Abstract
This event-related functional magnetic resonance imaging study compared neural correlates of executive function (cognitive set-shifting) in 28 healthy participants with either high (HIQ) or average (AIQ) intelligence. Despite comparable behavioral performance (except for slower reactions), the AIQ participants showed greater (especially prefrontal) activation during response selection; the HIQ participants showed greater activation (especially parietal) during feedback evaluation. HIQ participants appeared to engage cognitive resources to support more efficient strategies (planning during feedback in preparation for the upcoming response) which resulted in faster responses and less need for response inhibition and conflict resolution. Whether greater intelligence is associated with more or less brain activity (the “neural efficiency” debate) depends therefore on the specific component of the task being examined as well as the brain region recruited. One implication is that caution must be exercised when drawing conclusions from differences in activation between groups of individuals in whom IQ may differ (e.g., psychiatric vs. control samples).
Keywords: event-related, executive functioning, fMRI, intelligence, neural efficiency
Introduction
Executive function broadly defines higher cognitive abilities that enable individuals to strategically control and execute goal-directed behaviors in an uncertain and changing environment. Executive function has been conceptualized as a “supervisory attentional system” which plans, prioritizes, allocates attention, and recognizes corrective feedback to guide appropriate decisions (Norman and Shallice 1986). This concept has much in common with the “central executive” component of the working memory model which interacts with short-term verbal and visuospatial stores to manipulate information during complex decision making (Baddeley and Hitch 1974; Baddeley 2001).
Executive function and intelligence are thought to be related psychological constructs that underlie behavioral control and problem solving. The general factor or “g” of human intelligence is defined as underlying all cognitive abilities (Spearman 1928) and therefore correlates with psychometric tests of intelligence (Jensen 1998). Psychometric batteries such as the Wechsler Adult Intelligence Scale (WAIS) were devised to measure intelligence in general (i.e., both crystallized and fluid intelligence) and are an approximation of “g” plus the cognitive skills specific to each test used (Colom et al. 2002). In healthy participants, WAIS-Revised IQ scores moderately correlate (r = 0.38–0.63) with a wide variety of executive functioning tests (Obonsawin et al. 2002). Although there is an ongoing debate over the nature of the relationship between IQ and scoring on neuropsychological tests (Dodrill 1997, 1999; Horton 1999; Tremont 1999), more complex neuropsychological tests (which include measures of verbal and visual memory, complex attention, working memory and executive functioning) can be expected to strongly correspond to IQ (Jung et al. 2000). Thus, executive function and intelligence are thought to have strong psychometric associations and even share overlapping neural regions (Duncan 2005; Burgess et al. 2006). Indeed some authors regard executive function and fluid intelligence (“gF”) as one and the same (Duncan et al. 1995). Brain imaging studies indicate that high “g” tasks compared with low “g” (regardless of an individual's IQ) require greater recruitment of a network involving lateral prefrontal and posterior parietal cortices—regions that are also strongly implicated in executive function processes (Duncan and Owen 2000; Buchsbaum et al. 2005).
Of some controversy however is whether people with higher intelligence employ greater or lesser activation in these brain regions. According to the capacity-constrained view of cognition, decision-making processes rely on underlying neural systems whose efficiency is affected by several factors including the neurochemistry, interconnectivity and strategies they employ (Parks et al. 1989; Just and Carpenter 1992). Thus “neural efficiency” results from individual differences in the amount of neural resources available for cognitive processing (Haier et al. 1992; Rypma and D'Esposito 1999; Reichle et al. 2000; Rypma et al. 2002). Several studies using positron emission tomography (PET) to measure brain activity-induced changes in glucose metabolism have concluded an inverse correlation between neural activity and IQ score (or task performance) which is consistent with the neural efficiency hypothesis (Haier et al. 1992, 2000). This is not always the case however. Electroencephalography studies (which reflect summed electrical potentials across large neuronal populations) indicate that IQ-related task proficiency is sometimes associated with greater and other times lesser neural activity (Van Rooy et al. 2001; Jausovec and Jausovec 2004a; Neubauer et al. 2004) and may depend on the nature of the task being measured. In blood-oxygen-level–dependent (BOLD) functional magnetic resonance imaging (BOLD fMRI which reflects local changes in the ratio of deoxygenated to oxygenated hemoglobin), tasks that demand more complex reasoning typically result in greater neural activity for those individuals with higher IQ—which is inconsistent with the neural efficiency hypothesis (Gray et al. 2003; Lee et al. 2006). Although it is possible that methodological differences among these studies (i.e., each technique measures different neuronal phenomena) may contribute to the discrepancies in findings, it is still highly worthwhile investigating the controversial neural efficiency hypothesis. fMRI offers high spatial resolution and the possibility of event-related designs, hence the purpose of this study was to determine whether IQ-related differences in fMRI activation (increases or decreases) could be linked to specific cognitive subcomponents of an executive function task such as strategic response planning, goal-directed attention changes, working memory maintenance, response inhibition, and conflict resolution.
Methods
Participants
The study protocol was approved by the local institutional ethics committee and informed consent was obtained from 28 healthy right-handed volunteers who were proficient in English, free of medications or caffeine prior to scanning, and had no history of neurological or psychiatric disorders. These 28 participants were selected from a larger sample of 160, based on their performance on the Wechsler Abbreviated Scale of Intelligence. First, a group of participants whose IQ was close to the population average (100) was randomly selected. A second group was then chosen where IQ was at least one population standard deviation (15 points) above 100. Where possible we attempted to match on other factors such as age, years of education and demographics. The 2 groups differed only in terms of their Full Scale IQ (mean for average IQ = 98.9 ± 3.3, range = 93–104; mean for High IQ = 124.5 ± 8.4, range = 115–135; 2-tailed independent sample t-test, t(26) = 13.3, P < 0.0001). The 2 groups did not significantly differ in terms of age (t(26) = 0.8, P = 0.4) and years of education (t(26) = 1.12, P = 0.28): Average IQ group (mean age = 29.9 ± 11.9; mean years of education = 13.1 ± 2.2; 7 females) and High IQ group (mean IQ = 124.5 ± 8.4, range = 115–135; mean age = 26.3 ± 12.2; mean years of education = 13.8 ± 1.5; 9 females).
