Abstract
An important step in the process of metastasis from the primary tumor is invasive spread into the surrounding stroma. Using an in vivo invasion assay, we have previously shown that imposed gradients of EGF or CSF-1 can induce invasion through an EGF/CSF-1 paracrine loop between cancer cells and macrophages. We now report that invasion induced by other ligands also relies upon this EGF/CSF-1 paracrine invasive loop. Using an in vivo invasion assay, we show that MTLn3 breast cancer cells overexpressing ErbB3 exhibit enhanced invasion compared to control MTLn3 cells in response to the ErbB3 ligand HRG-β1. The invasive response of both MTLn3-ErbB3 and transgenic MMTV-Neu tumors to HRG-β1 is inhibited by blocking EGFR, CSF-1R, or macrophage function, indicating that invasiveness to HRG-β1 is dependent upon the EGF/CSF-1 paracrine loop. Furthermore, we show that CXCL12 also triggers in vivo invasion of transgenic MMTV-PyMT tumors in an EGF/CSF-1 dependent manner. Although the invasion induced by HRG-β1 or CXCL12 is dependent on the EGF/CSF-1 paracrine loop, invasion induced by EGF is not dependent upon HRG-β1 or CXCL12 signaling, demonstrating an asymmetric relationship between different ligand/receptor systems in driving invasion. Our results identify a stromal/tumor interaction that acts as an engine underlying invasion induced by multiple ligands.
INTRODUCTION
Metastasis is a multi-step process involving invasion of the basement membrane and surrounding stroma, intravasation, extravasation and survival/growth of cancer cells at new sites (1). Our research has focused on elucidating what drives cancer cells to leave the primary tumor, cross the basement membrane, resulting in invasion of the surrounding stroma, with the expectation of finding novel targets of metastasis that may be used to prevent the occurrence of this fatal process. With the development of an in vivo invasion assay in which invasive cells are collected from the primary tumor using needles preloaded with Matrigel and a chemoattractant (2), new insights into the process of invasion have been made. Employing this in vivo invasion assay to collect invasive cells from rat MTLn3 breast primary xenograft tumors as well as from transgenic mice in which mammary tumors are induced by the expression of the Polyoma Middle T oncoprotein from the MMTV promoter (PyMT) (3), it was observed that macrophages aided cancer cells in invading the surrounding tissue. This was due to a paracrine communication loop involving secretion of CSF-1 by cancer cells that would activate macrophages to secrete EGF, a chemoattractant for the cancer cells that would drive their invasion (4). Inhibition of either EGF or CSF-1 signaling resulted in decreased invasion to background levels (3), suggesting that EGF/CSF-1 signaling was the key to in vivo invasion in response to either EGF or CSF-1 in this model.
This research implicated the tumor microenvironment, especially stromal cells such as macrophages, in collaborating in the process of metastasis. Tumor associated macrophages, TAMs, have been correlated with poor prognosis in several cancers including breast, with high density of TAMs associated with metastasis (5, 6). Furthermore, overexpression of CSF-1, a major growth factor involved in the survival, differentiation and chemotaxis of macrophages has been shown to correlate with poor prognosis in breast cancer (7, 8). Studies using transgenic PyMT mice carrying a null mutation in the CSF-1 gene showed that recruitment of macrophages to the primary tumor was dramatically decreased in this model and accordingly, there was slower tumor progression with significantly reduced metastasis that was rescued upon expression of CSF-1 in the mammary tumor epithelium (9). Furthermore, imaging of PyMT tumors in which macrophages were labeled with Texas Red dextran showed that tumor cell motility was associated with the presence of macrophages, with more frequent motility associated with perivascular macrophages (10), suggesting again a close interaction between carcinoma cells and macrophages within the primary tumor that facilitates invasion.
In vitro characterization of invasion typically involves studying cancer cell traversal of a Matrigel barrier. Using this assay, several chemoattractants have been found to stimulate invasion, including CXCL12 (SDF-1) and Heregulin Beta 1 (HRGβ1). CXCL12 is a chemokine that binds to CXCR4. CXCR4 can be highly expressed on breast cancer cells as compared to normal breast tissue (11), and has been implicated as a predictor of poor prognosis in patients with breast cancer (12). In MDA-MB-231 breast cancer cells it has been shown that CXCL12 stimulates their invasion in vitro, as well as plays a key role in the metastatic behavior of these cells in vivo (11). HRGβ1, an EGF-like ligand that binds and activates ErbB3 and ErbB4, members of the ErbB family of receptor tyrosine kinases (13), has been implicated in modulating the invasive behavior of breast cancer cells. HRGβ1 stimulates the in vitro invasive behavior of MDA-MB-231, and downregulation of HRGβ1 expression results in impaired invasiveness in vitro and metastasis in vivo (14). HRGβ1 also has been shown to induce the migration and invasion of MCF-7 and T47D cells in vitro through activation of the ErbB2 and ErbB3 receptors (15). Overexpression of ErbB3 in MTLn3 cells results in enhanced chemotaxis to HRGβ1 as well as in increased metastatic behavior of MTLn3 cells, including leaving the primary tumor in greater numbers (16).
Since CXCL12 and HRGβ1 have been implicated in breast cancer cell invasion, we tested their ability to stimulate invasion in vivo in the tumor microenvironment using the in vivo invasion assay. Remarkably, we found that although HRGβ1 can stimulate the in vivo invasion of MTLn3 cells overexpressing the ErbB3 receptor, this invasion is inhibited by addition of Iressa, an EGFR tyrosine kinase inhibitor, or an EGF neutralizing antibody, as well as by a CSF-1R blocking antibody or by macrophage depletion using liposome encapsulated clodronate. This dependency on EGF/CSF-1 signaling to stimulate invasion in vivo was recapitulated in the MMTV-Neu transgenic animal model of mammary cancer in which ErbB3 is also expressed (17). In addition, we found that in vivo invasion towards a G-protein coupled receptor ligand, CXCL12, is also dependent upon the EGF/CSF-1 paracrine loop. Our results suggest that targeting EGF or CSF-1 signaling can be effective in blocking invasion in response to heterologous ligands through a novel dependence on tumor/stroma interactions revealed in vivo.
