Abstract
The detection of neuron-specific proteins in blood might allow quantification of the degree of neuropathology in experimental and clinical contexts. We have been studying a novel blood biomarker of axonal injury, the heavily phosphorylated axonal form of the high molecular weight neurofilament subunit NF-H (pNF-H). We hypothesized that this protein would be released from damaged and degenerating neurons following experimental traumatic brain injury (TBI) in amounts large enough to allow its detection in blood and that the levels detected would reflect the degree of injury severity. An enzyme-linked immunosorbent assay (ELISA) capture assay capable of detecting nanogram amounts of pNF-H was used to test blood of rats subjected to experimental TBI using a controlled cortical impact (CCI) device. Animals were subjected to a mild (1.0 mm), moderate (1.5 mm), or severe (2.0 mm) cortical contusion, and blood samples were taken at defined times post-injury. The assay detected the presence of pNF-H as early as 6 h post-injury; levels peaked at 24–48 h, and then slowly decreased to baseline over several days post-injury. No signal above baseline was detectable in control animals. Analysis of variance (ANOVA) showed a significant effect of lesion severity, and post hoc analysis revealed that animals given a moderate and severe contusion showed higher levels of blood pNF-H than controls. In addition, the peak levels of pNF-H detected at both 24 and 48 h post-injury correlated with the degree of injury as determined by volumetric analysis of spared cortical tissue. Relative amounts of pNF-H were also determined in different areas of the central nervous system (CNS) and were found to be highest in regions containing large-diameter axons, including spinal cord and brainstem, and lowest in the cerebral cortex and hippocampus. These findings suggest that the measurement of blood levels of pNF-H is a convenient method for assessing neuropathology following TBI.
Key words: cell death, cortical contusion, neurofilaments, serum
Introduction
Specific proteins are expected to be released from neurons and their processes following central nervous system (CNS) injury and disease. An assay that could reliably quantify the levels of these released proteins might provide useful information about the degree of neuronal injury occurring as a result of damage and disease states, and would be particularly useful if such proteins could be detected in blood. These assays could potentially be used to diagnose neurological disorders associated with neuronal loss, to track the progression of disease states, and to assess the effectiveness of appropriate neuroprotective drugs and other therapeutic interventions. There has been a growing appreciation that many kinds of CNS injury and disease states are the result of axonal injury and degeneration (Buki and Povlishock, 2006; Stys, 2005). Accordingly, a convenient method of detecting ongoing axonal loss might be particularly useful experimentally and clinically. An ideal biomarker of axonal injury would have several properties; it should be expressed specifically in axons, it should be abundant enough so that it can be readily detectable after the significant dilution that occurs following release into fluid compartments such as cerebrospinal fluid (CSF) and blood, and it should be resistant to proteases so that it is not degraded prior to or following release. Several lines of reasoning suggested that one of the subunits of neurofilaments, the major structural protein complexes of axons, met these criteria. Axonal neurofilaments consist predominantly of four subunits, namely NFL, NF-M, NF-H, and α-internexin (Shaw, 1998). The NF-H protein sequence contains unusual tandemly repeated 6–8-amino-acid sequences centered on the sequence lysine-serine-proline (KSP). These KSP repeats are very numerous, up to 60 in some mammalian species, and in axonal neurofilaments, essentially all of the serine residues are phosphorylated (Strong et al., 2001). In contrast, the dendritic and perikaryal form of NF-H is not normally phosphorylated on these sites (Sternberger and Sternberger, 1983). Thus, the KSP phosphorylated form is axon specific. This phosphorylated form of NF-H (here referred to as pNF-H) is known to be more resistant to calpain and other proteases than the other neurofilament subunits (Goldstein et al., 1987; Greenwood et al., 1993; Johnson et al., 1991; Pant, 1988; Schlaepfer and Zimmerman, 1985; Shaw et al., 2004). Finally, pNF-H has long been known to be highly immunogenic, and the multiple repeated phosphorylated KSP sites are an excellent target for antibody-based assays, allowing avid multiepitope capture and detection. Taken together, these findings suggest that pNF-H might be an unusually good candidate for a biomarker of axonal injury and degeneration. We previously developed a sensitive enzyme-linked immunosorbent assay (ELISA) for pNF-H that can reliably detect pNF-H as low as 50 pg/mL or 50 ng/L. We published the characterization of this ELISA and a preliminary report on the use of this assay to detect pNF-H in the blood of a small number of rats following experimental spinal cord injury (SCI) and traumatic brain injury (TBI) (Shaw et al., 2005). No pNF-H could be detected in control rat sera, but release of pNF-H into the blood was seen following both experimental SCI and TBI. More recent studies show that this ELISA can detect pHF-H in the CSF of individuals suffering from a variety of neurological disorders (Petzold and Shaw, 2007), and in both the CSF and blood of aneurysmal subarachnoid hemorrhage patients (Lewis et al., 2008). In the present study, we present a more detailed analysis of the time course of pNF-H release following a controlled cortical impact (CCI) model of TBI in rats. We found that pNF-H was readily detected in blood after experimental TBI, and that the levels detected reflect the severity of the injury. Furthermore, the peak levels of pNF-H seen at both 24 and 48 h after injury correlate with the volume of spared cortical tissue seen in these animals. These findings suggest that blood levels of pNF-H may be used to monitor neuronal damage following cortical injury.