Experimental Task and Design
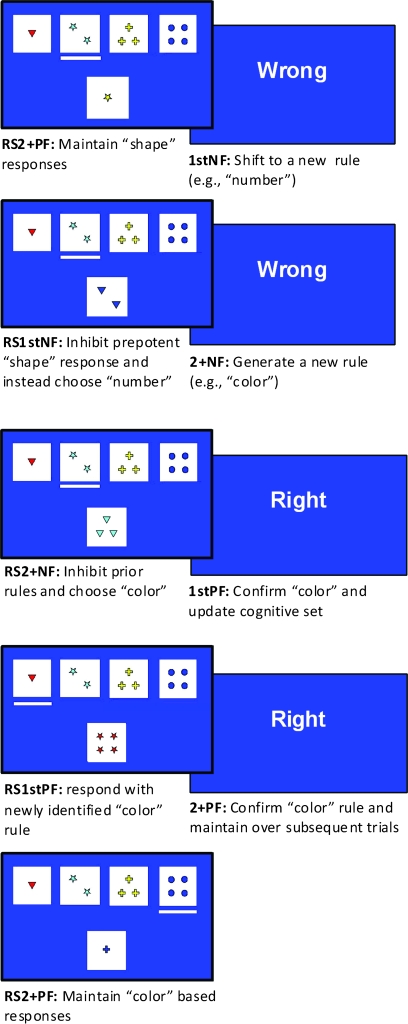
Participants were trained on a computerized set shifting task before entering the scanner to ensure that performance deficits were not attributable to misunderstandings about sorting criteria or the concept of sorting itself (Stuss et al. 1983). Briefly, each participant was trained for about 5 min to gain matching experience on all 3 rules (color, shape, and number) and was asked to comment on how they were solving the task to ensure appropriate understanding of the instructions. During response selection, 5 cards appeared on a blue screen. Four equally spaced reference cards appeared along the top of the screen and remained unchanged throughout the experiment (see Fig. 1). A target card appeared centrally and was to be matched with 1 of 4 reference cards, according to a randomly selected rule (colour, shape, or number). The target card was never identical to a reference card, but shared the same color, shape or number of composite items. The subject was allowed 4sec in which to respond, otherwise the words “too late” would appear and the trial would terminate. Following the subject's response, a bar appeared under the chosen reference card. At the end of the 4sec period, the stimuli disappeared and were replaced by fixation (a white cross centered on the blue background). After a further 5 s (9 s since the start of the trial), the feedback stimulus appeared: “Right” or “Wrong” in white letters centered on the blue background for correct or incorrect responses, respectively. The feedback stimulus appeared for 500 ms, the display then changed to fixation until the onset of the next trial. Variable periods of fixation (3, 6, or 9 s) were inserted between trials to allow sufficient separation and jittering of trials to facilitate deconvolution. The average trial onset asynchrony was 14sec. After a random number of between 3 and 5 successive correct feedback events (the first of which was declared an “update” event; the remaining trials were considered “maintenance” events), another rule was randomly selected. The next occurrence of negative feedback (the “shift” feedback) gave the subject the opportunity to realize that the rule had changed and to take appropriate action. All other trials on which negative feedback was presented were considered to be “generate” events. Each scanning session consisted of 5 runs and each run lasted for 8 min.
Figure 1.
On each trial the subject matched the card at the bottom of the screen (which varied on each trial) with 1 of the 4 top cards at the top of the screen. This figure depicts a typical sequence of the 8 event types during cognitive set shifting. In the first row responding was based on the rule established during previous trials, and confirmed by positive feedback; in the second row the rule continued to be maintained but feedback indicated that the rule had changed. In the third row a new rule had to be chosen from the 2 remaining candidates (and the previous rule inhibited). If positive feedback was received (1stPF) this would guide subsequent card selection. If negative feedback (2+NF) was received further attempts (RS2+NF) would be made until the correct rule was identified. The response (required within 4 s) was indicated by a white horizontal bar under the chosen card. A “fixation cross” then appeared for 5 s after which feedback (positive or negative) was presented for 0.5 s. After a variable fixation interval the next trial began. Note: “PF” = positive feedback; “NF” = negative feedback; “1st” = first; “2+” = subsequent; and “RS” = response selection following feedback.
Imaging Protocol
Functional imaging was performed on a 1.5-Tesla Symphony MRI scanner (Siemens, Erlangen, Germany) using blipped gradient-echo echoplanar imaging (time repetition = 3000 ms; flip angle = 90°; 64 × 64 pixel matrix; field of view [FOV] = 192 × 192 mm). Each run consisted of 156 whole brain acquisitions (32 oblique axial slices, 3-mm-thick, 0.3-mm gap between slices, descending interleaved slice acquisition) acquired in a plane parallel to the line between the anterior and posterior commissures on the sagittal scout images. A high-resolution (256 × 256 pixel matrix; FOV = 206 × 206 mm) T1-weighted anatomical reference was acquired using a magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence that yielded 80 slices of approximately 2-mm thickness in the coronal plane.
Image Data Analysis
The images were processed and analyzed using Brain Voyager QX (Version 1.10, Brain Innovation, the Netherlands). Slice scan time correction followed by motion correction, spatial smoothing (8-mm FWHM) and linear trend removal were performed. The functional images were registered to the MPRAGE images, and the resulting realigned data were then transformed into Talairach space prior to computation of a random effects general linear model with separate regressors (relative to a fixation baseline) for each condition. Each regressor was convolved with a canonical hemodynamic response function (HRF) peaking 6 s after presentation onset of the card stimuli or feedback, respectively. Jittering the fixation interval between feedback evaluation and the ensuing response selection aided in the deconvolution of events. Furthermore, the variable nature of the feedback (sometimes positive, other times negative) ensured that the specific type of response selection and feedback evaluation were not correlated in time. Deconvolution was further aided by the separation of first and subsequent instances of each event type. The inclusion of a fixation baseline also allowed the estimation of HRF predictors for each of these conditions of interest (rather than simply revealing a significant difference between 2 conditions). Thus, we examined whether each condition was activating or deactivating with respect to the fixation baseline. Regions of interest (ROIs) were selected based on group level differences, and the resulting maps were thresholded (P < 0.05 corrected) using minimum cluster size estimation (Goebel et al. 2006). After setting the voxel-level threshold to P < 0.01 (uncorrected), the maps were corrected at whole brain level using 1000 iterations of Monte Carlo simulation to estimate the minimum cluster size threshold that would yield a false positive rate of 5%. Voxels activated above the indicated threshold (P < 0.05 corrected) were selected and the peak of activation for each ROI was reported.
