Abstract
Introduction
Osteoblasts depend on a constant supply of prosurvival signals from their microenvironment. When trophic factors become limited by injury or disease, cells undergo apoptosis. This study establishes the regulation and function of Bim, Bak, and Bax in this response.
Materials and Methods
MBA-15.4 murine osteoblasts and primary human bone marrow stromal cells (hBMSCs) were subjected to growth factor depletion by serum starvation (1% FCS or serum withdrawal). Protein phosphorylation, activation, or expression was quantified by Western blotting and gene expression by real-time PCR. Regulation of apoptosis in response to serum depletion was determined using siRNA specific for Bim, Bak, or Bax, followed by TUNEL staining. Statistical significance was determined by one-way ANOVA after multiple experimental repeats.
Results
Serum depletion strongly induced expression of the proapoptotic protein Bim in both hBMSC and MBA-15.4 osteoblasts. Detailed analysis of the mouse line showed that both mRNA and protein levels rose from 2 h to peak between 16 and 24 h, in conjunction with activation of caspase 3 and rising levels of apoptosis. Both actinomycin D and cycloheximide prevented this increase in Bim, indicating transcriptional regulation. Serum deprivation caused immediate and sustained decreases in phosphorylation of prosurvival kinases, ERK and PKB, preceding upregulation of Bim. Pathway inhibitors, U0126 or LY294002, strongly increased both Bim mRNA and protein, confirming that both kinases regulate Bim. These inhibitors also induced osteoblast apoptosis within 24–72 h. JC-1 tracer detected mitochondrial potential disruption after serum deprivation, indicating involvement of the intrinsic pathway. Moreover, activation-associated conformational changes were detected in the channel-formers, Bax and Bak. Selective knockdown of Bim or Bak by siRNA protected osteoblasts from serum depletion-induced apoptosis by 50%, whereas knockdown of Bax alone or Bak and Bax together reduced apoptosis by 90%.
Conclusions
Our data indicate that Bim, Bak, and Bax actively mediate osteoblast apoptosis induced by trophic factor withdrawal. The complex upstream regulation of Bim may provide targets for therapeutic enhancement of osteoblast viability.
Keywords: osteoblast, Bim, Bak, Bax, apoptosis
INTRODUCTION
Development of novel bone anabolic therapies requires an understanding of the precise mechanisms controlling bone remodeling, fracture healing, and response to mechanical loading in the adult and elderly.(1,2) Growth factors are central to bone repair and regeneration and are used both directly and indirectly as anabolics. The efficacy of direct local application of growth factors in promotion of nonunion fracture healing has been well illustrated in animal models using bone morphogenetic protein-2 (BMP2), BMP7, insulin-like growth factor (IGF-I), TGF-β (particularly in combination with IGF-I), fibroblast growth factor-2 (FGF-2), platelet-derived growth factor (PDGF), thrombin, and vascular endothelial growth factor (VEGF).(3) Both BMP2 and BMP7 (OP-1) are licensed for this use in humans and are gaining favor as cost-effective accelerants of fracture healing.(3,4)
Growth factors also play an indirect role(5) in mediation of the effects of anabolic PTH [PTH(1-34) or teriparatide], which has revolutionized treatment of bone disorders with a basis in defective formation, rather than resorption, such as senile osteoporosis.(6) It is also very effective in reversing postmenopausal bone loss,(2) but it is unclear how this treatment works. There is a profound effect on bone formation without alteration in resorption, thought to be mediated in part by protection of mature osteoblasts and osteocytes from apoptosis and in part by the enhanced differentiation of osteoblasts.(2,5) An additional proposed mechanism is the secretion of paracrine growth factors by those cells in the bone microenvironment that express PTH receptors, especially mature osteoblasts and osteocytes, and circulation to the stromal precursor and bone lining compartments where enhanced osteoblast recruitment and differentiation ensues.(5)
Growth factors tapped for involvement include IGF-I, TGF β, and FGF-2, which are upregulated in the bone matrix compartment after systemic PTH administration.(5,7) Several members of the Wnt superfamily, associated with high bone mass disorders, are upregulated in vitro by PTH(8) and sclerostin, a potent inhibitor of bone formation that is secreted by the osteocytes, is strongly repressed in vivo and in vitro by PTH.(2,5) Further evidence for an indirect role for growth factors in mediating the bone anabolic effect of PTH comes from studies performed in mice deficient in IGF-I–, IGF receptor–, or insulin receptor substrate I (IRS-I)–deficient mice, all of which fail to produce an anabolic response to PTH.(9-11)
Unlike osteoclasts, which are heavily reliant on macrophage-colony stimulating factor (M-CSF) and RANKL (for their survival and differentiation),(12) osteoblasts are able to respond to a wide variety of trophic factors.(3,13) Osteoclasts, however, respond to M-CSF deprivation by upregulating Bim (Bcl2 interacting mediator of cell death), a BH3-only (Bcl2 homology 3) pro-apoptotic protein in the Bcl2 superfamily.(14-16) Bim translocates to the mitochondria, where it is thought to directly bind to and activate the channel formers, Bak (Bcl-2 homologous antagonist/killer) and Bax, by inducing conformational change.(17-19) Cytochrome c release and caspase 9 (aspartate-specific cysteine protease) activation follows, leading to caspase 3 activation and apoptotic death of the osteoclast. Bim knockout mice have large numbers of osteoclasts that survive in M-CSF–free culture for extended periods.(14) Akiyama et al.(14) reported that Bim is not expressed in fetal or adult osteoblasts and osteocytes. We have, however, recently found Bim to be upregulated in response to high-dose dexamethasone treatment in the MBA-15.4 osteoblastic cell line and also in human primary bone marrow–derived osteoblasts.(20)
The Bim knockout mouse has mild osteopetrosis, because although osteoclast numbers are greatly enhanced in the absence of Bim, these cells are less active.(14) This mouse has not yet been studied in old age or challenged with glucocorticoid overdose to establish the role of Bim in conditions dominated by osteoblastic formation deficits. However, it has recently been reported that the Bax-deficient female mouse ages better and has stronger bones in old age.(21) The bone phenotype of Bax-deficient female mice is complex, with knockout (KO) animals at maturity (7 mo) and even more in old age (20–22 mo) displaying elevated BMD in the whole body and both cortical (femoral) and trabecular (vertebral) bones, together with enhanced bone strength at both sites.(21) However, ovariectomy produced equivalent relative deficits in mature (7 mo) wildtype and KO animals in both cortical and trabecular BMD and trabecular structure, indicating that Bax deficiency does not protect against ovariectomy-induced bone loss.(21) This work shows the beneficial effect of sustained ovarian function on bone, but also highlights an ovary-independent protective effect of Bax deficiency in bone specifically.
