (2008) that activation in the left putamen covaried, across subjects, with that in aOT. However, covariation in regional activation, across subjects, does not indicate whether the regions are part of the same system within subject; how the regions interact with one another; or whether the interactions are special for reading. Moreover, by using an optimal implementation of the DCM analysis in a context that required overt responses and a carefully defined anterior OT, we aimed to reveal consistent interactions between aOT and putamen that were not identified in a recent DCM study (Booth et al. 2007).
The Different Pathways from aOT to PrC
The results of our DCM analyses confirmed our hypothesis that the putamen increased interactions with aOT during reading. Interestingly, our findings extend those from our previous functional covariance study (Seghier, Lee, et al. 2008), by showing that the interaction with aOT was mainly forward (i.e., from aOT to putamen). In addition, we demonstrated strong evidence, at the system level, for an involvement of the putamen in the interactions between aOT and PrC (i.e., model 2A). Critically, this observation was derived from a comparison of reading to object naming and other control tasks. We can therefore infer that the modulatory connections from aOT to articulatory areas via the putamen are stronger for reading than picture naming, even though activation per se was not greater for reading than picture naming. To the contrary, in aOT, activation was higher for picture naming than reading (see Fig. 1C), which illustrates the complementary types of inferences that can be drawn from DCM and univariate analyses. This rationale has also been shown in recent DCM studies that observed complementary results between standard main effect analysis and DCM analyses during semantic (Sonty et al. 2007) or lexical decision (Chow et al. 2008; Heim et al. 2009) tasks.
On the other hand, one may argue that the specific interactions observed here during reading might be driven by differences in task difficulty. In fact, as mentioned in the Results section, the accuracy of our subjects is lower during object naming than reading, indicating different difficulty levels for our 3 tasks (saying 123, reading and naming). However, we argue here that our winning models were not biased by task difficulty for the following reasons: 1) all our 27 models differed only in the modulated connections, and because only one modulatory factor was used (reading as one contextual input), it is therefore reasonable to assume that task difficulty differences were “constant” across all models; 2) difficulty level in reading is intermediate (between saying 123 and object naming); therefore, the winning models cannot be driven by a task being too easy or too difficult; and 3) the DCM, as a generative model, explains in a mechanistic way the observed responses in the GLM analysis. In other words, effects and factors of interest are those that are visible in the GLM. Because our effects of interest cannot be explained by task difficulty alone (e.g., activity in the thalamus and putamen did not correspond to the task difficulty level), it is unlikely that difficulty is causing changes in effective connectivity between aOT, PrC, and subcortical regions. Our selected ROIs are also different from the set of regions (e.g., frontal, insular, cingulate, and parietal regions) that have previously been shown to be correlated with task difficulty during reading different kinds of words (e.g., see Binder et al. 2005).
Over and above showing the role of the putamen in transferring information from visual recognition to articulatory areas, our system level (global) analyses also indicated that thalamic interactions were not selective to reading between aOT to PrC (model 3A). Thus, although thalamic activation is more common than putamen activation during reading, it was the putamen, not the thalamus, that was found to play a special role in reading (models 2A and 5A). Nevertheless, we must emphasize that the absence of reading modulations via the thalamus, in the global analysis, may indicate that the thalamic pathway was not selective for reading. It does not exclude the possibility that the same thalamic pathway was involved in picture naming as well as reading. Nor does it exclude the possibility that the thalamus is involved in a different reading pathway that does not involve aOT, PrC, or the putamen.
Finally, we show that the best fit of our data was not limited to an exclusive reading pathway through the putamen. There was also evidence for another reading pathway between aOT and PrC that does not involve either the putamen or thalamus but may involve other regions that were not included in our models (model 1A). Analysis of each subject's data independently illustrated that the best fit of the data involved the putamen pathway in some subjects and the direct pathway in other subjects (see Fig. 4). The individual subject analysis therefore indicated variability in reading preferences, as predicted on the basis of behavioral (e.g., Baron and Strawson 1976; Zevin and Balota 2000; Beech 2002; Snow 2002; Hyona and Nurminen 2006) and neuroimaging (Prat et al. 2007; Seghier, Lee, et al. 2008; Kherif et al. 2009) studies of reading. One challenging issue in the future will be to identify the plausible phenotype–genotype associations that may explain the differences in the preferred reading model.