Results
Behavioral Results
The number (mean ± SE) of rules identified did not differ significantly between the Average IQ (23.2 ± 1.1) and High IQ (25.6 ± 0.5) groups, nor did the average number of errors made when identifying each rule (1.5 ± 0.3 and 1.0 ± 0.1, respectively). The mean reaction times taken to match cards were however significantly slower (t(26) = 4.06, P < 0.001) in the Average IQ group (1806 ± 53 ms) compared the High IQ group (1470 ± 72 ms).
Imaging Data
To study how the activation pattern changed across different stages of cognitive set shifting, 8 experimental conditions were compared against a (fixation) baseline. These 8 conditions represented the progression of cognitive set shifting processes from the time of the first negative feedback signal (1stNF) indicating the rule change, to the response selection event (RS2+PF) associated with maintaining an already established rule (see Fig. 1).
Group Differences During Response Selection
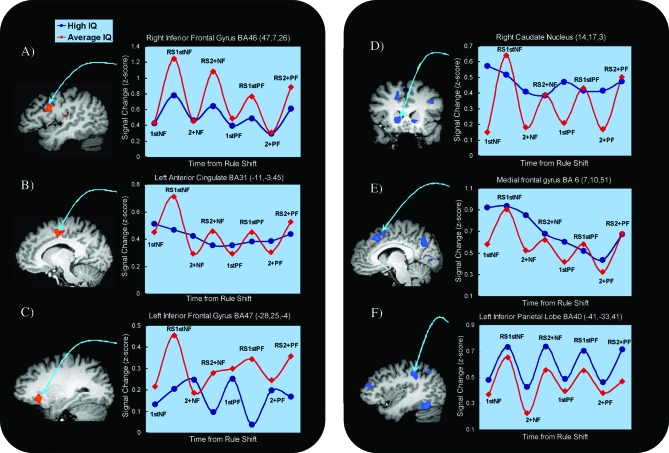
When the card stimuli were present during a response selection event, participants would need to select the appropriate response from competing alternatives, and after a rule shift, ignore the prepotent and now inappropriate stimulus-response contingency that had been established during recent trials (Graham et al. 2009). Response selection events would decrease in difficulty with increasing time since rule shift when the new rule had been established following confirmatory positive feedback. The main trend in group differences for response selection events across several regions was that the Average IQ group showed greater activation for response selection compared with the High IQ group (see Table 1). Consistent with the neural efficiency hypothesis, the function of some of these regions would suggest a greater cognitive challenge facing the Average IQ group. For example, the right inferior frontal gyrus (Brodmann Area [BA] 46) which research suggests is strongly implicated in inhibition of prepotent (but inappropriate) responses (Konishi et al. 1998) showed greater activation in Average IQ participants during response selection. Activation in this region peaked on response selection events immediately following negative feedback (when the need to inhibit the prepotent response would be greatest) and diminished with increasing time since rule shift (see Fig. 2A). A similar activation profile was observed in the anterior cingulate gyrus cortex (BA 31/32), which is thought to be involved in resolution of response conflict (Garavan et al. 1999; Braver et al. 2001) and again activation was most pronounced immediately after the rule change, that is, during the presence of more competing responses for selection when the identity of the new rule was unknown (Fig. 2B). If the Average IQ participants were to have experienced greater conflict (e.g., from competing response alternatives) then this could explain their relatively greater activation in these regions observed compared with the High IQ participants. Another area showing greater activation among Average IQ participants during response selection was the left inferior frontal gyrus (BA 47) (see Fig. 2C). This ventrolateral prefrontal region (VLPFC) is implicated in retrieving rule meanings and actively maintaining representations of the rule contingencies (Bunge 2004). A functionally related region (lateral BA 6) which was also more activated in the Average IQ group is thought to coordinate the rule and motor programs to allow execution of the response (Bunge et al. 2005). Thus greater activation in Average IQ participants could be explained by a greater need for response inhibition, conflict resolution and working memory to support the online maintenance and consideration of more response options and candidate rules during response selection.
Table 1.
High IQ versus Average IQ group differences in activation during across response selection events (RS1stNF, RS2+NF, RS1stPF, RS2+PF)
| Anatomical region | Talairach (x y z) | BA | mm3 | ||
| RS1stNF | |||||
| Average IQ > High IQ | |||||
| Left inferior frontal gyrus | −28 | 25 | −4 | 47 | 483 |
| Left anterior cingulate gyrus | −10 | 17 | 34 | 32 | 363 |
| Left superior temporal gyrus | −50 | 9 | −15 | 38 | 402 |
| Right inferior frontal gyrus | 47 | 7 | 26 | 46 | 1660 |
| Left superior temporal gyrus | −36 | 5 | −15 | 38 | 464 |
| Right middle frontal gyrus | 24 | −3 | 52 | 6 | 2046 |
| Left anterior cingulate gyrus | −11 | −3 | 45 | 31 | 660 |
| Right superior temporal gyrus | 54 | −6 | 5 | 42 | 4665 |
| Right postcentral gyrus | 54 | −8 | 19 | 3 | 3503 |
| Right putamen | 26 | −9 | 11 | — | 1147 |
| Left hippocampus | −29 | −12 | −18 | — | 687 |
| Left postcentral gyrus | −44 | −18 | 45 | 4 | 1365 |
| Right superior parietal lobule | 31 | −41 | 52 | 7 | 951 |
| RS2+NF | |||||
| High IQ > Average IQ | |||||
| Right superior frontal gyrus | 10 | 40 | 39 | 8/9 | 1172 |
| Right inferior occipital gyrus | 32 | −81 | −7 | 19 | 605 |
| Average IQ > High IQ | |||||
| Right inferior frontal gyrus | 45 | 7 | 26 | 46 | 1719 |
| Right insula | 39 | −4 | −10 | — | 409 |
| Left precentral gyrus | −54 | −12 | 47 | 6 | 325 |
| Left superior occipital gyrus | −33 | −73 | 25 | 19 | 389 |
| RS1stPF | |||||
| High IQ > Average IQ | |||||
| Right inferior occipital gyrus | 31 | −81 | −5 | 19 | 767 |
| Average IQ > High IQ | |||||
| Left inferior frontal gyrus | −25 | 25 | −6 | 47 | 388 |
| Right middle frontal gyrus | 25 | −7 | 50 | 6 | 1083 |
| Right postcentral gyrus | 34 | −26 | 49 | 3 | 486 |
| Left superior occipital gyrus | −38 | −75 | 24 | 19 | 598 |
| RS2+PF | |||||
| Average IQ > High IQ | |||||
| Left postcentral gyrus | −48 | −16 | 50 | 4 | 1104 |
| Left superior occipital gyrus | −38 | −75 | 25 | 19 | 735 |
Figure 2.