Methods
Head trauma
Young-adult male Sprague Dawley rats (Harlan, 200–225 g) were subjected to a mild (1.0 mm, n = 11), moderate (1.5 mm, n = 15), or severe (2.0 mm, n = 11) unilateral cortical contusion as previously described (Scheff and Sullivan, 1999; Sullivan et al., 1998, 2002). Eight rats served as crainiotomy controls and six as naive, unoperated controls. The moderate and severe cortical contusions used in these experiments results in severe behavioral deficits, significant loss of cortical tissue, blood–brain barrier (BBB) disruption, and loss of hippocampal neurons (Baldwin et al., 1996, 1997; Baldwin and Scheff, 1996; Beer et al., 2000; Scheff et al., 1997; Sullivan et al., 1998), mimicking the sequelae of human closed-head injury (CHI). In addition, the mild injury has been shown to produce behavioral deficits in the absence of obvious tissue loss (Scheff et al., 1997). All subjects were anesthetized with isoflurane (2%) and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA) prior to TBI. Following a midline incision, the skin was retracted, and a point midway between bregma and lambda and midway between the central suture and the temporalis muscle laterally was used as the central location of the 6-mm-diameter circular craniotomy that was made with a handheld Michele trephine (Miltex, York, PA). The skull cap was carefully removed without disruption of the underlying dura. Prior to the injury, the head of the animal was angled in a medial to lateral plane, so that the impacting tip was perpendicular to the exposed cortical surface. This was accomplished by rotating the entire stereotaxic frame in the transverse plane while leaving the nose bar at −5.0. The exposed brain was injured using a pneumatically controlled impacting device (TBI-0310 with soft-stop; Precision Systems & Instrumentation, Fairfax, VA) with a 5-mm beveled tip, which compressed the cortex at 3.5 m/sec for a duration of 500 msec (Baldwin and Scheff, 1996; Dixon et al., 1991; Sullivan et al., 2000). Following injury, Surgicel (Johnson and Johnson, Arlington, TX) was laid upon the dura and the skull cap replaced. A thin coat of dental acrylic was then spread over the craniotomy site and allowed to dry before the wound was stapled closed. During all surgical procedures and recovery, the core body temperature of the animals was maintained at 36–37°C using heating pads.
Blood collection
Blood was collected at 1, 6, 12, and 24 h, and 2, 3, 5, and 7 days post-injury. Approximately 250–500 μL of blood was collected in a sterile microcentrifuge tube from a tail-nick on each rat. Sterile gauze was held to the tail for 1–3 min to facilitate hemostasis. For subsequent blood collection, the clot was removed with sterile gauze, the tail gently massaged, and blood collected as above.
Analysis of cortical injury
Following the last blood collection, rats were overdosed with sodium pentobarbital (100 mg/kg) and transcardially perfused with 0.1 M phosphate-buffered saline (PBS), pH 7.4, followed by 4% paraformaldehyde (PF) in PBS. The brains were removed and postfixed in PF for 24 h at 4°C, and cryoprotected in 20% sucrose/PBS at 4°C. Brains were frozen in powdered dry ice, and serial 50-μm cryosections through the entire extent of the injury area were cut on a sliding microtome in the coronal plane and placed into PBS. Twelve equally spaced sections through the damaged area were mounted onto Fisher Superfrost Plus slides, dried on a slide warmer for 1 h, and stained with cresyl violet. Cortical tissue sparing analysis was performed as previously described (Scheff and Dhillon, 2004) to correlate with pNF-H levels at 24 and 48 h post-injury. Quantitative assessment of cortical tissue sparing employed the Cavalieri method (Michel and Cruz-Orive, 1988) to determine the volume of remaining cortical tissue both ipsilateral and contralateral to the injury. The percentage of cortical tissue spared was calculated by dividing the mean cortical volume for the injured side of the brain by the mean cortical volume for the control side of the brain (×100).