A Particular Role for the Putamen
We can ask whether the putamen pathway might correspond to a particular cognitive strategy. For example, unlike object naming, reading can proceed on the basis of nonsemantic sublexical relationships between orthography and phonology (Seidenberg and McClelland 1989; Coltheart et al. 1993; Plaut et al. 1996; Coltheart et al. 2001). Therefore, one might argue that the putamen supports phonological decoding in sublexical–nonsemantic reading. Consistent with this hypothesis, previous studies have shown that activity in the left putamen correlates with the demands (speed) on phonological processing (Tettamanti et al. 2005) and increases when verbal output must be monitored during syllable production (Riecker et al. 2002). These findings are also in line with a broader literature suggesting a role for the putamen in different phonological and speech output processes (Rosen et al. 2000; Murdoch 2001; Gil Robles et al. 2005; Riecker et al. 2005; Tettamanti et al. 2005; Bohland and Guenther 2006; Marchand et al. 2008), and with previous structural and functional connectivity studies that have observed significant connections between putamen and the primary and supplementary motor cortices, premotor cortex, and cerebellum (Henry et al. 2004; Wakana et al. 2004; Postuma and Dagher 2006; Leh et al. 2007; Di Martino et al. 2008; Draganski et al. 2008; Marchand et al. 2008).
On the basis of these findings, it is therefore tempting to propose that the reading pathway via the putamen is the signature for the extra coordination of complex motor sequences that is required when phonemes are assembled during sublexical reading. However, this interpretation is difficult to reconcile with observations that aOT activation is higher when phonological retrieval cannot proceed on the basis of sublexical processing. Thus, we observed greater aOT activation for object naming than reading (see Fig. 1) and several previous studies have reported greater aOT activation for reading exception words with irregular spellings than unfamiliar pseudowords (Herbster et al. 1997; Jobard et al. 2003; Mechelli et al. 2005; Price and Mechelli 2005). This conundrum of results may indicate that the putamen supports lexical or semantic access from aOT to the speech production system during reading. Consistent with this proposal, Sakurai et al. (1993) have suggested that the left putamen might be involved when semantic access is needed to read aloud Japanese phonograms (“kana” words).
An alternative perspective comes from considering studies that showed variation in subcortical activation across conditions. Here, the study by Ruz et al. (2005) is particularly relevant. These authors compared activation for written words versus consonant strings, superimposed on line drawings of familiar objects (Ruz et al. 2005). When the subjects attended to the letter strings, a typical pattern of reading activation was observed in frontal and temporal regions. However, when the subjects attended to pictures, leaving the letter strings unattended, the comparison of words to consonant strings resulted in thalamic and putamen activation that the authors associated with automatic reading processes. The results of the Ruz et al. (2005) study therefore lead us to suggest that intersubject variability in reading pathways may be a consequence of different levels of attention. However, further studies are required to test this and other interpretations because our paradigm was not designed to investigate functional differences in reading strategy across skilled readers.
Implication for Cognitive Models of Reading
One important implication of this study is the necessity to recognize the particular role of subcortical structures in cognitive models of reading. As far as we know, the only model of word reading that explicitly incorporated subcortical regions was that of Reichle et al. (2003) who suggested, in their “E-Z Reader” model, an important role for subcortical structures in modulating attentional resources during the control of eye movement in word reading (e.g., see Fig. 14 of Reichle et al. 2003). In a more general context, subcortical structures have been included in some language processing models (for review see Murdoch 2001; Whelan et al. 2003). For instance, Crosson's model (Crosson 1985, 1999) predicts an intermediate role for the basal ganglia linking language networks to speech output. Specifically, in this model called “the response release/semantic feedback model,” the thalamus played a pivotal role in semantic monitoring and the selection of lexical alternatives, whereas the basal ganglia (e.g., putamen and globus pallidus) played mainly an inhibitory role in the release of cortically formulated segments into the speech output system. In other words, the basal ganglia role is restricted to later language processes that require inhibition of competing alternatives (see also discussion in Longworth et al. 2005). Recently, in a more general framework, subcortical structures (mainly caudate nucleus, thalamus, and putamen) have been shown to be more involved when language processing cannot rely entirely on automatic processes but has to recruit controlled processes as well (Ketteler et al. 2008).
Based on these previous accounts, we hypothesize that the role of the left putamen in regular word reading is to control the release of phonological codes (e.g., articulation plans) to the speech output system. From our point of view, this hypothesis explains the interactions we observed between the left putamen and the word recognition (aOT) and articulation (PrC) systems. Although much further investigation is required to understand these interactions, we predict that the contribution of the putamen to reading depends on the strategy adopted by the reader.