To understand the response of osteoblasts to growth factors and the potential consequences of a reduced supply caused by trauma or disease, it would be helpful to model this in vitro. In this study, we examined the roles of Bim, Bak, and Bax in regulation of osteoblast survival and established the major regulatory mechanisms.
MATERIALS AND METHODS
Materials
All chemicals and reagents were obtained from Sigma (Poole, UK) except where otherwise stated.
Cell culture
Mouse bone marrow stromal cells (BMSCs) MBA-15.4 were a kind gift from Dr D Benayahu (Tel Aviv University, Tel Aviv, Israel). This well-characterized osteoblastic cell line expresses osteoblastic markers such as alkaline phosphatase and osteocalcin, undergoes progressive differentiation in vitro when treated with osteogenic supplements, and forms bone in vivo when transplanted under the kidney capsule in mice.(22,23) MBA 15.4 cells were grown in bicarbonate-buffered DMEM (Cambrex, Wokingham, UK) with 10% heat-inactivated FCS (Biosera, Ringmer, UK), 100 U/ml penicillin, and 100 mg/ml streptomycin. For serum starvation experiments, the cells were cultured in 1% FCS-containing medium or serum-free medium for the time indicated. Where inhibitors were used, the cells were treated with cycloheximide, actinomycin D, MG132, U0126, or LY294002 (Calbiochem, San, Diego, CA, USA) or the appropriate vehicle for the time indicated. Human primary bone marrow stromal cells (hBMSCs) were obtained from hematologically normal patients undergoing routine total hip replacement and were isolated as previously described.(24) The cells were culture in αMEM with 10% FCS and used at passage 1. Human tissue was obtained in compliance with COREC and HTA ethical procedures.
Apoptosis determination
An in situ cell death detection kit (Roche Applied Science, Penzberg, Germany) was used to perform the TUNEL reaction to identify apoptotic cells with fragmented DNA, according to the manufacturer’s instructions. 4′-6-diamidino-2-phenylindole (DAPI) was used to visualize nuclei. The cells were observed and photographed using an Olympus BX40 microscope and Olympus DP70 camera. TUNEL+ and DAPI+ cells were counted manually using cell^F program (Olympus, Soft Imaging System, Münster, Germany), and 20–30 fields were taken from each slide.
JC-1 staining
JC-1 molecular probe was used to visualize the mitochondrial membrane potential change during apoptosis induced by serum starvation. JC-1 (2 μl/ml) was introduced into the living cells before visualization using an Olympus BX40 microscope with FITC filter and Olympus DP70 camera.
Western blotting
Total cell protein was extracted from floating and adherent cells. Protein concentration was measured using the BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Equal protein samples were separated on 10% or 12% SDS-PAGE gels by electrophoresis and transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA, USA). Prestained Precision Plus molecular weight markers (BioRad, Hercules, CA, USA) were used to indicate molecular weight. Rabbit polyclonal primary antibodies, anti-Bim and cleaved caspase-3 antibodies, were purchased from Calbiochem (San Diego, CA, USA); anti-Bax, phospho-ERK (extracellular signal regulated kinase), phospho-PKB (protein kinase B), and PKB antibodies were from Cell Signaling Technologies (Beverley, MA, USA); anti-ERK 2 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-Bak antibody was from Upstate (Dundee, UK); and anti-GAPDH antibody was from Abcam (Cambridge, UK). Secondary antibody was goat anti-rabbit horseradish–peroxidase conjugated antibody (Abcam). Immunocomplexes were detected using enhanced chemiluminescence detection system or Supersignal West Dura extended duration substrate (Pierce Biotechnology, Rockford), and membranes were exposed to CL-Xposure film (Pierce Biotechnology). Image J 1.36b (NIH Freeware) was used to measure the density of bands of interest. GAPDH served as internal control.
Immunoprecipitation
Cells (2 × 10-cm plates for each sample) were lysed in 1% CHAPS-containing buffer (10 mM HEPES, 150 mM NaCl, 1% CHAPS, 1 mM EDTA, 1 mM PMSF) to maintain Bax and Bak in their native conformation.(25) Cells lysed in 1% Triton X-100–containing buffer to mimic the conformational change of Bax and Bak that occurs during apoptosis were used as positive control. The cell lysate was immuno-precipitated with active form-specific anti-Bax or Bak antibodies (anti-Bax-NT and anti-Bak-NT antibodies; Upstate) overnight at 4°C followed by Western blotting analysis using pan Bak (Upstate) or Bax (Cell Signaling Technologies) antibodies for detection. Total Bak or Bax was used as an internal control.
Immunocytochemistry for active Bax
Cells grown on coverglasses were subjected to serum deprivation and fixed in fresh 2% paraformaldehyde and permeabilized with 100% methanol for 1 min, followed by blocking of both endogenous peroxidases and nonspecific antibody binding. Cells were incubated with anti-active Bax antibody for 1 h at room temperature, followed by detection with the Vectastain Universal Elite ABC kit (Vector Laboratories) and metal DAB (Roche Applied Science). DAPI staining was used to visualize nuclei. Images were acquired using an Olympus BX40 microscope and Olympus DP70 camera.
Real-time quantitative PCR
Total mRNA was isolated from cells using RNeasy Mini kits (Qiagen, Hilden, Germany), according to the manufacturer’s protocol. RNase-Free DNase Set (Qiagen) was used to remove genomic DNA contamination. Total RNA (0.3 μg) in a 20-μl reaction volume was reverse-transcribed using SuperScript II Reverse Transcriptase (Invitrogen, Paisley, UK) in the presence of oligo-dT primer (Invitrogen). Samples without RT served as negative controls in real-time PCR reactions to exclude genomic DNA contamination. No-template control was always included to indicate there was no contamination in real-time PCR reagents. Primers were from MWG Biotech (London, UK). Mouse Bim forward primer was 5′-CGACAGTCTCAGGAGGAACC-3′ and reverse primer was 5′-CCTTCTCCATACCAGACGGA-3′. Mouse GAPDH forward primer was 5′-GGTCATCCCAGAGCTGAACG-3′ and reverse primer was 5′-TTGCTGTTGAAGTCGCAGGA-3′. Real-time quantitative PCR reactions were performed using a Corbett Rotor-Gene 3000 using QuantiTect SYBR Green PCR kit (Qiagen). Samples were run in duplicate, and the average value was used for analysis. Comparative quantitation analysis(26) (Corbett Rotor-Gene version 6.0.1) was used to compare the gene expression of Bim in treated samples relative to control cells and normalized to the housekeeping gene GAPDH. GAPDH has been described as a valid and stable control in other studies using serum deprivation.(27)
Transfection with Bim, Bak, and Bax small interfering RNA
Cells were preplated in 6-well plates in antibiotic-free medium containing 10% FCS 24 h before transfection and reached 40% confluence at the time of transfection. Optimal concentrations of small interfering RNA (siRNA) were determined in preliminary experiments, and it was found that Bim could be knocked down within 24 h, whereas Bak and Bax were more stable proteins and required 48 h after transfection (results not shown): 30 nM Bim (siBim), 20 nM Bak (siBak), and 30 nM Bax (siBax) siRNA as well as 30 nM scrambled control (siControl) siRNA (all from Ambion, Huntingdon-Cambridgeshire, UK) were introduced into the cells using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen), according to the manufacturer’s recommendations. The sense strand sequences were as follows: Bim 5′-GGUGGACAAUUGCAGCCUGtt-3′; Bak 5′-GCUUGCUCUCAUCGGAGAUtt-3′; Bax 5′-GGCCCUGUGCACUAAAGUGtt-3′. For the last 18 h of transfection, the cells were treated with serum starvation. Twenty-four hours after transfection with Bim siRNA or 48 h after transfection with Bak or Bax siRNA, protein levels of Bim, Bak, and Bax were determined in parallel to confirm specific gene knockdown using Western blotting. GAPDH was used as an internal control. TUNEL and DAPI staining were used to evaluate cell survival after transfection in the presence of 10% FCS.