The Animal Care and Use Committees at the University of Florida and the University of Kentucky approved all animal procedures. All efforts were made to minimize both the possible suffering and number of animals used.
ELISA procedures
The ELISA used here has been described in detail previously (Shaw et al., 2005). Briefly, the assay uses a chicken capture antibody and a rabbit detection antibody, both of which have been affinity purified on bovine pNF-H immobilized on cyanogen bromide activated Sepharose 4B. Both antibodies are available commercially from EnCor Biotechnology Inc. (Gainesville, FL), and ELISA kits similar to that used here are available from EnCor (catalog no. ELISA-pNF-H) and Millipore (Billerica, MA; catalog no. NS170). 100 μL of affinity-purified chicken anti pNF-H was incubated at a final concentration of 1 μg/mL in 50 mM sodium bicarbonate buffer at pH = 9.5 to each well of 96-well Maxisorb ELISA plates (Fisher Scientific, Pittsburg, PA). Plates were incubated overnight and then blocked for 1 h with 200 μL of ELISA buffer (5% Carnation instant non-fat dry milk in Tris buffer saline/Tween [TBST, 150mM NaCl, 10mM Tris/HCl, pH = 7.5, plus 0.1% Tween 20]). Plates could be stored at 4°C for several weeks in TBS plus 5mM azide. We assay in a 50-μL total volume, making up the sample to this volume using ELISA buffer. For the present experiments, we used 25 μL of blood, which had been subjected to centrifugation at 13,000 r.p.m. to pellet out the erythrocytes. Samples were run in duplicate on the same assay plate. After a 1-h incubation with shaking at room temperature, the plate was extensively washed in TBST using a Biorad microtiter plate washer. Affinity-purified rabbit anti pNF-H was dissolved in ELISA buffer, and 100 μL was applied to each well at a final concentration of 1 μg/mL, and incubated for 1 h at room temperature with shaking. After again washing with TBST, each well was incubated with 100 μL of ELISA buffer with 1:2,000 goat anti-rabbit alkaline phosphatase (Sigma-Aldrich, St. Louis, MO). After a 1-h incubation at room temperature with shaking, the plates were washed for a final time and developed with 100 μL/well of 0.1 M glycine, 1mM Mg, 1mM Zn, at pH = 10.4, containing 1 mg/mL p-nitrophenyl phosphate (Sigma-Aldrich). After 1 h, the reaction was stopped with 50 μL/well of 2M NaOH, and results were quantified on a Tecan Spectrafluor Plus ELISA plate reader at 405-nm absorbance. Data were normalized by subtraction of the background from each determination and division of the resulting signal by the OD difference between the background and the signal from full saturation of the assay.
Quantification of protein levels in normal CNS regions
Young-adult male Sprague-Dawley rats (Harlan; 200–225 g, n = 4) were deeply anesthetized (sodium pentobarbital 100 mg/kg, IP), decapitated, and tissue samples from different regions of the CNS were quickly dissected out, weighed, frozen in liquid nitrogen, and stored at −80°C. Samples were homogenized at 10 mg/mL wet weight in the buffer described by Hashimoto et al. (1999), which consists of 4M urea, 1mM EGTA, 1mM EDTA, 0.2mM PMSF, 10mM Tris/HCl (pH = 7.2). The homogenate was centrifuged for 5 min at top speed in an Eppendorf centrifuge, and the supernatant, here described as the urea-extractable protein fraction, was used for SDS-PAGE and ELISA. The small pellet that presumably contains extracellular matrix and other insoluble proteins was discarded. The extracts were also applied to ELISA plates as serial dilutions, as described for the serum samples. Protein levels of pure preparations of pNF-H were quantified using the Pierce micro-BCA assay.
Statistical analysis
Blood data were analyzed using a repeated-measures analysis of variance (ANOVA) with injury level and time post-injury as the independent variables. Data were then probed for differences between injury levels and controls by a one-tailed Dunnett's post hoc. Data from brain regions were probed with a one-way ANOVA and a Tukey-Kramer post hoc. Spared tissue correlation with blood data were modeled with Pearson's correlation. In all cases, significance was set at p = 0.05. All data are expressed as means ± SD.
