Abstract
The amygdala is consistently implicated in biologically relevant learning tasks such as Pavlovian conditioning. In humans, the ability to identify individual faces based on the social outcomes they have predicted in the past constitutes a critical form of associative learning that can be likened to “social conditioning.” To capture such learning in a laboratory setting, participants learned about faces that predicted negative, positive, or neutral social outcomes. Participants reported liking or disliking the faces in accordance with their learned social value. During acquisition, we observed differential functional magnetic resonance imaging activation across the human amygdaloid complex consistent with previous lesion, electrophysiological, and functional neuroimaging data. A region of the medial ventral amygdala and a region of the dorsal amygdala/substantia innominata showed signal increases to both Negative and Positive faces, whereas a lateral ventral region displayed a linear representation of the valence of faces such that Negative > Positive > Neutral. This lateral ventral locus also differed from the dorsal and medial loci in that the magnitude of these responses was more resistant to habituation. These findings document a role for the human amygdala in social learning and reveal coarse regional dissociations in amygdala activity that are consistent with previous human and nonhuman animal data.
Keywords: arousal, dorsal amygdala, fMRI, habituation, valence, ventral amygdala
Introduction
Studies of Pavlovian conditioning in both nonhuman animals (LeDoux 1996; Davis and Whalen 2001) and humans (Buchel et al. 1998, 1999; LaBar et al. 1998; Morris et al. 2001; Gottfried et al. 2002; Phelps et al. 2004) suggest that the amygdala plays an important role in learning the predictive value of biologically relevant stimuli. Facial expressions of emotion have also been shown to elicit increases in amygdala activity, presumably because they are salient, biologically relevant stimuli that have predicted important events in our environment (Whalen 1998). Indeed, the amygdala is more responsive to facial expressions embedded within an associative learning context than when presented in isolation (Hooker et al. 2006). Moreover, patients with bilateral amygdala damage judge strangers as being more approachable and trustworthy than controls (Adolphs et al. 1998) suggesting that the amygdala plays an important role in representing the social reinforcement value of other individuals (Adolphs 2001). Given that we form preferences for other people based on our previous experiences with them, the goal of the present study was to characterize the human amygdala's role in social conditioning, defined here as the associative process whereby we learn to identify individuals that have predicted threats or rewards in the past.
Work in nonhuman animals suggests that subnuclei within the amygdaloid complex differentially contribute to associative learning (see Discussion), and functional magnetic resonance imaging (fMRI) research has begun to use functional response profiles to spatially dissociate regions of the human amygdaloid complex. For example, Morris et al. (2001) showed that although responses within a ventral region of the amygdala did not habituate across trials during a traditional Pavlovian conditioning study, responses in a dorsal region did. These findings could be consistent with animal data showing that although some cells in the amygdala show early and transient increases in activity during learning, other cells maintain sustained representations of conditioned stimuli (Repa et al. 2001; Radwanska et al. 2002). In a separate line of inquiry, Whalen and colleagues reported that a lateral ventral portion of the human amygdala showed the greatest responses to facial expressions communicating negative valence, whereas responses in a medial ventral and a dorsal amygdala region were equally responsive to facial expressions communicating both positive and negative valence (Kim et al. 2003). These data were interpreted to suggest that different portions of the human amygdaloid complex show greater specialization for processing valence versus arousal, respectively.
Taken together, these studies predict that it should be possible to dissociate regional differences in responses across the human amygdaloid complex during learning at routinely utilized fMRI spatial resolutions. In this experiment, we monitored these regional differences in amygdala response during a social conditioning paradigm. We chose this modification of more traditional Pavlovian conditioning paradigms for several reasons. Our primary aim was to relate an extensive literature on the amygdala's role in associative learning tasks (i.e., Pavolovian conditioning) to a growing literature that implicates the amygdala in human social and emotional learning (Ohman and Mineka 2001; Hooker et al. 2006; Olsson et al. 2007; Olsson and Phelps 2007; Schiller et al. 2009). In humans, the ability to identify individual faces based on the social outcomes they have predicted in the past constitutes a critical form of association that, at a very basic level, mirrors other learning processes such as associating certain cues with biologically relevant outcomes. Moreover, the social reinforcers employed in the current paradigm allow us to manipulate valence while controlling for arousal. This is more difficult to do in traditional Pavlovian learning studies because primary reinforcers (such as the presence or absence of shock) elicit different levels of emotional arousal, making it difficult to dissociate amygdala response to valence vs. arousal.
During fMRI scanning, participants viewed faces that predicted either negative, positive, or neutral social outcomes. We predicted that dorsal and medial ventral regions would show signal increases to faces predicting negative and positive outcomes, consistent with previous human (Breiter et al. 1996; Whalen 1998; Kim et al. 2003; Somerville et al. 2006; Hare et al. 2008; Levita et al. 2009) and nonhuman animal studies (Gallagher et al. 1990; Baxter and Murray 2002; Paton et al. 2006). Additionally, we predicted that a lateral ventral region of the amygdala would differentially represent the learned valence of faces, with greatest sensitivity to faces predicting negative social outcomes (Repa et al. 2001; Radwanska et al. 2002; Kim et al. 2003). Finally, because human amygdala fMRI responses tend to habituate over time (Buchel et al. 1998; LaBar et al. 1998; Buchel et al. 1999; Morris et al. 2001; Phelps et al. 2001; Cheng et al. 2007; Hare et al. 2008), and animal data suggest it is possible to detect both habituating and nonhabituating responses within the amygdala (Repa et al. 2001; Radwanska et al. 2002), we also assessed variations in habituation rates within these regions of the amygdaloid complex.
Materials and Methods
Subjects
Forty-seven healthy adult participants were recruited for this experiment. Three participants were excluded because of technical problems during data collection and 2 were excluded based on excessive head motion. Thus, 42 healthy adult participants (21 males, mean age: 24.3 ± 3.96) were included in this analysis. All subjects were right-handed as assessed by the Edinburgh Handedness Inventory (Oldfield 1971) and free from psychiatric, neurological, and medical illness. Psychiatric history was assessed using an abbreviated version of the Non-Patient edition of the Structured Clinical Interview for DSM Disorders (First et al. 1995), which assessed for current and past history of major depressive disorder, dysthymia, hypomania, bipolar disorder, specific phobia, social anxiety disorder, generalized anxiety disorder, and obsessive compulsive disorder. Neurological and medical histories were assessed through self-report. This investigation was conducted in accordance with the guidelines of the Human Subjects Committee of the University of Wisconsin-Madison.