Statistical analysis
Data are presented as means ± SE from three or more separate experiments, as indicated. Data were analyzed using GraphPad Prism v 4.03 (GraphPad Software). Student t-test (for single comparison) or one-way ANOVA (for multigroup comparisons) with either the Dunnett or Tukey posthoc test were used as appropriate. p < 0.05 was regarded as denoting statistical significance.
RESULTS
Mitochondrial involvement in serum depletion–induced apoptosis in osteoblasts
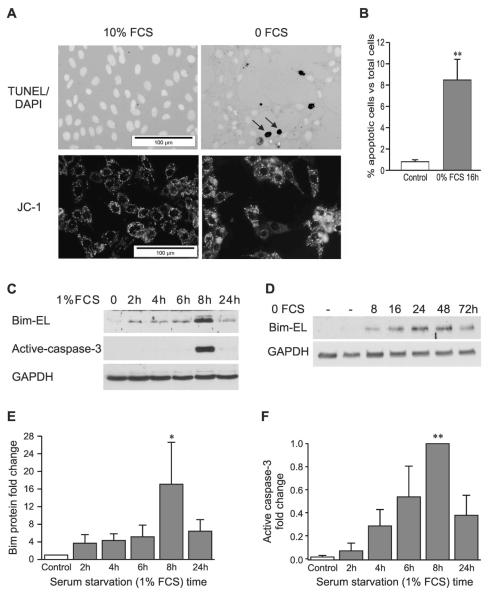
TUNEL and DAPI double-staining of cells grown on coverslips was used to evaluate apoptosis induced by serum starvation. In 10% FCS culture medium, the osteoblasts appeared healthy and well attached (Fig. 1A, top left panel). Few TUNEL+ cells were observed, and DAPI staining showed normal morphology of nuclei. Withdrawal of serum or reduction to 1% FCS triggered apoptosis within 24 h in osteoblasts, with progressive detachment and disintegration of cells. The remaining cells tended to form clumps, and an increased number of TUNEL+ cells and cell fragments were observed after serum starvation for 16 h (Fig. 1A, top right panel). The percentage of adherent apoptotic cells was increased from 1% in control cells to 8% in cells treated with serum starvation for 16 h (Fig. 1B). The total number of apoptotic cells including attached and floating cells is higher but was not quantified in this study because adherent cells provided sufficiently high numbers to discriminate effects.
FIG. 1.
Serum starvation induces apoptosis involving disruption of mitochondrial integrity, upregulation of Bim expression, and activation of caspase-3 in osteoblasts. (A) TUNEL and DAPI double staining (top panels) or JC-1 staining (bottom panels) in the presence of 10% FCS (left) or in serum depletion medium (no serum) for 16 h (right). The open arrows indicate TUNEL+ cells. (B) Percentage of TUNEL+ cells vs. total cells (mean ± SE, n = 5). (C) Representative Western blots for Bim-EL, active caspase-3, and internal control, GAPDH, after serum starvation (1% FCS) for 2, 4, 6, 8 and 24 h. (D) Representative Western blot of Bim-EL and GAPDH in normal human bone marrow stromal cells subjected to serum depletion for 8–72 h. Fold change of Bim (E) and active caspase-3 (F) in MBA-15.4 osteoblasts are presented as mean ± SE (n = 3). *p < 0.05 and **p < 0.01 vs. control.
JC-1 staining was used to confirm that serum starvation–induced apoptosis involves the mitochondrial pathway in osteoblasts. This dye is sensitive to mitochondrial membrane potential, staining mitochondria with high membrane potential orange and those with low membrane potential green. Furthermore, JC-1 is concentrated into aggregates in intact mitochondrial membrane, producing a punctate staining pattern, whereas in cells with collapsed mitochondrial membrane, JC-1 forms monomers and produces a diffuse green fluorescence. Using a FITC filter, punctate distribution of mitochondrial fluorescence was observed as intense yellow spots in unstressed cells (Fig. 1A, bottom left panel). JC-1 dye was dispersed throughout the entire cell, with a diffuse, green cytoplasmic stain (Fig. 1A, bottom right panel) in the serum-starved cells, indicating disruption of mitochondrial potential.
Serum starvation upregulates Bim protein expression and activates Bak, Bax, and caspase 3
Bim protein levels were very low in control osteoblasts (mouse and human) cultured in 10% FCS medium and increased in cells cultured with 1% FCS in a time-dependent manner, peaking between 8 and 24 h for murine MBA-15.4 (Figs. 1C and 1E) and between 8 and 48 h in primary hBMSCs (Fig. 1B). Activation of caspase 3 was detectable by Western blotting from 4 h onward in MBA-15.4 cells (Fig. 1F). Both Bim and active caspase-3 levels reduced after 24 h of treatment, by which time many of the MBA-15.4 cells had died. Similar results were obtained from cells treated with serum-free medium (0% FCS), and both Bim expression and activation of caspase-3 peaked between 8 and 16 h (data not shown).