Results
The specificity of the ELISA assay used in these experiments has been previously described in detail (Petzold et al., 2007; Shaw et al., 2005). Briefly, both the capture and detection antibodies are directed against the heavily phosphorylated, axonal forms of NF-H, and the limits of detection are approximately 0.05 ng/mL pNF-H.
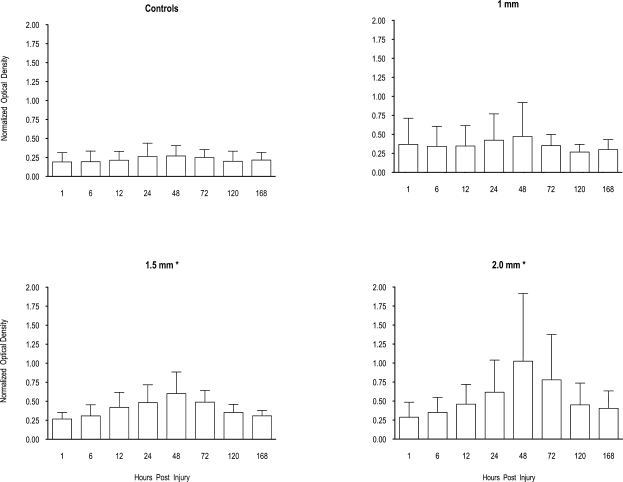
The levels of pNF-H seen in the sera of controls and rats subjected to experimental TBI are shown in Figure 1. Initial comparison of craniotomy controls and naive controls showed no significant differences between these groups; therefore, they were combined for further statistical comparisons. ANOVA showed a significant effect of injury severity (F = 5.34, df 3, 43; p < 0.003). There was also a significant effect of days post-injury (F = 12.94, df 3, 43; p < 0.001) and an injury severity by days post-injury interaction (F = 2.85, df 3, 43; p < 0.0001). In all experimental groups, NF-H expression reached a peak at 2 days post-injury and had declined to near control levels by 7 days post-injury. The moderate and severe injury groups showed significantly stronger pNF-H expression from controls. Although animals with a 1.0-mm injury showed a trend of increased levels of pNF-H, this was not statistically significant. We did notice some variability in the response of individual animals, as indicated by the relatively large variance.
FIG. 1.
Serum pNF-H levels from rats that have received three levels of traumatic brain injury (TBI). Analysis of variance (ANOVA) showed that there was as significant effect of injury level, and post hoc analysis revealed that the 1.5- and 2.0-mm groups were significantly different from controls (*p < 0.05). Data are mean ± SD.
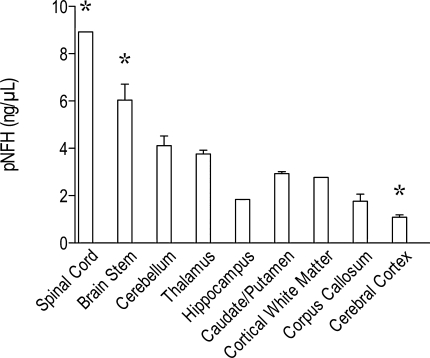
To test the hypothesis that pNF-H is found in high concentration in brain areas that have a predominance of large diameter axons, we also used the ELISA to quantify levels of pNF-H within different subregions of the brain and spinal cord (Fig. 2). Our data showed that pNF-H was highest in the spinal cord and brain stem, intermediate levels in the cerebellum, thalamus, caudate/putamen and subcortical white matter, with lowest levels in the hippocampus, cerebral cortex, and corpus callosum. When these data were subjected to a one-way ANOVA, a significant effect of brain region was seen (F = 20.78, df 8, 27; p < 0.0001). In addition, post hoc analysis showed that the spinal cord and brain stem had significantly higher levels of pNF-H than all the other brain regions, and the cerebral cortex had significantly lower levels of pNF-H than the other brain regions.
FIG. 2.
pNF-H in various regions of the brain of naive rats (n = 4). Highest levels are seen in brain regions relatively rich in large diameter axons. Analysis of variance (ANOVA) showed a significant effect of brain region (p < 0.001). Data are mean ± SD. Asterisk indicates that these brain regions were significantly different from all other brain regions (p < 0.05).
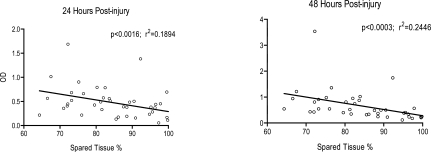
To further examine the association between serum pNF-H and injury severity, we plotted the percentage of spared cortical tissue versus pNF-H concentration at 24 and 48 h post-injury (Fig. 3). Pearson's correlation coefficient showed a strong association between the levels of pNF-H and spared cortical tissue at 24 h (F = 11.22, df 1, 48; p < 0.0016; r2 = 0.189) and at 48 h (F = 15.56, df 1, 48; p < 0.0003; r2 = 0.245).
