Abstract
Nitric oxide (NO) has been shown to reduce thrombogenicity by decreasing platelet and monocyte activation by the surface glycoprotein, P-selectin and the integrin, CD11b, respectively. In order to prevent platelet and monocyte activation with exposure to an extracorporeal circulation (ECC), a nitric oxide releasing (NORel) polymeric coating composed of plasticized polyvinyl chloride (PVC) blended with a lipophilic N-diazeniumdiolate was evaluated in a 4 hour rabbit thrombogenicity model using flow cytometry. The NORel polymer significantly reduced ECC thrombus formation compared to polymer control after 4 hours blood exposure (2.8 ± 0.7 NORel vs 6.7 ± 0.4 pixels/cm2 control). Platelet count (3.4 ± 0.3 NORel vs 2.3 ± 0.3 × 108/ml control) and function as measured by aggregometry (71 ± 3 NORel vs 17 ± 6 % control) were preserved after 4 hours exposure in NORel versus control ECC. Plasma fibrinogen levels significantly decreased in both NORel and control groups. Platelet P-selectin mean fluorescence intensity (MFI) as measured by flow cytometry was attenuated after 4 hours on ECC to ex vivo collagen stimulation (27 ± 1 NORel vs 40 ± 2 MFI control). Monocyte CD11b expression was reduced after 4 hours on ECC with NORel polymer (87 ± 14 NORel vs 162 ± 30 MFI control). These results suggest that the NORel polymer coatings attenuate the increase in both platelet P-selectin and monocytic CD11b integrin expression in blood exposure to ECCs. These NO-mediated platelet and monocytic changes were shown to improve thromboresistance of these NORel-polymer-coated ECCs for biomedical devices.
Keywords: Nitric oxide, Monocyte CD11b, Platelet P-selectin, Extracorporeal circulation, Hemocompatible polymer coating, Diazeniumdiolates, Rabbit thrombogenicity model
1. Introduction
Hemocompatibility of various biomaterials is still elusive in spite of over 5 decades of investigation[1–5]. The biocompatibility of polymeric tubing used in extracorporeal circulation is highly dependent on the suppression of both the contact coagulation pathway (i.e., fibrin formation) and the activation of circulating blood platelets. It has been recognized that preventing fibrin formation and platelet as well as monocyte activation, while patients are on chronic extracorporeal life support (ECLS), is needed for successful clinical outcomes and provides the motivation to suppress thrombogenicity in ECLS circuits. In the normal blood vessel the endothelial lining provides a thromboresistive surface by releasing molecules such as the anticoagulants thrombomodulin and heparan sulfates as well as potent inhibitors of platelet activation prostacyclin (PGI2), surface ADP-destroying ectoenzyme (ADPase) and the vasodilator, nitric oxide (NO) [6–8]. The possible role of nitric oxide released locally from polymeric coatings in mediating the level of platelet membrane glycoprotein, P-selectin and the influence on the monocyte integrin, CD11b, remains unexplored in thrombogenicity.
Nitric oxide, has been extensively studied for its inhibitory effects via the cGMP signaling pathway on circulating platelet and monocyte activation which leads to aggregation and ultimately thrombosis initiation [9–18]. The normal endothelial lining releases nitric oxide as an endogenous inhibitor of platelet activation and prevent intravascular thrombosis. Recent in vitro research has tried to mimic this ‘endothelial function’ through polymeric coatings of artificial surfaces that can prevent platelet [19–25]and possibly monocyte activation through the slow release of NO from polymer-embedded NO donor molecules. In an in vivo study with the NORel polymer, Skrzypchak et al. reported that the level of NO release per polymer surface area (i.e., NO flux) needed to be 14 × 10−10 mol·cm−2·min−1 or greater to prevent platelet activation and preserve platelet count in a rabbit thrombogenicity model [26]. This level of NO flux was achieved using 25 wt% of the NO donor, diazeniumdiolated dibutylhexanediamine (DBHD/N2O2). However, this previous work did not investigate the other important aspect of contact-induced thrombosis and that is the proinflammatory response elicited by activated monocytes. Preserving platelet count and function as well as maintaining monocyte inactivation is the role NO serves in normal blood vessels and proposed when exposed to a NO releasing artificial surface. However, by what cellular mechanism does NO attenuate these platelet and monocyte activated events needs further investigation. In extension of the results presented by Skrzypchak et al. this report will determine if the NO flux is maintained over the 4 hour period and if the NO released from the DBHD/N2O2 polymer coating preserves platelet count and function by attenuating platelet and monocyte activation as measured by P-selectin and CD11b upregulations, respectively.
To test the hypothesis that the level of ECC thrombogenicity is mediated through both platelet (via surface molecule P-selectin) and monocyte (by the surface integrin CD11b) activation, the present study was designed to evaluate if platelet P-selectin and monocyte CD11b expressions, as measured by flow cytometry, are mediated by NO released from DBHD/N2O2 polymer-coated ECC. Additionally, we investigated the correlation of platelet P-selectin and monocyte CD11b expressions with platelet quiescence and anti-thrombogencity in the presence of the NORel ECC.
2. Materials and Methods
2.1. Materials
Tygon® poly(vinyl chloride) (PVC) tubing was purchased from Fisher Healthcare (Houston, TX). Potassium tetrakis(4-chlorophenyl) borate (KTpClPB) and dioctyl sebacate (DOS) were obtained from Fluka (Ronkonkoma, NY). PVC (average MW = 106,000) was purchased from Aldrich (Milwaukee, WI). Tetrahydrofuran (THF) was distilled over sodium and benzophenone prior to use. (Z)- 1 -[N-Butyl-N-[6[(N-butylammoniohexl)amino]]-diazen-1-ium-1,2-diolate (diazeniumdiolated dibutylhexanediamine (DBHD/N2O2)) was synthesized by treating N,N′-dibutyl-1,6-hexanediamine (Aldrich) with nitric oxide (NO) (80 psi) at room temperature for 17–24 h as previously described [24]. All other reagents were of the highest purity available. The mouse antibodies for sheep CD41/CD61 (GPIIb/IIIa) FITC and human P-selectin glycoprotein (CD62P) PE were obtained from AbD Serotec (Raleigh, NC) as well as Mouse isotype controls for IgG1 FITC and IgG1 PE. For monocyte surface receptor determination of the integrin, CD11b, and the surface antigen CD14, the rat antibody for mouse CD11b Alexa Flour 488 and mouse antibody for human CD 14 PE, respectively from AbD Serotec were used. The antibody clones for P-selectin, IIb/IIIa receptor, CD11b and CD14 have been previously shown to have cross-reactivity to the rabbit [27–30]. Rat isotype control for IgG2b Alexa Fluor 488 and mouse isotype control for IgG2a PE were purchased from AbD Serotec. Human plasma fibrinogen containing ≥ 90% clottable proteins was obtained from Calbiochem (La Jolla, CA)and fluorescein-labeled goat IgG (polyclonal) to denatured human fibrinogen was purchased from MP Biomedicals, LLC (Solon, OH) and the 96-well microtiter plates (black, polypropylene) were obtained from Nalge Nunc International (Rochester, NY). For intracellular labeling of human platelets used with the in vitro platelet adhesion assay, a fluorescent stain, calcein AM (Sigma-Aldrich Chem. St. Louis, MO), was utilized.
2.2. Preparation of NORel polymeric coatings and ECC construction
A plasticized PVC coating containing 25 wt% DBHD/N2O2, a NO donor, was prepared using a method previously reported [20]. Briefly, triple layers of polymeric coatings which included a base layer, active layer and top layer were individually coated into the Tygon® tubing that formed the ECC. The base layer was prepared by dissolving 7 g PVC in 120 ml THF (Soln A). The top layer was synthesized using 543 mg PVC, 1057 mg DOS plasticizer and 30 ml THF (Soln B). The active layer containing the NO donor was prepared by dissolving 702 mg PVC, 360 mg DOS and 458 mg KTpClPB in 10 ml THF. Two hundred sixty seven milligrams DBHD/N2O2 was then dispered within the polymer cocktail by sonication for 10 min to obtain a slightly cloudy dispersion of the diazeniumdiolate in the solution (Soln C). The trilayer configuration had a total thickness of approx. 100–150 μm.
The ECC construction used in this study was previously described but with some modifications [26]. The ECC consisted with a 16-gauge and 14-gauge IV polyurethane angiocatheters (Kendall Monoject Tyco Healthcare Mansfield, MA), two 16 cm in length of ¼ inch inner diameter (ID) Tygon® tubing and a 8 cm length of 3/8 inch ID Tygon® tubing which created a thrombogenicity chamber where thrombus could form more easily due to more turbulent blood flow. The ECC was pieced together, starting at the left carotid artery side, with the 16-gauge angiocatheter, one 15 cm length ¼ inch ID tubing, the 8 cm length thrombogenicity chamber, the second 15 cm length ¼ inch ID tubing and finally the 14-gauge angiocatheter. The angiocatheters were interfaced with tubing using 2 luer-lock PVC connectors. The 3/8 inch ID tubing and the ¼ inch tubing were welded together using THF.