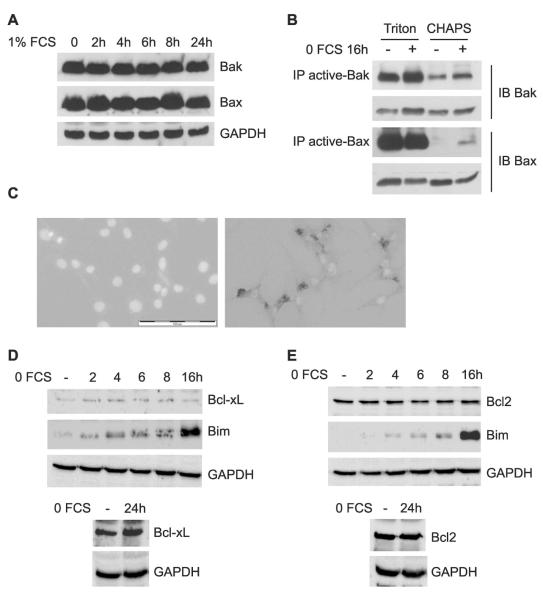
In contrast, Bak and Bax expression remained unchanged throughout 24 h of serum reduction (Fig. 2A). However, immunoprecipitation with antibodies specific for the active conformational state of Bak or Bax showed that serum depletion activated Bak slightly and Bax strongly within 16 h, although total Bak and Bax levels remained unchanged (Fig. 2B). Because SDS-PAGE eliminates protein conformation, inactive versus active state was discriminated using immunoprecipitation with anti-active state antibodies before electrophoresis and immunodetection with pan antibodies.(25) Cells were lysed using CHAPS detergent before immunoprecipitation to maintain the inactive conformation of Bak and Bax, where present. Inactive Bax, and to a lesser extent Bak, failed to immunoprecipitate from osteoblasts growing in 10% FCS because they were not recognized by the anti-active antibody. As a positive control, cells were also lysed using Triton X-100 detergent, which disrupts conformation so that Bak and Bax seem active regardless of serum treatment (Fig. 2B). To confirm the activation of Bax, immunocytochemistry was used to detect activated Bax in cells treated for 16 h with 1% FCS (Fig. 2C, right panel), with no evidence of activation in control cells (left panel).
FIG. 2.
Serum starvation induces Bak and Bax activity, but does not alter Bak, Bax, Bcl2, or Bcl-xL expression. (A) Western blots for total Bak and Bax expression, as well as internal control, GAPDH, after serum starvation (1% FCS) for the time indicated. (B) Immunoprecipitation with anti-active (conformation-dependent) Bak or Bax antibodies in 1% Triton X-100 lysis buffer as a control or in 1% CHAPS lysis buffer to maintain the native conformation of Bak or Bax after serum withdrawal. Immunodetection was performed using pan (conformation independent) Bak or Bax antibodies and total Bak or Bax served as internal controls, respectively. (C) Immunostaining with active-Bax antibody of cells in 10% FCS (left) or treated with 1% FCS for 16 h (right). Scale bar is 100 μm. Representative Western blots of Bcl-xL (D) and Bcl2 (E) in comparison with Bim and/or GAPDH in the same samples after serum starvation for 2, 4, 6, 8 and 16 h (top panels) or for 24 h (bottom panels).
Apoptosis may also be induced by a decrease in expression of anti-apoptotic proteins such as Bcl2 or Bcl-XL, which protect mitochondria from the actions of Bim, Bak, and Bax. However, serum deprivation had no effect on the levels of Bcl2 (Fig. 2D) or Bcl-xL (Fig. 2E) over the period of 24 h during which most apoptosis occurs. The same samples display progressively rising levels of Bim (Figs. 2D and 2E). Longer exposures confirmed that there was no difference at 24 h between levels of these proteins in 10% and 1% serum conditions (bottom panels).
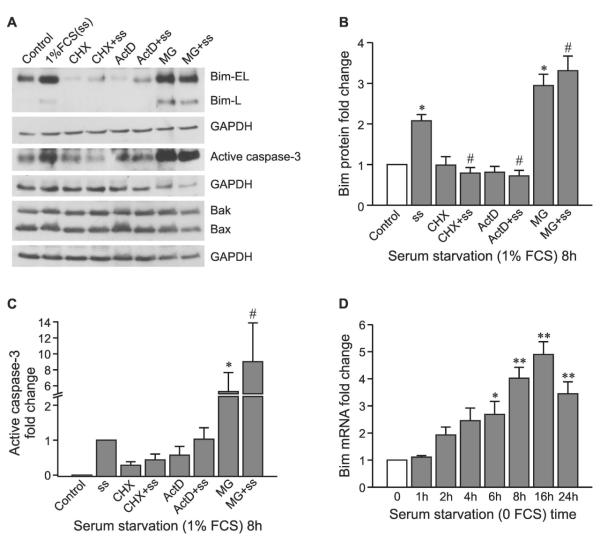
Bim levels are regulated by transcription, protein synthesis, and protein degradation in osteoblasts
Induction of Bim protein by 8 h of serum withdrawal was completely prevented by the protein translation inhibitor, cycloheximide, or the transcription inhibitor, actinomycin D (Figs. 3A and 3B). Treatment with cycloheximide or actinomycin D alone did not affect Bim expression. This indicates that the upregulation of Bim at 8 h requires rapid, de novo protein synthesis. Treating the cells with proteasome inhibitor, MG132, for 8 h results in Bim accumulation with or without serum starvation, suggesting that Bim is a rapid turnover protein in osteoblasts and that degradation is through the ubiquitin-proteasome pathway. Total Bak and Bax expression was unchanged by any of these inhibitors (Fig. 3A), further confirming that Bak and Bax are stable proteins in osteoblasts. Serum withdrawal induced caspase-3 activity, and this effect was blocked by co-treatment with cycloheximide but not actinomycin D (Fig. 3C). Actinomycin D has also been reported to increase caspase-3 activity in Jurkat cells, with 10 μM, the concentration used in our study, inducing maximum caspase activity.(28) Caspase 3 activity increased strongly in the presence of MG132, in keeping with the strong accumulation of Bim (Fig. 3C), although it should be noted that activated caspase 3 is itself subject to proteasomal degradation.(29) To confirm that Bim is regulated at the transcriptional level by serum deprivation, real-time RT-PCR was performed and showed that Bim mRNA increased after serum depletion in a time-dependent manner and reached a peak between 6 and 24 h (Fig. 3D), in agreement with the protein data.
FIG. 3.
Serum withdrawal regulates Bim levels through gene transcription, protein synthesis, and protein degradation. (A) Representative Western blots for Bim (Bim-EL and L), active caspase-3, Bak, and Bax in the presence of cycloheximide (CHX), actinomycin D (ActD) or MG132 with or without serum starvation (ss; 1% FCS) for 8 h. Fold change of Bim (B) and active caspase-3 (C) are presented as mean ± SE (n = 4). (D) Real-time quantitative PCR was used to compare the relative gene expression of Bim after serum starvation (0 FCS) for the time indicated and normalised to the housekeeping gene, GAPDH (mean ± SE, between three and five experimental repeats). *p < 0.05 and **p < 0.01 vs. control; #p < 0.05 vs. serum starvation alone.