Stimuli
Visual stimuli were presented using E-Prime software (Psychology Software Tools, Pittsburgh, PA) through a fiber-optic goggle system (AVOTEC, Stuart, FL) mounted on a quadrature head coil. Face stimuli (Ekman and Friesen 1976) consisted of 3 male faces with neutral facial expressions (Identities: G.S., J.J., P.E.). Sentence stimuli were derived from an independent pilot study in which participants rated the valence (−4, very negative to +4, very positive) and arousal (1, “the least amount of emotional arousal I have ever felt” to 9, “the greatest amount of emotional arousal I have ever felt”) of self-relevant sentences. Based on these ratings, we selected 4 negative (e.g., He thinks you're stupid), 4 positive (e.g., He thinks you're smart), and 4 neutral (e.g., He thinks your shirt is blue) sentences (see Supplementary Materials) that were matched on several dimensions. The Negative and Positive sentences were matched for opposing content (i.e., stupid vs. smart), and were not significantly different in the absolute mean value of valence (Negative: −2.12 ± 0.30; Positive: 2.21 ± 0.05; t(6) = 0.545, P > 0.05) or arousal (Negative: 4.44 ± 0.29; Positive: 4.48 ± 0.16; t(6) = 0.245, P > 0.05). The Neutral sentences were rated as neither negative nor positive (valence: 0.17 ± 0.07) and were less emotionally arousing than both Negative and Positive sentences (arousal: 1.81 ± 0.14).
Paradigm
Orienting Phase
Participants initially viewed each face 20 times in a pseudorandomized order and separated by a fixation crosshair on an otherwise blank screen. This was done to acclimate subjects to the “to be conditioned” faces.
Conditioning Phase
Next, each face identity was paired with one category of self-relevant sentences (Negative, Positive, Neutral) and these pairs were presented in a pseudorandomized order. The face presentation always preceded the sentence presentation. Each of the 4 face–sentence pairings per valence was repeated 3 times, for a total of 12 trials per condition. Thus, one individual face always predicted negative social outcomes (i.e., insults), another always predicted positive social outcomes (i.e., compliments), whereas the third always predicted neutral social outcomes. Face identity–sentence category pairings were counterbalanced across participants. Participants were told that they would see a series of faces and sentences, and that each sentence provided information about the preceding face. Participants were not required to make any responses during this learning phase.
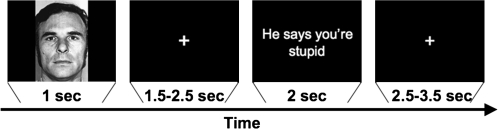
In each trial, a face was presented for 1 s, followed by a sentence presented for 2 s (Fig. 1). The interval between the onset of the face (CS) and the onset of the sentence (US) varied between 2.5 and 3.5 s (mean = 3.0 s), and the interval between the onset of the sentence and the onset of a face in the next trial varied between 4.5 and 5.5 s (mean = 5.0 s). This “jittered” timing allowed us to separately model overlapping hemodynamic responses to face and sentence stimuli (Buckner et al. 1996; Friston et al. 1999).
Figure 1.
Experimental paradigm. In a single trial, participants viewed each face for 1 s, followed by a variable interstimulus interval, and then a sentence for 2 s. Trials were separated by a variable intertrial interval averaging 3 s.
To document that subjects associated the intended social value with the face identities, subjects viewed each face and provided likeability and arousal ratings after each phase (i.e., Orienting, Conditioning) of the task. Each face was presented for 1 s in the absence of the sentences, followed by a rating screen asking, “How much do you like this person?” (−4, “not at all” to +4 “very much”) and “How much emotional arousal do you feel when looking at this face?” (1, “the least amount of emotional arousal I have ever felt” to 9 “the most amount of emotional arousal I have ever felt”).
fMRI Image Acquisition
Images were acquired on a 3.0 Tesla MRI scanner (General Electric Signa; Waukesha, WI) with high speed imaging gradients and a quadrature head coil. Anatomical images were whole brain high-resolution T1-weighted scans (3D Inversion Recovery [IR]; 256 × 256 in-plane resolution; 240-mm field of view (FOV); 124 × 1.1 mm axial slices). Functional scans consisted of an echo planar sequence with a 2000-ms repetition time, 33-ms echo time, and a 60° flip angle with 18 contiguous 3-mm-thick interleaved coronal slices (0.5-mm interslice gap; 64 × 64 in-plane resolution, 180-mm FOV). Due to our a priori focus on the amygdala, slices were centered over the medial temporal lobe, covering most of the frontal cortex (missing only the most anterior aspects of the frontal pole) and temporal cortex (including the amygdala and hippocampus), but did not include the majority of the parietal and occipital cortices. This acquisition scheme utilizes a voxel size that strikes a balance between adequate signal to noise ratio while reducing voxel-dephasing which contributes to susceptibility signal dropout. These parameters were based on our previous studies demonstrating strong functional signal reliability (Johnstone et al. 2005) and high signal to noise ratios (Whalen et al. 2004) in the amygdaloid region.
Behavioral Data Analysis
To assess whether subjects learned about the predictive nature of the 3 neutral face identities, we computed a within subjects ANOVA (Condition (Negative, Positive, Neutral) × Time (Before conditioning, After conditioning) on self-reported likeability and arousal ratings of the faces. Because subjects provided these ratings both before and after the conditioning phase of the experiment, we were able to examine changes due to learning. Because the faces all had neutral facial expressions, we predicted no differences in either arousal or likeability before conditioning, but specific differences in these ratings after conditioning. That is, we predicted that after conditioning subjects would report not liking the Negative faces, liking the Positive faces, and have no real preference for the Neutral faces. In addition, we predicted that after conditioning subjects would rate both the Negative and Positive faces as more arousing than the Neutral faces. Such changes would be consistent with pilot ratings of the sentences, and would indicate that subjects successfully imbued the individual faces with the social value associated with the sentences.
fMRI Data Analysis
All fMRI data analyses were performed using AFNI (Cox 1996). Raw functional blood-oxygen-level–dependent (BOLD) images were slice time-corrected, motion-corrected, and smoothed using a Gaussian kernel of 6-mm full width at half maximum (FWHM). Based on the matched filter theorem the smoothing kernel was chosen to match the size of expected activations as small as approximately 100–200 mm3, which corresponds to a sphere of diameter 6–7 mm. Subsequent measurement of the resulting spatial smoothness of data yielded a FWHM of 7 mm. All subjects included in these analyses yielded estimated head motion of less than 1.5 mm.