The assembled ECC was coated first with a base coating of Soln A, followed by two coats of the active layer of Soln C and the top coat (Soln B). The circuitry was filled with each solution then removed. Each coat was allowed to dry for at least 1 h. The finshed ECCs were allowed to cure under nitrogen conditions for 20 min then dried under vacuum for 2 days. (Figure 1).
Figure 1.
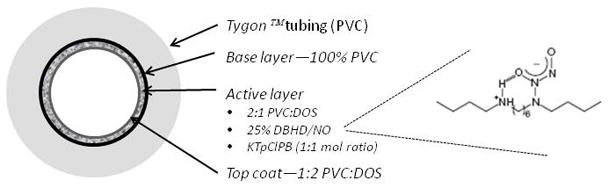
Schematic of plasticized poly(vinyl chloride) (PVC) layer doped with a lipophilic N-diazeniumdiolate (diazeniumdiolated dibutylhexanediamine (DBHD/N2O2)) nitric oxide (NO) donor. A top coat of plasticized PVC over the active layer limited the NO release and provided a consistent NO level over the 4 hour in vivo evaluation.
2.3. NO release measurements
NO released from Norel coated ciruits was measured via a Sievers chemiluminescence NO Analyzer® (NOA), model 280 (Boulder, CO) as described previously [24]. Briefly, a ~1/2 sample of the circuitry was placed in PBS buffer at 37C. NO was continuously swept from the headspace of the sample vessel and purged from the bathing solution with a nitrogen sweep gas and bubbler into the chemiluminescence detection chamber. The flow rate was set to 200 ml/min with a chamber pressure of 5.4 Torr and an oxygen pressure of 6.0 psi. Using this method, a uniform segment of NORel polymer was tested for NO release prior to and 4 hours after blood exposure.
2.4. In vitro fluorescence assays
The in vitro fibrinogen adsorption immunofluorescence assay was performed in a 96-well format. The NORel and control polymer solutions used in preparing the ECC was also used to coat microwells of the 96-well microtiter plates. In addition, similar polymer drying conditions were done between the ECC and 96-well preparations. Briefly, human fibrinogen was diluted to 3 mg/ml with Dulbecco’s phosphate-buffered saline (dPBS) without CaCl2 and MgCl2 (Gibco Invitrogen Grand Island, NY), equivalent to the human plasma concentration, and then used for the adsorption experiments [31]. Of this diluted solution, 100 μl were added to each well for 1.5 hours at 37°C, followed by eight washing steps using 100 μl of wash buffer which consisted of a 10-fold dilution of AbD Serotec Block ACE buffer (Raleigh, NC) and 0.05% Tween 20 (Calbiochem La Jolla, CA). To block nonspecific antibody binding, wells were incubated with 100 μl blocking buffer (4-fold dilution of Serotec Block ACE buffer) for 30 min at 37°C. After rinsing 3 times with wash buffer (100 μl per well) a background fluorescence measurement of the plates was performed at 485 nm (excitation) and 528 nm (emission) on a Synergy 2 fluorescence microplate reader (Biotek Winooski, VT). To detect the adsorbed fibrinogen, goat antihuman fibrinogen was diluted (1:10) in antibody diluents (10-fold dilution of Serotec Block ACE buffer) and 100 μl was added to each well. The antibody was allowed to bind to the surface-adsorbed fibrinogen for 1.5 hours at 37°C. Human fibrinogen adsorption to noncoated polypropylene was used as an internal control to normalize the fluorescence signals within different plates. Also, a standard curve for fibrinogen was obtained on each plate from 0 to 3000 μg/ml. All measurements were done in triplicate and performed with the Synergy 2 fluorescence reader.
To ascertain the platelet adhesive characteristics of the NORel polymer, an in vitro platelet adhesion fluorescence assay was used. This assay was modified from a previous in vitro adhesion assay which used a measurement of released lactate dehydrogenase [32]. Briefly, polymer-coated 96-well plates were prepared similarly as to the in vitro fibrinogen adsorption assay. To prepare fluorescently labeled human platelets for the in vitro platelet adhesion assay, washed human platelets were obtained from whole blood by centrifugation at 110 × g for 15 min. Platelet-rich plasma was transferred to new tube with 10 ml Hanks balanced salt solution (HBSS) without Ca2+ and Mg2+ plus 10% citrate-dextrose solution (ACD) and then centrifuged at 1200 × g for 10 min. The resulting platelet pellet was resuspended in HEPES-buffered Tyrode’s solution (137 mM NaCl, 3 mM KCl, 0.42 mM NaH2PO4, 6 mM NaHCO3, 1 mM MgCl2, 5 mM HEPES, 5.5 mM D-glucose and 0.35% bovine serum albumin (BSA), pH 7.4) to a level of 10 × 108 platelets/ml. A final concentration of 5 μM calcein AM was added to platelet suspension and then incubated for 30 min at 37°C in the dark. After incubation, 10 ml of HBSS-ACD solution was added to labeled platelet suspension and then centrifuged at 1200 × g for 10 min. The resulting pellet was resuspended in HEPES-buffered Tyrode’s solution at 10 × 108 platelets/ml. After the 96-well plates were incubated with 3 mg/ml human fibrinogen at 37°C for 1.5 hr and washed with 100 μl dPBS 8 times, 10 × 106 platelets/well was added. The plate was then incubated for 60 min at 37°C. After incubation, nonadherent platelets were carefully removed by washing with 100 μl dPBS 3 times and then 100 μl HEPES-buffered Tyrode’s solution was added. In separate wells on each plate, a standard curve for platelet count was obtained on each plate from 0 to 10 × 107 platelets/well. All measurements were done in triplicate and performed with the Synergy 2 fluorescence reader with excitation filter of 485 nm and and emission filter of 535 nm.
2.5. The rabbit thrombogenicity model
The animal handling and surgical procedures were approved by the University Committee on the Use and Care of Animals in accordance with university and federal regulations. A total of 18 New Zealand white rabbits (Myrtle’s Rabbitry, Thompson’s Station, TN) were used in this study. All rabbits (2.5–3.5 kg) were initially anesthetized with intramuscular injections of 5 mg/kg xylazine injectable (AnaSed® Lloyd Laboratories Shenandoah, Iowa) and 30 mg/kg ketamine hydrochloride (Hospira, Inc. Lake Forest, IL). Maintenance anesthesia was administered via a diluted intravenous (IV) infusion of ketamine (2 mg/ml) at a rate of 1.53 mg/kg/hr. In order to maintain blood pressure stability, IV fluids of Lactated Ringer’s were given at a rate of 33 ml/kg/hr. The paralytic, pancuronium bromide (0.33 mg/kg, IV), was administered to have animal totally dependent upon mechanical ventilation which was done via a tracheotomy and using a Sechrist Infant Ventilator Model IV-100 (Sechrist, Anaheim, CA). For monitoring blood pressure and collecting blood samples, the rabbit right carotid artery was cannulated using a 16-gauge IV angiocatheter (Jelco®, Johnson & Johnson, Cincinnati, OH). Blood pressure and derived heart rate were monitored with a Series 7000 Monitor (Marquette Electronics Milwaukee, WI). Body temperature was monitored with a rectal probe and maintained at 37°C using a water-jacketed heating blanket. Prior to placement of the arteriovenous (AV) custom-built extracorporeal circuit (ECC), the rabbit left carotid artery and right external jugular vein were isolated and baseline hemodynamics as well as arterial blood pH, pCO2, pO2, total hemoglobin and methemoglobin using an ABL 725 blood gas analyzer and OSM3 Hemoximeter (Radiometer Copenhagen Copenhagen, DK). In addition, baseline blood samples were collected for platelet and total white blood cell (WBC) counts utilizing a Coulter Counter Z1 (Coulter Electronics Hialeah, FL), plasma fibrinogen levels measured by a BBL Fibrosystem fibrometer (Becton Dickinson Cockeysville, MD), activated clotting time (ACT) using a Hemochron Blood Coagulation System Model 801 (International Technidyne Corp. Edison, NJ), platelet function using a Chrono-Log optical aggregometer model 490 (Havertown, PA) and platelet P-selectin and monocyte CD11b expression utilizing fluorescent-activated cell sorting (FACS) with a Becton Dickinson FACSCalibur flow cytometer (San Jose, CA).
After baseline blood measurements, the AV custom-built ECC was placed into position by cannulating the left carotid artery for ECC inflow and the right external jugular vein for ECC outflow. The flow through the ECC was started by unclamping the arterial and venous sides of ECC and blood flow in circuit was monitored with an ultrasonic flow probe and flow meter (Transonic HT207 Ithaca, NY). Animals had no systemic anticoagulation throughout the experiment.
After four hours on ECC, the circuits were clamped, removed from animal, rinsed with 60 ml of saline and drained. Any residual thrombus in the larger tubing of ECC (i.e., thrombogenicity chamber) was photographed and thrombus image was quantitated using Image J imaging software from National Institutes of Health (Bethesda, MD). All animals prior to euthanasia received a dose of 400 U/kg sodium heparin to prevent necrotic thrombosis. The animals were euthanized using a dose of Fatal Plus (130 mg/kg sodium pentobarbital) (Vortech Pharmaceuticals Dearborn, MI). All animals underwent gross necropsy after being euthanized and included examination of the lungs, heart, liver and spleen for any signs of thromboembolic events.
