Abstract
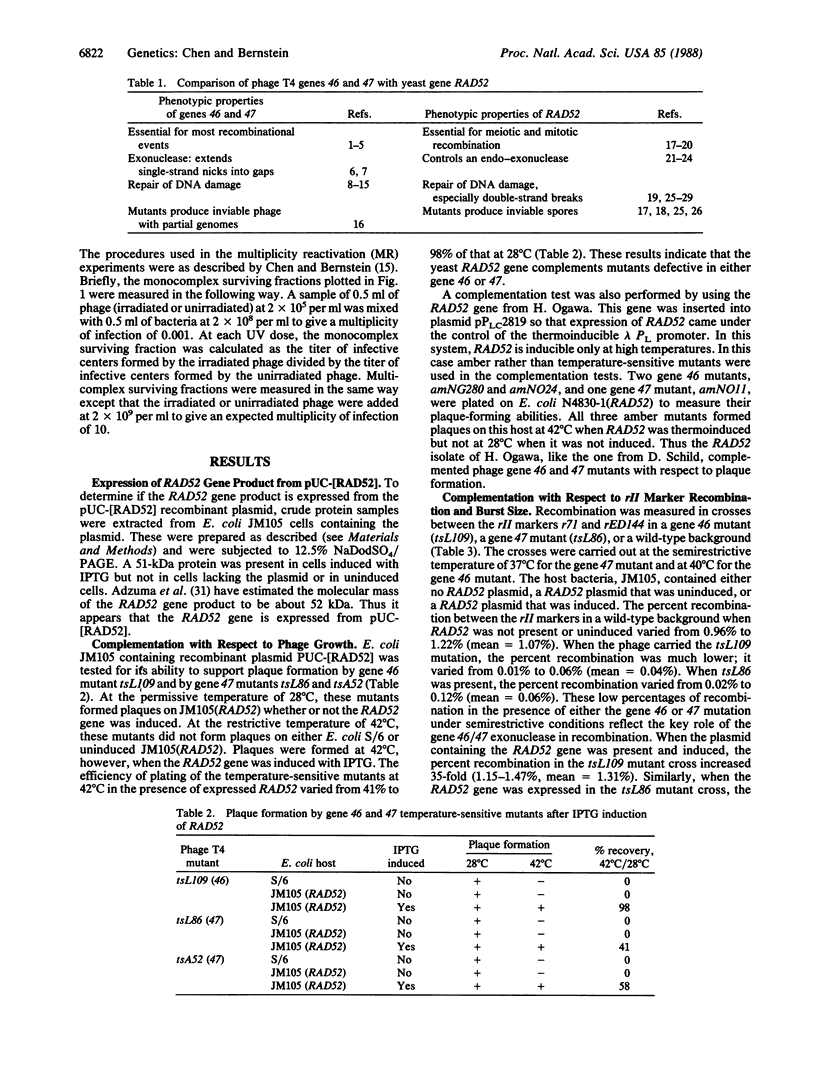
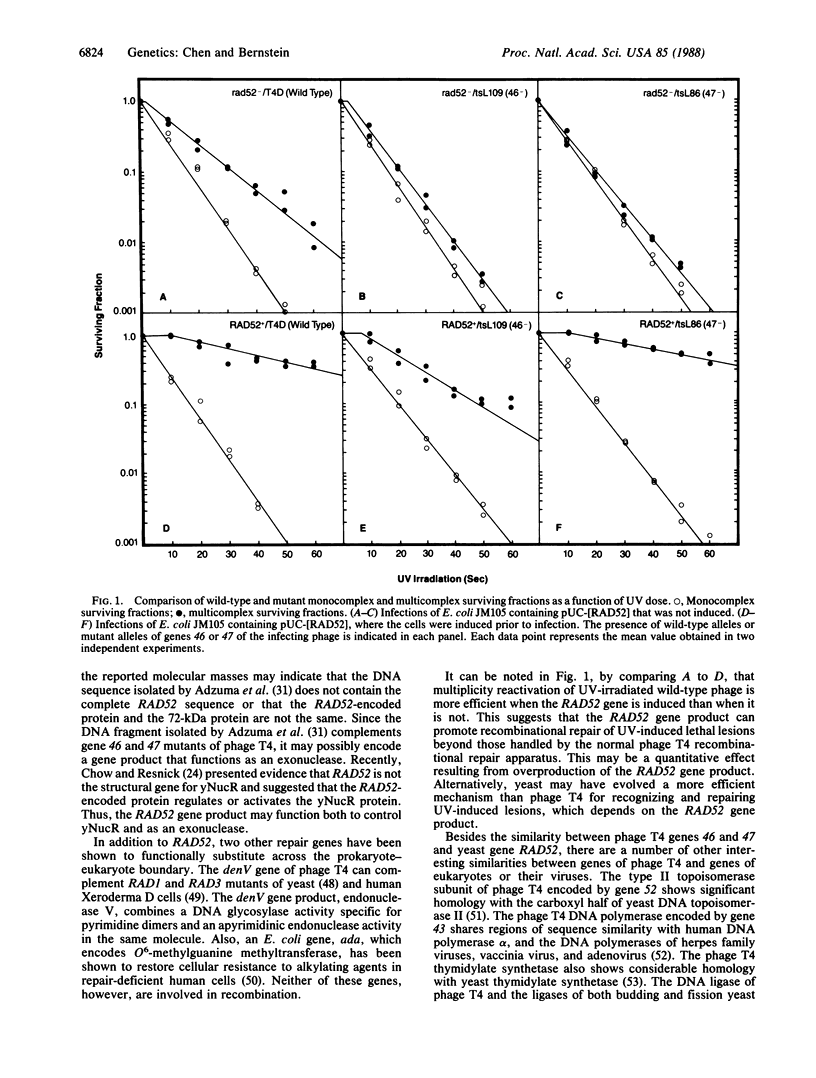
The RAD52 gene of Saccharomyces cerevisiae and genes 46 and 47 of bacteriophage T4 are essential for most recombination and recombinational repair in their respective organisms. The RAD52 gene was introduced into expression vectors that were used to transform Escherichia coli. The expression of RAD52 was then induced, and the ability of RAD52 to complement phage mutants defective in gene 46 or 47 was determined with respect to the three criteria of phage growth, recombination, and recombinational repair. RAD52 gene expression was found to allow growth of gene 46 and 47 mutants under otherwise restrictive conditions, as measured by plaque formation and burst size. Expression of the RAD52 gene also restored the ability of gene 46 and 47 mutants to undergo recombination of rII markers. Furthermore, the RAD52 gene restored the ability of gene 46 and 47 mutants to undergo recombinational repair after UV irradiation. The published DNA sequence of gene RAD52 was compared with the published sequences of genes 46 and 47. Although overall sequence similarities were only marginally significant, RAD52 and gene 46 had substantial sequence similarity over a limited region.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adzuma K., Ogawa T., Ogawa H. Primary structure of the RAD52 gene in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2735–2744. doi: 10.1128/mcb.4.12.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B. M. The DNA enzymology of protein machines. Cold Spring Harb Symp Quant Biol. 1984;49:1–12. doi: 10.1101/sqb.1984.049.01.003. [DOI] [PubMed] [Google Scholar]
- Arrand J. E., Squires S., Bone N. M., Johnson R. T. Restoration of u.v.-induced excision repair in Xeroderma D cells transfected with the denV gene of bacteriophage T4. EMBO J. 1987 Oct;6(10):3125–3131. doi: 10.1002/j.1460-2075.1987.tb02622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldy M. W. The UV sensitivity of some early-function temperature-sensitive mutants of phage T4. Virology. 1970 Feb;40(2):272–287. doi: 10.1016/0042-6822(70)90403-4. [DOI] [PubMed] [Google Scholar]
- Barker D. G., White J. H., Johnston L. H. Molecular characterisation of the DNA ligase gene, CDC17, from the fission yeast Schizosaccharomyces pombe. Eur J Biochem. 1987 Feb 2;162(3):659–667. doi: 10.1111/j.1432-1033.1987.tb10688.x. [DOI] [PubMed] [Google Scholar]