MATERIALS AND METHODS
Cell lines and animal models
All procedures involving mice were conducted in accordance with the National Institutes of Health regulations concerning the use and care of experimental animals. The study of mice was approved by the Albert Einstein College of Medicine animal use committee. The rat mammary carcinoma MTLn3 cells transduced with the empty pLXSN retroviral vector or vector containing human ErbB3 receptor (16) were grown in α-MEM supplemented with 5% fetal bovine serum (FBS) and penicillin/streptomycin solution (Life Technologies). The tumor cells were grown to 70–85% confluence before being harvested. Cells were detached using PBS-EDTA and 5 scraped using a rubber policeman. 5×105 cells were injected into the right fourth mammary fat pad from the head of 5-to-7-week-old female severe combined immunodeficient (SCID) mice (National Cancer Institute, Bethesda, MD). Tumors were allowed to grow for 4 weeks before cell collection. FVB mice transgenic for the polyoma virus middle T (PyVT or PyMT) oncogene under the mouse mammary tumor virus (MMTV) long terminal repeat 206 (LTR), generated as previously described (18), were used for in vivo invasion assays at 12–14 weeks of age. FVB mice transgenic for Neu driven by the MMTV LTR containing an activating deletion in the extracellular region (17) were used in in vivo invasion assays and for primary tumor harvest at 31–33 weeks of age.
In vivo invasion assay
Cell collection into needles placed in the primary tumor of anesthetized mice was carried out as described previously (2). Invasive cells were collected into 33-gauge Hamilton needles (Fisher 14-815-423) filled with Matrigel (Beckton Dickinson 356234) diluted 1:10 with L15-BSA and a chemoattractant for 4 hours. At the end of collection, the contents of the needles were extruded with approximately 30ul DAPI using a syringe onto a coverslip. The chemoattractants used in this assay include HRGβ1 at a concentration of 50nM (R & D Systems 396-HB), EGF at 25nM (Life Technologies), and CXCL12α at 62.5nM (R & D Systems 460-SD). To inhibit activation of the EGF receptor through EGF binding, an anti-mouse EGF neutralizing antibody (R & D Systems AF2028) was used at a concentration of 20μg/ml. To inhibit activity of the EGF receptor, Iressa (AstraZeneca), a tyrosine kinase inhibitor specific for the EGF receptor, was used at 1 μM. For inhibiting the CSF-1 receptor, a purified monoclonal anti-mouse CSF-1 receptor antibody (AFS98) (19) was used at 15μg/ml. To block activation of CXCR4, AMD3100 was used at 100nM (Sigma A5602). To block HRGβ1 mediated signaling, an ErbB3 antibody that blocks binding of HRGβ1 to the ErbB3 receptor was used (Fisher HER-3 Ab-5). As a control antibody for the experiments involving signaling inhibition using blocking/neutralizing antibodies, a mouse IgG antibody was used at the same concentration used for the experimental antibodies (Jackson ImmunoResearch 015-000-003). To functionally impair macrophages in mice bearing tumors, clodronate liposomes were administered systemically. Empty (control) and clodronate-containing liposomes were administered by tail vein into mice 48 hours prior to the in vivo invasion assay. Liposomes were prepared as detailed in (20) using clodronate at a concentration of 2.5 grams per 10 ml PBS. Clodronate (or Cl2MDP) was a gift of Roche Diagnostics GmbH, Mannheim, Germany. Phosphatidylcholine (LIPOID E PC) was obtained from Lipoid GmbH, Ludwigshafen, Germany. Cholesterol was purchased from SIGMA Chem.Co., USA. Animals were injected with 100μl of liposome solution per 10g of weight.
Determination of cell types collected in the in vivo invasion assay
After 4 hour collection, the invasive cells were extruded from the needles using 10% paraformaldehyde into poly-L-lysine coated MatTek dishes and fixed for 1 hour at room temp. To block non-specific binding the samples were incubated overnight at 4°C in Tris-buffered saline (TBS)-1% FBS. The blocking solution was removed, cells washed with TBS-1% BSA and incubated with a primary antibody mixture of mouse anti-ErbB3 antibody (Neomarkers MS-303) for carcinoma cells and rat anti-F4/80 (21) for macrophages in TBS-1% BSA. After 1 hour incubation with the primary antibodies, the cells were washed with TBS-1% BSA and incubated in a mixture of goat anti-mouse Cy3 and sheep anti-rat FITC. The cells were rinsed and left in TBS-1% BSA with 4′,6 -diamidino-2-phenylindole (DAPI) and counted using a fluorescence microscope.
Microchemotaxis chamber assay
A 48-well microchemotaxis chamber (Neuroprobe, Cabin John, MD) was used as described previously (16). Briefly, cells were starved for 3 hours in L15 media supplemented with 0.35% BSA (L15-BSA) at 37°C, then detached using PBS-EDTA and loaded into the top wells of the microchemotaxis chamber, 20,000 cells per well. Cells were allowed to migrate for 4 hours at 37°C through a collagen-coated 8um pore filter (Neuroprobe) towards bottom wells filled with either EGF or HRGβ1 diluted in L15-BSA with or without EGF neutralizing antibody (R & D Systems AF2028), or to L15-BSA alone. The filter was then taken out, fixed in 10% paraformaldehyde for 1 hour, nonmigrating cells were removed from the top surface of the filter, and the remaining cells on the lower surface were stained overnight in hematoxylin staining solution (Fisher, CS402-1D). The membrane was then washed in de-ionized water and migrated cells were counted using a light microscope.