2.6. Blood sampling
Rabbit whole blood samples were collected in non-anticoagulated 1 cc syringes for ACT, 10% anticoagulant containing sodium citrate, sodium phosphate and dextrose (ACD) (Hospira, Inc. Lake Forest, IL) in 3 cc syringes for cell counts, aggregometry and FACS analysis and 1 cc syringes containing 40 U/ml of sodium heparin (APP Pharmaceuticals, LLC Schaumburg, IL) for blood gas analysis. Every hour up to 4 hours, following the initiation of ECC blood flow, blood samples were collected for in vitro measurements. Samples were used within 2 hours of collection to avoid any artifactual activation of platelets and monocytes.
2.7. Platelet aggregometry
Rabbit platelet aggregation was assayed based on Born’s turbidimetric method using a Chrono-Log optical aggregometer. Citrated blood (1:10 blood to ACD) was collected (6 mls) and platelet-rich plasma (PRP) was obtained by centrifugation at 110 × g for 15 min. Platelet-poor plasma (PPP) was obtained by another centrifugation of the PRP-removed blood sample at 2730 × g for 15 min and was used as the blank for aggregation. PRP was incubated for 10 min at 37°C and then 40 μg/ml collagen (Chrono-PAR #385 Havertown, PA) was added. The percentage of aggregation was determined 3 min after the addition of collagen using Chrono-Log Aggrolink software.
2.8. Flow cytometry
To determine platelet P-selectin (CD62P) and IIb/IIIa (fibrinogen receptor) expression, 100 ul diluted blood aliquots (1:100 dilution of blood to Hank’s Balanced Salt Solution (HBSS) without CaCl2 and MgCl2) were directly prepared for cell surface staining of P-selectin and IIb/IIIa. In four 12 × 75 polypropylene tubes containing 100 μl of diluted blood, 40 μg/ml collagen (4 μl 1000 μg/ml) is added to two tubes and 4 μl saline is added to the other two tubes. At this point, saturating concentrations (10 μl) of monoclonal antisheep IIb/IIIa FITC and monoclonal antihuman CD62P PE antibodies were added to one collagen and one saline treated tubes and incubated for 15 min at room temperature (RT) in the dark. In the other two tubes containing collagen and saline, 10 μl each of antimouse IgG1 FITC and PE were added as nonbinding isotype controls and also incubated for 15 min at RT in the dark. After the antibody incubation, each tube received 700 μl of freshly prepared 1% formaldehyde buffer (in dPBS) and stored at 4°C until ready for flow cytometric analysis. To determine monocyte CD11b and CD14 expression, 100 μl undiluted blood aliquots were directly prepared for cell surface staining of CD11b and CD14. At this point, saturating concentrations (10 μl) of rat antimouse CD11b Alexa Fluor 488 and monoclonal antihuman CD14 PE antibodies were added to one tube and 10 μl each of antirat IgG2b Alexa Fluor 488 and antimouse IgG2a PE were added as nonbinding isotype controls. All tubes were incubated for 30 min at 4°C in the dark. After the antibody incubation, lysing of red blood cells was accomplished by adding 2 ml of FACSLysing Buffer (Becton Dickinson San Jose, CA), gentle vortexing and then incubating for 10 minutes at room temperature in the dark. After red blood cell lysing, centrifugation at 250 × g for 5 minutes at 4°C pelleted the stained leukocytes. After aspirating supernatant, one wash step was done with wash buffer containing dPBS, 0.1% sodium azide and 0.5% bovine serum albumin, centrifuged again and then supernatant was aspirated. The cells were then resuspended in 250 ul of freshly prepared 1% formaldehyde buffer and stored at 4°C until ready for flow cytometric analysis. A FACSCalibur flow cytometer (Becton Dickinson San Jose, CA) was used for the acquisition of flow data and the CellQuest software used for data analysis. Cell populations were identified for data collection by their forward scatter (FSC) and side scatter (SSC) light profiles. For each sample, 30,000 total events were collected. Fluorescence intensity of immunostaining was quantitated by histogram log plot analysis. Mean fluorescent intensity (MFI) was expressed as the geometric mean channel fluorescence minus the appropriate isotype control.
Statistical Analysis
Data are expressed as mean ± SEM. Comparison between the NORel and polymer control groups were analyzed by the one-way ANOVA with the multiple comparison of means using Student’s t-test. All statistical analyses were performed using the statistical program SAS JMP (SAS Institute Cary, NC). Values of P<0.05 were considered statistically significant.
3. Results
3.1. NO releasing and protein adsorption characteristics of NORel polymer
The NORel coating used in these studies has some key features which makes it an ideal material for investigating the interactions between NO and biological cells and proteins. First, the material continuously releases NO under physiological conditions at levels above that which is suggested to be needed to passivate platelets [26]. As shown in a representative NORel ECC (Figure 2A), the NO release as measured via a NO analyzer showed a sustained NO flux of approx. 12.5 × 10−10 mol cm−2 min−1. This NO flux generates a level of NO release that exceeds the physiological NO release level from endothelial cells (0.5– 4 × 10−10 mol cm−2 min−1) [29]. The level of NO was intentionally designed to maintain a steady state NO flux during the entire 4 hours of the experiment. As shown in Figure 2A, the ability of the coating to release steady state levels of NO in physiological buffer was achieved for up to 5 hours. Most importantly, it was confirmed that the NO release from the NORel ECC was not reduced by exposure to flowing blood after 4 hours as shown in the circled region of Figure 2A. A piece of tubing was removed from the circuit post-experiment and measured for NO release. The post-4 hour blood-exposed sample was observed to superimpose onto the pre-blood exposed NO release curve. Further, it is important that the NO release levels were essentially the same after exposure to blood because this suggests that the blood environment (i.e., proteins, cells, etc.) may not alter the kinetics of the NO release from the NOrel coating. Another adverse characteristic, the issue of NO donor leaching, was avoided in the NORel coating by using a more lipophilic NO donor such as DBHD/N2O2 that would preferentially stay in the organic polymer phase based on favorable partition coefficients. Our lab previously measured formulations of high plasticizer with no top coat for 24 hours to try to obtain leaching of the DBHD/NO and only detected 0.19% of the diamine leaching. Therefore, it is highly unlikely that in only 4 hours with a 2:1 PVC/DOS active coat plus a top coat that any leaching would occur.
Figure 2.
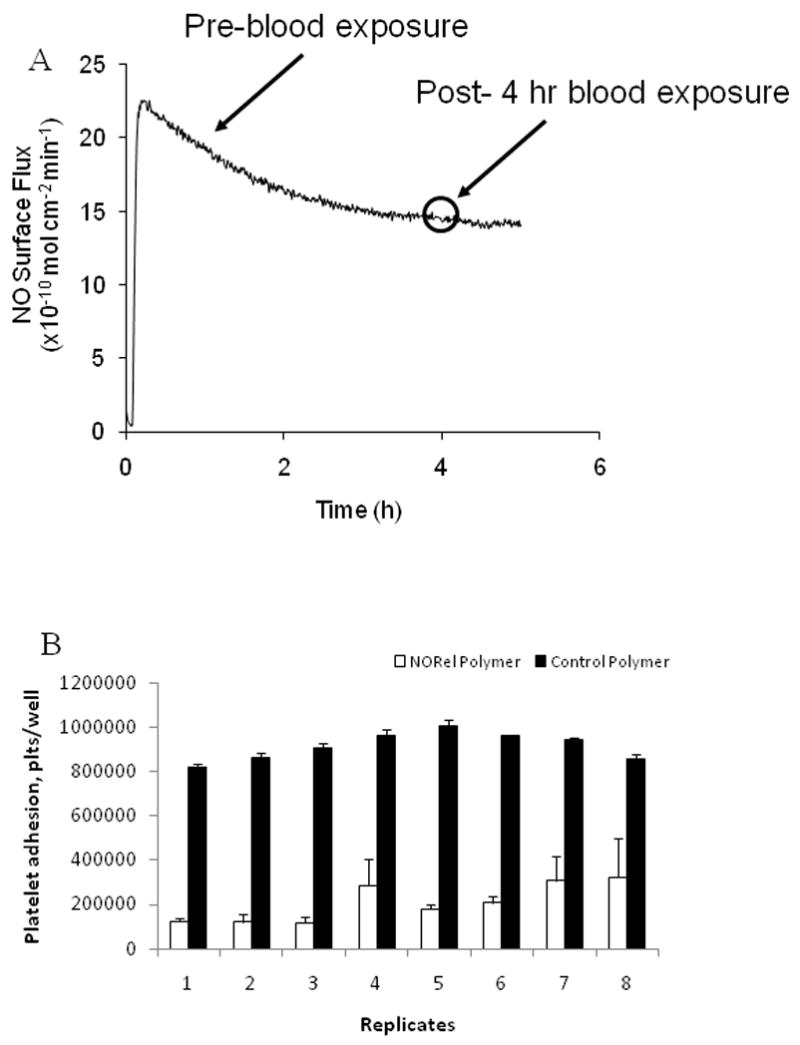
NO releasing character and human platelet adhesion properties of NO releasing polymer-coated extracorporeal circuits (NORel polymer ECC). (A) Representative NO surface flux profiles from NORel polymer ECC containing DBHD/N2O2 at 37°C and pH 7.4 obtained via chemiluminescence detection and a NO analyzer. A comparison of NO release prior to and after blood exposure. (B) In vitro fluorescent assay with calcein-labeled human platelets utilizing preadsorbed human fibrinogen (3 mg/ml) to NORel (white bars) and control (black bars) polymers. Platelet adhesion quantitated using a platelet number vs fluoresecence standard curve with a linear relationship between platelet number and fluorescence.