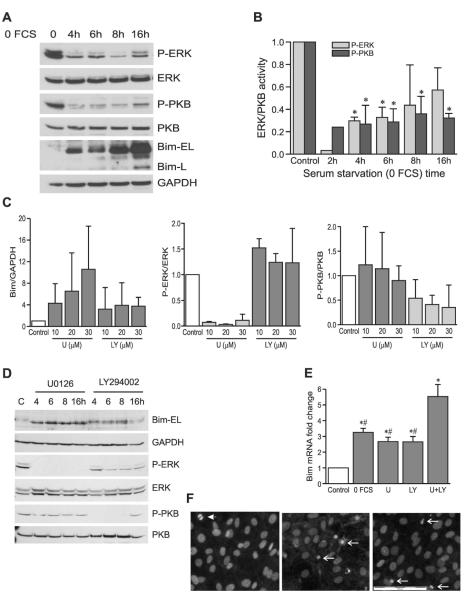
Bim mRNA and protein levels are regulated by prosurvival kinases, ERK and PKB
It has been reported that prosurvival kinases ERK and/or PKB can regulate Bim expression either by targeting protein for ubiquitin-mediated degradation or by repressing gene transcription in certain cells.(14,30-33) Both PKB and ERK became rapidly dephosphorylated after serum starvation in osteoblasts, preceding Bim upregulation (Figs. 4A and 4B).To establish which of these kinases regulate Bim in osteoblasts, the MAPK/ERK kinase (MEK)-ERK inhibitor, U0126, or the phosphatidylinositol-3-kinase (PI3K)-PKB inhibitor, LY294002, was used (Figs. 4C and 4D). After 24 h, U0126 fully inhibited ERK activity across a range of concentrations without affecting PKB activity (Figs. 4C, middle panel, and 4D), whereas LY294002 dose-dependently blocked PKB activity and had no effect on ERK activity (Figs. 4C, right panel, and 4D). Both inhibitors strongly induced Bim protein expression (Figs. 4C, left panel, and 4D), and active, phosphorylated PKB and ERK levels also decreased within 4 h of serum starvation. Moreover, inhibition of either active PKB or ERK activity for 16 h increased Bim mRNA 3-fold (Fig. 4E) and inhibition of both kinases together caused a 5-fold increase in Bim mRNA, indicating that both of these kinases can repress Bim gene transcription in osteoblasts.
FIG. 4.
Prosurvival kinases, ERK and PKB, regulate Bim protein and gene expression. (A) Representative Western blots for phosphorylated-ERK (P-ERK), phosphorylated-PKB (P-PKB), and Bim-EL and L after serum depletion for the time indicated. Blots are from the same set of samples, stripped and reprobed for total ERK, PKB, and GAPDH, respectively. (B) Relative expression of phosphorylated-ERK and PKB compared with total ERK and PKB, respectively. Data are presented as mean ± SE (n = 3 except 2-h data). *p < 0.05 vs. control. (C) Expression of Bim (left), P-ERK (middle), and P-PKB (right) in the presence of U0126 or LY294002 (10, 20, and 30 μM, respectively) for 24 h (mean ± SE, n = 3). (D) Western blots for P-ERK, P-PKB, and Bim-EL in the presence of 20 μM U0126 or 20 μM LY294002 for 4, 6, 8, and 16 h. Blots are from the same set of samples, stripped and reprobed for total ERK, PKB, and GAPDH, respectively. (E) Real-time quantitative PCR shows the relative gene expression of Bim after treatment with 20 μM U0126 (U) and/or 20 μM LY294002 (LY) for 16 h under normal (10%) serum conditions. Serum starvation sample served as a positive control, and data are presented as fold change (mean ± SE, n = 4). *p < 0.05 vs. control and ##p < 0.01 vs. U + LY. (F) DAPI staining of cells grown in 10% medium alone (left) or with addition of 20 μM LY294002 (middle) or 20 μM U0126 (right) for 72 h. Arrowhead indicates mitotic body; arrows indicate apoptotic nuclear morphology. Scale bar is 100 μm.
To confirm that inhibition of these prosurvival signaling pathways negatively affected cell viability, DAPI staining was used to evaluate apoptosis after inhibition of PI3K-PKB activity by 20 μM LY294002 and of ERK by 20 μMof U0126 for 24, 48, and 72 h. LY294002 induced increasing levels of apoptosis from 24 to 72 h (shown for 72 h; Fig. 4F, center panel), whereas U0126 induced overt apoptosis within 48 h, increasing even more by 72 h (shown for 72 h; Fig. 4F, right panel).
siRNA transfection specifically knocked down Bim, Bak, and Bax gene expression, resulting in the protection of osteoblasts from apoptosis induced by serum depletion
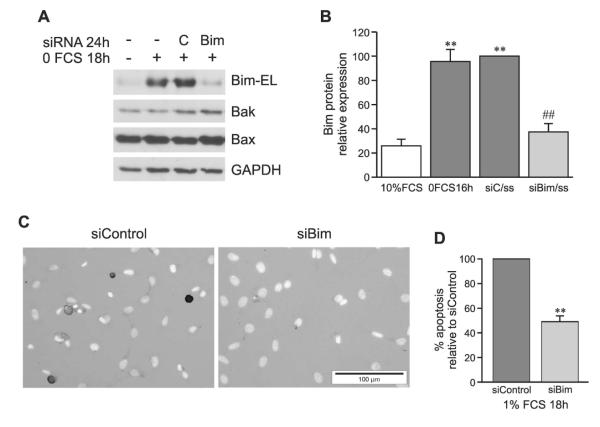
To confirm that Bim, Bak, and Bax play a causal role in induction of osteoblast apoptosis, siRNA transfection techniques were optimized to achieve specific, nontoxic knockdown of protein expression. Knockdown of Bak and Bax was optimized and tested under both serum replete and serum reduced conditions, whereas Bim could only be knocked down once induced by serum reduction. Silencing of Bim had no effect on expression of Bak or Bax (shown for low serum conditions in Fig. 5A), and silencing of Bak or Bax or both Bak and Bax together had no effect on Bim expression (shown for low serum conditions in Fig. 6A). Western blotting was used to evaluate gene knockdown efficiency after siRNA transfection. Serum depletion, either 0% (Figs. 5A and 5B) or 1% (Fig. 1C), upregulated Bim and transfection with scrambled control siRNA was unable to block this. However, transfection with Bim siRNA decreased Bim protein expression by 65%, which effectively returned Bim to basal levels (Figs. 5A and 5B). Bim siRNA did not affect the expression of other Bcl2 family members, Bak and Bax, or the house keeping protein, GAPDH, indicating specific knockdown. TUNEL and DAPI double staining showed that knockdown of Bim expression inhibited apoptosis induced by serum starvation of osteoblasts by 50% (Figs. 5C and 5D).