Primary tumor cell culturing and CSF-1 ELISA
After the animal was euthanized, the primary tumor was removed, rinsed briefly in 70% ethanol and washed with PBS. In a petri dish with PBS, the tumor was minced until only small clumps remained. The minced tumor was transferred into a 50 ml conical tube into 10ml digestion medium consisting of growth medium supplemented with 300 U/ml collagenase (Sigma C5138) and 100 U/ml hyaluronidase (Sigma H3506), and incubated in a 37°C shaker for 30 mins. Tumor cells and clumps were centrifuged at 1000rpm, washed 3X with PBS and resuspended in growth medium (DMEM/F12 (Fisher MT10092CV) supplemented with 10% FBS, and 50U/ml Pen/Strep, 10μg/ml insulin (Sigma I5523), 1mg/ml BSA, 5μg/ml linoleic acid complex (Sigma L1376), 20U/ml Fungizone (Invitrogen 15290-018), 50μg/ml Gentamicin (Sigma G-1272), and 1.2g sodium bicarbonate per 1000 ml medium) and plated at densities varying from 1 to 5 × 106 on 10cm plates (22). The following day the cultures were washed with growth medium and medium was changed every day following that. Cells were cultured for 5–10 days before they were used for in vitro stimulations. Cells were grown to 85–90% confluency, starved for 3 hours in L15-BSA and stimulated for 4 hours with 12.5nM HRGβ1 in L15-BSA. After 4 hours, the supernatants were collected, spun down to remove debris and stored at −20°C. The samples were tested for the presence of secreted CSF-1 using a mouse CSF-1 ELISA kit (R & D Systems MMC00).
Statistical analysis
Multiple comparisons were performed using ANOVA and 2 condition comparisons performed using t-test.
RESULTS
HRGβ1 stimulates the in vivo invasion of MTLn3-ErbB3 cells
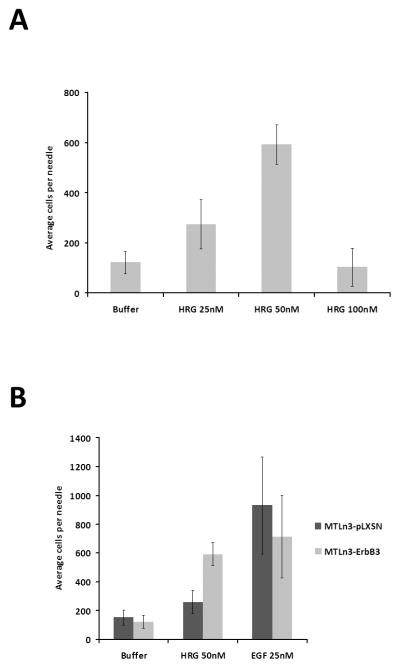
ErbB3 overexpression in MTLn3 cells increases their in vitro chemotactic response to HRGβ1 through the formation of active ErbB2-ErbB3 heterodimers (16), as well as their metastatic behavior in vivo. To investigate whether HRGβ1 could also stimulate MTLn3 rat mammary adenocarcinoma cell chemotaxis and invasion in vivo, cells expressing increased levels of ErbB3 (MTLn3-ErbB3) or empty vector control cells (MTLn3-pLXSN) were injected into the mammary fat pads of SCID mice to form orthotopic tumors. Four weeks post-injection, microneedles containing Matrigel and a chemoattractant were inserted into the primary tumors to collect invasive cells. HRGβ1 induced in vivo invasion in MTLn3-ErbB3 tumors (Fig. 1A) in a dose dependent fashion, with the optimal concentration being 50nM HRGβ1. Using EGF as a chemoattractant, comparable invasiveness of MTLn3-pLXSN and MTLn3-ErbB3 cells was observed, indicating that overexpression of ErbB3 did not affect the ability of MTLn3 cells to respond to EGF (Fig. 1B). However, MTLn3-ErbB3 tumors showed a greater in vivo invasion in response to HRGβ1 compared to control pLXSN tumors (Fig 1B).
Figure 1. HRGβ1 induces in vivo invasion of MTLn3-ErbB3 cells.
A. Dose-response for HRGβ1-stimulated in vivo invasion in MTLn3-ErbB3 primary tumors (p<.002 by ANOVA). B. In vivo invasion of MTLn3-ErbB3 (light gray) and MTLn3-pLXSN (dark gray) primary tumors in response to 50nM HRGβ1 and 25nM EGF (p<.0004 for pLXSN and p<.02 for ErbB3 by ANOVA). Means and standard deviations are shown.