Regions of interest graphs depicting the signal change across the events from rule shift (A) right inferior frontal gyrus, (B) left anterior cingulate, (C) left inferior frontal gyrus, (D) caudate nucleus, (E) medial frontal gyrus, (F) left inferior parietal lobe.
Another region consistent with neural efficiency that showed greater activity during response selection for the Average IQ participants was the right superior temporal gyrus (BA 38/42). The lateral temporal lobes represent semantic features of abstract rules (Bunge 2004), and greater activation in Average IQ participants is consistent with our observations that Average IQ participants were using more verbal strategies (e.g., “match on color”) to guide response selection on the ensuing trial. In contrast, the postexperiment debriefing revealed that many High IQ (but not Average IQ) participants reported detailed spatial response strategies in relation to the abstract rules (e.g., first select a candidate dimension “color” and when the card stimuli appear during the next response selection, press the left key if the card is red; see Fig. 1). This observation is consistent with another study in which higher IQ participants reported greater use of spatial strategies compared with those with lower IQ who reported greater use of verbal strategies (Jausovec and Jausovec 2004b).
Two regions however showed greater activation in High IQ compared with Average IQ participants (inconsistent with the neural efficiency hypothesis). These included the right superior frontal gyrus (BA 8/9) and right inferior occipital gyrus (BA 19) which is considered to support the memory mechanism for low-level visual attributes (Magnussen 2000). This could have facilitated visual scanning of the candidate and reference card at response selection to enable a faster response.
Group Differences during Feedback Evaluation
The feedback stimulus provided negative or positive reinforcement about the last response thus enabling the participant to adapt their response strategy on subsequent trials. The first negative feedback (1stNF) presented after a series of positive feedback events signaled the need for participants to shift from the previously correct and prepotent rule to a strategy based on 1 of the 2 remaining rule candidates. Any subsequent negative feedback (2+NF) would prompt participants to continue generating rule candidates, until the new rule was identified. The first positive feedback (1stPF) would cue participants to remember and continue performing based on this new rule. Subsequent confirmatory positive feedback (2+PF) would confirm the established rule and its continued rehearsal in memory. This rule would then be maintained until another rule change occurred as indicated by the next 1stNF signal. In general, activation during feedback evaluation should therefore reflect cognitive processes that support planning of a response strategy in preparation for the ensuing trial.
In contrast to the trend observed during response selection, presentation of feedback (positive or negative) resulted in greater activation for the High IQ compared with the Average IQ group (see Table 2). Regions showing group differences included 1) bilateral caudate nuclei; 2) left inferior (BA 40), bilateral superior (BA 7) parietal lobe and precuneus (BA 7); 3) medial frontal gyrus (BA 6); 4) posterior cingulate (BA 23), and 5) bilateral lingual gyri (BA 17) and bilateral fusiform gyri (BA 37/19). Inspection of the parameter estimates for most of these ROIs (see Fig. 2D–F) indicated that the Average IQ group failed to produce as much activation during feedback evaluation as did the High IQ group. These group differences are inconsistent with the neural efficiency hypothesis and instead suggest that the High IQ individuals were engaged in greater cognitive processing which we will argue reflects more strategic response planning at the time of feedback evaluation.
Table 2.
High IQ versus Average IQ group differences in activation during feedback evaluation events (1stNF, 2+NF, 1stPF, 2+PF)
| Anatomical region | Talairach (x y z) | BA | mm3 | ||
| 1stNF | |||||
| High IQ > Average IQ | |||||
| Right caudate nucleus | 14 | 17 | 3 | — | 4241 |
| Left caudate nucleus | −14 | 17 | 3 | — | 3405 |
| Medial frontal gyrus | ±7 | 10 | 51 | 6 | 5882 |
| Right thalamus | 11 | −11 | 9 | — | 3768 |
| Left postcentral gyrus | −29 | −32 | 42 | 3 | 4913 |
| Right superior parietal lobule | 23 | −55 | 45 | 7 | 6946 |
| Left fusiform gyrus | −48 | −58 | −12 | 37 | 4474 |
| Right fusiform gyrus | 25 | −65 | −10 | 19 | 3663 |
| Lingual gyrus | ±4 | −80 | −3 | 18 | 4325 |
| 2+NF | |||||
| High IQ > Average IQ | |||||
| Right medial frontal gyrus | 7 | 23 | 45 | 6 | 3787 |
| Posterior cingulate gyrus | ±3 | −28 | 30 | 23 | 1573 |
| Left inferior parietal lobule | −41 | −33 | 41 | 40 | 3042 |
| Left fusiform gyrus | −43 | −53 | −15 | 37 | 1477 |
| Left superior parietal lobule | −26 | −61 | 46 | 7 | 3190 |
| Right superior parietal lobule | 23 | −62 | 49 | 7 | 2892 |
| Left precuneus | −4 | −62 | 49 | 7 | 2300 |
| Right precuneus | 6 | −65 | 40 | 7 | 2071 |
| Lingual gyrus | ±3 | −80 | 5 | 17 | 1872 |
| 1stPF | |||||
| High IQ > Average IQ | |||||
| Left fusiform gyrus | −36 | −53 | −15 | 37 | 3419 |
| Right fusiform gyrus | 29 | −66 | −6 | 37 | 2539 |
| Lingual gyrus | ±3 | −76 | 5 | 17 | 4427 |
| 2+PF | |||||
| High IQ > Average IQ | |||||
| Right caudate nucleus | 13 | 21 | 4 | — | 1867 |
| Right superior parietal lobule | 24 | −58 | 48 | 7 | 3851 |
| Left superior parietal lobule | −25 | −67 | 48 | 7 | 5031 |
| Right fusiform gyrus | 43 | −70 | −8 | 19 | 6669 |
| Left fusiform gyrus | −33 | −70 | −8 | 19 | 5136 |
| Lingual gyrus | ±3 | −76 | 5 | 17 | 3887 |
Discussion
The present fMRI study compared the event-related fMRI activation during cognitive set shifting performance between High IQ and Average IQ participants. Despite comparable behavioral performance (except for faster reaction times among the High IQ participants) there were striking imaging differences between the High and Average IQ groups during response selection and feedback evaluation events. During response selection the Average IQ group showed relatively greater activation in the prefrontal and anterior cingulate regions. Conversely, during feedback evaluation, the High IQ group showed relatively greater activation in parietal, caudate, fusiform and occipital regions. The findings provide evidence both consistent with (during response selection) and inconsistent with (during feedback evaluation) the neural efficiency hypothesis, indicating that the brain region being recruited and the level of activation are dependent on the specific cognitive set-shifting component of the task. We suggest that the Average IQ group was less strategic in evaluating feedback about the preceding trial than the High IQ group and as a result experienced greater response conflict from competing options during the ensuing response selection.