- Barker D. G., White J. H., Johnston L. H. The nucleotide sequence of the DNA ligase gene (CDC9) from Saccharomyces cerevisiae: a gene which is cell-cycle regulated and induced in response to DNA damage. Nucleic Acids Res. 1985 Dec 9;13(23):8323–8337. doi: 10.1093/nar/13.23.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Warren A. J., Fry K. E. Variations in genetic recombination due to amber mutations in T4D bacteriophage. J Virol. 1969 Feb;3(2):171–175. doi: 10.1128/jvi.3.2.171-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein C. Deoxyribonucleic acid repair in bacteriophage. Microbiol Rev. 1981 Mar;45(1):72–98. doi: 10.1128/mr.45.1.72-98.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H. Repair and recombination in phage T4. I. Genes affecting recombination. Cold Spring Harb Symp Quant Biol. 1968;33:325–331. doi: 10.1101/sqb.1968.033.01.037. [DOI] [PubMed] [Google Scholar]
- Bernstein H. The effect on recombination of mutational defects in the DNA-polymerase and deoxycytidylate hydroxymethylase of phage T4D. Genetics. 1967 Aug;56(4):755–769. doi: 10.1093/genetics/56.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broker T. R. An electron microscopic analysis of pathways for bacteriophage T4 DNA recombination. J Mol Biol. 1973 Nov 25;81(1):1–16. doi: 10.1016/0022-2836(73)90243-x. [DOI] [PubMed] [Google Scholar]
- Chen D., Bernstein C. Recombinational repair of hydrogen peroxide-induced damages in DNA of phage T4. Mutat Res. 1987 Sep;184(2):87–98. doi: 10.1016/0167-8817(87)90064-2. [DOI] [PubMed] [Google Scholar]
- Chow T. Y., Resnick M. A. An endo-exonuclease activity of yeast that requires a functional RAD52 gene. Mol Gen Genet. 1988 Jan;211(1):41–48. doi: 10.1007/BF00338391. [DOI] [PubMed] [Google Scholar]
- Chow T. Y., Resnick M. A. Purification and characterization of an endo-exonuclease from Saccharomyces cerevisiae that is influenced by the RAD52 gene. J Biol Chem. 1987 Dec 25;262(36):17659–17667. [PubMed] [Google Scholar]
- Chu F. K., Maley G. F., Maley F. RNA splicing in the T-even bacteriophage. FASEB J. 1988 Mar 1;2(3):216–223. doi: 10.1096/fasebj.2.3.3280375. [DOI] [PubMed] [Google Scholar]
- Chu F. K., Maley G. F., West D. K., Belfort M., Maley F. Characterization of the intron in the phage T4 thymidylate synthase gene and evidence for its self-excision from the primary transcript. Cell. 1986 Apr 25;45(2):157–166. doi: 10.1016/0092-8674(86)90379-x. [DOI] [PubMed] [Google Scholar]