Previous studies have shown that macrophages co-migrate with breast cancer cells in response to EGF stimulation in the needle collection assay (3) To assess whether macrophages also co-migrate with cancer cells in HRGβ1-mediated in vivo invasion, the invasive cells collected upon HRGβ1 stimulation from MTLn3-ErbB3 tumors were fixed and stained with an ErbB3 antibody to detect MTLn3-ErbB3 cells and an F4/80 antibody to detect macrophages. MTLn3-ErbB3 cells represented 75% (±5%) of the invasive population and macrophages 25% (±5%), with no other cell types present in significant amounts, similar to what we have previously reported for the in vivo invasion in response to EGF (3). To test whether macrophages play a role in the invasive behavior of MTLn3-ErbB3 cells in response to HRGβ1, macrophages were functionally impaired using clodronate-containing-liposomes (20). SCID mice bearing MTLn3-ErbB3 tumors were injected i.v. with clodronate-containing-liposomes or empty liposomes as a negative control 48 hours prior to performing the in vivo invasion assay. As described in the Supplemental Data and Supplemental Fig. 1, although the number of macrophages in the primary tumor is not decreased, clodronate liposome treatment results in reduced macrophage function as measured by macropinocytosis of 70kD dextran. There was a significantly reduced invasive response to HRGβ1 in tumors that had been pretreated with clodronate, compared to those pretreated with empty liposomes (ratio of HRGβ1-induced in vivo invasion in the presence of clodronate-containing liposomes to empty liposomes: 0.6 ±0.1, p<0.01); while basal invasion in the absence of attractant was not affected. This indicates that macrophages contribute to the invasive behavior of MTLn3-ErbB3 cells in response to HRGβ1 stimulation.
The EGF/CSF-1 paracrine loop is required for the in vivo invasion of MTLn3-ErbB3 cells in response to HRGβ1
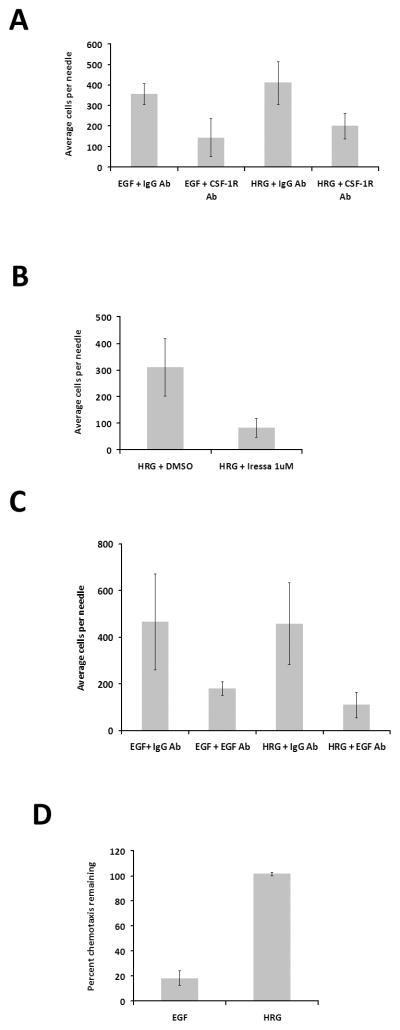
The previous section suggested that macrophages could play an active role in HRGβ1-induced in vivo invasion of MTLn3-ErbB3 cells. We therefore tested whether the EGF/CSF-1 paracrine loop was necessary for HRGβ1-induced in vivo invasion. We first evaluated the role of CSF-1 signaling by blocking the CSF-1 receptor on macrophages using a CSF-1 receptor blocking antibody. As a positive control, we confirmed that the invasion of MTLn3-ErbB3 tumor cells towards EGF was inhibited upon blockage of the CSF-1 receptor (p<0.05) (Fig. 2A, left side). Similarly, addition of the CSF-1 receptor antibody also inhibited HRGβ1-stimulated in vivo invasion by MTLn3-ErbB3 tumors (p<0.005) (Fig. 2A, right side), indicating that activation of the CSF-1 receptor in macrophages is required for MTLn3-ErbB3 cells to invade in response to HRGβ1.
Figure 2. EGF/CSF-1 signaling is required for in vivo invasion of MTLn3-ErbB3 tumors in response to HRGβ1 stimulation.
A. In vivo invasion in response to 25 nM EGF or 50 nM HRGβ1 in the presence of a control antibody, (IgG Ab), or a CSF-1 receptor-blocking antibody (CSF-1R Ab). B. The effect of 1μM Iressa or vehicle (DMSO) on in vivo invasion of MTLn3-ErbB3 primary tumors in response to 50 nM HRGβ1. C. In vivo invasion in response to 25 nM EGF or 50 nM HRGβ1 in the presence of a control antibody, (IgG Ab), or an EGF-binding antibody (EGF Ab). D. The effect of an EGF neutralizing antibody on chemotaxis of MTLn3-ErbB3 cells to 5 nM EGF or 12.5 nM HRGβ1 determined using a Boyden chamber. Results are shown as the percent of the chemotaxis response for each ligand remaining in the presence of the inhibitor compared to the chemotactic response for that ligand in the absence of the inhibitor. Means and standard deviations are shown. Pairwise comparisons by t-test are provided in the text.
To determine whether EGF signaling also plays a role in the invasive response of MTLn3-ErbB3 cells to HRGβ1 stimulation, an EGFR- specific tyrosine kinase inhibitor, Iressa (23), was added to microneedles loaded with HRGβ1 (Fig. 2B). Addition of Iressa inhibited the in vivo invasion of MTLn3-ErbB3 cells to HRGβ1, bringing the response down to basal levels (p<0.05). This suggests that EGFR signaling is required for MTLn3-ErbB3 cells to invade when stimulated with HRGβ1. To test whether EGF specifically was required, we determined the effect of an EGF antibody that blocks the binding of EGF to its receptor, EGFR (Fig. 2C). The EGF antibody significantly inhibited the in vivo invasion of MTLn3-ErbB3 cells to EGF as anticipated (p<0.05, Fig. 2C, left side), but also resulted in inhibition of the in vivo invasion of MTLn3-ErbB3 cells to HRGβ1 (p<0.005, Fig. 2C, right side). To check that the neutralizing EGF antibody was specific in blocking EGF-mediated signaling and does not affect HRGβ1 binding and chemotaxis, a Boyden chamber was used to analyze the in vitro chemotaxis behavior of MTLn3-ErbB3 cells in the presence of the EGF antibody. While the EGF antibody itself had no effect in the basal motility of MTLn3-ErbB3 cells (p<0.6, data not shown), the EGF antibody blocked the chemotaxis of MTLn3-ErbB3 cells to EGF (p<0.005) but not to HRGβ1 (p<0.15) (Fig. 2D), demonstrating that the antibody was not directly affecting HRGβ1 chemotaxis. This result indicates that HRGβ1-induced in vivo invasion is dependent on the secretion of EGF within the primary tumor. In summary, these results indicate that HRGβ1-mediated in vivo invasion of MTLn3-ErbB3 tumors is dependent on EGF/CSF-1 signaling within the primary tumor.