A general linear model was then used to estimate each regressor's unique contribution to variance. Twelve regressors of interest were included in the model. For both early (first half) and late (last half) trials, we separately modeled Negative, Positive, and Neutral faces, as well as Negative, Positive, and Neutral sentences, using a train of stimulus square waves convolved with a gamma-variate ideal hemodynamic response function (see Supplementary Materials for a detailed discussion of our ability to separate responses to the faces and sentences). Individual motion estimates were included in the general linear model as 6 covariates of no interest to control for variability in the signal that was highly correlated with head motion (Friston et al. 1996; Johnstone et al. 2006). We calculated a separate baseline comprising the fixation periods between trials and those at the beginning and end of each functional run using a second degree polynomial to allow for slow signal drift over the course of the run.
Contrast images were then transformed into percent signal change maps and spatially normalized into standard space for group analysis. Spatial normalization was performed using AFNI software according to Cox (1996) by manually identifying 11 landmarks on each subject's high-resolution image, then using a linear interpolation algorithm to transform the images to Talairach space (Talairach and Tournoux 1988). Functional images aligned with the anatomical images were warped to Talairach space and resampled to 2 mm × 2 mm × 2 mm voxels using parameters from the anatomical transformation, and were then masked with a 3-dimensional binary volume created from the subject-averaged baseline signal in order to remove activation in regions without strong signal in all subjects.
Drawing from previous human and nonhuman animal studies, we performed planned a priori voxelwise contrasts based upon 3 specific hypotheses.
Hypothesis 1: Regions of the amygdala will show differential habituation rates. Numerous studies show that human amygdala fMRI responses tend to habituate over time (Buchel et al. 1998, 1999; LaBar et al. 1998; Morris et al. 2001; Phelps et al. 2001; Cheng et al. 2007; Hare et al. 2008), and animal data suggest that it should also be possible to detect responses that do not habituate within the lateral ventral amygdala (Repa et al. 2001; Medina et al. 2002; Radwanska et al. 2002). Therefore, all data were contrasted as Early versus Late responses to capture any differences over time, a strategy that has proven useful in previous neuroimaging studies across numerous laboratories (e.g., Buchel et al. 1998, 1999; Cheng et al. 2007; LaBar et al. 1998; Morris et al. 2001; Phelps et al. 2001).
We chose this strategy of modeling Early vs. Late trials compared with specifically modeling time-related changes as a covariate in order to increase our ability to detect gross changes over time. This strategy reduces our ability to detect subtle individual differences in habituation rates. However, BOLD response in the amygdala is not sufficiently robust to permit modeling of nonlinear trends on a trial-by-trial basis due to the inherently low signal to noise ratio in this region (Johnstone et al. 2005). Averaging over 6 trials increases reliability and allows us to detect gross changes in activity Early in learning as compared with Late in learning. In addition, modeling time-related change as a covariate is especially advantageous when decreases in amygdala activation are uniformly linear (for a linear covariate) or otherwise conform to an a priori nonlinear model. We do not necessarily expect to see a linear decrease in activity over time because it is possible that activity in the dorsal and ventral medial amygdala will remain flat or even increase while subjects are learning the contingencies between stimuli, but that once these contingencies are learned, activity will begin to habituate. Moreover, because the dorsal and ventral medial subregions will likely represent different aspects of learning, we would not expect habituation patterns in these areas to show similar response patterns (e.g., response habituation consistent with some additional measure of learning such as electrodermal activity).
Hypothesis 2: Areas of the dorsal amygdala/substantia innominata region will show similar sensitivity to negatively and positively valenced stimuli. We tested this using the contrast Negative and Positive > Neutral, based upon studies showing that the amygdala is responsive to both negatively and positively valenced stimuli (Breiter et al. 1996; Whalen 1998; Kim et al. 2003), and that this sensitivity to negative and positive stimuli is increased compared with the amygdala's response to neutral stimuli (Somerville et al. 2006).
Hypothesis 3: The ventral region of the human amygdala will show greatest sensitivity to negatively valenced stimuli. We tested this hypothesis using 2 linear contrasts: The first contrast assessed amygdala responses modeled as Negative > Positive > Neutral, and was based upon aversive conditioning data in animals showing that amygdala responses to a CS+ that faithfully predicts shock are greater than those to a CS− that predicts the absence of shock, and that activity during CS− presentations is greater than the pretrial baseline periods. Seligman (1968) and Rescorla (1969) have suggested that the CS− is usefully considered a positive stimulus because it signals the absence of shock (see also Ohman 2009). Thus, our prediction of Negative > Positive > Neutral is based upon the observations that 1) in animal conditioning studies amygdala activity also increases to the positive CS−, but to a lesser degree than that observed to the CS+ (see e.g., Kapp et al. 1992) and 2) numerous human neuroimaging studies document signal increases in the amygdala to positively valenced (compared with neutral stimuli) that are of a lower magnitude than those observed to negative stimuli (e.g., Breiter et al. 1996; Whalen et al. 1998; Fitzgerald et al. 2006). Although the Negative > Positive > Neutral contrast was the focus of this study of conditioning, other human neuroimaging data show that when human neural responses to facial expressions are assessed, amygdala activity can show a Negative > Neutral > Positive profile (Kim et al. 2003). For completeness, we also assessed this linear contrast within the current study design.
Evaluation of Gender Effects
In order to ensure that the observed effects did not include gender differences, we computed post hoc t-tests to determine whether there were any significant differences between males and females for each of our planned contrasts.
Relationship between Neural Activity and Self-Report Measures
In order to determine whether individual differences in neural activity during conditioning predicted subsequent likeability and emotional arousal reports, we computed contrasts on likeability and arousal data that mirrored the contrasts that identified amygdala activity based upon valence (lateral ventral amygdala; Negative > Positive > Neutral) and arousal (dorsal amygdala; Negative and Positive > Neutral). These were then correlated with Regions of interest (ROIs) extracted from the voxelwise statistical maps as described above.
ROIs were extracted from voxelwise statistical maps calculated using the contrasts outlined above. All amygdala clusters in the reported results survive statistical thresholding at P < 0.05, corrected for multiple comparisons, stipulated by Monte Carlo simulations using the AFNI program Alphasim. To determine a correction threshold appropriate to our a priori ROI, we calculated a search volume of 3500 mm3 as measured in Talairach space in the Mai et al. (2004) atlas. This bilateral volume comprises BLA (lateral and basal nuclei) as well as the central nucleus as it extends into the substantia innominata (SI) region of the ventral basal forebrain and intermingles with other neuronal groups (e.g., nucleus basalis of Meynert; NBM). We included within this search volume the portion of the SI that is contiguous and immediately dorsal and medial to the central nucleus, where 1) NBM is located and 2) we have previously observed activation to faces (Kim et al. 2003). Thus, we are using the same correction volume defined in previous studies from our laboratory (Kim et al. 2003, 2004; Whalen et al. 2004) and peak activations observed in the present study were within the confines of this search volume (see Supplementary Fig. 1 for anatomical depictions of these subnuclei).