Average IQ Participants Required Greater Response Inhibition and Experienced Greater Conflict during Response Selection
The overall pattern of higher activation among the Average IQ participants during response selection may reflect the experience of greater cognitive demands (or the use of more inefficient strategies) compared with High IQ participants. As discussed earlier, greater activation in the right inferior frontal gyrus and anterior cingulate (BA 32) is implicated in inhibitory control of behavior, monitoring of information, error detection and conflict processing, and could explain greater activation in the Average IQ participants (Konishi et al. 1998; Garavan et al. 1999; Konishi et al. 1999; Braver et al. 2001). Research findings support the involvement of the VLPLC in working memory (Owen et al. 2005). Greater VLPFC activation could also reflect greater demands on working memory to support the consideration of more response options and candidate rules during response selection. These results are consistent with earlier studies that found greater VLPFC activity during working memory maintenance in subjects who performed more slowly and less accurately (Rypma et al. 2002) and in low memory span relative to high memory span participants during a simple reading task (Osaka et al. 2004). Thus greater activation among the Average IQ participants is consistent with the neural efficiency hypothesis, and our interpretation is that this reflects a more inefficient cognitive strategy. Inconsistent with the neural efficiency hypothesis, the superior frontal gyrus (BA 8/9) which borders on the cingulate sulcus and BA 19 (inferior occipital gyrus) showed relatively greater activation in the High IQ participants. Functional connectivity between these 2 regions has been considered an important component of “g” and is thought to help resolve competition among incoming visual stimuli (Haier et al. 2003).
High IQ Participants Plan Future Response Strategies during Feedback Evaluation
During feedback evaluation, the High IQ group marshaled greater activation in many regions which are associated with complex reasoning and strategic response preparation. For example, greater caudate activity in High IQ participants could reflect the formulation of response strategies during feedback evaluation in preparation for the next trial. A study of associative conditioning in which monkeys learned to discriminate between visual cues showed that caudate activity preceded activity in the prefrontal cortex, thus reflecting more rapid anticipation of the correct discrimination choice; activation in the prefrontal cortex in contrast reflected a slower learning mechanism (Pasupathy and Miller 2005). One interpretation is that the caudate nuclei rapidly support the formation of a heuristic response strategy, which is useful under time pressure. Thus in the present study, greater caudate activation in High IQ participants could have enabled advance planning of the forthcoming response which would be consistent with the detailed response strategies they verbalized during the postexperiment debriefing. At the feedback stage, greater activation was also observed in the medial frontal gyrus (BA 6)—a region which has also been implicated in motor planning of tasks such as decision making, discrimination, and reasoning (Talati and Hirsch 2005). In the present study employing such strategies during feedback evaluation would have enabled the High IQ participants to respond faster and more automatically during response selection. The bilaterial superior parietal regions (BA 7) are implicated in task set reconfiguration (Brass and von Cramon 2004; Crone et al. 2006) in the present study and also showed greater activation in High IQ participants during feedback evaluation. Under task switching demands, the stimulus–response associations would need to be re-mapped to respond appropriately and higher activation during feedback evaluation would be expected if the High IQ participants attempted to reconfigure these mappings before they were required to make a response. Again this is consistent with the detailed response planning reported by the High IQ participants during the postexperiment debriefing sessions.
The inferior parietal lobe (BA 40) which was also more strongly activated by High IQ participants during feedback evaluation has been related to an attentional role during cognitive set shifting tasks (Berman et al. 1995; Lauber et al. 1996; Smith et al. 2004). One interpretation of the current findings is that as a consequence of greater parietal activation during feedback evaluation (contradictory to the neural efficiency hypothesis), relatively less activation was required for High IQ participants during response selection (consistent with the neural efficiency hypothesis). In other words, High IQ individuals may have launched greater cognitive resources to support advanced planning of the next response. In a review of structural imaging studies, BA 40 featured frequently as a discrete Brodmann area associated with intelligence/reasoning (Jung and Haier 2007). Our interpretation is that the inferior parietal regions (together with caudate nuclei) support strategizing in the High IQ participants who as a consequence (i.e., having hypothesized a candidate rule and planned forthcoming response mappings) faced less difficulty with prepotent responses and responded faster than the Average IQ participants on the next trial. Consistent with this notion, EEG studies of problem solving show development of better strategies and more parietal activity in high ability participants compared with lower ability participants who rely more on frontal regions (Gevins and Smith 2000) and working memory (Jausovec and Jausovec 2004b). It has been proposed that as memory retrieval becomes more automatic it is processed in the posterior temporal and parietal perceptual system rather than the frontal cortex which supports more effortful retrieval (Petrides 1998; Koch et al. 2006).