To determine if plasma protein adsorption and in particular, human fibrinogen, occurs with this polymer, an in vitro immunofluorescence assay was performed. The NORel coating had significant human fibrinogen adsorption (2767 ± 59% of the 3000 μg/ml fibrinogen control; p< 0.05, n= 8) compared to the control polymer (120 ± 3% of the 3000 μg/ml fibrinogen control, n= 8). The adsorption of the human fibrinogen antibody without the presence of exogenous human fibrinogen was 28 ± 1% of the 3000 μg/ml fibrinogen control.
As a further characterization of the NORel polymer, platelet adhesion was determined in an in vitro 96-well microplate assay utilizing fluorescently-labeled human platelets and preadsorbed human fibrinogen. Platelet adhesion to preadsorbed fibrinogen (3 mg/ml) on the NORel and control polymers were significantly decreased on the NORel polymer (178185 ± 3801 platelets/well, p< 0.05) compared to the control (886239 ± 22583 platelets/well). This represented a 80% decrease in adhering platelets in this non-flow assay.
3.2. NORel polymer effects on rabbit hemodynamics and thrombus formation
Hemodynamic effects of NORel- and control-coated ECC over 4 hours blood exposure in the rabbit model of thrombogenicity are shown in Table 1. Mean arterial blood pressure (MAP) within the first two hours on ECC significantly fell in both the NORel and control polymer groups but returned to baseline levels after 4 hours. This returned in MAP to baseline is due to the continuous IV fluid maintenance at 10 ml/kg/min. Heart rate for the NORel and control ECC groups were unchanged over the 4 hours. The blood flow through the ECC was dependent upon MAP and after 4 hours increased due to the added IV fluids. More important is that the number of control polymer ECCs that had maintained blood flow after 4 hours was 4 out of 10 experiments while 7 out of 7 rabbits in the NORel polymer ECCs were patent (Table 1). Not unexpected the activated clotting time (ACT) after 4 hours on the NORel or control polymer ECCs, probably due to the increase in intravascular fluids, i.e., hemodilution effect. The total white blood cell (WBC) count remained unchanged over the 4 hours on either ECC group. The total WBC count is composed of lymphocytes, monocytes and granulocytes.
TABLE 1.
Effects of NO releasing polymer (NORel) on hemodynamic parameters in the rabbit model of extracorporeal circuits (ECC).
| Time on ECC (hours) |
||||||
|---|---|---|---|---|---|---|
| Treatment | Parameter | Baselinet | 1 | 2 | 3 | 4 |
| Control polymer ECC | MAP | 74 ± 3 (17) | 53 ± 3 (10)* | 61 ± 5 (10)* | 78 ± 6 (9) | 86 ± 10 (5) |
| HR | 207 ± 6 (17) | 205 ± 5 (10) | 210 ± 4 (10) | 212 ± 9 (9) | 246 ± 15 (5)* | |
| ECC BF | 107 ± 6 (17) | 90 ± 10 (10) | 124 ± 7 (9) | 145 ± 14 (6)* | 153 ± 14 (4)* | |
| ACT | 158 ± 5 (17) | 224 ± 20 (10)* | 249 ± 14 (10)* | 303 ± 18 (9)* | 325 ± 12 (5)* | |
| WBC count | 3303 ± 188 (17) | 3029 ± 338 (10) | 2761 ± 430 (10) | 2608 ± 509 (9) | 2730 ± 894 (5) | |
| NORel polymer ECC | MAP | 74 ± 3 (17) | 47 ± 6 (7)* | 48 ± 1 (7)* | 66 ± 4 (7) | 87 ± 6 (7)* |
| HR | 207 ± 6 (17) | 206 ± 4 (7) | 201 ± 6 (7) | 200 ± 5 (7) | 229 ± 9 (7)* | |
| ECC BF | 107 ± 6 (17) | 112 ± 14 (7) | 118 ± 8 (7) | 160 ± 16 (7)* | 180 ± 9 (7)* | |
| ACT | 158 ± 5 (17) | 218 ± 10 (7)* | 233 ± 14 (7)* | 240 ± 13 (7)* | 253 ± 17 (7)* | |
| WBC count | 3303 ± 188 (17) | 2939 ± 348 (7) | 3311 ± 706 (7) | 3172 ± 689 (7) | 3068 ± 572 (7) | |
Values are means ± SEM. ()= n size.
Values are prior to ECC placement. MAP= mean arterial pressure (mmHg), HR= heart rate (beats/min), BF= blood flow (ml/min), ACT= activated clotting time (sec), WBC= white blood cells.
p<0.05 vs baseline; ANOVA with Student’s t multirange test.
To ascertain the differential formation of thrombus in the thrombogenicity chamber (i.e., the 3/8 inch ID Tygon tubing 8 cm in length) of the NORel or control polymer-coated ECC, 2-dimensional (2D) image analysis was performed after 4 hours of blood exposure. In Figures 3A and 3B, representative images of the control and NORel circuits, respectively, are shown. The red colorized region was obtained by using the threshold feature in the Image J imaging software and is representative of the 2D area of thrombus formation (pixels/cm2) in each tubing chamber. These thrombi area measurements were quantitated and, as shown in Figure 3C, the thrombus area of the NORel polymer ECC was significantly smaller as compared to the control polymer ECC.
Figure 3.
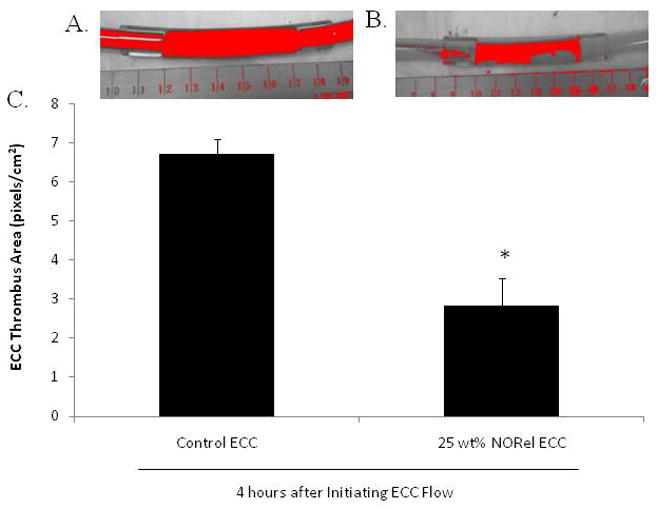
Two-dimensional representation of thrombus formation NORel and control polymer ECCs after 4 hours blood exposure in rabbit thrombogenicity model. (A) Colorized image of thrombus in 3/8 inch I.D. tubing (red region) in the control ECC. (B) Colorized image of thrombus in 3/8 inch I.D. tubing (red region) in the NORel polymer ECC. (C) Quantitation of thrombus area as calculated using ImageJ software from NIH. The data are means ± SEM. * = p<0.05, control ECC vs 25 NORel ECC after 4 hours.
3.3. Effects of NORel polymer on rabbit platelet function and surface receptor expressions
Platelet function during exposure to NORel and control polymer-coated ECC was assessed by observing platelet count (Figure 4A), percent of aggregation, as determined by ex vivo collagen (40 μg/ml) stimulation of PRP (Figure 4B), and the level of plasma fibrinogen, to which activated platelets bind (Figure 4C). Both platelet count and plasma fibrinogen levels were corrected for any hemodilution due to added IV fluids into the rabbits. The NORel polymer ECC showed a greater preservation of platelet count and collagen-stimulated platelet aggregation over the course of the 4 hour blood exposure while the control polymer ECC showed a time-dependent loss in platelet count and ability to aggregate upon stimulation. The NORel ECC was able to maintain platelet counts 80% or greater even at 3 and 4 hours of blood exposure when compared to the control polymer ECC (Figure 4A). The ability to aggregate upon exogenous collagen stimulation was essentially maintained in the NORel polymer ECC whereas platelets from the control ECC were nearly unresponsive to collagen, especially at 3 and 4 hours (Figure 4B). In Figure 4C, plasma fibrinogen levels are shown to significantly decrease in both the NORel and control ECCs time-dependently. Interestingly, there was no difference between the NORel and control polymers in their ability to bind fibrinogen at any time point. Thus, the preservation of platelet count in the NORel ECC can not be explained by the plasma fibrinogen levels because both NORel and the control ECCs lost nearly 50% of the soluble fibrinogen after 4 hours of blood exposure.
Figure 4.