FIG. 5.
siRNA specifically knocks down Bim gene expression and inhibits apoptosis in osteoblasts. (A) 24 h after transfection with Bim (siBim) or control (siC) siRNA and after 18 h of serum starvation, Bim-EL, Bak, and Bax protein levels were determined by Western blotting. (B) Bim expression relative to control siRNA (siC) transfection (mean ± SE, n = 4). (C) TUNEL and DAPI double-staining 24 h after transfection with control (left) or Bim (right) siRNA in the presence of serum starvation (1% FCS) for the last 18 h. (D) Percentage of apoptotic cells vs. total cells, normalized to siC treatment (mean ± SE, n = 3). **p < 0.01 vs. control; ##p < 0.01 vs. siControl + serum starvation.
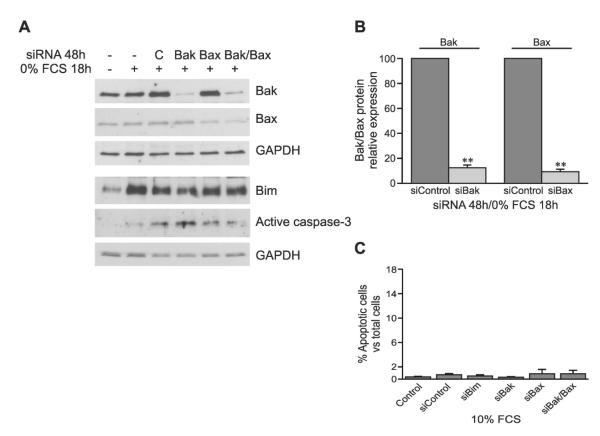
FIG. 6.
Transfection with siRNA specifically knocks down Bak or Bax gene expression without affecting cell survival under normal serum (10%) conditions. (A) 48 h after siRNA transfection with Bak/Bax in the presence of serum starvation for the last 18 h, Bak, Bax, Bim-EL, and active caspase-3 were detected by Western blotting. (B) Relative Bak and Bax expression compared with control siRNA (siControl) transfection (mean ± SE, n = 4–5). (C) TUNEL and DAPI double-staining to show the percentage of apoptotic cells vs. total cells (mean ± SE, n = 3) 48 h after siRNA transfection in the presence of 10% FCS. **p < 0.01 vs. siControl.
Bak and Bax are widely described to mediate mitochondrial apoptotic response downstream of Bim. Transfection with Bak or Bax siRNA alone specifically knocked down Bak or Bax expression, respectively, by 90% compared with control siRNA transfection, without changing Bim and GAPDH expression (Figs. 6A and 6B). Double knockdown of Bak and Bax together was also achieved without effect on Bim or GAPDH. Moreover, activity of caspase-3 decreased after knockdown of Bax or Bak and Bax together (Fig. 6A).
TUNEL and DAPI double staining was used to evaluate the effect of transfection alone on the viability of cells. In 10% FCS medium, the apoptotic ratio in cells transfected with scrambled control, Bim, Bak, and/or Bax was similar to that in control cells without transfection (Fig. 6C), indicating that the transfection process did not affect cell survival.
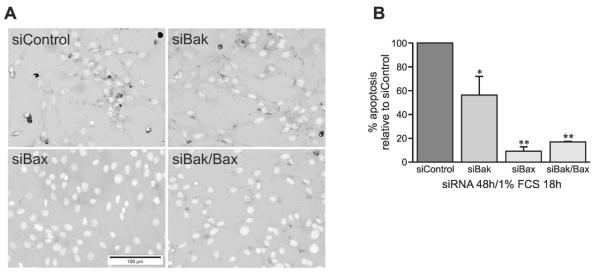
Double staining also showed that knockdown of Bak expression alone decreased serum deprivation induced osteo-blast apoptosis by 45% (Figs. 7A and 7B). Knockdown of Bax alone or double knockdown of Bak and Bax strongly inhibited apoptosis by 85–90% (Figs. 7A and 7B). Taken together, this indicates that Bim, Bak, and Bax play a dominant role in mediation of growth factor deprivation–induced apoptosis in osteoblasts.
FIG. 7.
Knock down of Bak and/or Bax gene expression inhibits apoptosis in osteoblasts. (A) TUNEL and DAPI double-staining 48 h after transfection with control (top left panel), Bak (top right panel), Bax (bottom left panel), or Bak plus Bax (bottom right panel) siRNA in the presence of serum starvation (1% FCS) for the last 18 h. (B) Percentage of apoptotic cells vs. total cells, normalized to siC treatment (mean ± SE, n = 3). *p < 0.05 and **p < 0.01 vs. siControl.
DISCUSSION
We found in this study that knockdown of Bim alone is able to partially protect osteoblasts against apoptosis induced by withdrawal of trophic factors and that knockdown of the mitochondrial effectors of Bim signaling, Bak and Bax, is strongly protective. This, together with the recent report of improved bone aging in the Bax knockout mouse,(21) highlights this pathway as a relevant mechanism in control of osteoblast survival and a potential therapeutic target.
Bim has not been regarded as a regulator of osteoblast function because it has been reported in the bone literature to be restricted in expression to osteoclasts.(14) Our group has been the first to report expression and regulation of Bim in both osteoblastic cell lines and primary osteoblasts under pro-apoptotic conditions such as high-dose glucocorticoid treatment.(20) Knockdown of Bim or Bax protein expression also reduced glucocorticoid-induced apoptosis in MBA-15.4 osteoblasts.(20) This study provides novel mechanistic insight into the function and regulation of Bim in both human BMSCs and mouse MBA-15.4 osteoblasts in response to serum withdrawal, with more rapid kinetics than after glucocorticoid treatment and with higher levels of apoptosis. Furthermore, two of the key regulatory kinases and the downstream regulatory pathways are identified. The anti-apoptotic proteins Bcl2 and Bcl-xL maintain stable levels during 24 h of serum reduction when the peak of apoptosis occurs, suggesting that their depletion is not an additional factor in this process.
To target Bim or Bax, a clear understanding is needed of upstream regulatory control pathways in osteoblasts. Osteoblasts receive survival stimuli from a variety of growth factors, unlike osteoclasts, which rely heavily on M-CSF and die when this specific factor is not added to the culture.(14) Several other tissues, including neurons, HEK 293, T lymphocytes, and brown fat cells show multifactor dependence, and serum deprivation has commonly been used to model their response to trophic factor depletion.(34-37) Osteoblasts die in response to reduction or removal of serum. The MBA-15.4 mouse osteoblast cell line displays accumulation of Bim within 2 h of serum removal. This is followed by activation of Bax by conformational change, loss of mitochondrial potential, and activation of caspase 3, followed by morphological changes consistent with apoptosis and DNA fragmentation that becomes readily detectable within 16 h. RNA interference at the level of Bim, Bak, or Bax is protective, with Bax knockdown most effective. The 50% protection conferred by Bim knockdown may indicate involvement of another BH3-only family member, such as Bad, Bmf, or Puma.(15,35,38) However, our siBim sequence based on those published(39,40) did not produce a knockdown below basal levels of expression or sustained beyond 24 h, in contrast to the Bax siRNA, which was 90% protective. A more effective siBim sequence would be helpful in clarifying this, as would use of viral vectors capable of delivering sustained knockdown.