FIG. 3.
Correlation between spared cortical tissue versus pNF-H levels at 24 and 48 h post-injury. Pearson's test showed that, at both time points, there was a significant correlation. Regression line added for illustrative purposes.
Discussion
In this study, we have demonstrated that pNF-H can be detected in the blood of rats subjected to an experimental cortical contusion. Given that neurofilaments are located exclusively in neurons, their presence in blood is indicative of the damage and/or death of neurons following brain injury. In addition, the ELISA used in this study exclusively detects the heavily phosphorylated axonal form of this neuron-specific protein. Since blood collection is faster and safer than collection of CSF, and the ELISA assay is rapid and fairly simple, this suggests that analysis of blood for pNF-H could prove to be a useful research and clinical tool to conveniently assess axonal damage. Recent data have emphasized that the unusual morphology of vertebrate neurons renders the axon unusually sensitive to mechanical damage and metabolic perturbations. Finally, a major portion of the pathophysiology following TBI is due to axonal damage (Buki and Povlishock, 2006). Therefore, a convenient blood assay of axonal loss could be of great utility.
The level of serum pNF-H we detected was correlated with the severity of the brain injury. The peak of pNF-H was seen at 2 days post-injury in all groups, with the severe injury group (2.0 mm) showing the highest amounts of pNF-H. Previous studies using a similar injury model reported that a mild TBI injury without extensive neuronal loss resulted in significant behavioral changes (Scheff et al., 1997). The present assay appears capable of detecting moderate and severe axonal injury in the cerebral cortex. It will be of interest to compare the results obtained here on rats with the results of cortical injury on larger mammals and humans. Larger mammals have much thicker and usually folded cortices containing a greater proportion of white matter that is expected to be rich in pNF-H containing axons. In fact, about 42% of the human neocortex is white matter compared to less than 10% in the rat and other small lissencephalic mammals (Bush and Allman, 2003; Frahm et al., 1982). Our preliminary studies indicate that experimental brain injury in pigs and humans suffering from TBI show more robust blood pNF-H signals than detected here (in preparation). It is also possible that more sensitive assays than those utilized in the present study may show better differentiation between craniotomy controls and animals with a mild injury. The sensitivity of the current assay is about 50 pg/mL, which is close to the levels of pNF-H detected in many of the samples here. We are currently developing pNF-H assays of improved sensitivity that may address this issue.
Several other proteins have been proposed as potential biomarkers in TBI (Ingebrigtsen and Romner, 2002; Ingebrigtsen and Romner, 2003). For example, the small Ca2+-binding modulator protein S-100β has been extensively studied in both animal models of TBI and human TBI victims (Ingebrigtsen and Romner, 2002, 2003). While S-100β has been shown to be elevated following TBI in humans and rats, its usefulness as a specific marker of brain injury has been debated since it appears to be elevated in humans with other forms of trauma (Anderson et al., 2001). In addition, S-100β levels have been shown to be elevated following femoral fractures in rats without any accompanying brain injury (Pelinka et al., 2003) and is elevated in human marathon runners without clinical or laboratory signs of brain damage (Hasselblatt et al., 2004). Furthermore, in a rat CCI study, S-100β was elevated for only 24 h post-injury and levels did not differ based on severity of the injury (Rothoerl et al., 2000). Previous studies have also examined serum levels of c-tau as a marker of TBI in both humans (Shaw et al., 2002) and rats (Gabbita et al., 2005). However, the level of c-tau in the serum of injured animals is significantly less than pNF-H. Furthermore, tau is not neuronal specific; it is expressed in astroglia (Togo and Dickson, 2002) and in non-neuronal tissues such as heart, skeletal muscle, lung, kidney, and testis (Gu et al., 1996). Further studies of pNF-H will be required before we understand if similar problems will occur with the use of this protein as a biomarker. However, as noted above, pNF-H is located solely in axons, and axonal loss is a major problem in many kinds of human neurological damage and disease states, such as TBI, multiple sclerosis, and amyotrophic lateral sclerosis (Buki and Povlishock, 2006; Stys, 2005).