An ErbB2 transgenic model recapitulates the MTLn3-ErbB3 xenograft model of in vivo invasion to HRGβ1
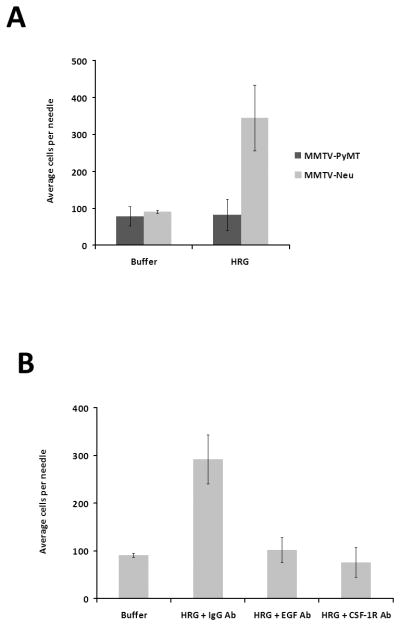
To determine whether HRGβ1 mediated in vivo invasion is dependent on EGF and CSF-1 signaling in other breast cancer models, we compared the MMTV-PyMT and MMTV-Neu transgenic models (17, 18). We have previously shown that HRGβ1 did not induce invasion of MMTV-PyMT tumors (3), but MMTV-Neu tumors show high levels of expression of ErbB3 (17). Consistent with their ErbB3 expression, MMTV-Neu tumors showed enhanced in vivo invasion in response to HRGβ1 (p<0.005) whereas MMTV-PyMT tumors did not (Fig. 3A). We next tested the effect of blocking EGF and CSF-1 receptor signaling on HRGβ1-induced in vivo invasion (Fig. 3B). Addition of the EGF neutralizing antibody (p<0.005) or CSF-1 receptor blocking antibody (p<0.005) resulted in inhibition of MMTV-Neu tumor invasion in response to HRGβ1. We conclude that HRGβ1 stimulates in vivo invasion in MMTV-Neu tumors also by activating the EGF/CSF-1 paracrine loop. To test how HRGβ1 could trigger the EGF/CSF-1 paracrine loop we stimulated MMTV-Neu primary tumor cells in vitro with HRGβ1 and tested the supernatants for CSF-1 secretion. Cultured Neu primary tumor cells were stimulated for 4 hours with HRGβ1 (the same time used for the in vivo invasion assay), and the supernatants were collected and assayed for the presence of CSF-1 protein using a commercially available ELISA. There was a modest but significant increase in CSF-1 secretion upon HRGβ1 stimulation of Neu tumor cells (ratio of HRGβ1 stimulated to buffer stimulated: 1.60+/−0.66, mean and standard deviation, N=6, p<.05). Thus, HRGβ1 induction of CSF-1 production could induce the EGF/CSF-1 paracrine loop.
Figure 3. MMTV-Neu tumors show in vivo invasion in response to HRGβ1 which requires EGF/CSF-1 signaling.
A. MMTV-PyMT (dark gray) and MMTV-Neu (light gray) tumors were tested for their ability to invade in vivo in response to 50 nM HRGβ1. B. MMTV-Neu tumor in vivo invasive response to 50 nM HRGβ1 in the presence of either control antibody (IgG Ab), an EGF neutralizing antibody (EGF Ab) or a CSF-1 R blocking antibody (CSF-1R Ab) (p<5×10−13 by ANOVA). Means and standard deviations are shown.
CXCL12 mediated in vivo invasion in MMTV-PyMT tumors is also dependent on activation of EGF/CSF-1 signaling
We had previously found that CXCL12 also induced in vivo invasion in MMTV-PyMT tumors and that this invasive response could be blocked upon addition of Iressa (J. Wyckoff and D. Cox, unpublished, Supplemental Fig. 2). Given our studies showing that HRGβ1-induced invasion was dependent on the EGF/CSF-1 paracrine loop, we decided to test whether a different type of ligand, such as CXCL12, which activates the G-protein coupled receptor CXCR4, could also activate the EGF/CSF-1 paracrine loop. Similar to our results with HRGβ1-induced invasion, both the neutralizing EGF antibody (p<0.005) and the CSF-1R blocking antibody (p<0.005) resulted in decreased in vivo invasion of MMTV-PyMT tumors in response to CXCL12 (Fig. 4). To evaluate the contribution of macrophages to CXCL12 in vivo invasion, MMTV-PyMT mice were injected with either empty or clodronate-containing liposomes via tail vein 48 hours prior to measurement. Clodronate treatment resulted in significantly reduced in vivo invasion to CXCL12 in PyMT tumors (the ratio of in vivo invasion to CXCL12 in tumors pretreated with clodronate containing liposomes to empty liposomes was 0.4 ±0.2, p<0.04).
Figure 4. MMTV-PyMT tumors invade in vivo in response to CXCL12 stimulation and this invasion is dependent on EGF/CSF-1 signaling and macrophages.