Practical Issues
As detailed above, DCM offers a flexible framework to estimate effective connectivity at the neuronal level with high sensitivity in different contexts and between different regions. However, some practical issues should be acknowledged when using DCM to assess effective connectivity. Specifically, 1) the estimated model is context dependent, which means that interactions between 2 regions may depend on other included regions and connections, in addition to the specified driving and modulatory inputs; 2) significant interactions between 2 regions do not necessarily mean a direct effect between the 2 regions as this does not preclude the mediation of other regions that are not explicitly included in the DCM analysis; 3) a significant effective connection between 2 regions is independent from whether anatomical connections exist or not between the 2 regions; 4) effective connectivity, like functional responses, vary across subjects, and this is why connectivity results at the subject level were treated here as random variables and submitted to t-test analyses and we also estimated their consistency across subjects (see Fig. 4) due to the size of ROIs and their variable localizations across subjects, it was not possible to select specific putamen subdivisions and thalamic nuclei that have different functional properties; and 4) due to the practical limitation of the allowed maximum number of ROIs in DCM analysis, several interesting regions that are typically activated during reading aloud have not been considered in our model, including for instance the superior temporal gyrus.
In the same way, by restricting our analyses to a subset of subjects, one might argue about the generalizability of our findings. As a rule, DCM requires data from all regions in all subjects; however, there are numerous reasons why fMRI data may not be consistent across subjects. Most relate to noise in the data due to either the subject themselves (e.g., movement, attention, and weak hemodynamic responses) or the scanner acquisition protocols (e.g., signal stability). Over and above these data acquisition inconsistencies, subjects may use different strategies for the same task. This means that not all subjects will show robust activation in all the expected areas (e.g., see discussion in Seghier, Lazeyras, et al. 2008). To minimize noise in the data and accommodate intersubject variance in fMRI activation, previous DCM studies have focused their analysis on a subsample of their total subjects (e.g., Stephan, Marshall, et al. 2007). Others have used liberal distances from the area of interest (e.g., 20 mm in Bitan et al. 2005; Booth et al. 2007) in order to find robust activation in all their subjects. There are pros and cons to each of these solutions. In our paper, we wanted to ensure high spatial definition (e.g., to segregate aOT and pOT in all subjects); therefore, we only considered data that were within 4 mm of our selected group coordinates. By restricting our sample to those with the most robust and consistent data, we have produced strong evidence for how putamen activation is functionally connected to other regions of the reading system. However, it is also important to point out that our results only pertain to the subjects included in the analysis. For the remaining subjects who did not show a significant activation (at P < 0.05) in all of our ROIs, alternative models should be considered with different ROIs. However, the BMS procedure cannot be used in this context as model comparison can only operate on models with exactly the same nodes.
Another important methodological issue is the difference between the local and global approaches. The local approach allows us to determine the parameters that are significant and consistent across our subjects, whereas the global approach determines the best/optimal combination of local parameters. Several previous studies have used the findings of the local approach as representative of the best model at the system level (e.g., Bitan et al. 2005; Booth et al. 2007). The local approach can indeed be useful when no hypotheses are actually available to build plausible and valid models that can be tested with the global approach or when the number of all possible models (i.e., the search space) is very high to be practically tested (but see Leff et al. 2008 for DCM with few ROIs). In other words, by combining both approaches we guaranteed the following issues: 1) a reasonable number of possibilities to build plausible models for the global approach. For instance, the local approach revealed that only the backward connection between PrC and PUT was significant (tested in model 27), which reduced significantly the number of models with all possible combinations of backward connections; 2) all modulations that were combined at the system level (models 1–27) were all significant and consistent across subjects. This helps to avoid the situation of a winning model that contained inconsistent effects across subjects. However, we argue here against generalizing these local effects to the system level. For instance, our results indicated that the output model from the local approach that contained all consistent intrinsic and modulatory effects (Model 26) is not the model with the highest evidence at the global level (see Table 3). In other words, the local effects that were found to be highly consistent across our 28 subjects did not combine into the most effective way at the system level because the local approach by definition ignored all the dependencies between the estimated parameters (i.e., for each subject, these dependencies were approximated in the negative free energy as a measure of the model evidence). This is an important conceptual issue to keep in mind when comparing different DCM studies that used different approaches.