The CCI model of TBI preferentially damages the cerebral cortex, which consists of neuronal perikarya and dendrites, mixed glial populations, endothelia, and local dispersed axons. Lesser injury occurs to regions adjacent to the impact site and even less to the contralateral cortex. The ipsilateral hippocampus is mildly damaged, and some injury of the contralateral cortex may be detected. The subcortical white matter is also likely injured in a more variable manner in this type of injury, and as shown here, this region is relatively richer in pNF-H when compared to the cerebral cortex. It is also possible that the CCI model used in this study variably disrupts the BBB and therefore release of pNF-H into the blood following trauma is differentially affected. Previous studies have shown that, following a 2.0-mm CCI, the BBB at the injury site opens in a biphasic manner with two distinct peaks occurring at the injury site early (5 m to 6 h post-contusion) and a later phase (1–3 days post-contusion) (Baldwin and Scheff, 1996; Baskaya et al., 1997). However, only a transient and more moderate opening is seen in the perifocal zone (Stroop et al., 1998). Interestingly, the BBB is significantly disrupted as early as 5 min and as late as 7 days post-injury when compared with controls. Thus, the rise, peak, and decline of blood pNF-H seen in the present study are within the time frame of BBB disruption reported previously. We noticed the same basic profile of pNF-H release following milder 1.5-mm and 1.0-mm CCI, though with lower amounts of pNF-H released. These signals showed that the BBB must be significantly and reproducibly perturbed even by these mild injuries. Clearly, it would be of interest to directly correlate the pNF-H blood signal with the degree of BBB disruption experienced by each individual animal. It is also likely that the animals with the highest blood pNF-H signals would have the poorest recoveries. Future experiments will address these interesting issues.
In summary, we have demonstrated that pNF-H is elevated in the blood of animals subjected to a CCI model of TBI and described time-dependent changes in the detectable levels of pNF-H. The levels of pNF-H detected correspond with the severity of the injury and the amount of cortical damage. Recent data (Petzold and Shaw, 2007) show that the assay used here will detect pNF-H in human CSF samples, with the highest levels in patients with acute neurological disorders such as stroke and brain tumors. We also studied blood and CSF of a cohort of aneurismal subarchnoid hemorrhage patients, and detected several correlations between blood and CSF levels of pNF-H and patient outcome (Lewis et al., 2008). We are currently developing assays that can provide data on blood levels of pHF-H in a much more rapid manner than the current ELISA. With such refinements, pNF-H determinations may eventually be useful in a clinical setting to determine levels of brain injury in trauma victims and to provide convenient diagnostic and prognostic information about a variety of other CNS damage and disease states.
Acknowledgments
We would like to thank Chris Horrell for his technical assistance. This study was supported by the Kentucky Spinal Cord and Head Injury Research Trust (project no. 5A), the Spinal Cord Research Foundation, the Department of Veteran Affairs, and the NIH (grants NS39828 and AG21981).
Author Disclosure Statement
Gerry Shaw holds equity in EnCor Biotechnology Inc., a company commercializing the ELISA used in this paper, and may benefit by receiving royalties or equity growth.
References
- Anderson R.E. Hansson L.O. Nilsson O. Dijlai-Merzoug R. Settergren G. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001;48:1255–1260. doi: 10.1097/00006123-200106000-00012. [DOI] [PubMed] [Google Scholar]
- Baldwin S.A. Gibson T. Callihan C.T. Sullivan P.G. Palmer E. Scheff S.W. Neuronal cell loss in the CA3 subfield of the hippocampus following cortical contusion utilizing the optical disector method for cell counting. J. Neurotrauma. 1997;14:385–398. doi: 10.1089/neu.1997.14.385. [DOI] [PubMed] [Google Scholar]