Reconciliation of Extant Neural Efficiency Literature
In general, PET and fMRI studies involving working memory and mental imagery/rotation show reduced activation in more proficient participants (Haier et al. 1988; Haier et al. 1992; Kosslyn et al. 1996; Rypma and D'Esposito 1999; Rypma et al. 2002; Rypma et al. 2005) and are often taken as support for the neural efficiency hypothesis. Close scrutiny, however, suggests that such an interpretation is sometimes less straight-forward. For example, Haier et al. (1992) observed negative correlations between the learning-related change in glucose metabolism rates during a visual object rotation (“Tetris”) task performed before and after practice (consistent with neural efficiency) but positive correlations between IQ score and glucose metabolism rates during the initial task performance session (inconsistent with neural efficiency). In addition, Rypma et al. (2002) showed that task difficulty can affect activation within the working memory-related dorsolateral prefrontal (DLPFC) and ventrolateral prefrontal (VLPFC) regions. Higher performing participants showed less DLPFC activation during delay and retrieval periods for the easier (2-item) trials compared with the more difficult (8-item) trials. A different pattern was observed in lower performing participants during the encoding periods in whom there was greater VLPFC activation for the easier compared with the more difficult items (Rypma et al. 2002). Task difficulty can therefore influence patterns of neural activity. For example, a task difficulty by group interaction was observed in a working memory study in which high-capacity participants showed larger activation differences in anterior cingulate and inferior frontal gyrus (BA 44/45) between simple and more complex reading tasks compared with low-capacity participants (Osaka et al. 2004)—specifically, a simple task produced less activation, whereas the more complex task produced more activation in the left inferior frontal gyrus in high but not low working memory capacity individuals. It therefore appears that the neural efficiency hypothesis receives support when tasks are relatively simple to perform (but not when task complexity increases).
Imaging findings from tasks demanding more complex reasoning tend to go against the neural efficiency hypothesis. For example, individuals with higher fluid intelligence (Raven's Advanced Progressive Matrices scores) demonstrated stronger recruitment of the lateral prefrontal (bilateral BA 44/45/46/9/10) and bilateral parietal lobes (BA 40) during the more challenging trials of a 3-back working memory task (Gray et al. 2003). No relationship between “gF” and brain activity was observed for the simpler trials, but activity during correct responses to the more difficult lure trials was positively correlated with individuals’ fluid “gF” scores. In addition timing differences in activation profiles were noted (e.g., left lateral prefrontal): in particular a stronger but shorter duration of activation among higher ability participants.
Thus the difficulty of the cognitive task as well as the point and period of measurement appears to influence the degree to which differences between high and low ability participants are detectable. Furthermore, IQ-related activation differences in the present study appear to underlie the adoption of different strategies between High IQ and Average IQ individuals in an attempt to optimize task performance. This is consistent with interpretations in earlier studies of individual differences (Haier et al. 1992; Rypma and D'Esposito 1999; Reichle et al. 2000; Ruff et al. 2003; Rypma et al. 2005), which proposed greater strategy-shifting ability (Prabhakaran et al. 1997) or better prioritizing of cognitive processes (Rypma et al. 2002) in higher performers. Some authors also consider neural efficiency to reflect nonuse of brain regions irrelevant for good task performance and more focused use of specific task-relevant areas (Jausovec and Jausovec 2004b) or entirely different neural circuits (involving less prefrontal regions) in more intelligent individuals (Haier et al. 2003). Also, a recent study showed that individuals with greater working memory capacity capitalized on the additional processing time available during a delay interval in order to achieve faster performance on subsequent trials (Rypma and Prabhakaran 2009). This is entirely consistent with the pattern of results observed in the present study—higher IQ participants appear to have made better advantage of the delay between feedback and the start of the next trial to help plan their actions and thus execute their responses more quickly.
Implications for Imaging Research on Executive Function in Disordered Populations
Understanding the way in which IQ influences executive functioning has important implications for research on disorders such as schizophrenia and autism where frontal/executive deficits are core and enduring clinical features (Weinberger and Berman 1988; Frith 1992; Russell et al. 1997). Although it is widely accepted that schizophrenia and autism are associated with deficits in executive function (Johnson-Selfridge and Zalewski 2001; Hill 2004), the present study confirms that intellectual function too could have an influential role in the patterns of brain activation. Some of the current lack of consensus about brain activation during executive functioning in schizophrenia (Barch 2006; Pomarol-Clotet et al. 2008) could therefore be due to a potential confound of different IQ levels in between schizophrenia patients and healthy controls. When studying executive function deficits in clinical populations, neuropsychological and imaging studies of schizophrenia typically match patients and comparison participants on number of years of education, socioeconomic status and premorbid IQ. The present study indicates that current IQ differences alone can lead to radically different fMRI activation patterns as well as different response strategies. This implies that studies of disordered populations could benefit from more careful consideration of differences in current IQ. One approach has been to “partial out” the effect of current IQ as a covariate in an attempt to increase the power of detecting imaging differences due to the disorder itself. As the mean of these current IQ covariates would likely differ between the patients (who typically have relatively lower current IQ) and controls, analysis of covariance (ANCOVA) would both reduce the error term as well as adjust the mean on the dependent variable (imaging regressor). The present study however shows that using ANCOVA would not be advisable as the covariate interacts with the within-subject imaging conditions. Consider for example a voxel located in the caudate nucleus (see Fig. 2D) whose activity during feedback events is greater for High IQ compared to Average IQ participants, but whose activity during response selection conditions does not differ between High IQ and Average IQ participants. Because there is only one covariate value for a given participant, the ANCOVA model would not be able to appropriately use the covariate to adjust the means for such a voxel. If for example, the covariate adjusted the means upward for the Average IQ participants, then this would appear to “partial out” the IQ effect for the feedback conditions, but it would also adjust the means upward and introduce spurious group differences for the response selection conditions. Thus under the ANCOVA model, activation in this voxel might disappear for the feedback conditions but appear for response selection conditions. To illustrate this further, we calculated the correlations between participants’ IQ scores and signal change in caudate nuclei for each of their imaging conditions. There were positive correlations (average r = 0.47 ± 0.06) between IQ and caudate activation for each of the feedback conditions, however correlations for the response selection conditions were either low or negative (average r = −0.10 ± 0.08). Thus although it would be possible to compute ANCOVA models of the data, the adjusted means for the imaging parameters and resultant activation differences might be rather difficult to interpret in any meaningful way. A better approach might therefore be to include separate control groups matched on current IQ or premorbid IQ and to use the pattern of results obtained to decide which imaging differences were related to IQ decline and which were related to the disorder itself.