Nevertheless, despite the practical limitations detailed above, we ensured here an optimal implementation for DCM analysis by 1) using strict criteria for ROI selection that guaranteed comparable models across subjects, 2) limiting the generated models (hypotheses) to the most consistent modulatory effects across our 28 subjects, 3) ensuring that the estimated parameters were precise and uncorrelated by using sensitive criterion, based on the negative free energy to measure the relevance of each tested model, and 4) using the random-effects BMS to minimize the contribution of outlier subjects.
Conclusion
In conclusion, we demonstrated here, with DCM, how the left putamen played a pivotal role during reading aloud familiar words in skilled readers. Our DCM included critical reading areas in the left ventral occipito-temporal cortex and the effect of reading was compared with carefully designed control conditions that included articulation and object naming. The comparison of competitive models that differed in the pathways from word recognition to speech output yielded 2 possible pathways, one of which involved the putamen. Our findings bring additional support to the importance of incorporating subcortical structures in cognitive models of reading aloud. Future studies are now needed to see how the dynamics of subcortical structures change with the type of word and reading strategy in both skilled and unskilled readers.
Funding
Welcome Trust and the James S. MacDonnell Foundation (conducted as part of the Brain Network Recovery Group initiative).
Acknowledgments
We would like to thank our 3 radiographers (Amanda Brennan, Janice Glensman, and David Bradbury) as well as Clare Shakeshaft, Laura Stewart, and Tom Schofield for their help with fMRI data collection, Caroline Ellis for her help with data analysis, and Hwee Ling Lee and Sue Ramsden for their valuable help setting up the fMRI database. Conflict of Interest: None declared.
References
- Allen P, Mechelli A, Stephan KE, Day F, Dalton J, Williams S, McGuire PK. Fronto-temporal interactions during overt verbal initiation and suppression. J Cogn Neurosci. 2008;20:1656–1669. doi: 10.1162/jocn.2008.20107. [DOI] [PubMed] [Google Scholar]
- Baron J, Strawson C. Use of orthographic and word-specific knowledge in reading words aloud. J Exp Psychol Hum Percep Perfom. 1976;2:386–393. [Google Scholar]
- Beech JR. Individual differences in mature readers in reading, spelling, and grapheme–phoneme conversion. Curr Psychol. 2002;21:121–132. [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. Neuroimage. 2005;27:677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam MM. Shifts of effective connectivity within a language network during rhyming and spelling. J Neurosci. 2005;25:5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32:821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Tagamets MA, Friedman RB, Horwitz B. Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron. 2001;30:609–617. doi: 10.1016/s0896-6273(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA. Regional cerebral blood flow during object naming and word reading. Human Brain Mapp. 1995;3:93–106. [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007;1133:136–144. doi: 10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky R, Cummine J, Owen WJ, Friesen CK, Shih F, Sarty GE. FMRI of ventral and dorsal processing streams in basic reading processes: insular sensitivity to phonology. Brain Topogr. 2006;18:233–239. doi: 10.1007/s10548-006-0001-2. [DOI] [PubMed] [Google Scholar]
- Cao F, Bitan T, Booth JR. Effective brain connectivity in children with reading difficulties during phonological processing. Brain Lang. 2008;107:91–101. doi: 10.1016/j.bandl.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow HM, Kaup P, Raabe M, Greenlee MW. Evidence of fronto-temporal interactions for strategic inference processes during language comprehension. Neuroimage. 2008;40:940–954. doi: 10.1016/j.neuroimage.2007.11.044. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Curtis B, Atkins P, Haller M. Models of reading aloud: dual-route and parallel-distributed processing approaches. Psychol Rev. 1993;100:589–608. [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: a dual route cascaded model of visual word recognition and reading aloud. Psychol Rev. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Crosson B. Subcortical functions in language: a working model. Brain Lang. 1985;25:257–292. doi: 10.1016/0093-934x(85)90085-9. [DOI] [PubMed] [Google Scholar]