- Davis K. J., Symonds N. The pathway of recombination in phage T4. A genetic study. Mol Gen Genet. 1974;132(2):173–180. doi: 10.1007/BF00272183. [DOI] [PubMed] [Google Scholar]
- Fry S. E. Stimulation of recombination in phage T4 by nitrous acid-induced lesions. J Gen Virol. 1979 Jun;43(3):719–722. doi: 10.1099/0022-1317-43-3-719. [DOI] [PubMed] [Google Scholar]
- Game J. C., Mortimer R. K. A genetic study of x-ray sensitive mutants in yeast. Mutat Res. 1974 Sep;24(3):281–292. doi: 10.1016/0027-5107(74)90176-6. [DOI] [PubMed] [Google Scholar]
- Game J. C., Zamb T. J., Braun R. J., Resnick M., Roth R. M. The Role of Radiation (rad) Genes in Meiotic Recombination in Yeast. Genetics. 1980 Jan;94(1):51–68. doi: 10.1093/genetics/94.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. E., Adhya S., Das A. Transcription antitermination by bacteriophage lambda N gene product. J Mol Biol. 1980 Jun 15;140(1):57–75. doi: 10.1016/0022-2836(80)90356-3. [DOI] [PubMed] [Google Scholar]
- Gram H., Rüger W. Genes 55, alpha gt, 47 and 46 of bacteriophage T4: the genomic organization as deduced by sequence analysis. EMBO J. 1985 Jan;4(1):257–264. doi: 10.1002/j.1460-2075.1985.tb02344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K. S. Induction of DNA double-strand breaks by X-rays in a radiosensitive strain of the yeast Saccharomyces cerevisiae. Mutat Res. 1975 Dec;30(3):327–334. [PubMed] [Google Scholar]
- Holmes G. E., Schneider S., Bernstein C., Bernstein H. Recombinational repair of mitomycin C lesions in phage T4. Virology. 1980 Jun;103(2):299–310. doi: 10.1016/0042-6822(80)90189-0. [DOI] [PubMed] [Google Scholar]
- Hosoda J. Role of genes 46 and 47 in bacteriophage T4 reproduction. III. Formation of joint molecules in biparental recombination. J Mol Biol. 1976 Sep 15;106(2):277–284. doi: 10.1016/0022-2836(76)90085-1. [DOI] [PubMed] [Google Scholar]
- Huang W. M. The 52-protein subunit of T4 DNA topoisomerase is homologous to the gyrA-protein of gyrase. Nucleic Acids Res. 1986 Sep 25;14(18):7379–7390. [PMC free article] [PubMed] [Google Scholar]
- Ishizaki K., Tsujimura T., Fujio C., Zhang Y. P., Yawata H., Nakabeppu Y., Sekiguchi M., Ikenaga M. Expression of the truncated E. coli O6-methylguanine methyltransferase gene in repair-deficient human cells and restoration of cellular resistance to alkylating agents. Mutat Res. 1987 Sep;184(2):121–128. doi: 10.1016/0167-8817(87)90068-x. [DOI] [PubMed] [Google Scholar]