Conclusion
Functional imaging studies have consistently implicated a network of prefrontal and parietal regions associated with better performance on measures of intelligence and complex reasoning (Colom et al. 2006; Jung and Haier 2007). In the present study, differences in frontal, parietal, occipital and basal ganglia activation patterns suggest that an individual's level of intelligence can influence the strategy used to perform complex tasks. The event-related design of the present study unveiled how isolated processes involved in set-shifting (strategic planning, working memory, maintenance, conflict resolution, and response inhibition) were associated with IQ-related imaging differences. Different conclusions about neural efficiency could easily have been drawn from a block design experiment where subtle but significant differences would have been masked. The implications of these findings are that the brain region, task complexity (g load), and experimental design used to image specific cognitive processes require careful consideration before any definitive conclusions about IQ and neural efficiency can emerge.
Given the important differences in neural activity and strategy employed by individuals in the present study, care should be taken when extending findings from fMRI decision-making studies across samples whose IQ differs by 1 or 2 standard deviations. For future studies, researchers investigating populations with potentially different IQ levels should therefore consider controlling for these IQ differences which may otherwise cloud interpretations.
Funding
National University of Singapore (R581-000-053-112, NUS R581-000-061-112); A*STAR Biomedical Research Council (R581-000-056-305).
Acknowledgments
We thank Ho New Fei and the anonymous reviewers for their helpful comments. Conflict of Interest: None declared.
References
- Baddeley AD. The concept of episodic memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1345–1350. doi: 10.1098/rstb.2001.0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJL. Working memory. In: Bower GA, editor. The psychology of learning and motivation: advances in research and theory. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Barch DM. What can research on schizophrenia tell us about the cognitive neuroscience of working memory? Neuroscience. 2006;139:73–84. doi: 10.1016/j.neuroscience.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, Carson RE, Herscovitch P, Weinberger DR. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: a positron emission tomography study. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Selection for cognitive control: a functional magnetic resonance imaging study on the selection of task-relevant information. J Neurosci. 2004;24:8847–8852. doi: 10.1523/JNEUROSCI.2513-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA. How we use rules to select actions: a review of evidence from cognitive neuroscience. Cogn Affect Behav Neurosci. 2004;4:564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD. Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cereb Cortex. 2005;15:239–249. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- Burgess GC, Braver TS, Gray JR. Exactly how are fluid intelligence, working memory, and executive function related? Cognitive neuroscience approaches to investigating the mechanisms of fluid cognition. Behav Brain Sci. 2006;29:128–129. [Google Scholar]
- Colom R, Abad FJ, Garcia LF, Juan-Espinosa M. Education, Wechsler's Full Scale IQ, and g. Intelligence. 2002;30:449–462. [Google Scholar]
- Colom R, Jung RE, Haier RJ. Distributed brain sites for the g-factor of intelligence. Neuroimage. 2006;31:1359–1365. doi: 10.1016/j.neuroimage.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Dodrill CB. Myths of neuropsychology. Clin Neuropsychol. 1997;11:1–17. doi: 10.1076/1385-4046(199911)13:04;1-Y;FT562. [DOI] [PubMed] [Google Scholar]
- Dodrill CB. Myths of neuropsychology: further considerations. Clin Neuropsychol. 1999;13:562–572. doi: 10.1076/1385-4046(199911)13:04;1-Y;FT562. [DOI] [PubMed] [Google Scholar]
- Duncan J. Frontal lobe function and general intelligence: why it matters. Cortex. 2005;41:215–217. doi: 10.1016/s0010-9452(08)70896-7. [DOI] [PubMed] [Google Scholar]
- Duncan J, Burgess P, Emslie H. Fluid intelligence after frontal lobe lesions. Neuropsychologia. 1995;33:261–268. doi: 10.1016/0028-3932(94)00124-8. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Frith CD. The cognitive neuropsychology of schizophrenia. Hove: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A, Smith ME. Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cereb Cortex. 2000;10:829–839. doi: 10.1093/cercor/10.9.829. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S, Phua E, Soon CS, Oh T, Au C, Shuter B, Wang SC, Yeh IB. Role of medial cortical, hippocampal and striatal interactions during cognitive set-shifting. Neuroimage. 2009;45:1359–1367. doi: 10.1016/j.neuroimage.2008.12.040. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Buchsbaum MS, DeMet E, Wu J. Biological vulnerability to depression: replication of MAO and evoked potentials as risk factors. Neuropsychobiology. 1988;20:62–66. doi: 10.1159/000118474. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Siegel B, Tang C, Abel L, Buchsbaum MS. Intelligence and changes in regional cerebral glucose metabolic rate following learning. Intelligence. 1992;16:415–426. [Google Scholar]
- Haier RJ, White NS, Alkire MT. Individual differences in general intelligence correlate with brain function during nonreasoning tasks. Intelligence. 2003;31:429–441. [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Horton AM. Above-average intelligence and neuropsychological test score performance. Int J Neurosci. 1999;99:221–231. doi: 10.3109/00207459908994326. [DOI] [PubMed] [Google Scholar]
- Jausovec N, Jausovec K. Intelligence related differences in induced brain activity during the performance of memory tasks. Pers Individ Diff. 2004a;36:597–612. doi: 10.1016/s0278-2626(03)00263-x. [DOI] [PubMed] [Google Scholar]
- Jausovec N, Jausovec K. Differences in induced brain activity during the performance of learning and working-memory tasks related to intelligence. Brain Cogn. 2004b;54:65–74. doi: 10.1016/s0278-2626(03)00263-x. [DOI] [PubMed] [Google Scholar]
- Jensen AR. The g factor: the science of mental ability. Santa Barbara (CA): Praeger; 1998. [Google Scholar]