- Crosson B. Subcortical mechanisms in language: lexical-semantic mechanisms and the thalamus. Brain Cogn. 1999;40:414–438. doi: 10.1006/brcg.1999.1088. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Wlaters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state fMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dietz NA, Jones KM, Gareau L, Zeffiro TA, Eden GF. Phonological decoding involves left posterior fusiform gyrus. Hum Brain Mapp. 2005;26:81–93. doi: 10.1002/hbm.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethofer T, Anders S, Erb M, Herbert C, Wiethoff S, Kissler J, Grodd W, Wildgruber D. Cerebral pathways in processing of affective prosody: a dynamic causal modeling study. Neuroimage. 2006;30:580–587. doi: 10.1016/j.neuroimage.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, Von Cramon DY. fMRI evidence for dual routes to the mental lexicon in visual word recognition. J Cogn Neurosci. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Gandini D, Lemaire P, Anton JL, Nazarian B. Neural correlates of approximate quantification strategies in young and older adults: an fMRI study. Brain Res. 2008;1246:144–157. doi: 10.1016/j.brainres.2008.09.096. [DOI] [PubMed] [Google Scholar]
- Gil Robles S, Gatignol P, Capelle L, Mitchell MC, Duffau H. The role of dominant striatum in language: a study using intraoperative electrical stimulations. J Neurol Neurosurg Psychiatry. 2005;76:940–946. doi: 10.1136/jnnp.2004.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser WR, Glaser MO. Context effects in stroop-like word and picture processing. J Exp Psychol Gen. 1989;118:13–42. doi: 10.1037//0096-3445.118.1.13. [DOI] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca's area varies with reading ability. Neuroimage. 2006;31:513–519. doi: 10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Ischebeck AK, Friederici AD, Stephan KE, Amunts K. Effective connectivity of the left BA 44, BA 45, and inferior temporal gyrus during lexical and phonological decisions identified with DCM. Hum Brain Mapp. 2009;30:392–402. doi: 10.1002/hbm.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RG, Berman JI, Nagarajan SS, Mukherjee P, Berger MS. Subcortical pathways serving cortical language sites: initial experience with diffusion tensor imaging fiber tracking combined with intraoperative language mapping. Neuroimage. 2004;21:616–622. doi: 10.1016/j.neuroimage.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbster AN, Mintun MA, Nebes RD, Becker JT. Regional cerebral blood-flow during word and non-word reading. Human Brain Mapp. 1997;5:84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Fiebach CJ. The brain bases of reading late learned words: evidence from functional MRI. Visual Cogn. 2006;13:1027–1043. [Google Scholar]
- Hinton SC, Harrington DL, Binder JR, Durgerian S, Rao SM. Neural systems supporting timing and chronometric counting: an FMRI study. Brain Res Cogn Brain Res. 2004;21:183–192. doi: 10.1016/j.cogbrainres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Huang J, Carr TH, Cao Y. Comparing cortical activations for silent and overt speech using event-related fMRI. Hum Brain Mapp. 2001;15:39–53. doi: 10.1002/hbm.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyona J, Nurminen AM. Do adult readers know how they read? Evidence from eye movement patterns and verbal reports. Br J Psychol. 2006;97:31–50. doi: 10.1348/000712605X53678. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Joseph JE, Gathers AD, Piper GA. Shared and dissociated cortical regions for object and letter processing. Cogn Brain Res. 2003;17:56–67. doi: 10.1016/s0926-6410(03)00080-6. [DOI] [PubMed] [Google Scholar]
- Kerr DL, Gusnard DA, Snyder AZ, Raichle ME. Effect of practice on reading performance and brain function. Neuroreport. 2004;15:607–610. doi: 10.1097/00001756-200403220-00007. [DOI] [PubMed] [Google Scholar]
- Ketteler D, Kastrau F, Vohn R, Huber W. The subcortical role of language processing. High level linguistic features such as ambiguity-resolution and the human brain; an fMRI study. Neuroimage. 2008;39:2002–2009. doi: 10.1016/j.neuroimage.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Kherif F, Josse G, Seghier ML, Price CJ. The main sources of inter-subject variability in neuronal activation for reading aloud. J Cogn Neurosci. 2009;21:654–668. doi: 10.1162/jocn.2009.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljic-Obradovic DC. Subcortical aphasia: three different language disorder syndromes? Eur J Neurol. 2003;10:445–448. doi: 10.1046/j.1468-1331.2003.00604.x. [DOI] [PubMed] [Google Scholar]