We report that serum deprivation induces de novo expression of Bim mRNA and protein, because inhibitors of transcription and translation were equally effective in preventing Bim accumulation after serum deprivation, and real-time PCR detected rapidly increasing Bim mRNA levels within 4 h of serum depletion. As in all cell types described to date, Bim is also degraded by the proteasome in osteoblasts and accumulates strongly in response to proteasome inhibitor, indicating that it is a high turnover protein, constantly synthesized and degraded provided prosurvival signaling remains strong. After serum depletion of osteoblasts, activity of two of the major prosurvival kinases, ERK and PKB, drops off. This kinase inactivation precedes upregulation of Bim, and we provide evidence that both of these kinases directly regulate Bim levels, because inhibitors of MEK and PI3 kinase, upstream of ERK and PKB, respectively, also induce accumulation of Bim protein. It has been found in different cell types that these kinases phosphorylate and target Bim protein for ubiquitin-mediated proteasomal degradation(30,37,39,41) but may also act to repress Bim transcription by inhibiting activity of the forkhead transcription factors that drive Bim expression.(32,35,42) We find that inhibition of either ERK or PKB results in increased Bim mRNA synthesis, suggesting that, in osteoblasts, both kinases are capable of repressing transcription. The increase in Bim protein seen with use of either inhibitor may be entirely due to this de novo expression or to the combined effect of increased transcription with decreased proteasomal degradation. The importance of these kinases as prosurvival mediators is highlighted by the induction of apoptosis after sustained treatment with inhibitors of either pathway. However, given the slower kinetics of apoptosis induction by these inhibitors in comparison with serum depletion, it seems likely that other kinases may also be involved in the osteoblast apoptotic response to trophic factor depletion.
Bim can be transiently repressed in cultured cells by the addition of insulin or IGF-I and -II,(34,36) NGF,(43) or bFGF.(37) This might be achieved in vivo using local application of growth factors, and in fact, this mechanism may partly explain the positive acceleration of bone fracture healing seen with local application of growth factors such as BMP2, BMP7, IGF-I, TGF-β, FGF-2, PDGF, thrombin, and VEGF,(3) all of which can activate ERK and may also activate PKB. In addition, anabolic PTH therapy is strongly anti-apoptotic in both osteoblasts and osteocytes,(2,5,44) although the involvement of paracrine induction of growth factors by PTH in this protective effect is unclear. PTH is dependent for some of its bone anabolic effects on the insulin/IGF signaling pathway,(9-11) which activates both ERK and PKB. Repression of Bim in osteoblasts may therefore explain some of the beneficial effects seen in vivo with direct and indirect growth factor therapies.
In conclusion, we provide evidence that Bim plays a causal role in osteoblast apoptosis. It is highly regulated by both ERK and PKB signaling pathways in this cell type and may therefore be amenable to therapeutic targeting. Furthermore, we provide evidence that activation of Bak and Bax by trophic factor deprivation causes osteoblast apoptosis and that knock down of these proteins is highly protective.
ACKNOWLEDGMENTS
This study was funded by an Arthritis Research Campaign Fellowship 16343 (PH) and by the Vergottis Foundation (ML).
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Giannoudis P, Tzioupis C, Almalki T, Buckley R. Fracture healing in osteoporotic fractures: Is it really different? A basic science perspective. Injury. 2007;38(Suppl 1):S90–S99. doi: 10.1016/j.injury.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Martin TJ, Seeman E. New mechanisms and targets in the treatment of bone fragility. Clin Sci (Lond) 2007;112:77–91. doi: 10.1042/CS20060046. [DOI] [PubMed] [Google Scholar]
- 3.Giannoudis P, Psarakis S, Kontakis G. Can we accelerate fracture healing? A critical analysis of the literature. Injury. 2007;38(Suppl 1):S81–S89. doi: 10.1016/j.injury.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Dahabreh Z, Dimitriou R, Giannoudis PV. Health economics: A cost analysis of treatment of persistent fracture non-unions using bone morphogenetic protein-7. Injury. 2007;38:371–377. doi: 10.1016/j.injury.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 5.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girotra M, Rubin MR, Bilezikian JP. The use of parathyroid hormone in the treatment of osteoporosis. Rev Endocr Metab Disord. 2006;7:113–121. doi: 10.1007/s11154-006-9007-z. [DOI] [PubMed] [Google Scholar]
- 7.Pfeilschifter J, Laukhuf F, Muller-Beckmann B, Blum WF, Pfister T, Ziegler R. Parathyroid hormone increases the concentration of insulin-like growth factor-I and transforming growth factor beta 1 in rat bone. J Clin Invest. 1995;96:767–774. doi: 10.1172/JCI118121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin L, Qiu P, Wang L, Li X, Swarthout JT, Soteropoulos P, Tolias P, Partridge NC. Gene expression profiles and transcription factors involved in parathyroid hormone signaling in osteoblasts revealed by microarray and bioinformatics. J Biol Chem. 2003;278:19723–19731. doi: 10.1074/jbc.M212226200. [DOI] [PubMed] [Google Scholar]
- 9.Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002;17:1570–1578. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi M, Ogata N, Shinoda Y, Akune T, Kamekura S, Terauchi Y, Kadowaki T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H. Insulin receptor substrate-1 is required for bone anabolic function of parathyroid hormone in mice. Endocrinology. 2005;146:2620–2628. doi: 10.1210/en.2004-1511. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Nishida S, Boudignon BM, Burghardt A, Elalieh HZ, Hamilton MM, Majumdar S, Halloran BP, Clemens TL, Bikle DD. The IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res. 2007;22:1329–1337. doi: 10.1359/jbmr.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka S, Nakamura I, Inoue J, Oda H, Nakamura K. Signal transduction pathways regulating osteoclast differentiation and function. J Bone Miner Metab. 2003;21:123–133. doi: 10.1007/s007740300021. [DOI] [PubMed] [Google Scholar]