- Jackson J. A., Fink G. R. Gene conversion between duplicated genetic elements in yeast. Nature. 1981 Jul 23;292(5821):306–311. doi: 10.1038/292306a0. [DOI] [PubMed] [Google Scholar]
- Johns V., Bernstein C., Bernstein H. Recombinational repair of alkylation lesions in phage T4. II. Ethyl methanesulfonate. Mol Gen Genet. 1978 Nov 29;167(2):197–207. doi: 10.1007/BF00266913. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Wilbur W. J., Smith T. F., Waterman M. S. On the statistical significance of nucleic acid similarities. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):215–226. doi: 10.1093/nar/12.1part1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Dujon B. Genetic exchanges between bacteriophage T4 and filamentous fungi? Cell. 1986 Aug 1;46(3):323–323. doi: 10.1016/0092-8674(86)90651-3. [DOI] [PubMed] [Google Scholar]
- Mickelson C., Wiberg J. S. Membrane-associated DNase activity controlled by genes 46 and 47 of bacteriophage T4D and elevated DNase activity associated with the T4 das mutation. J Virol. 1981 Oct;40(1):65–77. doi: 10.1128/jvi.40.1.65-77.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonn E. M., Bernstein C. Multiplicity reactivation and repair of nitrous acid-induced lesions in bacteriophage T4. J Mol Biol. 1977 Oct 15;116(1):31–47. doi: 10.1016/0022-2836(77)90117-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen-Lane J., Belfort M. Variable occurrence of the nrdB intron in the T-even phages suggests intron mobility. Science. 1987 Jul 10;237(4811):182–184. doi: 10.1126/science.3037701. [DOI] [PubMed] [Google Scholar]
- Prakash S., Prakash L., Burke W., Montelone B. A. Effects of the RAD52 Gene on Recombination in SACCHAROMYCES CEREVISIAE. Genetics. 1980 Jan;94(1):31–50. doi: 10.1093/genetics/94.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashad N., Hosoda J. Role of genes 46 and 47 in bacteriophage T4 reproduction. II. Formation of gaps on parental DNA of polynucleotide ligase defective mutants. J Mol Biol. 1972 Oct 14;70(3):617–635. doi: 10.1016/0022-2836(72)90562-1. [DOI] [PubMed] [Google Scholar]