- Leff AP, Schofield TM, Stephan KE, Crinion JT, Friston KJ, Price CJ. The cortical dynamics of intelligible speech. J Neurosci. 2008;28:13209–13215. doi: 10.1523/JNEUROSCI.2903-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leh SE, Ptito A, Chakravarty MM, Strafella AP. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett. 2007;419:113–118. doi: 10.1016/j.neulet.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J, Pernet C, Treserras S, Boulanouar K, Berry I, Aubry F, Demonet JF, Celsis P. Piecemeal recruitment of left-lateralized brain areas during reading: a spatio-functional account. Neuroimage. 2008;43:581–591. doi: 10.1016/j.neuroimage.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Longworth CE, Keenan SE, Barker RA, Marslen-Wilson WD, Tyler LK. The basal ganglia and rule-governed language use: evidence from vascular and degenerative conditions. Brain. 2005;128:584–596. doi: 10.1093/brain/awh387. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Lee JN, Thatcher JW, Hsu EW, Rashkin E, Suchy Y, Chelune G, Starr J, Barbera SS. Putamen coactivation during motor task execution. Neuroreport. 2008;19:957–960. doi: 10.1097/WNR.0b013e328302c873. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Lambon Ralph MA, Patterson K, McClelland JL, Price CJ. Dissociating reading processes on the basis of neuronal interactions. J Cogn Neurosci. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Penny WD, Price CJ, Gitelman DR, Friston KJ. Effective connectivity and intersubject variability: using a multisubject network to test differences and commonalities. Neuroimage. 2002;17:1459–1469. doi: 10.1006/nimg.2002.1231. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Price CJ. Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage. 1999;10:181–192. doi: 10.1006/nimg.1999.0450. [DOI] [PubMed] [Google Scholar]
- Murdoch BE. Subcortical brain mechanisms in speech and language. Folia Phoniatr Logop. 2001;53:233–251. doi: 10.1159/000052679. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Dehaene S, Jobert A, Le Bihan D, Kouider S. Task-specific change of unconscious neural priming in the cerebral language network. Proc Natl Acad Sci USA. 2007;107:19643–19648. doi: 10.1073/pnas.0704487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer ED, Rosen HJ, Ojemann JG, Buckner RL, Kelley WM, Petersen SE. An event-related fMRI study of overt and covert word stem completion. Neuroimage. 2001;14:182–193. doi: 10.1006/nimg.2001.0779. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Comparing dynamic causal models. Neuroimage. 2004a;22:1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Modelling functional integration: a comparison of structural equation and dynamic causal models. Neuroimage. 2004b;23:S264–S274. doi: 10.1016/j.neuroimage.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson K. Understanding normal and impaired word-reading: computational principles in quasi-regular domains. Psychol Rev. 1996;103:56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Prat CS, Keller TA, Just MA. Individual differences in sentence comprehension: a functional magnetic resonance Imaging investigation of syntactic and lexical processing demands. J Cogn Neurosci. 2007;19:1950–1963. doi: 10.1162/jocn.2007.19.12.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. NeuroImage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Price CJ, McCrory E, Noppeney U, Mechelli A, Moore CJ, Biggio N, Devlin JT. How reading differs from object naming at the neuronal level. Neuroimage. 2006;29:643–648. doi: 10.1016/j.neuroimage.2005.07.044. [DOI] [PubMed] [Google Scholar]
- Price CJ, Mechelli A. Reading and reading disturbance. Curr Opin Neurobiol. 2005;15:231–238. doi: 10.1016/j.conb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Frackowiak C. The effect of varying stimulus rate and duration on brain activity during reading. NeuroImage. 1996;3:40–52. doi: 10.1006/nimg.1996.0005. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Watson JD, Patterson K, Howard D, Frackowiak RS. Brain activity during reading. The effects of exposure duration and task. Brain. 1994;117(Pt 6):1255–1269. doi: 10.1093/brain/117.6.1255. [DOI] [PubMed] [Google Scholar]
- Reichle ED, Rayner K, Pollatsek A. The E-Z reader model of eye-movement control in reading: comparisons to other models. Behav Brain Sci. 2003;26:445–526. doi: 10.1017/s0140525x03000104. [DOI] [PubMed] [Google Scholar]