- Baldwin S.A. Scheff S.W. Intermediate filament change in astrocytes following mild cortical contusion. Glia. 1996;16:266–275. doi: 10.1002/(SICI)1098-1136(199603)16:3<266::AID-GLIA9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Baskaya M.K. Rao A.M. Dogan A. Donaldson D. Dempsey R.J. The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci. Lett. 1997;226:33–36. doi: 10.1016/s0304-3940(97)00239-5. [DOI] [PubMed] [Google Scholar]
- Beer R. Franz G. Srinivasan A. Hayes R.L. Pike B.R. Newcomb J.K. Zhao X. Schmutzhard E. Poewe W. Kampfl Et A. Temporal profile and cell subtype distribution of activated caspase-3 following experimental traumatic brain injury. J. Neurochem. 2000;75:1264–1273. doi: 10.1046/j.1471-4159.2000.0751264.x. [DOI] [PubMed] [Google Scholar]
- Buki A. Povlishock J.T. All roads lead to disconnection?–Traumatic axonal injury revisited. Acta Neurochir. (Wien) 2006;148:181–193. doi: 10.1007/s00701-005-0674-4. [DOI] [PubMed] [Google Scholar]
- Bush E.C. Allman J.M. The scaling of white matter to gray matter in cerebellum and neocortex. Brain Behav. Evol. 2003;61:1–5. doi: 10.1159/000068880. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Clifton G.L. Lighthall J.W. Yaghmai A.A. Hayes R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Frahm H.D. Stephan H. Stephan M. Comparison of brain structure volumes in Insectivora and Primates. I. Neocortex. J. Hirnforsch. 1982;23:375–389. [PubMed] [Google Scholar]
- Gabbita S.P. Scheff S.W. Menard R.M. Roberts K. Fugaccia I. Zemlan F.P. Cleaved-tau: a biomarker of neuronal damage after traumatic brain injury. J. Neurotrauma. 2005;22:83–94. doi: 10.1089/neu.2005.22.83. [DOI] [PubMed] [Google Scholar]
- Goldstein M.E. Sternberger N.H. Sternberger L.A. Phosphorylation protects neurofilaments against proteolysis. J. Neuroimmunol. 1987;14:149–160. doi: 10.1016/0165-5728(87)90049-x. [DOI] [PubMed] [Google Scholar]
- Greenwood J.A. Troncoso J.C. Costello A.C. Johnson G.V. Phosphorylation modulates calpain-mediated proteolysis and calmodulin binding of the 200–kDa and 160–kDa neurofilament proteins. J. Neurochem. 1993;61:191–199. doi: 10.1111/j.1471-4159.1993.tb03555.x. [DOI] [PubMed] [Google Scholar]
- Gu Y. Oyama F. Ihara Y. Tau is widely expressed in rat tissues. J. Neurochem. 1996;67:1235–1244. doi: 10.1046/j.1471-4159.1996.67031235.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto R. Nakamura Y. Tsujio I. Tanimukai H. Kudo T. Takeda M. Quantitative analysis of neurofilament proteins in Alzheimer brain by enzyme-linked immunosorbent assay system. Psychiatry Clin. Neurosci. 1999;53:587–591. doi: 10.1046/j.1440-1819.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- Hasselblatt M. Mooren F.C. Von Ahsen N. Keyvani K. Fromme A. Schwarze-Eicker K. Senner V. Paulus W. Serum S100beta increases in marathon runners reflect extracranial release rather than glial damage. Neurology. 2004;62:1634–1636. doi: 10.1212/01.wnl.0000123092.97047.b1. [DOI] [PubMed] [Google Scholar]
- Ingebrigtsen T. Romner B. Biochemical serum markers of traumatic brain injury. J. Trauma. 2002;52:798–808. doi: 10.1097/00005373-200204000-00038. [DOI] [PubMed] [Google Scholar]
- Ingebrigtsen T. Romner B. Biochemical serum markers for brain damage: a short review with emphasis on clinical utility in mild head injury. Restor. Neurol. Neurosci. 2003;21:171–176. [PubMed] [Google Scholar]
- Johnson G.V. Greenwood J.A. Costello A.C. Troncoso J.C. The regulatory role of calmodulin in the proteolysis of individual neurofilament proteins by calpain. Neurochem. Res. 1991;16:869–873. doi: 10.1007/BF00965535. [DOI] [PubMed] [Google Scholar]
- Lewis S.B. Wolper R.A. Miralia L. Yang C. Shaw G. Detection of phosphorylated NF-H in the cerebrospinal fluid and blood of aneurysmal subarachnoid hemorrhage patients. J. Cereb. Blood Flow Metab. 2008;28:1261–1271. doi: 10.1038/jcbfm.2008.12. [Erratum in: J. Cereb. Blood Flow Metab. 2008;28:1274.] [DOI] [PubMed] [Google Scholar]
- Pant H.C. Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem. J. 1988;256:665–668. doi: 10.1042/bj2560665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelinka L.E. Szalay L. Jafarmadar M. Schmidhammer R. Redl H. Bahrami S. Circulating S100B is increased after bilateral femur fracture without brain injury in the rat. Br. J. Anaesth. 2003;91:595–597. doi: 10.1093/bja/aeg225. [DOI] [PubMed] [Google Scholar]