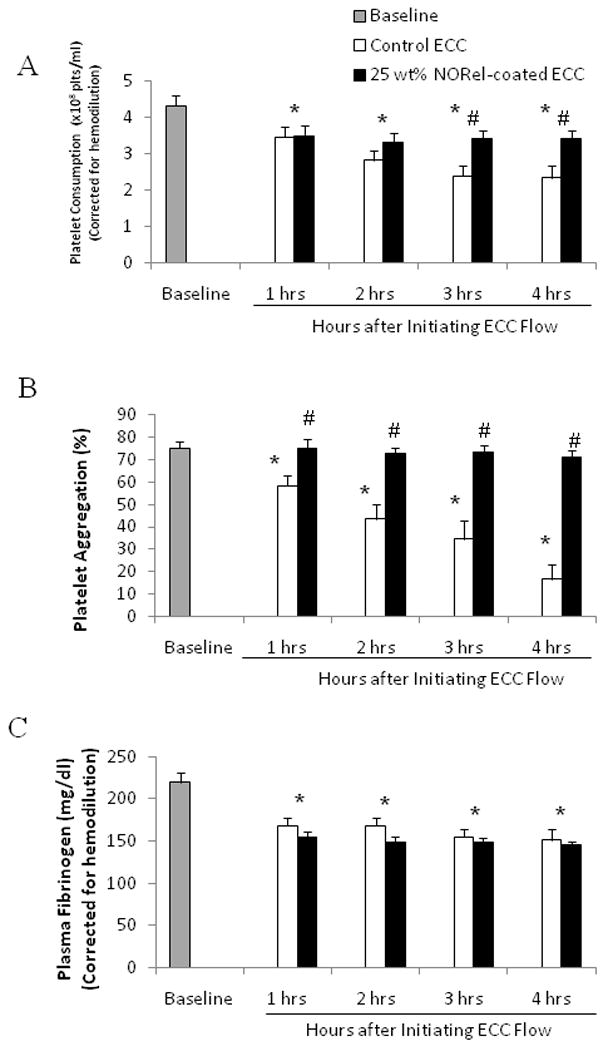
Time-dependent effects of NORel polymer ECC on rabbit platelet count (i.e., consumption) (A), platelet function as measured via aggregometry (B) and plasma fibrinogen levels (C) after 4 hours of blood exposure in rabbit thrombogenicity model. Data is the mean ± SEM. * = p<0.05, baseline vs control and NORel ECC. # = p<0.05, control ECC vs NORel ECC.
To ascertain the differential expression of the platelet adhesion glycoprotein, P-selectin (CD62P), between the NORel and control polymer ECC, fluorescence-activated cell sorting (FACS) analysis was performed on rabbit circulating platelets. As shown in Figure 5A, a representative forward scatter (FSC) versus side scatter (SSC) dot plot is shown for diluted rabbit whole blood. The gated region is the representative of the circulating platelet population and this gate was used in all subsequent FACS analysis for CD62P expression (Figures 5B and 5C). When platelets become activated, the surface expression of P-selectin increases [34]. The histogram plot (Figure 5B) demonstrates that 40 μg/ml collagen stimulation of rabbit blood platelets does indeed increase the expression of surface P-selectin (blue filled area) as compared to the unstimulated condition as denoted by the red filled area. The green line shows nonspecific binding of the mouse isotype control antibody used for P-selectin. The histogram data is then quantified in mean fluorescence intensity (MFI) for platelet P-selectin surface expression (Figure 5C). P-selectin expression was unchanged after 4 hours on either the control or NORel ECC as compared to the unstimulated (i.e., no exogenous collagen) baseline (no ECC present). However, 40 μg/ml collagen significantly stimulated an increase in P-selectin expression at baseline and after 4 hours on control or NORel ECCs. In addition, the collagen-stimulated P-selectin expression for the 4 hour control and NORel ECCs were significantly increased and decreased, respectively, from the collagen-stimulated baseline levels.
Figure 5.
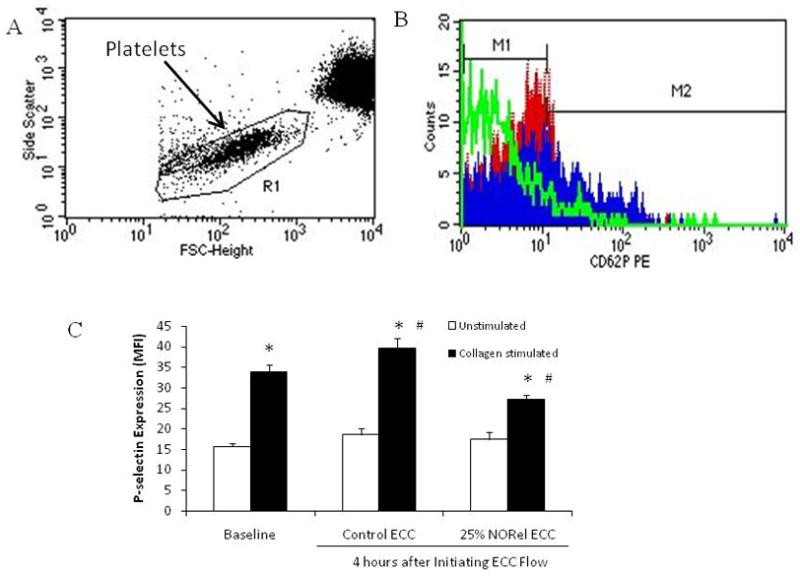
Fluorescent-activated cell sorting (FACS) analysis for circulating platelets after 4 hour blood exposure with NORel or control polymer-coated ECC. (A), Representative FACS analysis graph for unstained circulating platelets. FSC= forward scatter and SSC= side scatter (B) Histogram of P-selectin (CD62P) platelet expression using mouse anti-human CD62P PE antibody conjugate (baseline, no ECC, red filled area). Blue filled area is P-selectin mean fluorescence intensity (MFI) after 40 μg/ml collagen stimulation while the green line denotes the isotype control for P-selectin. (C). Platelet P-selectin mean fluorescence intensity (MFI) after 4 hours on ECC with or without 40 ug/ml collagen stimulation in NORel and control polymers. The data are means ± SEM. * = p<0.05, unstimulated vs collagen-stimulated for baseline, control ECC and NORel ECC. # = p<0.05, collagen-stimulated for baseline vs collagen-stimulated for control ECC and collagen-stimulated for NORel ECC. The specific MFI data is after the isotype control value was subtracted from each P-selectin MFI value. All FACS analyses used the gated FSC/SSC plot for platelets and 100 μl of diluted whole rabbit blood (1:100) was used for each determination.
In order to understand if the NORel polymer can induce changes in the IIb/IIIa (fibrinogen) receptor, a specific antibody to IIb/IIIa (CD41/CD61) was used to evaluate platelet surface expression of IIb/IIIa via flow cytometry. As shown in Figure 6 the platelet IIb/IIIa surface expression after 4 hours on either control or NORel ECC was not changed compared to the no ECC baseline. The IIb/IIIa expression, however, significantly increased with 40 μg/ml exogenous collagen at baseline and after 4 hours on control or NORel ECC. Interestingly, the NORel polymer-coated ECC significantly decreased the collagen-induced IIb/IIIa expression from both the collagen-induced IIb/IIIa levels in the baseline and control ECC groups.
Figure 6.
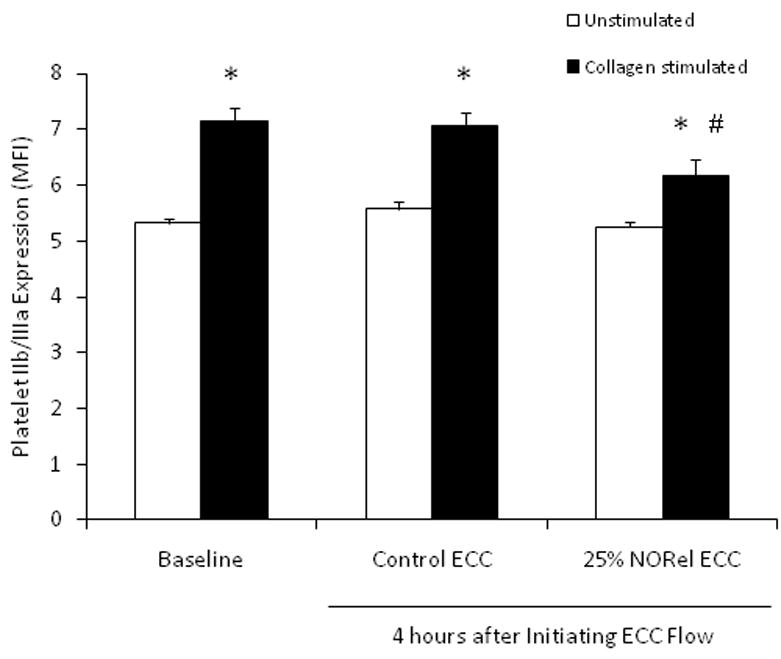
Platelet IIb/IIIa (fibrinogen receptor) mean fluorescence intensity (MFI) after 4 hours on ECC with or without 40 ug/ml collagen stimulation in NORel and control polymers as measured via flow cytometry. The data are means ± SEM. * = p<0.05, unstimulated vs collagen stimulated for baseline, control ECC and NORel ECC. The specific MFI data is after the isotype control value was subtracted from each IIb/IIIa MFI value.
All FACS analyses used the gated FSC/SSC plot for platelets and 100 μl of diluted whole rabbit blood (1:100) was used for each determination.