- Johnson-Selfridge M, Zalewski C. Moderator Variables of executive functioning in schizophrenia: meta-analytic findings. Schizophr Bull. 2001;27:305–316. doi: 10.1093/oxfordjournals.schbul.a006876. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Jung RE, Yeo RA, Chiulli SJ, Sibbitt WL, Jr, Brooks WM. Myths of neuropsychology: intelligence, neurometabolism, and cognitive ability. Clin Neuropsychol. 2000;14:535–545. doi: 10.1076/clin.14.4.535.7198. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension: individual differences in working memory. Psychol Rev. 1992;99:122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Koch K, Wagner G, von Consbruch K, Nenadic I, Schultz C, Ehle C, Reichenbach J, Sauer H, Schlosser R. Temporal changes in neural activation during practice of information retrieval from short-term memory: an fMRI study. Brain Res. 2006;1107:140–150. doi: 10.1016/j.brainres.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat Neurosci. 1998;1:80–84. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Shin LM, Thompson WL, McNally RJ, Rauch SL, Pitman RK, Alpert NM. Neural effects of visualizing and perceiving aversive stimuli: a PET investigation. Neuroreport. 1996;7:1569–1576. doi: 10.1097/00001756-199607080-00007. [DOI] [PubMed] [Google Scholar]
- Lauber EJ, Meyer DE, Evans JE, Rubinstein J, Gmeindl L, Junck I, Koeppe RA. The brain areas involved in the executive control of task switching as revealed by PET. Neuroimage. 1996;3:247. [Google Scholar]
- Lee KH, Choi YY, Gray JR, Cho SH, Chae J-H, Lee S, Kim K. Neural correlates of superior intelligence: stronger recruitment of posterior parietal cortex. Neuroimage. 2006;29:578–586. doi: 10.1016/j.neuroimage.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Magnussen S. Low-level memory processes in vision. Trends Neurosci. 2000;23:247–251. doi: 10.1016/s0166-2236(00)01569-1. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Grabner RH, Freudenthaler HH, Beckmann JF, Guthke J. Intelligence and individual differences in becoming neurally efficient. Acta Psychol. 2004;116:55–74. doi: 10.1016/j.actpsy.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to Action: Willed and automatic control of behaviour. In: Norman DA, Shallice T, editors. Consciousness and self-regulation: advances in research and theory. New York: Plenum Press; 1986. pp. 1–18. [Google Scholar]
- Obonsawin MC, Crawford JR, Page J, Chalmers P, Cochrane R, Low G. Performance on tests of frontal lobe function reflect general intellectual ability. Neuropsychologia. 2002;40:970–977. doi: 10.1016/s0028-3932(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Osaka N, Osaka M, Kondo H, Morishita M, Fukuyama H, Shibasaki H. The neural basis of executive function in working memory: an fMRI study based on individual differences. Neuroimage. 2004;21:623–631. doi: 10.1016/j.neuroimage.2003.09.069. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks RW, Crockett DJ, Tuokko H, Beattie BL, Ashford JW, Coburn KL, Zec RF, Becker RE, McGeer PL, McGeer EG. Neuropsychological “systems efficiency” and positron emission tomography. J Neuropsychiatry Clin Neurosci. 1989;1:269–282. doi: 10.1176/jnp.1.3.269. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex. In: Roberts AC, Robbins TW, Weiskrantz L, editors. The prefrontal cortex. Oxford: Oxford University Press; 1998. pp. 103–116. [Google Scholar]
- Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, Guerrero A, Ortiz-Gil J, Sans-Sansa B, Capdevila A, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med. 2008;38:1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Smith JA, Desmond JE, Glover GH, Gabrieli JD. Neural substrates of fluid reasoning: an fMRI study of neocortical activation during performance of the Raven's Progressive Matrices Test. Cognit Psychol. 1997;33:43–63. doi: 10.1006/cogp.1997.0659. [DOI] [PubMed] [Google Scholar]
- Reichle ED, Carpenter PA, Just MA. The neural bases of strategy and skill in sentence-picture verification. Cognit Psychol. 2000;40:261–295. doi: 10.1006/cogp.2000.0733. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Knauff M, Fangmeier T, Spreer J. Reasoning and working memory: common and distinct neuronal processes. Neuropsychologia. 2003;41:1241–1253. doi: 10.1016/s0028-3932(03)00016-2. [DOI] [PubMed] [Google Scholar]
- Russell AJ, Munro JC, Jones PB, Hemsley DR, Murray RM. Schizophrenia and the myth of intellectual declines. Am J Psychiatry. 1997;154:635–639. doi: 10.1176/ajp.154.5.635. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D'Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci. 2002;14:721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Genova HM, Rebbechi D, D'Esposito M. Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI. Cortex. 2005;41:582–594. doi: 10.1016/s0010-9452(08)70198-9. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Natl Acad Sci USA. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V. When less is more and when more is more: the mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37:207–222. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Rubia K. Neural correlates of switching set as measured in fast, event-related functional magnetic resonance imaging. Hum Brain Mapp. 2004;21:247–256. doi: 10.1002/hbm.20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman C. The abilities of man. Science. 1928;68:38. doi: 10.1126/science.68.1750.38-a. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF, Kaplan EF, Weir WS, Naeser MA, Lieberman I, Ferrill D. The involvement of orbitofrontal cerebrum in cognitive tasks. Neuropsychologia. 1983;21:235–248. doi: 10.1016/0028-3932(83)90040-4. [DOI] [PubMed] [Google Scholar]
- Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on “what,” “when,” and “where” related information: an fMRI study. J Cogn Neurosci. 2005;17:981–993. doi: 10.1162/0898929054475226. [DOI] [PubMed] [Google Scholar]
- Tremont G. Effect of intellectual level on neuropsychological test performance: a response to Dodrill. 1997. Clin Neuropsychol. 1999;12:560–567. [Google Scholar]
- Van Rooy C, Stough C, Pipingas A, Hocking C, Silberstein RB. Spatial working memory and intelligence: biological correlates. Intelligence. 2001;29:275–292. [Google Scholar]
- Weinberger DR, Berman KF. Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophr Bull. 1988;14:157–158. doi: 10.1093/schbul/14.2.157. [DOI] [PubMed] [Google Scholar]