- Petzold A. Keir G. Warren J. Fox N. Rossor M.N. A systematic review and meta-analysis of CSF neurofilament protein levels as biomarkers in dementia. Neurodegener. Dis. 2007;4:185–194. doi: 10.1159/000101843. [DOI] [PubMed] [Google Scholar]
- Petzold A. Shaw G. Comparison of two ELISA methods for measuring levels of the phosphorylated neurofilament heavy chain. J. Immunol. Methods. 2007;319:34–40. doi: 10.1016/j.jim.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Rothoerl R.D. Brawanski A. Woertgen C. S-100B protein serum levels after controlled cortical impact injury in the rat. Acta Neurochir. (Wien) 2000;142:199–203. doi: 10.1007/s007010050024. [DOI] [PubMed] [Google Scholar]
- Scheff S.W. Baldwin S.A. Brown R.W. Kraemer P.J. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J. Neurotrauma. 1997;14:615–627. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- Scheff S.W. Sullivan P.G. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J. Neurotrauma. 1999;16:783–792. doi: 10.1089/neu.1999.16.783. [DOI] [PubMed] [Google Scholar]
- Schlaepfer W.W. Zimmerman U.J. Calcium-activated proteolysis of intermediate filaments. Ann. NY Acad. Sci. 1985;455:552–562. doi: 10.1111/j.1749-6632.1985.tb50435.x. [DOI] [PubMed] [Google Scholar]
- Shaw G. Neurofilaments. Springer-Verlag; New York: 1998. [Google Scholar]
- Shaw G. Yang C. Ellis R. Anderson K. Mickle J. Scheff S. Pike B. Anderson D. Howland D. Hyperphosphorylated neurofilament NF-H is a serum biomarker of axonal injury. Biochem. Biophys. Res. Commun. 2005;336:1268–1277. doi: 10.1016/j.bbrc.2005.08.252. [DOI] [PubMed] [Google Scholar]
- Shaw G. Yang C. Zhang L. Cook P. Pike B. Hill W.D. Characterization of the bovine neurofilament NF-M protein and cDNA sequence, and identification of in vitro and in vivo calpain cleavage sites. Biochem. Biophys. Res. Commun. 2004;325:619–625. doi: 10.1016/j.bbrc.2004.09.223. [DOI] [PubMed] [Google Scholar]
- Shaw G.J. Jauch E.C. Zemlan F.P. Serum cleaved tau protein levels and clinical outcome in adult patients with closed head injury. Ann. Emerg. Med. 2002;39:254–257. doi: 10.1067/mem.2002.121214. [DOI] [PubMed] [Google Scholar]
- Sternberger L.A. Sternberger N.H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc. Natl. Acad. Sci. USA. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong M.J. Strong W.L. Jaffe H. Traggert B. Sopper M.M. Pant H.C. Phosphorylation state of the native high-molecular-weight neurofilament subunit protein from cervical spinal cord in sporadic amyotrophic lateral sclerosis. J. Neurochem. 2001;76:1315–1325. doi: 10.1046/j.1471-4159.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- Stroop R. Thomale U.W. Pauser S. Bernarding J. Vollmann W. Wolf K.J. Lanksch W.R. Unterberg A.W. Magnetic resonance imaging studies with cluster algorithm for characterization of brain edema after controlled cortical impact injury (CCII) Acta Neurochir. Suppl. 1998;71:303–305. doi: 10.1007/978-3-7091-6475-4_88. [DOI] [PubMed] [Google Scholar]
- Stys P.K. General mechanisms of axonal damage and its prevention. J. Neurol. Sci. 2005;233:3–13. doi: 10.1016/j.jns.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Keller J.N. Bussen W.L. Scheff S.W. Cytochrome c release and caspase activation after traumatic brain injury. Brain Res. 2002;949:88–96. doi: 10.1016/s0006-8993(02)02968-2. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Keller J.N. Mattson M.P. Scheff S.W. Traumatic brain injury alters synaptic homeostasis: implications for impaired mitochondrial and transport function. J. Neurotrauma. 1998;15:789–798. doi: 10.1089/neu.1998.15.789. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Rabchevsky A.G. Hicks R.R. Gibson T.R. Fletcher-Turner A. Scheff S.W. Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience. 2000;101:289–295. doi: 10.1016/s0306-4522(00)00380-8. [DOI] [PubMed] [Google Scholar]
- Togo T. Dickson D.W. Tau accumulation in astrocytes in progressive supranuclear palsy is a degenerative rather than a reactive process. Acta Neuropathol. (Berl.) 2002;104:398–402. doi: 10.1007/s00401-002-0569-x. [DOI] [PubMed] [Google Scholar]