Because we had strong a priori hypotheses regarding patterns of activation in different subregions of the amygdala, careful anatomical localization was performed by examining the clusters obtained from group data in relation to each individual subjects’ anatomy. First, the spatial location of each ROI was verified by comparing the Talairach coordinates of both the peak voxel and cluster boundaries to an atlas in standard space (Mai et al. 2004). Each ROI was also overlaid on an averaged anatomical image as well as each subject's individual high-resolution anatomical image. These images were compared with the atlas in order to ensure anatomical consistency between individual data, group data, and standardized atlas images.
Results
Behavioral Results
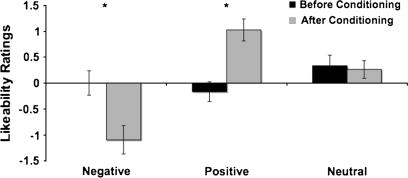
Likeability and arousal ratings of the faces collected before and after conditioning (see Methods) showed that subjects learned about the predictive nature of the 3 neutral face identities. A within subjects ANOVA (Condition (Negative, Positive, Neutral) X Time (Before conditioning, After conditioning) showed a significant interaction between Condition and Time for both types of ratings (Arousal: F2,82 = 4.433, P < 0.05; Likeability: F2,82 = 3.176, P < 0.05). Univariate ANOVAs (Condition: Negative, Positive, Neutral) revealed no difference in ratings for either likeability (F2,123 = 1.448, P > 0.20) or arousal (F2,123 = 0.698, P > 0.40) before conditioning. As predicted, after conditioning both Negative (t (41) = 2.595, P = 0.01) and Positive (t (41) = 2.760, P < 0.01) faces were rated as more emotionally arousing than the Neutral faces (mean Negative = 3.79, Positive = 3.64, Neutral = 3.14). There was no significant difference in arousal ratings for the faces that predicted negative and positive social outcomes (t (41) = −0.771, P > 0.05). A univariate (Condition: Negative, Positive, Neutral) ANOVA revealed a significant effect of Condition (F2,123 = 23.367, P < 0.001) in the postconditioning likeability ratings. In accordance with our predictions, subjects disliked faces predicting Negative social outcomes (−1.10 ± 1.76), liked the faces predicting positive social outcomes (1.02 ± 1.37), and showed no strong preference toward the faces predicting neutral social outcomes (0.26 ± 1.11) (Fig. 2).
Figure 2.
Likeability ratings before and after conditioning. Subjects indicated how much they liked each face (−4, Not at all to +4, Very much) after the orienting and conditioning phases of the task. Subjects liked the negative faces less after conditioning, and the positive faces ore after conditioning. There was no change in likeability ratings for neutral faces.
fMRI Results
Responses to Faces
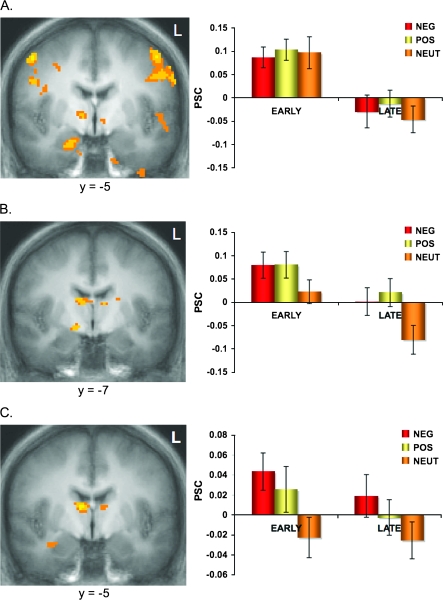
Greater activity for Early compared with Late trials during learning was observed in the medial basal amygdala (Fig. 3A). Voxels within this cluster responded to all stimulus conditions (Negative, Positive & Neutral) initially, and these responses returned to baseline during the last half of conditioning. The peak voxel (x = 19, y = −5, z = −19) maps to the BLA, and the cluster extends primarily over the medial portion of the BLA where the basal nuclei are located (Mai et al. 2004). Visual inspection of the placement of this cluster in terms of each individual's anatomy showed that this mapping was consistent across all 42 subjects (i.e., the cluster did not extend outside amygdala and extended over the medial aspects of the amygdala in all subjects).
Figure 3.
Differential amygdala response to conditioned stimuli. PSC, Percent Signal Change. (A) Activity in the medial ventral amygdala increased in response to all 3 faces, and this response habituated over time. (B) Activity in the dorsal amygdala/SI increased to Neg and Pos faces when compared with Neu, and this habituated over time but maintained differential representation of Neg and Pos compared with Neu. (C) Linear representation of valence in the lateral ventral amygdala with Neg > Pos > Neu, which did not habituate over time.
Increased activity to Negative and Positive stimuli compared with Neutral stimuli was observed in the dorsal amygdala/SI region (Fig. 3B) where the superior border of the temporal lobe meets the ventral border of the basal forebrain. The peak voxel (x = 13, y = −6, z = −5) maps to an area just dorsal to the central and medial nuclei of the amygdala (Mai et al. 2004), in an area where scattered congregations of sublenticular extended amygdala (SLEA) neurons as well as nucleus basalis of Meynert (NBM) neurons are also known to be located (see Discussion). Visual inspection of the placement of this cluster in terms of each individual's anatomy showed a consistent spatial profile within all 42 subjects (i.e., the cluster does not cross into the immediately superior globus pallidus in any subject). Paired t-tests on average values extracted from this ROI revealed no difference between dorsal amygdala/SI activity to the Negative compared with Positive faces during both Early (t (41) = −0.025, P = 0.98) and Late (t (41) = −0.624, P = 0.536) trials.
In order to determine areas of the amygdala that differentially represented the predictive valence of the faces, we computed 2 linear contrasts (see Methods). No voxels in the amygdala/SI region survived statistical correction (P > 0.1) in the first linear contrast, where we modeled valence as Negative > Neutral > Positive. However, as predicted for this conditioning paradigm, we did find a significant linear representation of valence (Negative > Positive > Neutral) in a region overlapping the lateral ventral amygdala (Fig. 3C). The peak voxel (x = 33, y = −5, z = −17) mapped to the lateral ventral border of the amygdala. The significant cluster comprised voxels that mapped to the lateral portion of the BLA as well as immediately adjacent voxels that mapped to the amygdala–striatal transition zone (Mai et al. 2004). Visual inspection of the placement of this cluster in terms of each individual's anatomy showed a similarly consistent spatial profile with all 42 subjects, such that this cluster overlapped the lateral ventral amygdala in all subjects.