- Remaut E., Tsao H., Fiers W. Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene. 1983 Apr;22(1):103–113. doi: 10.1016/0378-1119(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Resnick M. A., Chow T., Nitiss J., Game J. Changes in the chromosomal DNA of yeast during meiosis in repair mutants and the possible role of a deoxyribonuclease. Cold Spring Harb Symp Quant Biol. 1984;49:639–649. doi: 10.1101/sqb.1984.049.01.072. [DOI] [PubMed] [Google Scholar]
- Resnick M. A. Genetic control of radiation sensitivity in Saccharomyces cerevisiae. Genetics. 1969 Jul;62(3):519–531. doi: 10.1093/genetics/62.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Martin P. The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol Gen Genet. 1976 Jan 16;143(2):119–129. doi: 10.1007/BF00266917. [DOI] [PubMed] [Google Scholar]
- Saeki T., Machida I., Nakai S. Genetic control of diploid recovery after gamma-irradiation in the yeast Saccharomyces cerevisiae. Mutat Res. 1980 Dec;73(2):251–265. doi: 10.1016/0027-5107(80)90192-x. [DOI] [PubMed] [Google Scholar]
- Schneider S., Bernstein C., Bernstein H. Recombinational repair of alkylation lesions in phage T4. I. N-methyl-N'-nitro-N-nitrosoguanidine. Mol Gen Genet. 1978 Nov 29;167(2):185–195. doi: 10.1007/BF00266912. [DOI] [PubMed] [Google Scholar]
- Shalitin C., Kahana S. Conversion of T4 gene 46 mutant deoxyribonucleic acid into nonviable bacteriophage particles. J Virol. 1970 Sep;6(3):353–362. doi: 10.1128/jvi.6.3.353-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. R., Lagosky P. A., Storms R. K., Haynes R. H. Molecular characterization of the cell cycle-regulated thymidylate synthase gene of Saccharomyces cerevisiae. J Biol Chem. 1987 Apr 15;262(11):5298–5307. [PubMed] [Google Scholar]
- Valerie K., Fronko G., Henderson E. E., de Riel J. K. Expression of the denV gene of coliphage T4 in UV-sensitive rad mutants of Saccharomyces cerevisiae. Mol Cell Biol. 1986 Oct;6(10):3559–3562. doi: 10.1128/mcb.6.10.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. W., Wahl A. F., Yuan P. M., Arai N., Pearson B. E., Arai K., Korn D., Hunkapiller M. W., Wang T. S. Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J. 1988 Jan;7(1):37–47. doi: 10.1002/j.1460-2075.1988.tb02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yarosh D. B., Johns V., Mufti S., Bernstein C., Bernstein H. Inhibition of UV and psoralen-plus-light mutagenesis in phage T4 by gene 43 antimutator polymerase alleles. Photochem Photobiol. 1980 Apr;31(4):341–350. doi: 10.1111/j.1751-1097.1980.tb02551.x. [DOI] [PubMed] [Google Scholar]