In vivo invasion of MMTV-PyMT tumors in response to 62.5 nM CXCL12 in the presence of a control antibody (IgG Ab), a CSF-1 receptor antibody (CSF-1R Ab), or an EGF neutralizing antibody (EGF Ab) (p< 2 × 10−8 by ANOVA). Means and standard deviations are shown.
CXCL12 and HRGβ1 signaling are not required for invasion in response to EGF
Given that CXCL12 and HRGβ1-induced invasion are dependent on EGF/CSF-1 signaling, we then wished to determine whether EGF/CSF-1 induced invasion was in turn dependent upon CXCL12 or HRGβ1. We first tested whether CXCL12 signaling was necessary for EGF/CSF-1 in vivo invasion. We added the CXCR4 inhibitor AMD3100 (24) to needles containing CXCL12 as a positive control to confirm that AMD3100 could block CXCL12 mediated in vivo invasion from PyMT tumors. As expected, addition of AMD3100 resulted in inhibition of CXCL12 mediated in vivo invasion from PyMT tumors (p<0.005) (Fig. 5A); however, addition of AMD3100 to EGF containing needles did not result in impaired in vivo invasion to EGF in PyMT tumors (p<0.4) (Fig. 5B). These results demonstrate that although CXCL12-induced in vivo invasion is dependent on EGF/CSF-1 signaling, EGF-induced in vivo invasion is not dependent on CXCL12 signaling.
Figure 5. EGF induced in vivo invasion is not dependent on CXCL12 or HRGβ1 signaling.
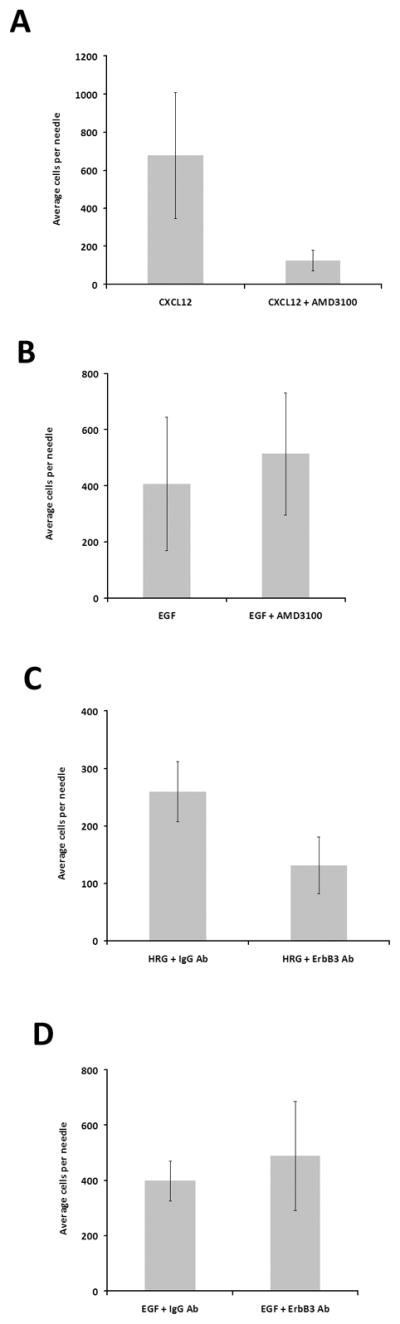
A. The effect of CXCR4 inhibition (AMD3100, 100 nM) on invasion of MMTV-PyMT tumors in response to 62.5 nM CXCL12. B. The effect of CXCR4 inhibition (AMD3100, 100 nM) on invasion of MMTV-PyMT tumors in response to EGF. C. In vivo invasion of MTLn3-ErbB3 tumors to 50 nM HRGβ1 in the presence of a control antibody (IgG Ab), or an ErbB3 blocking antibody (ErbB3 Ab). D. In vivo invasion of MTLn3-ErbB3 tumors to 25 nM EGF in the presence of a control antibody (IgG Ab), or an ErbB3 blocking antibody (ErbB3 Ab). Means and standard deviations are shown. Pairwise comparisons by t-test are provided in the text.
Similarly, to test the role of HRGβ1 in EGF-induced in vivo invasion, we used an anti-ErbB3 blocking antibody. ErbB3 is the major receptor for HRGβ1 in MTLn3-ErbB3 cells (16), and this antibody blocks the binding site for HRGβ1 on ErbB3. Addition of the ErbB3 blocking antibody inhibited invasion of MTLn3-ErbB3 tumors to HRGβ1 (p<0.005) (Fig. 5C) but not to EGF (p<0.4) (Fig. 5D), indicating that although HRGβ1-induced in vivo invasion is dependent on EGF signaling, EGF-induced in vivo invasion is not dependent on HRGβ1.
DISCUSSION
We have previously shown that breast cancer cells invade surrounding breast tissue with the help of macrophages (3). This process involves a paracrine communication loop between cancer cells and macrophages in which cancer cells secrete CSF-1, a chemoattractant for macrophages. Macrophages are in turn stimulated by CSF-1 and secrete EGF, a ligand that binds to the EGF receptor in breast cancer cells and directs their chemotaxis (4). Within the primary tumor, externally imposed gradients of EGF or CSF-1 will induce cancer cell and macrophage invasion up the gradient. We report here for the first time that other ligand/receptor systems can induce in vivo invasion responses that are dependent upon this EGF/CSF1 paracrine loop. Both a xenograft model (MTLn3-ErbB3 (16)) overexpressing the receptor for heregulin, ErbB3, and a transgenic model (MMTV-Neu (17)) that expresses ErbB3, show heregulin-induced invasion. This heregulin-induced invasion is dependent on macrophage function as indicated by clodronate liposome inhibition, as well as by inhibition using a blocking CSF-1R antibody. Both an EGFR-specific inhibitor, Iressa, as well as an anti-EGF antibody, demonstrate that EGFR function and signaling via extracellular EGF are required for the heregulin-induced invasion, but not for heregulin chemotaxis in vitro. A ligand for CXCR4, CXCL12 (SDF-1), induced invasion effectively in the MMTV-PyMT model (which showed no invasion in response to heregulin). CXCL12-induced in vivo invasion was also dependent upon macrophage function, CSF-1R function, and extracellular EGF signaling.