Critically, post hoc paired t-tests suggest that Early versus Late trial responses within the lateral ventral amygdala ROI were resistant to habituation over the course of conditioning trials (t(41) = 0.817, P > 0.10). This is in contrast to Early versus Late habituation observed in the medial ventral (voxelwise analysis) and dorsal amygdala/SI (t(41) = 2.445, P < 0.05) loci. Post hoc t-tests confirmed that there were no significant differences between Early and Late trials in this lateral ventral locus in any condition [Negative condition (t(41) = 0.972, P > 0.30); Positive condition (t(41) = 0.881, P > 0.30); Neutral condition (t(41) = 0.107, P > 0.90)]. In order to directly compare regional differences in habituation rates, we conducted a within subjects ANOVA with amygdala subregion (medial ventral, lateral ventral, and dorsal amygdala) as the independent variable and habituation value (i.e., Early–Late) as the dependent variable. As predicted, there was a significant effect of amygdala subregion (F2,82 = 6.861, P < 0.01). Paired t-tests confirm that there is no difference in habituation values (Early vs. Late) between the medial ventral and dorsal amygdala/SI regions (t(41) = 1.251, P > 0.1). In contrast, habituation rate within the lateral ventral region was significantly different from the medial ventral region (t(41) = 4.338, P < 0.001) and the dorsal amygdal/SI region (t(41) = 2.478, P < 0.05). That is, both the medial ventral and dorsal amygdala regions showed significantly greater habituation than the lateral ventral amygdala.
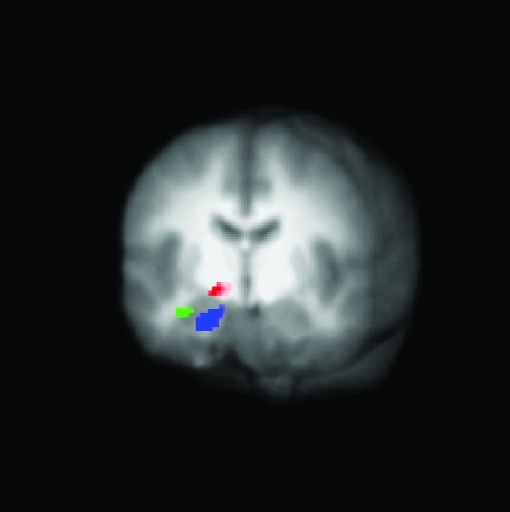
The 3 activation peaks reported here were from 13 to 23 mm apart (Fig. 4). The ability to separately measure BOLD signal change from proximal brain structures is ultimately limited by the intrinsic spatial smoothness of fMRI in these regions. Estimates of spatial smoothness of our sample in the amygdala/SI region yielded a FWHM of 7 mm, well below the interpeak distances reported. In addition, none of the clusters overlapped. Thus, these data show that we can confidently dissociate these coarse spatial clusters at the spatial resolution parameters utilized here based on their functional profiles.
Figure 4.
Three-dimensional representation of amygdala activation during learning. Activations are superimposed on an averaged anatomical brain (42 subjects) to show their location relative to one another. Three clusters were obtained from different a priori contrasts. Activity in the dorsal amygdala/SI and medial ventral amygdala clusters habituate over time, whereas activity in the lateral ventral amygdala is sustained throughout learning.
Responses to Sentences
We also included estimates of subjects’ responses to each of the sentence categories (Negative, Positive, Neutral) in our general linear model. In order to ensure that the observed responses to faces were different from responses to the sentences, we computed the same statistical contrasts on cluster means extracted from each of the functional ROIs specified above for responses to sentences. We found that activity in the medial ventral amygdala to all sentences was similar to that observed to faces [i.e., was greater during Early trials compared with Late trials (t(41) = 4.373, P < 0.001)]. Critically, responses to the sentences did not mirror responses to the faces in the dorsal amygdala/SI ROI showing Negative and Positive > Neutral faces (t(41) = −0.24, P > 0.1), or the lateral ventral ROI showing a linear representation of valence modeled as Negative > Positive > Neutral faces (t(41) = −0.503, P > 0.1).
Evaluation of Gender Differences
Post hoc t-tests were performed on cluster means extracted from individual subjects to evaluate potential influences of gender for each ROI. No significant differences between males and females were observed in the ventral medial locus (Early vs. Late: t(40) = 1.143, P > 0.2), the dorsal locus (Neg/Pos > Neu: t(40) = 1.208, P > 0.2), or the ventral lateral locus (Neg > Pos > Neu: t(40) = −1.923, P > 0.05).
Relationship between Neural Activity and Self-Report Measures
We collected postacquisition likeability and arousal ratings to confirm that subjects learned the value associated with the 3 face identities. We did not expect that subjective ratings of the faces taken postacquisition would correlate with amygdala response during acquisition. For completeness, we note that subjective arousal ratings were not correlated with dorsal amygdala/SI responses based on arousal (r = 0.201, P > 0.1). In addition, subjective likeability ratings did not correlate with neural responses observed in the lateral ventral amygdala (r = 0.058, P > 0.1).
Discussion
Here we showed human amygdala activation during a social conditioning task where subjects learned that different individuals predicted different social outcomes. Consistent with previous work (Buchel et al. 1998; LaBar et al. 1998; Morris et al. 1998; Buchel et al. 1999; Morris et al. 2001; Phelps et al. 2004; Cheng et al. 2007; Hare et al. 2008), we observed robust habituation of amygdala responses across the course of a relatively short conditioning phase. In the medial ventral amygdala, this effect comprised responses that were initially strong across all stimulus categories (Negative/Positive/Neutral) that then returned to baseline. In the dorsal amygdala/SI, this effect comprised responses that were higher to Negative and Positive compared with Neutral faces, and these differences between conditions were maintained even as the overall response magnitude for all conditions decreased over time. Finally, in the lateral ventral amygdala, we observed a linear representation of valence such that Negative > Positive > Neutral. This locus differed from the dorsal and medial loci in that the magnitude of these responses did not significantly decrease over the acquisition phase studied here (Fig. 3). Gender differences were not observed for any of the reported effects.