- Reinke K, Fernandes M, Schwindt G, O'Craven K, Grady CL. Functional specificity of the visual word form area: general activation for words and symbols but specific network activation for words. Brain Lang. 2008;104:180–189. doi: 10.1016/j.bandl.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Riecker A, Kassubek J, Groschel K, Grodd W, Ackermann H. The cerebral control of speech tempo: opposite relationship between speaking rate and BOLD signal changes at striatal and cerebellar structures. Neuroimage. 2006;29:46–53. doi: 10.1016/j.neuroimage.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64:700–706. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Riecker A, Wildgruber D, Dogil G, Grodd W, Ackermann H. Hemispheric lateralization effects of rhythm implementation during syllable repetitions: an fMRI study. Neuroimage. 2002;16:169–176. doi: 10.1006/nimg.2002.1068. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Ojemann JG, Ollinger JM, Petersen SE. Comparison of brain activation during word retrieval done silently and aloud using fMRI. Brain Cogn. 2000;42:201–217. doi: 10.1006/brcg.1999.1100. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B. Phonological and orthographic components of word recognition: a PET-rCBF study. Brain. 1997;120:739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Ruz M, Wolmetz ME, Tudela P, McCandliss BD. Two brain pathways for attended and ignored words. Neuroimage. 2005;27:852–861. doi: 10.1016/j.neuroimage.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Momose T, Iwata M, Sudo Y, Ohtomo K, Kanazawa I. Cortical activity associated with vocalization and reading proper. Brain Res Cogn Brain Res. 2001;12:161–165. doi: 10.1016/s0926-6410(01)00023-4. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Momose T, Iwata M, Watanabe T, Ishikawa T, Kanazawa I. Semantic process in kana word reading: activation studies with positron emission tomography. Neuroreport. 1993;4:327–330. doi: 10.1097/00001756-199303000-00026. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Momose T, Iwata M, Watanabe T, Ishikawa T, Takeda K, Kanazawa I. Kanji word reading process analysed by positron emission tomography. Neuroreport. 1992;3:445–448. doi: 10.1097/00001756-199205000-00017. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Pegna AJ, Annoni JM, Khateb A. Group analysis and the subject factor in functional magnetic resonance imaging: analysis of fifty right-handed healthy subjects in a semantic language task. Hum Brain Mapp. 2008;29:461–477. doi: 10.1002/hbm.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Lee HL, Schofield T, Ellis CL, Price CJ. Inter-subject variability in the use of two different neuronal networks for reading aloud familiar words. Neuroimage. 2008;42:1226–1236. doi: 10.1016/j.neuroimage.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychol Rev. 1989;96:523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Snow CE. 2002. An expanded review of the research on variability in reading comprehension In: Reading for understanding: toward a research and development program in reading comprehension. In: Snow CE, editor. pp 75–110. Santa Monica, CA: RAND. [Google Scholar]
- Sonty SP, Mesulam MM, Weintraub S, Johnson NA, Parrish TB, Gitelman DR. Altered effective connectivity within the language network in primary progressive aphasia. J Neurosci. 2007;27:1334–1345. doi: 10.1523/JNEUROSCI.4127-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrfelt R, Gerlach C. The visual what for area: words and pictures in the left fusiform gyrus. Neuroimage. 2007;35:334–342. doi: 10.1016/j.neuroimage.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Harrison LM, Kiebel SJ, David O, Penny WD, Friston KJ. Dynamic causal models of neural system dynamics:current state and future extensions. J Biosci. 2007;32:129–144. doi: 10.1007/s12038-007-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Marshall JC, Penny WD, Friston KJ, Fink GR. Interhemispheric integration of visual processing during task-driven lateralization. J Neurosci. 2007;27:3512–3522. doi: 10.1523/JNEUROSCI.4766-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ. Bayesian model selection for group studies. Neuroimage. 2009;46:1004–1017. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettamanti M, Moro A, Messa C, Moresco RM, Rizzo G, Carpinelli A, Matarrese M, Fazio F, Perani D. Basal ganglia and language: phonology modulates dopaminergic release. Neuroreport. 2005;16:397–401. doi: 10.1097/00001756-200503150-00018. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Whelan BM, Murdoch B, Theodoros D, Hall B, Silburn P. Building upon working theories of subcortical participation in language: integration of intrinsic basal ganglia circuitry. Acta Neuropsychol. 2003;1:174–193. [Google Scholar]
- Wright ND, Mechelli A, Noppeney U, Veltman DJ, Rombouts SA, Glensman J, Haynes JD, Price CJ. Selective activation around the left occipito-temporal sulcus for words relative to pictures: individual variability or false positives? Hum Brain Mapp. 2008;29:986–1000. doi: 10.1002/hbm.20443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevin JD, Balota DA. Priming and attentional control of lexical and sublexical pathways during naming. J Exp Psychol Learn Mem Cogn. 2000;26:121–135. doi: 10.1037//0278-7393.26.1.121. [DOI] [PubMed] [Google Scholar]