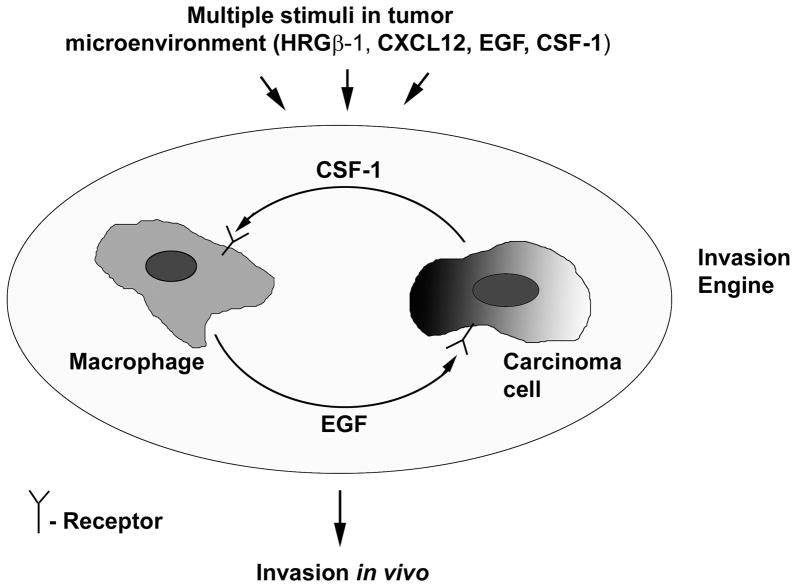
While inhibition of the EGF/CSF-1 paracrine loop blocked in vivo invasion in response to HRGβ1 and CXCL12, inhibition of either CXCR4 or ErbB3 did not block in vivo invasion induced by EGF. These results demonstrate that the EGF/CSF-1 in vivo paracrine invasion loop is independent of HRGβ1 and CXCL12, but can be triggered by these chemoattractants. Based on these results, we propose a novel model for in vivo invasion (Fig. 6), in which the EGF/CSF-1 paracrine loop is an important driver of invasion within the primary tumor. In its simplest form, invasion is induced by ligands of EGFR or CSF-1R present in the tumor microenvironment, which directly feed into this “invasion engine”. In addition, if the tumor cells (or potentially the macrophages) express other receptors such as ErbB3 or CXCR4, the corresponding ligands such as heregulin or CXCL12 can activate the engine, which can then dramatically enhance the invasion response to the ligand. Both CXCL12 and heregulin are expressed in breast cancers (25–29). CXCL12 can be secreted by cancer-activated fibroblasts (30, 31) while heregulin has been reported to be expressed by endothelial cells (32–34). In consequence, the invasion induced in response to the ligands secreted by these cells in the local tumor microenvironment can contribute to enhanced intravasation and metastasis by directing invasion out into the stroma and towards blood vessels. We have previously reported that the MTLn3-ErbB3 cells show enhanced intravasation and metastasis (16), and CXCR4 has also been reported to enhance breast cancer metastasis (11, 35), consistent with this hypothesis. However, not all ligands are able to trigger the invasion response in the models we have tested; the macrophage chemoattractant VEGF did not induce invasion (3).
Figure 6. Model for in vivo invasion.
The primary tumor expresses receptors for a number of chemoattractants, including the EGF receptor. Chemoattractants present in the tumor microenvironment can feed into the EGF/CSF-1 paracrine loop, which then drives invasion in vivo.
A clinical implication of these results is that inhibition of EGFR or CSF-1R signaling could provide a broader inhibition of tumor cell invasiveness than might be anticipated based simply on EGFR expression. EGFR expression levels in MTLn3 cells are slightly elevated (about 50,000 per cell (36)), but are not overexpressed to the level associated with typical EGFR overexpressors such as the MDA-MB-231 cells (700,000 receptors per cell (37)) or MDA-MB-468 cells (1.9 × 106 receptors per cell (38)). Similarly, CSF1R expression in MTLn3 cells is low (4), and MTLn3 cells do not show CSF-1 induced lamellipod extension responses (data not shown). Thus, our data suggest that invasion by breast tumors that do not show high EGFR or CSF-1R expression can still be sensitive to inhibition of these receptors due to in vivo paracrine interactions that can occur in the tumor microenvironment.
Supplementary Material
Acknowledgments
Funding was provided by CA107050 (D.C.), CA110269 (L.H.), CA77522 (T.S. and J.E.S.) and CA100324 (E.R.S., J.W., J.W.P. and J.E.S.), CBCRA and Terry Fox Foundation (W.M.), CA131270 (J.P.). J.E.S. is the Betty and Sheldon Feinberg Senior Faculty Scholar in Cancer Research.