Amygdala Responses during Acquisition of an Associative Social Learning Task
In the current study, we reduced the complex process of assigning reinforcement value to others based on prior social interactions to a simple associative learning paradigm. We based the design of this paradigm on more traditional Pavlovian conditioning studies showing that the human amygdala is sensitive to signals that predict biologically relevant outcomes (e.g., Buchel et al. 1998; LaBar et al. 1998; Buchel et al. 1999; Morris et al. 2001; Phelps et al. 2001). Studies showing human amygdala involvement in processing facial expressions also informed our predictions because facial expressions communicate the emotional states of others and allow for the prediction of social outcomes (see Whalen et al. 2009). In fact, a growing body of literature suggests that the amygdala is involved in such subtle social and emotional processes just as it is involved in processing stimuli that induce stronger emotional states, such as tones predicting shock (e.g., Buchel et al. 1998, 1999; LaBar et al. 1998; Morris et al. 2001; Phelps et al. 2001), threatening scenes (e.g., Canli et al. 2000) or aversive odors (Zald and Pardo 1997; see Whalen 1998 for discussion). Here we extend traditional models of Pavlovian conditioning to characterize the amygdala's role in assigning reinforcement value to other people, and suggest that its role in effective social interaction may be best understood through its more elemental role in forming biologically relevant associations (Adolphs 2003; Amaral et al. 2003).
The human amygdala has been previously implicated in other extensions of Pavlovian conditioning, such as abstract representations of fear acquired through social communication (Phelps et al. 2001) or through social observation (Olsson and Phelps 2007). Indeed, the exact relationship between the current model of learning and traditional forms of Pavlovian conditioning requires further elucidation. One possibility is that the current paradigm is related to evaluative conditioning, which is an associative process whereby a previously neutral stimulus acquires the affective value of an unconditioned stimulus, rather than predicts its occurrence (De Houwer et al. 2001). Future studies might seek to directly compare the present paradigm with more traditional conditioning tasks to document the differences between the two.
Dissociating fMRI Responses across the Human Amygdaloid Complex
In the present study, we observed that both the dorsal amygdala/SI and the medial ventral amygdala showed signal increases to faces predicting both negative and positive social outcomes. This finding is consistent with data in monkeys showing that neurons throughout the basal and centromedial amygdaloid nuclei code visual stimuli predicting either negative or positive outcomes (Paton et al. 2006). These data support the assertion that the amygdala is involved in linking negative as well as positive value to stimuli that predict threat and reward, respectively (Holland and Gallagher 1999; Murray 2007).
In contrast, a lateral ventral region of the amygdala differentiated between conditioned stimuli based upon their valence, with the largest responses observed to faces predicting negative social outcomes. This spatial dissociation converges with an earlier study of amygdala responses to surprised facial expressions (Kim et al. 2003). In that study, a strikingly similar region of the lateral ventral amygdala showed the greatest response to negatively interpreted surprised faces. Critically, in these same subjects, a dorsal amygdala/SI region and a medial ventral region of the amygdala showed comparable signal increases to positively as well as negatively interpreted surprised faces (Kim et al. 2003), consistent with the responses observed within the dorsal amygdala/SI and ventral medial amygdala in the present study. Together, these fMRI data support the notion that it is possible to dissociate activity across coarsely defined subregions of the human amygdala at resolution levels routinely employed by numerous neuroimaging laboratories (i.e., 3 mm3 voxels). Such results suggest that an appreciation for regional differences might help resolve apparent discrepancies across studies of the amygdala where some show amygdala activity clearly tracking valence (Kim et al. 2003; Pessoa et al. 2005; Straube et al. 2008) while others show responses related to arousal value (Garavan et al. 2001; Anderson et al. 2003; Kensinger and Schacter 2006; Lewis et al. 2007; Demos et al. 2008).
Response Habituation Profiles Observed during Acquisition
Activity within the lateral ventral amygdala showed differential activity to all face categories based upon their learned valence, with the greatest signal increases observed to the face identity predicting negative social outcomes. In addition, these responses were sustained across the acquisition phase studied here. These data are potentially consistent with Pavlovian conditioning data in rats (Repa et al. 2001; Radwanska et al. 2002) showing that a population of cells in the lateral nucleus of the amygdala maintained differential representations of conditioned stimuli (CS+ vs. CS−) throughout learning (see also Medina et al. 2002). These findings converge with another human neuroimaging report showing that a region of the human ventral amygdala was more resistant to habituation during Pavlovian conditioning (Morris et al. 2001). Here we localize such an effect to the most lateral aspects of the ventral amygdaloid complex.
This effect was in stark contrast to responses observed within the medial ventral amygdala, which showed a clear pattern of habituation (i.e., early signal increases that returned to baseline). Noteably, the habituation profile observed in the dorsal amygdala/SI region was more complex. Here, like the medial ventral amygdala, early signal increases to positive and negative stimuli returned to baseline over time. However, like the lateral ventral amygdala, response magnitude differences between valenced (Negative and Positive) and Neutral face identities were maintained within the dorsal amygdala/SI through Early and Late trials. Based on these findings, we tentatively suggest that during acquisition, the lateral ventral amygdala maintained differential representations of valence while the dorsal amygdala/SI region maintained representations related to the similar arousal value of Negative and Positive identities. Further, regional differences in habituation rates are at least consistent with the possibility that parts of the amygdala maintain learned representations while others play a more time-limited role during learning (Repa et al. 2001; Medina et al. 2002; Radwanska et al. 2002).
Relating Regional fMRI Responses to Amygdala Anatomy
Though we are careful to refer to these differential activations in a regional sense, the predictions for the present study were inspired by data in animals showing that amygdala subnuclei can be dissociated both functionally and anatomically. In humans, fMRI activations within the ventral region of the amygdaloid complex (e.g., z = −10 to z = −28) clearly map to the BLA as defined in Mai et al. (2004). Within the BLA, the basal nuclei are located medially and the lateral nucleus is located laterally. Here, we report differences between these 2 ventral amygdala regions in habituation rates and valence discrimination that suggests it may be possible to functionally dissociate these portions of BLA using BOLD.
The central nucleus lies dorsal to the BLA in humans (see Supplemental Fig. 1). fMRI activations located within dorsal amygdala regions (z = −3 to z = −9) are more difficult to localize to a specific neuronal source due to this region's complicated intermingled anatomy. For example, neurons similar to those found in the central nucleus of the amygdala extend from the amygdala “proper” in a superior and medial direction through the ventral basal forebrain, and are referred to as the SLEA. Also intermingled within this same region are the corticopetal cholinergic neurons of the nucleus basalis of Meynert (NBM) (Heimer and Van Hoesen 2006). Indeed, the intermingled nature of the neurons in this region of the ventral basal forebrain is why it is referred to as the SI. Thus, fMRI activations within the dorsal amygdala/SI region detailed here, as well as spatially similar activations documented previously (Breiter et al. 1996; Whalen et al. 1998; Canli et al. 2000; Morris et al. 2001; Phelps et al. 2001; Whalen et al. 2001; Pessoa et al. 2002, 2005, 2006; Kim et al. 2003) could reflect activity of dorsal amygdala nuclei, SLEA and/or NBM neurons. Concerns that these neuronal groups cannot be discerned with fMRI are mitigated by the fact that they are anatomically interconnected and together have been implicated in alerting other neural systems (e.g., cortex) to the arousal value of predictive stimuli (Kapp et al. 1992, 1994; Whalen et al. 1994; Holland and Gallagher 1999). This theoretical stance is consistent with the presently observed signal increases within the dorsal amygdala/SI region to both positively and negatively valenced faces (see also Kim et al. 2003), as well as the significantly increased subjective arousal ratings ascribed to these 2 stimulus categories following learning.