3.4. Effects of NORel polymer on rabbit surface receptor expressions of integrin, CD11b, and monocyte-specific receptor, CD14
To determine if the NORel polymer in ECC can increase the surface integrin, CD11b, expression on circulating monocytes translates into activated monocytes, FACS analysis of rabbit whole blood at baseline (no ECC) and after 4 hours of circuit blood exposure was performed. A representative FSC versus SSC dot plot is shown in Figure 7A for rabbit whole blood. The gated region, R5, is representative of the circulating monocyte subpopulation of the blood white blood cells and this gate was used in all subsequent FACS analysis for CD11b expression (Figures 7B and 7C). The CD11b expression on rabbit monocytes was observed to significantly increase 3-fold after 4 hour blood exposure to control polymer ECCs but the NORel polymer ECC reduced the CD11b expression back toward baseline (Figure 7B). The CD11b expression observed with the NORel polymer 4 hours after blood exposure was also significantly reduced from that observed after 4 hours on control polymer ECC. If the circulating monocytes are becoming activated as indicated by the increase in CD11b expression, then, as shown in Figure 7C, the monocyte-specific surface receptor, CD14, should be decreased. After 4 hours of blood exposure the control ECC did show a least a 50% decrease in CD14 expression indicating a loss of circulating monocytes compared to baseline levels. The NORel ECC after 4 hours blood exposure was still significantly reduced from baseline but there was a trend for a greater number of circulating monocytes.
Figure 7.
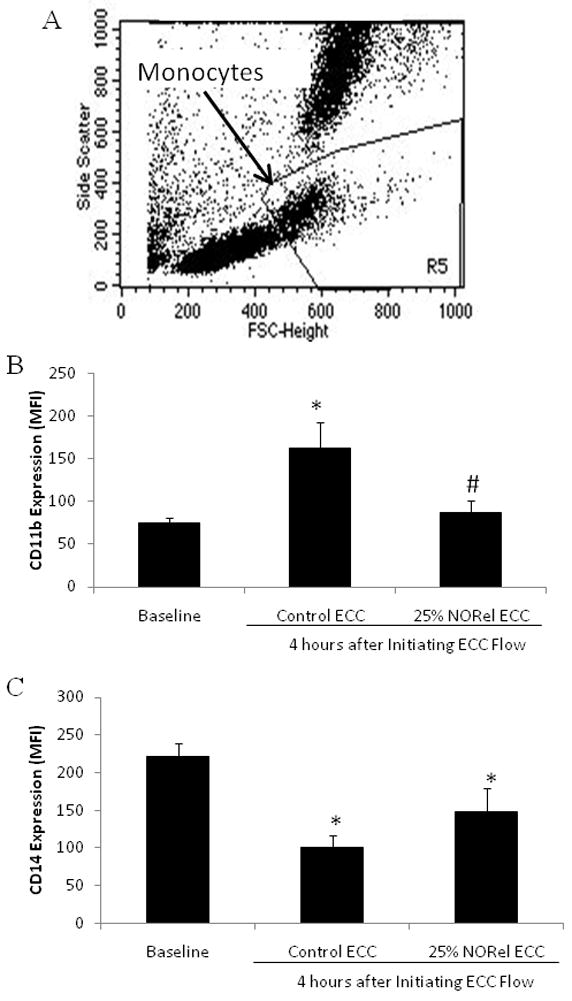
Fluorescent-activated cell sorting (FACS) analysis for circulating monocytes after 4 hour blood exposure with NORel or control polymer-coated ECC. (A), Representative FACS analysis graph for unstained circulating monocytes. FSC= forward scatter and SSC= side scatter. (B) Monocyte CD11b mean fluorescence intensity (MFI) after 4 hours on ECC in NORel and control polymers. (C) Monocyte CD14 (monocyte specific marker) mean fluorescence intensity (MFI) after 4 hours on ECC in NORel and control polymers. The data are means ± SEM. * = p<0.05, baseline vs control ECC or NORel ECC. The specific MFI data is after the isotype control value was subtracted from each CD11b or CD14 MFI value. All FACS analyses used the gated FSC/SSC plot for monocytes and 100 μl of whole rabbit blood was used for each determination.
4. Discussion
We demonstrated that extracorporeal circuits containing a NO releasing polymer can release NO, preserve platelet count and function as well as maintain monocyte inactivation after at least 4 hours of blood exposure in a rabbit model of thrombogenicity. These platelet and monocyte physiological events have been shown for the first time to strongly correlate with molecular changes in the platelet surface glycoprotein, P-selectin, and the monocyte surface integrin, CD11b, expressions which can be utilized to determine the level of platelet and monocyte activation, respectively. Based on these platelet and monocyte membrane protein changes, platelet P-selectin and monocyte CD11b could serve as non-invasive clinical markers for hemocompatibility of extracorporeal life support (ECLS) systems.
Platelet and monocyte physiological changes are of immense importance in defining the hemocompatibility of biomaterials used in ECLS and have been extensively investigated [3–5,13,35–55]. However, even though the activation status of platelets and monocytes in their macro response, i.e., counts and function, is relatively understood, the early stimulatory effects of exposure to an extracorporeal circulation (ECC) have not been clearly elucidated. The present paper shows, for the first time, that the regulation of platelet surface P-selectin and monocyte membrane integrin CD11b by blood contact with artificial surfaces is, in part, dependent on the polymer release of the platelet and monocyte inactivator, NO. By utilizing NO as the agent being released from the diazeniumdiolated polymer in this study, the degree of thrombosis and ECC-induced expression of platelet P-selectin and monocyte CD11b were attenuated. Much of ECLS research has previously shown a decrease in the levels of thrombosis and an underlying threshold level of locally-released NO from either polyvinyl chloride (PVC) or polyurethane (PU)-based polymers [25,26,56–58]. Skrzypchak et al, in particular, had shown that the NO donor, DBHD/N2O2 released an amount of NO from the PVC polymer that preserved platelet number and maintained their ability to aggregate upon stimulation with exogenous collagen [26]. Using this level of NO donor, our results confirm their data and, in addition, showed that this amount of NO flux, i.e., approx. 15 × 10−10 mol cm−2 min−1, was maintained over the 4 hour test period and that the surface proteins platelet P-selectin and monocyte CD11b, protein markers of cellular activation, were maintained quiescent. Interestingly, NO alone was able to maintain a thromboresistive state in the NORel ECC even in the face of significant adsorption of fibrinogen. The level of plasma fibrinogen was reduced in rabbits with either the NORel or control ECCs. The in vitro human fibrinogen immunoassay showed that fibrinogen adsorbed significantly to the NORel polymer surface and confirmed that the loss of rabbit plasma fibrinogen was more in likely being adsorbed to the ECC. Even though significant levels of fibrinogen bound to the NORel polymer, static platelet adhesion in an in vitro 96-well assay showed that 50% or less of the labeled platelets bound to the fibrinogen-adsorbed polymer surface. The effectiveness of NO released from polymers to promote platelet quiescence has confirmed previous in vitro platelet adhesion results, which had also shown that NO released from polymers could attenuate platelet binding to adsorbed plasma fibrinogen and ultimately prevent their activation [59]. Platelet P-selectin plays a key role in mediating the adhesion of platelets to the ECC-adsorbed fibrinogen but the mechanism of the inhibitory effects of NO on the platelet upregulation of P-selectin and its binding to fibrinogen still needs investigation.
The major cause of the strong in vitro fibrinogen binding is probably due to both the hydrophobicity of the DBHD/N2O2/PVC polymer and the small positive charge that develops with the DBHD compound after it releases NO. Zhou et al [20] had shown that the contact angle for PVC was 99.4° and the lipophilic DBHD/N2O2 would only increase this angle. In addition, our polymer coating had a top coat of PVC/DOS which would maintain hydrophobicity and keep the contact angle near 100°. Based on the high hydrophobicity and possible positive charge of the NORel polymer, this would represent poor hemocompatibility of the ECC. However, one must not forget that as long as the polymer is continuously releasing NO at a level that can keep circulating platelets quiescent even in the face of high fibrinogen adsorption, the anti-platelet effects of NO will be present as observed in the in vitro platelet adhesion assay.
However, even though the high hydrophobicity and positive surface charge of the NORel-coated ECC are also present in vivo, other considerations have to be taken into account, in particular, the response of platelets in flowing blood and their interaction with not only ECC-bound fibrinogen but also bound von Willebrand’s factor. In a shear flow system of platelet rich plasma, Zhang et al [60] showed that platelets adhere to biomaterials through a mechanism mediated by both fibrinogen and von Willebrand’s factor. The presence of plasma proteins like von Willebrand’s factor augment the ability of activated platelets to adhere to artificial surfaces. The ability of released NO from the NORel-coated ECC confirms the important role NO plays in the attenuating platelet activation in the blood flowing system when several activators of platelets are present and not in the in vitro systems.