- 13.Chan GK, Duque G. Age-related bone loss: Old bone, new facts. Gerontology. 2002;48:62–71. doi: 10.1159/000048929. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama T, Bouillet P, Miyazaki T, Kadono Y, Chikuda H, Chung UI, Fukuda A, Hikita A, Seto H, Okada T, Inaba T, Sanjay A, Baron R, Kawaguchi H, Oda H, Nakamura K, Strasser A, Tanaka S. Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J. 2003;22:6653–6664. doi: 10.1093/emboj/cdg635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villunger A, Scott C, Bouillet P, Strasser A. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood. 2003;101:2393–2400. doi: 10.1182/blood-2002-07-2132. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths GJ, Corfe BM, Savory P, Leech S, Esposti MD, Hickman JA, Dive C. Cellular damage signals promote sequential changes at the N-terminus and BH-1 domain of the proapoptotic protein Bak. Oncogene. 2001;20:7668–7676. doi: 10.1038/sj.onc.1204995. [DOI] [PubMed] [Google Scholar]
- 18.Cartron PF, Arokium H, Oliver L, Meflah K, Manon S, Vallette FM. Distinct domains control the addressing and the insertion of Bax into mitochondria. J Biol Chem. 2005;280:10587–10598. doi: 10.1074/jbc.M409714200. [DOI] [PubMed] [Google Scholar]
- 19.Taylor JM, Quilty D, Banadyga L, Barry M. The vaccinia virus protein F1L interacts with Bim and inhibits activation of the pro-apoptotic protein Bax. J Biol Chem. 2006;281:39728–39739. doi: 10.1074/jbc.M607465200. [DOI] [PubMed] [Google Scholar]
- 20.Espina B, Liang M, Russell RG, Hulley PA. Regulation of bim in glucocorticoid-mediated osteoblast apoptosis. J Cell Physiol. 2008;215:488–496. doi: 10.1002/jcp.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez GI, Jurisicova A, Wise L, Lipina T, Kanisek M, Bechard A, Takai Y, Hunt P, Roder J, Grynpas M, Tilly JL. Absence of the proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. Proc Natl Acad Sci USA. 2007;104:5229–5234. doi: 10.1073/pnas.0608557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benayahu D, Kletter Y, Zipori D, Wientroub S. Bone marrow-derived stromal cell line expressing osteoblastic phenotype in vitro and osteogenic capacity in vivo. J Cell Physiol. 1989;140:1–7. doi: 10.1002/jcp.1041400102. [DOI] [PubMed] [Google Scholar]
- 23.Benayahu D, Sela J. Histomorphometric analysis of heterotopic bone formed by stromal-osteogenic subpopulations. Calcif Tissue Int. 1996;59:254–258. doi: 10.1007/s002239900119. [DOI] [PubMed] [Google Scholar]
- 24.Locklin RM, Oreffo RO, Triffitt JT. Effects of TGFbeta and bFGF on the differentiation of human bone marrow stromal fibroblasts. Cell Biol Int. 1999;23:185–194. doi: 10.1006/cbir.1998.0338. [DOI] [PubMed] [Google Scholar]
- 25.Chandra D, Choy G, Daniel PT, Tang DG. Bax-dependent regulation of Bak by voltage-dependent anion channel 2. J Biol Chem. 2005;280:19051–19061. doi: 10.1074/jbc.M501391200. [DOI] [PubMed] [Google Scholar]
- 26.Warton K, Foster NC, Gold WA, Stanley KK. A novel gene family induced by acute inflammation in endothelial cells. Gene. 2004;342:85–95. doi: 10.1016/j.gene.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 27.Ihbe A, Baumann G, Heinzmann U, Atkinson MJ. Loss of the differentiated phenotype precedes apoptosis of ROS 17/2.8 osteoblast-like cells. Calcif Tissue Int. 1998;63:208–213. doi: 10.1007/s002239900516. [DOI] [PubMed] [Google Scholar]
- 28.Tao Z, Goodisman J, Penefsky HS, Souid AK. Caspase activation by anticancer drugs: The caspase storm. Mol Pharmacol. 2007;4:583–595. doi: 10.1021/mp070002r. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci USA. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley R, Ewings KE, Hadfield K, Howes E, Balmanno K, Cook SJ. Extracellular signal-regulated kinases 1/2 are serum-stimulated “Bim(EL) kinases” that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J Biol Chem. 2004;279:8837–8847. doi: 10.1074/jbc.M311578200. [DOI] [PubMed] [Google Scholar]
- 31.Meller R, Cameron JA, Torrey DJ, Clayton CE, Ordonez AN, Henshall DC, Minami M, Schindler CK, Saugstad JA, Simon RP. Rapid degradation of Bim by the ubiquitinproteasome pathway mediates short-term ischemic tolerance in cultured neurons. J Biol Chem. 2006;281:7429–7436. doi: 10.1074/jbc.M512138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dijkers PF, Birkenkamp KU, Lam EW, Thomas NS, Lammers JW, Koenderman L, Coffer PJ. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: Protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J Cell Biol. 2002;156:531–542. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan TT, Degenhardt K, Nelson DA, Beaudoin B, Nieves-Neira W, Bouillet P, Villunger A, Adams JM, White E. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell. 2005;7:227–238. doi: 10.1016/j.ccr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Valverde AM, Mur C, Brownlee M, Benito M. Susceptibility to apoptosis in insulin-like growth factor-I receptor-deficient brown adipocytes. Mol Biol Cell. 2004;15:5101–5117. doi: 10.1091/mbc.E03-11-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rukenstein A, Rydel RE, Greene LA. Multiple agents rescue PC12 cells from serum-free cell death by translation-and transcription-independent mechanisms. J Neurosci. 1991;11:2552–2563. doi: 10.1523/JNEUROSCI.11-08-02552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graos M, Almeida AD, Chatterjee S. Growth-factor-dependent phosphorylation of Bim in mitosis. Biochem J. 2005;388:185–194. doi: 10.1042/BJ20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adachi M, Zhang YB, Imai K. Mutation of BAD within the BH3 domain impairs its phosphorylation-mediated regulation. FEBS Lett. 2003;551:147–152. doi: 10.1016/s0014-5793(03)00915-3. [DOI] [PubMed] [Google Scholar]
- 39.Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK, Brugge JS. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 40.Wang P, Gilmore AP, Streuli CH. Bim is an apoptosis sensor that responds to loss of survival signals delivered by epidermal growth factor but not those provided by integrins. J Biol Chem. 2004;279:41280–41285. doi: 10.1074/jbc.C400248200. [DOI] [PubMed] [Google Scholar]
- 41.Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem. 2006;281:813–823. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- 42.Zheng WH, Kar S, Quirion R. Insulin-like growth factor-1-induced phosphorylation of the forkhead family transcription factor FKHRL1 is mediated by Akt kinase in PC12 cells. J Biol Chem. 2000;275:39152–39158. doi: 10.1074/jbc.M002417200. [DOI] [PubMed] [Google Scholar]
- 43.Biswas SC, Greene LA. Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. J Biol Chem. 2002;277:49511–49516. doi: 10.1074/jbc.M208086200. [DOI] [PubMed] [Google Scholar]
- 44.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]