Although subregions of the amygdaloid complex are heavily interconnected, differences in intrinsic and extrinsic connectivity suggest that the subregions identified here should support different aspects of associative learning tasks. The lateral nucleus receives the majority of the amgydala's sensory input, with heavy projections from higher order unimodal sensory cortices as well as multimodal sensory projections (Freese and Amaral 2009). As such, the lateral nucleus is commonly thought of as a sensory input and convergent processing center that detects incoming sensory input and then integrates this input across modalities (LeDoux et al. 1990; Pitkanen 2000). In primates, the lateral nucleus receives considerable input from visual area TE (Aggleton et al. 1980) which is a high level visual area situated at the end of the ventral visual stream (commonly referred to as the “what” pathway). Information within the amygdala flows from the lateral nucleus to more medially situated nuclei, such as the basal and accessory basal nuclei. These nuclei are heavily interconnected with the orbital and medial prefrontal cortices, as well as the rostral aspects of the anterior cingulate cortex (see Freese and Amaral 2009). These connections allow the basal nuclei to integrate contextual and motivational information with the sensory information passed on from the lateral nucleus. Although the basal nuclei can act as one output source for the amygdala (i.e., sending outputs to prefrontal cortical regions), they also project to the central and medial nuclei of the amygdala. These nuclei, located dorsally in the human, project directly to all of the major neuromodulatory centers in the brain (Price and Amaral 1981; Price 1986; Price et al. 1987). Thus, these nuclei, in conjunction with the intermingled cell groups of the SLEA, have the ability to nonspecifically increase arousal throughout the brain.
These patterns of intrinsic and extrinsic connectivity are potentially consistent with the differential patterns of activation observed in the current paradigm. That is, the lateral aspects of the amygdala maintained differential representations of the valenced identities of the faces, consistent with its role in detecting and representing highly processed sensory information (LeDoux et al. 1990; Pitkänen 2000) such as that emanating from the ventral visual stream. In contrast, activity in the medial ventral amygdala did not discriminate between valenced face identities, but rather showed an overall increase in activity to all faces that decreased over the course of learning. This could be consistent with the idea that the basal nuclei, via their reciprocal connections with the prefrontal cortex (Freese and Amaral 2009), are integrating the self-relevant contextual information provided by the sentences with existing representations of the individual faces. Finally, the dorsal aspects of the amygdala, consistently implicated in increasing nonspecific arousal (see Kapp et al. 1992; Whalen 1998), show increased responsivity to both negative and positive stimuli (which are associated with equivalent levels of emotional arousal in this task) compared with the neutral stimulus.
Caveats and Limitations
Because classical Pavlovian conditioning studies inspired the current design, future studies employing additional dependent measures (e.g., electrodermal activity, startle, etc.) will allow us to more carefully compare our paradigm with previous work to elucidate the overlap in brain circuitry mediating these forms of learning. Moreover, such implicit indices of learning might allow us to use individual differences in learning to predict differences in amygdala activity across the subregions studied here, and to more carefully assess exactly where in the acquisition phase learning occurred. Indeed, though self-reported likeability and arousal ratings collected in the present study provided evidence that subjects learned about the value of the presented individual faces, these ratings did not correlate with amygdala responses. This could be due to the fact that the self-report data were collected post-training, or to the fact that these ratings showed a relatively restricted range that was not optimal for examining individual differences in learning. Collecting implicit measures of learning on-line could provide future studies with the variability necessary to examine such differences.
The present study utilized a relatively short acquisition phase. This was based on previous human neuroimaging studies of conditioning which used a similarly small number of trials (Buchel et al. 1998, 1999; LaBar et al. 1998; Morris et al. 2001; Phelps et al. 2004; Cheng et al. 2007), presumably because fMRI response habituation within the amygdala is a robust phenomenon. Thus, future studies will be needed to determine how long the observed responses in the lateral amygdala are sustained (e.g., would they have eventually habituated with more acquisition trials?). Further, the limited number of trials used here did not allow us to address when exactly the amygdala began to show evidence of learning on an individual subject basis. Therefore, we look forward to design modifications that might extend amygdala activity during learning to address these issues. Such modifications would allow for the determination of any regulatory role the prefrontal cortex might have in this process as predicted by the nonhuman animal literature (e.g., Likhtik et al. 2005).
Finally, caution should be exercised in asserting regional differences in functional activation across a small volume such as the amygdaloid complex. The compelling nature of the present spatial dissociations is their 1) nonoverlapping nature (Fig. 4), 2) distinct response profiles (Fig. 3), and 3) consistency with previous research. One goal of the present design was to show it is possible to make crude spatial dissociations at the spatial resolution levels commonly employed by many neuroimaging laboratories. Future studies using higher resolution imaging will allow for the delineation of anatomical ROIs based on human amygdala subnuclei and can be used to verify the response profiles observed here.
Table 1.
Differential regional activation of the amygdala during associative learning, based on clusters identified from voxelwise tests and surviving correction for multiple comparisons at P < 0.05
| Contrast | Location (Mai et al. 2004) | x | y | z | Cluster size |
| Early > Late | Medial ventral amygdala | 19 | −5 | −19 | 105 |
| Neg/Pos > Neu | Dorsal amygdala/SI | 13 | −6 | −5 | 23 |
| Neg > Pos > Neu | Lateral ventral amygdala | 33 | −5 | −17 | 9 |
Note: Cluster size is reported as number of 2 mm × 2 mm × 2 mm voxels and x, y, z coordinates represent the peak voxel within each cluster.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Institute of Mental Health (MH080716 and MH069315).
Supplementary Material
Acknowledgments
The authors would like to thank Michael Anderle, Ron Fisher, Justin Kim, and Ashly McLean for technical assistance. Special thanks go to Wayne Kerr for providing the inspiration for this study. Conflict of Interest: None declared.