Not only does ECLS increase thrombus formation and upregulate platelet P-selectin expression upon collagen stimulation but our results in this paper also shows a strong enhancement of the monocyte surface integrin, CD11b. The NORel polymer-coated ECC, however, has been shown to attenuate the activation of both platelets and monocytes after exposure to the artificial surfaces. The activated platelet due to exposure to an artificial surface trigger autocrine and paracrine activation processes that lead to leukocyte (i.e., monocytes) recruitment and activation at areas of platelet adhesion [61]. The activated platelets and the upregulation of the surface ligand, P-selectin, can attach and interact with monocytes through a P-selectin glycoprotein (PSGL-1) [16]. The attraction of monocytes to activated platelets is hypothesized to be through the secretion of the monocyte chemoattractant protein-1 (MCP-1) which has been recently shown to be markedly induced by activated platelets [62]. The monocyte-platelet interaction activates the monocyte into a proinflammatory state and upregulation of the surface integrin, CD11b mediates the firm adhesion to platelets [12,63]. Our results show a significant increase in monocyte CD11b upon exposure to ECC and NO released from the polymer-coated ECC reduced this enhanced CD11b expression. Our results plus the known effects of activated monocytes initiate monocyte secretion of chemokines, cytokines and procoagulatory tissue factor ultimately leading to thrombus formation. By NO attenuating the expression of the proinflammatory surface marker on monocytes, CD11b, the monocytic effect on thrombus formation is greatly reduced. However, the exact contribution of NO and its effects on platelet P-selectin and monocyte CD11b expressions as well as the platelet/monocyte molecular interaction still remain to be elucidated.
5. Conclusions
This study has shown that the coating of plasticized PVC containing DBHD/N2O2 in PVC tubing can continuously release NO at least 4 hours in a rabbit model of extracorporeal circulation. This release of NO from the polymer coating has been shown to attenuate the activation of both platelets, as measured by a decrease in P-selectin expression, and monocytes by the adhesion molecule, CD11b, downregulation. These results indicate the potential of locally released NO to increase hemocompatibility of extracorporeal circuits and devices by reducing their thrombogenicity and relieve the use of systemic anticoagulation and all its inherent risks. Studies combining the anti-platelet activity of NO with the well-known anticoagulant activity of immobilized heparin are under investigation and could provide the ultimate endothelial-like preservation of circulating platelets and monocytes when exposed to biomaterials.
Acknowledgments
The authors declare this work is supported by the National Institutes of Health, Grant #R01 HD 01534. The authors except Dr. Robert Bartlett confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. Dr. Robert Bartlett has an equity interest in Accord Biomaterials, Inc. but no financial support from this company was utilized in any of this paper’s work. The authors would like to thank Charles Burney for his technical assistance and Candice Hall for her administration of resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ranucci M, Balduini A, Ditta A, Boncilli A, Brozzi S. A systematic review of biocompatible cardiopulmonary bypass circuits and clinical outcome. Ann Thorac Surg. 2009;87:1311–1319. doi: 10.1016/j.athoracsur.2008.09.076. [DOI] [PubMed] [Google Scholar]
- 2.Sin DC, Kei HL, Miao X. Surface coatings for ventricular assist devices. Expert Rev Med Devices. 2009;6:51–60. doi: 10.1586/17434440.6.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Ratner BD. The catastrophe revisited: blood compatibility in the 21st Century. Biomaterials. 2007;28:5144–5147. doi: 10.1016/j.biomaterials.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stegemann JP, Kaszuba SN, Rowe SL. Review: advances in vascular tissue engineering using protein-based biomaterials. Tissue Eng. 2007;13:2601–2613. doi: 10.1089/ten.2007.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmermann AK, Weber N, Aebert H, Ziemer G, Wendel HP. Effect of biopassive and bioactive surface-coatings on the hemocompatibility of membrane oxygenators. J Biomed Mater Res B Appl Biomater. 2007;80:433–439. doi: 10.1002/jbm.b.30614. [DOI] [PubMed] [Google Scholar]
- 6.Maruyama I. Biology of endothelium. Lupus. 1998;7 (Suppl 2):S41–S43. doi: 10.1177/096120339800700210. [DOI] [PubMed] [Google Scholar]
- 7.Osto E, Coppolino G, Volpe M, Cosentino F. Restoring the dysfunctional endothelium. Curr Pharm Des. 2007;13:1053–1068. doi: 10.2174/138161207780487566. [DOI] [PubMed] [Google Scholar]
- 8.Cohen JD. Overview of physiology, vascular biology, and mechanisms of hypertension. J Manag Care Pharm. 2007;13:S6–S8. doi: 10.18553/jmcp.2007.13.s5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, et al. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood. 2008;111:613–623. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aktas B, Utz A, Hoenig-Liedl P, Walter U, Geiger J. Dipyridamole enhances NO/cGMP-mediated vasodilator-stimulated phosphoprotein phosphorylation and signaling in human platelets: in vitro and in vivo/ex vivo studies. Stroke. 2003;34:764–769. doi: 10.1161/01.STR.0000056527.34434.59. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen BL, Saitoh M, Ware JA. Interaction of nitric oxide and cGMP with signal transduction in activated platelets. Am J Physiol. 1991;261:H1043–H1052. doi: 10.1152/ajpheart.1991.261.4.H1043. [DOI] [PubMed] [Google Scholar]
- 12.Sato Y, Hiramatsu Y, Homma S, Sato M, Sato S, Endo S, Sohara Y. Phosphodiesterase type 4 inhibitor rolipram inhibits activation of monocytes during extracorporeal circulation. J Thorac Cardiovasc Surg. 2005;130:346–350. doi: 10.1016/j.jtcvs.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann AK, Aebert H, Reiz A, Freitag M, Husseini M, Ziemer G, et al. Hemocompatibility of PMEA coated oxygenators used for extracorporeal circulation procedures. ASAIO J. 2004;50:193–199. doi: 10.1097/01.mat.0000123638.41808.59. [DOI] [PubMed] [Google Scholar]
- 14.Edmunds LH., Jr Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1998;66:S12–S16. doi: 10.1016/s0003-4975(98)00967-9. [DOI] [PubMed] [Google Scholar]
- 15.Furie B, Furie BC. Leukocyte crosstalk at the vascular wall. Thromb Haemost. 1997;78:306–309. [PubMed] [Google Scholar]
- 16.Furie B, Furie BC. The molecular basis of platelet and endothelial cell interaction with neutrophils and monocytes: role of P-selectin and the P-selectin ligand, PSGL-1. Thromb Haemost. 1995;74:224–227. [PubMed] [Google Scholar]
- 17.Palabrica T, Lobb R, Furie BC, Aronovitz M, Benjamin C, Hsu YM, et al. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992;359:848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 18.Larsen E, Celi A, Gilbert GE, Furie BC, Erban JK, Bonfanti R, et al. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59:305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- 19.Frost MC, Meyerhoff ME. Synthesis, characterization, and controlled nitric oxide release from S-nitrosothiol-derivatized fumed silica polymer filler particles. J Biomed Mater Res A. 2005;72:409–419. doi: 10.1002/jbm.a.30275. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Z, Meyerhoff ME. Preparation and characterization of polymeric coatings with combined nitric oxide release and immobilized active heparin. Biomaterials. 2005;26:6506–6517. doi: 10.1016/j.biomaterials.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 21.Frost MC, Reynolds MM, Meyerhoff ME. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatibility of blood-contacting medical devices. Biomaterials. 2005;26:1685–1693. doi: 10.1016/j.biomaterials.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Fleser PS, Nuthakki VK, Malinzak LE, Callahan RE, Seymour ML, Reynolds MM, et al. Nitric oxide-releasing biopolymers inhibit thrombus formation in a sheep model of arteriovenous bridge grafts. J Vasc Surg. 2004;40:803–811. doi: 10.1016/j.jvs.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds MM, Frost MC, Meyerhoff ME. Nitric oxide-releasing hydrophobic polymers: preparation, characterization, and potential biomedical applications. Free Radic Biol Med. 2004;37:926–936. doi: 10.1016/j.freeradbiomed.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Batchelor MM, Reoma SL, Fleser PS, Nuthakki VK, Callahan RE, Shanley CJ, et al. More lipophilic dialkyldiamine-based diazeniumdiolates: synthesis, characterization, and application in preparing thromboresistant nitric oxide release polymeric coatings. J Med Chem. 2003;46:5153–5161. doi: 10.1021/jm030286t. [DOI] [PubMed] [Google Scholar]