References
- 1.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 2.Wyckoff JB, Segall JE, Condeelis JS. The collection of the motile population of cells from a living tumor. Cancer Res. 2000;60:5401–4. [PubMed] [Google Scholar]
- 3.Wyckoff J, Wang W, Lin EY, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 4.Goswami S, Sahai E, Wyckoff JB, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–83. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 5.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 6.Scholl SM, Pallud C, Beuvon F, et al. Anti-colony-stimulating factor-1 antibody staining in primary breast adenocarcinomas correlates with marked inflammatory cell infiltrates and prognosis. J Natl Cancer Inst. 1994;86:120–6. doi: 10.1093/jnci/86.2.120. [DOI] [PubMed] [Google Scholar]
- 7.Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia. 2002;7:147–62. doi: 10.1023/a:1020399802795. [DOI] [PubMed] [Google Scholar]
- 8.Scholl SM, Lidereau R, de la Rochefordiere A, et al. Circulating levels of the macrophage colony stimulating factor CSF-1 in primary and metastatic breast cancer patients. A pilot study. Breast Cancer Res Treat. 1996;39:275–83. doi: 10.1007/BF01806155. [DOI] [PubMed] [Google Scholar]
- 9.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–40. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyckoff JB, Wang Y, Lin EY, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–56. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 11.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 12.Kato M, Kitayama J, Kazama S, Nagawa H. Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res. 2003;5:R144–50. doi: 10.1186/bcr627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breuleux M. Role of heregulin in human cancer. Cell Mol Life Sci. 2007;64:2358–77. doi: 10.1007/s00018-007-7120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai MS, Shamon-Taylor LA, Mehmi I, Tang CK, Lupu R. Blockage of heregulin expression inhibits tumorigenicity and metastasis of breast cancer. Oncogene. 2003;22:761–8. doi: 10.1038/sj.onc.1206130. [DOI] [PubMed] [Google Scholar]
- 15.Spencer KS, Graus-Porta D, Leng J, Hynes NE, Klemke RL. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J Cell Biol. 2000;148:385–97. doi: 10.1083/jcb.148.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue C, Liang F, Mahmood R, et al. ErbB3-dependent motility and intravasation in breast cancer metastasis. Cancer Res. 2006;66:1418–26. doi: 10.1158/0008-5472.CAN-05-0550. [DOI] [PubMed] [Google Scholar]
- 17.Siegel PM, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. Embo J. 1999;18:2149–64. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–61. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sudo T, Nishikawa S, Ogawa M, et al. Functional hierarchy of c-kit and c-fms in intramarrow production of CFU-M. Oncogene. 1995;11:2469–76. [PubMed] [Google Scholar]
- 20.van Rooijen N, van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide”. Methods Enzymol. 2003;373:3–16. doi: 10.1016/s0076-6879(03)73001-8. [DOI] [PubMed] [Google Scholar]
- 21.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–15. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 22.Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Wakeling AE, Guy SP, Woodburn JR, et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–54. [PubMed] [Google Scholar]
- 24.Hatse S, Princen K, Bridger G, De Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–62. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- 25.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–7. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 26.Kang H, Watkins G, Parr C, Douglas-Jones A, Mansel RE, Jiang WG. Stromal cell derived factor-1: its influence on invasiveness and migration of breast cancer cells in vitro, and its association with prognosis and survival in human breast cancer. Breast Cancer Res. 2005;7:R402–10. doi: 10.1186/bcr1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn M, Sinha P, Campbell R, et al. Co-expression of neuregulins 1, 2, 3 and 4 in human breast cancer. J Pathol. 2004;203:672–80. doi: 10.1002/path.1561. [DOI] [PubMed] [Google Scholar]
- 28.Atlas E, Cardillo M, Mehmi I, Zahedkargaran H, Tang C, Lupu R. Heregulin is sufficient for the promotion of tumorigenicity and metastasis of breast cancer cells in vivo. Mol Cancer Res. 2003;1:165–75. [PubMed] [Google Scholar]
- 29.Stove C, Bracke M. Roles for neuregulins in human cancer. Clin Exp Metastasis. 2004;21:665–84. doi: 10.1007/s10585-004-6917-6. [DOI] [PubMed] [Google Scholar]
- 30.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 31.Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Iivanainen E, Paatero I, Heikkinen SM, et al. Intra- and extracellular signaling by endothelial neuregulin-1. Exp Cell Res. 2007;313:2896–909. doi: 10.1016/j.yexcr.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 33.Lemmens K, Segers VF, Demolder M, De Keulenaer GW. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem. 2006;281:19469–77. doi: 10.1074/jbc.M600399200. [DOI] [PubMed] [Google Scholar]
- 34.Cote GM, Miller TA, Lebrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp Cell Res. 2005;311:135–46. doi: 10.1016/j.yexcr.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Liang Z, Wu H, Reddy S, et al. Blockade of invasion and metastasis of breast cancer cells via targeting CXCR4 with an artificial microRNA. Biochem Biophys Res Commun. 2007;363:542–6. doi: 10.1016/j.bbrc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Lichtner RB, Wiedemuth M, Kittmann A, Ullrich A, Schirrmacher V, Khazaie K. Ligand-induced activation of epidermal growth factor receptor in intact rat mammary adenocarcinoma cells without detectable receptor phosphorylation. J Biol Chem. 1992;267:11872–80. [PubMed] [Google Scholar]
- 37.Fitzpatrick SL, LaChance MP, Schultz GS. Characterization of epidermal growth factor receptor and action on human breast cancer cells in culture. Cancer Res. 1984;44:3442–7. [PubMed] [Google Scholar]
- 38.Filmus J, Pollak MN, Cailleau R, Buick RN. MDA-468, a human breast cancer cell line with a high number of epidermal growth factor (EGF) receptors, has an amplified EGF receptor gene and is growth inhibited by EGF. Biochem Biophys Res Commun. 1985;128:898–905. doi: 10.1016/0006-291x(85)90131-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.