References
- Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Is the human amygdala specialized for processing social information? Ann N Y Acad Sci. 2003;985:326–340. doi: 10.1111/j.1749-6632.2003.tb07091.x. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Capitanio JP, Lavenex P, Mason WA, Mauldin-Jourdain ML, Mendoza SP. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:517–522. doi: 10.1016/s0028-3932(02)00310-x. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Baxter M, Murray E. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J Neurosci. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Bandettini PA, O'Craven KM, Savoy RL, Petersen SE, Raichle ME, Rosen BR. Detection of cortical activation during averaged single trials of a cognitive task using functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93:14878–14883. doi: 10.1073/pnas.93.25.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learn Mem. 2007;14:485–490. doi: 10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De Houwer J, Thomas S, Baeyens F. Associative learning of likes and dislikes: a review of 25 years of research on human evaluative conditioning. Psychol Bull. 2001;127:853–869. doi: 10.1037/0033-2909.127.6.853. [DOI] [PubMed] [Google Scholar]
- Demos KE, Kelley WM, Ryan SL, Davis FC, Whalen PJ. Human amygdala sensitivity to the pupil size of others. Cereb Cortex. 2008;18:2729–2734. doi: 10.1093/cercor/bhn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto (CA): Consulting Psychologists Press; 1976. [Google Scholar]
- First M, Spitzer M, Williams J, Gibbon M. Structured clinical interview for DSM-V (SCID) Washington (DC): American Psychiatric Association; 1995. [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Freese J, Amaral D. Neuroanatomy of the primate amygdala. In: Whalen P, Phelps E, editors. The human amygdala. New York: Guilford; 2009. p. 3–42. [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AM. Stochastic designs in event-related fMRI. Neuroimage. 1999;10:607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC. Amygdala response to both positively and negatively valenced stimuli. Neuroreport. 2001;12:2779–2783. doi: 10.1097/00001756-200108280-00036. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW. The limbic lobe and its output channels: Implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Hooker C, Germine L, Knight R, D'Esposito M. Amygdala response to facial expressions reflects emotional learning. J Neurosci. 2006;26:8915–8922. doi: 10.1523/JNEUROSCI.3048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR. Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Hum Brain Mapp. 2006;27:779–788. doi: 10.1002/hbm.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, Whalen PJ. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage. 2005;25:1112–1123. doi: 10.1016/j.neuroimage.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Supple WF, Jr, Whalen PJ. Effects of electrical stimulation of the amygdaloid central nucleus on neocortical arousal in the rabbit. Behav Neurosci. 1994;108:81–93. doi: 10.1037//0735-7044.108.1.81. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Whalen PJ, Supple WF, Pascoe JP. Amygdaloid contributions to conditioned arousal and sensory information processing. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 229–254. [Google Scholar]
- Kensinger EA, Schacter DL. Processing emotional pictures and words: effects of valence and arousal. Cogn Affect Behav Neurosci. 2006;6:110–126. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville L, Johnstone T, Polis S, Alexander A, Shin L, Whalen P. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16:1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain: the mysterious underpinnings of emotional life. New York: Touchstone; 1996. [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hare TA, Voss HU, Glover G, Ballon DJ, Casey BJ. The bivalent side of the nucleus accumbens. Neuroimage. 2009;44:1178–1187. doi: 10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Rotshtein P, Dolan RJ. Neural correlates of processing valence and arousal in affective words. Cereb Cortex. 2007;17:742–748. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J, Assheuer J, Paxinos G. Atlas of the human brain. New York: Thieme; 2004. [Google Scholar]
- Medina JF, Repa JC, Mauk MD, LeDoux JE. Parallels between cerebellum- and amygdala-dependent conditioning. Nat Rev Neurosci. 2002;3:122–131. doi: 10.1038/nrn728. [DOI] [PubMed] [Google Scholar]
- Morris JS, Buchel C, Dolan RJ. Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. Neuroimage. 2001;13:1044–1052. doi: 10.1006/nimg.2000.0721. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Ohman A. Human fear conditioning and the amygdala. In: Whalen PJ, Phelps EA, editors. The human amygdala. New York (NY): The Guilford Press; 2009. pp. 118–154. [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Social learning of fear. Nat Neurosci. 2007;10:1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Japee S, Sturman D, Ungerleider LG. Target visibility and visual awareness modulate amygdala responses to fearful faces. Cereb Cortex. 2006;16:366–375. doi: 10.1093/cercor/bhi115. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28:249–255. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The amygdala. A functional analysis. New York: Oxford University Press; 2000. pp. 31–117. [Google Scholar]
- Price JL. Subcortical projections from the amygdaloid complex. Adv Exp Med Biol. 1986;203:19–33. doi: 10.1007/978-1-4684-7971-3_2. [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The limbic region II: the amygdaloid complex. In: Bjorklund A, Hokfelt T, editors. Handbook of chemical neuroanatomy. Amsterdam: Elsevier; 1987. pp. 279–388. [Google Scholar]
- Radwanska K, Nikolaev E, Knapska E, Kaczmarek L. Differential response of two subdivisions of lateral amygdala to aversive conditioning as revealed by c-Fos and P-ERK mapping. Neuroreport. 2002;13:2241–2246. doi: 10.1097/00001756-200212030-00015. [DOI] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Establishment of a positive reinforcer through contrast with shock. J Comp Physiol Psychol. 1969;67:260–263. doi: 10.1037/h0026789. [DOI] [PubMed] [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nat Neurosci. 2009;12:508–514. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- Seligman ME. Chronic fear produced by unpredictable electric shock. J Comp Physiol Psychol. 1968;66:402–411. doi: 10.1037/h0026355. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Wig GS, Whalen PJ, Kelley WM. Dissociable medial temporal lobe contributions to social memory. J Cogn Neurosci. 2006;18:1253–1265. doi: 10.1162/jocn.2006.18.8.1253. [DOI] [PubMed] [Google Scholar]
- Straube T, Pohlack S, Mentzel HJ, Miltner WH. Differential amygdala activation to negative and positive emotional pictures during an indirect task. Behav Brain Res. 2008;191:285–288. doi: 10.1016/j.bbr.2008.03.040. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr Directions Psychol Sci. 1998;7:177–188. [Google Scholar]
- Whalen PJ, Davis FC, Oler JA, Kim H, Kim JM, Neta M. Human amygdala responses to facial expressions of emotion. In: Phelps EA, Whalen PJ, editors. The human amygdala. New York: Guilford Press; 2009. [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, McLaren DG, Somerville LH, McLean AA, Maxwell JS, Johnstone T. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kapp BS, Pascoe JP. Neuronal activity within the nucleus basalis and conditioned neocortical electroencephalographic activation. J Neurosci. 1994;14:1623–1633. doi: 10.1523/JNEUROSCI.14-03-01623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.