- 25.Annich GM, Meinhardt JP, Mowery KA, Ashton BA, Merz SI, Hirschl RB, et al. Reduced platelet activation and thrombosis in extracorporeal circuits coated with nitric oxide release polymers. Crit Care Med. 2000;28:915–920. doi: 10.1097/00003246-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Skrzypchak AM, Lafayette NG, Bartlett RH, Zhou Z, Frost MC, Meyerhoff ME, et al. Effect of varying nitric oxide release to prevent platelet consumption and preserve platelet function in an in vivo model of extracorporeal circulation. Perfusion. 2007;22:193–200. doi: 10.1177/0267659107080877. [DOI] [PubMed] [Google Scholar]
- 27.Massaguer A, Engel P, Perez-del-Pulgar S, Bosch J, Pizcueta P. Production and characterization of monoclonal antibodies against conserved epitopes of P-selectin (CD62P) Tissue Antigens. 2000;56:117–128. doi: 10.1034/j.1399-0039.2000.560202.x. [DOI] [PubMed] [Google Scholar]
- 28.Welt FG, Edelman ER, Simon DI, Rogers C. Neutrophil, not macrophage, infiltration precedes neointimal thickening in balloon-injured arteries. Arterioscler Thromb Vasc Biol. 2000;20:2553–2558. doi: 10.1161/01.atv.20.12.2553. [DOI] [PubMed] [Google Scholar]
- 29.Brodersen R, Bijlsma F, Gori K, Jensen KT, Chen W, Dominguez J, Haverson K, Moore PF, Saalmuller A, Sachs D, Slierendrecht WJ, Stokes C, Vainio O, Zuckermann F, Aasted B. Analysis of the immunological cross reactivities of 213 well characterized monoclonal antibodies with specificities against various leucocyte surface antigens of human and 11 animal species. Vet Immunol Immunopathol. 1998;64:1–13. doi: 10.1016/s0165-2427(98)00117-2. [DOI] [PubMed] [Google Scholar]
- 30.Jacobsen CN, Aasted B, Broe MK, Petersen JL. Reactivities of 20 anti-human monoclonal antibodies with leucocytes from ten different animal species. Vet Immunol Immunopathol. 1993;39:461–466. doi: 10.1016/0165-2427(93)90075-f. [DOI] [PubMed] [Google Scholar]
- 31.Grunkemeier JM, Tsai WB, McFarland CD, Horbett TA. The effect of adsorbed fibrinogen, fibronectin, von Willebrand factor and vitronectin on the procoagulant state of adherent platelets. Biomaterials. 2000;21:2243–2252. doi: 10.1016/s0142-9612(00)00150-2. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, Simonovsky FI, Ratner BD, Horbett TA. The role of adsorbed fibrinogen in platelet adhesion to polyurethane surfaces: a comparison of surface hydrophobicity, protein adsorption, monoclonal antibody binding, and platelet adhesion. J Biomed Mater Res A. 2005;74:722–738. doi: 10.1002/jbm.a.30381. [DOI] [PubMed] [Google Scholar]
- 33.Vaughn MW, Kuo L, Liao JC. Estimation of nitric oxide production and reaction rates in tissue by use of a mathematical model. Am J Physiol. 1998;274:H2163–H2176. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 34.Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb Res. 2004;114:447–453. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 36.Kher N, Marsh JD. Pathobiology of atherosclerosis--a brief review. Semin Thromb Hemost. 2004;30:665–672. doi: 10.1055/s-2004-861509. [DOI] [PubMed] [Google Scholar]
- 37.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 38.Ito T, Ikeda U. Inflammatory cytokines and cardiovascular disease. Curr Drug Targets Inflamm Allergy. 2003;2:257–265. doi: 10.2174/1568010033484106. [DOI] [PubMed] [Google Scholar]
- 39.Inoue S, Egashira K, Ni W, Kitamoto S, Usui M, Otani K, et al. Anti-monocyte chemoattractant protein-1 gene therapy limits progression and destabilization of established atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2002;106:2700–2706. doi: 10.1161/01.cir.0000038140.80105.ad. [DOI] [PubMed] [Google Scholar]
- 40.Brasier AR, Recinos A, III, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22:1257–1266. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- 41.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 42.Reape TJ, Groot PH. Chemokines and atherosclerosis. Atherosclerosis. 1999;147:213–225. doi: 10.1016/s0021-9150(99)00346-9. [DOI] [PubMed] [Google Scholar]
- 43.Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM. Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res. 1998;83:952–959. doi: 10.1161/01.res.83.9.952. [DOI] [PubMed] [Google Scholar]
- 44.Han KH, Tangirala RK, Green SR, Quehenberger O. Chemokine receptor CCR2 expression and monocyte chemoattractant protein-1-mediated chemotaxis in human monocytes. A regulatory role for plasma LDL. Arterioscler Thromb Vasc Biol. 1998;18:1983–1991. doi: 10.1161/01.atv.18.12.1983. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe T, Fan J. Atherosclerosis and inflammation mononuclear cell recruitment and adhesion molecules with reference to the implication of ICAM-1/LFA-1 pathway in atherogenesis. Int J Cardiol. 1998;66 (Suppl 1):S45–S53. doi: 10.1016/s0167-5273(98)00147-8. [DOI] [PubMed] [Google Scholar]
- 46.Gu L, Rutledge B, Fiorillo J, Ernst C, Grewal I, Flavell R, et al. In vivo properties of monocyte chemoattractant protein-1. J Leukoc Biol. 1997;62:577–580. doi: 10.1002/jlb.62.5.577. [DOI] [PubMed] [Google Scholar]
- 47.Wilcox JN, Nelken NA, Coughlin SR, Gordon D, Schall TJ. Local expression of inflammatory cytokines in human atherosclerotic plaques. J Atheroscler Thromb. 1994;1 (Suppl 1):S10–S13. doi: 10.5551/jat1994.1.supplemment1_s10. [DOI] [PubMed] [Google Scholar]
- 48.Clinton SK, Libby P. Cytokines and growth factors in atherogenesis. Arch Pathol Lab Med. 1992;116:1292–1300. [PubMed] [Google Scholar]
- 49.Wettero J, Askendal A, Tengvall P, Bengtsson T. Interactions between surface-bound actin and complement, platelets, and neutrophils. J Biomed Mater Res A. 2003;66:162–175. doi: 10.1002/jbm.a.10591. [DOI] [PubMed] [Google Scholar]
- 50.Balasubramanian V, Slack SM. The effect of fluid shear and co-adsorbed proteins on the stability of immobilized fibrinogen and subsequent platelet interactions. J Biomater Sci Polym Ed. 2002;13:543–561. doi: 10.1163/15685620260178391. [DOI] [PubMed] [Google Scholar]
- 51.Tsai WB, Grunkemeier JM, Horbett TA. Human plasma fibrinogen adsorption and platelet adhesion to polystyrene. J Biomed Mater Res. 1999;44:130–139. doi: 10.1002/(sici)1097-4636(199902)44:2<130::aid-jbm2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 52.Hasper D, Hummel M, Hetzer R, Volk HD. Blood contact with artificial surfaces during BVAD support. Int J Artif Organs. 1996;19:590–596. [PubMed] [Google Scholar]
- 53.Groth T, Campbell EJ, Herrmann K, Seifert B. Application of enzyme immunoassays for testing haemocompatibility of biomedical polymers. Biomaterials. 1995;16:1009–1015. doi: 10.1016/0142-9612(95)94909-5. [DOI] [PubMed] [Google Scholar]
- 54.Chinn JA, Ratner BD, Horbett TA. Adsorption of baboon fibrinogen and the adhesion of platelets to a thin film polymer deposited by radio-frequency glow discharge of allylamine. Biomaterials. 1992;13:322–332. doi: 10.1016/0142-9612(92)90057-u. [DOI] [PubMed] [Google Scholar]
- 55.Wu B, Gerlitz B, Grinnell BW, Meyerhoff ME. Polymeric coatings that mimic the endothelium: combining nitric oxide release with surface-bound active thrombomodulin and heparin. Biomaterials. 2007;28:4047–4055. doi: 10.1016/j.biomaterials.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Annich GM, Miskulin J, Osterholzer K, Merz SI, Bartlett RH, et al. Nitric oxide releasing silicone rubbers with improved blood compatibility: preparation, characterization, and in vivo evaluation. Biomaterials. 2002;23:1485–1494. doi: 10.1016/s0142-9612(01)00274-5. [DOI] [PubMed] [Google Scholar]
- 57.Mellgren K, Friberg LG, Mellgren G, Hedner T, Wennmalm A, Wadenvik H. Nitric oxide in the oxygenator sweep gas reduces platelet activation during experimental perfusion. Ann Thorac Surg. 1996;61:1194–1198. doi: 10.1016/0003-4975(96)00017-3. [DOI] [PubMed] [Google Scholar]
- 58.Mellgren K, Friberg LG, Hedner T, Mellgren G, Wadenvik H. Blood platelet activation and membrane glycoprotein changes during extracorporeal life support (ECLS). In vitro studies. Int J Artif Organs. 1995;18:315–321. [PubMed] [Google Scholar]
- 59.Wu Y, Zhou Z, Meyerhoff ME. In vitro platelet adhesion on polymeric surfaces with varying fluxes of continuous nitric oxide release. J Biomed Mater Res A. 2007;81:956–963. doi: 10.1002/jbm.a.31105. [DOI] [PubMed] [Google Scholar]
- 60.Zhang M, Wu Y, Hauch K, Horbett TA. Fibrinogen and von Willebrand factor mediated platelet adhesion to polystyrene under flow conditions. J Biomater Sci Polym Ed. 2008;19:1383–1410. doi: 10.1163/156856208786052353. [DOI] [PubMed] [Google Scholar]
- 61.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Hsu MY, Steinbacher TE, Monticello TM, Schumacher WA. Quantification of platelet composition in experimental venous thrombosis by real-time polymerase chain reaction. Thromb Res. 2007;119:593–600. doi: 10.1016/j.thromres.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 63.Ilmakunnas M, Pesonen EJ, Ahonen J, Ramo J, Siitonen S, Repo H. Activation of neutrophils and monocytes by a leukocyte-depleting filter used throughout cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005;129:851–859. doi: 10.1016/j.jtcvs.2004.07.061. [DOI] [PubMed] [Google Scholar]


