Abstract
Voltage-gated proton channels and NADPH oxidase function cooperatively in phagocytes during the respiratory burst, when reactive oxygen species are produced to kill microbial invaders. Agents that activate NADPH oxidase also enhance proton channel gating profoundly, facilitating its roles in charge compensation and pHi regulation. The “enhanced gating mode” appears to reflect protein kinase C (PKC) phosphorylation. Here we examine two candidates for PKC-δ phosphorylation sites in the human voltage-gated proton channel, HV1 (Hvcn1), Thr29 and Ser97, both in the intracellular N terminus. Channel phosphorylation was reduced in single mutants S97A or T29A, and further in the double mutant T29A/S97A, by an in vitro kinase assay with PKC-δ. Enhanced gating was evaluated by expressing wild-type (WT) or mutant HV1 channels in LK35.2 cells, a B cell hybridoma. Stimulation by phorbol myristate acetate enhanced WT channel gating, and this effect was reversed by treatment with the PKC inhibitor GF109203X. The single mutant T29A or double mutant T29A/S97A failed to respond to phorbol myristate acetate or GF109203X. In contrast, the S97A mutant responded like cells transfected with WT HV1. We conclude that under these conditions, direct phosphorylation of the proton channel molecule at Thr29 is primarily responsible for the enhancement of proton channel gating. This phosphorylation is crucial to activation of the proton conductance during the respiratory burst in phagocytes.
Keywords: Cell/Phagocytosis, Channel/Other, Immunology/Innate Immunity, Membrane/Channels, Phosphorylation, Signal Transduction, Proton Transport, Proton Channels
Introduction
Voltage-gated proton channels enable sustained superoxide anion (O2˙̄) production by NADPH oxidase during the respiratory burst in phagocytes. NADPH oxidase generates O2˙̄ by transferring electrons from NADPH in the cytosol across the membrane to reduce extracellular or phagosomal O2 to O2˙̄. O2˙̄ is the precursor to other reactive oxygen species that contribute to killing microbial invaders. The consequences of NADPH oxidase activity, in particular decreased internal pH (pHi) and membrane depolarization, are counteracted by H+ efflux through open voltage-gated proton channels (1–6). The activities of NADPH oxidase and voltage-gated proton channels are coordinated in several ways. The depolarization and pHi decrease resulting from NADPH oxidase activity both directly promote proton channel opening. In addition, interventions that activate NADPH oxidase profoundly enhance the gating properties of proton channels (3, 7). This “enhanced gating mode” consists of four changes in proton channel properties, each of which increases the likelihood of channel opening under any given set of conditions. The channels open faster (smaller activation time constant, τact)3 and close more slowly (larger deactivation time constant, τtail), display increased maximum proton conductance (gH,max), and manifest a 40-mV hyperpolarizing shift of the entire proton conductance-voltage relationship (gH-V). The enhanced gating mode improves the efficiency of NADPH oxidase by minimizing the depolarization required to open enough proton channels to fully compensate the electrical consequences of NADPH oxidase activity (i.e. the electron current) (5). Depolarization directly inhibits NADPH oxidase (4, 8).
The enhanced gating mode is induced by PMA, an activator of PKC, and is prevented and at least partially reversed by the PKC inhibitor GFX (9, 10). Although these results suggest regulation by PKC phosphorylation, they do not clarify whether the target of PKC is an accessory protein or the channel itself. This study identifies phosphorylation sites on the human proton channel molecule and determines their involvement in converting the proton channel to the enhanced gating mode. We find that a single residue, Thr29, in the intracellular N-terminal domain appears to be responsible for inducing enhanced gating. Evidently, enhanced gating reflects a phosphorylated state of the proton channel and does not require accessory proteins.
EXPERIMENTAL PROCEDURES
Plasmids and Retroviral Infection
Myc-tagged Hvcn1 was cloned by PCR in green fluorescent protein-bicistronic MigRI retroviral vector. Hvcn1 T29A/S97A, T29A, T29D, S97A, and S97D mutants were generated by site-directed mutagenesis of wild-type Hvcn1 sequence in MigRI vector using the Stratagene (La Jolla, CA) QuikChange technology. Sequences of primers used for mutagenesis are available upon request.
Lentiviral particles were prepared as follows. Phoenix α packaging cell line was transfected with empty vector control and Hvcn1 MigRI plasmids by Ca2+ phosphate transfection. Viral supernatants were collected after 24, 36, and 48 h and frozen at −80 °C until use. LK35.2 cells were infected by spinoculation at 2300 rpm for 90 min in the presence of 4 μg/ml Polybrene (Sigma Aldrich, Dorset, UK) three times over a period of 2 days. At day 3, highly green fluorescent-protein-positive cells were sorted on a FACSVantage with CellQuest software (Becton Dickinson, Oxford, UK) and used for patch clamp studies.
In Vitro Kinase Assay
HEK-293 cells were transfected with Myc-tagged Hvcn1, Hvcn1 T29A/S97A, Hvcn1 T29A, and Hvcn1 S97A MigRI constructs by Ca2+ phosphate. 24 h after transfection, cells were harvested and lysed (1% Triton X-100, 20 mm Hepes, 137 mm NaCl, 2.5 mm β-glycerophosphate, 1 mm Na3O4, 2 mm EDTA, protease inhibitor mixture (Sigma Aldrich)) prior to immunoprecipitation with anti-Myc antibody (Cell Signaling Technology) conjugated to protein G-Sepharose beads. Beads were then incubated in kinase assay buffer (20 mm Hepes, 1 mm EGTA, 0.4 mm EDTA, 5 mm MgCl2, 0.1 mm CaCl2, 0.05 mm dithiothreitol, 0.2 mg/ml phosphatidylinositol, 2.5 mm β-glycerophosphate, 1 μm PMA, 40 mm PKC-δ (Cell Signaling Technology), 10 μCi of [γ-32P]ATP (GE Healthcare)) for 20 min at 30 °C, prior to being suspended in 2× Laemmli buffer. Four-fifths of samples were loaded on an SDS-PAGE that was then dried in a gel dryer prior to exposure to x-ray film; one-fifth of samples was loaded on a separate SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with anti-Myc as loading control.
Electrophysiology
The recording and data analysis setups were as described previously (11). Pipettes were made from 7052 glass (Garner Glass Co., Claremont, CA). Seals were formed with Ringer's solution (in mm: 160 NaCl, 4.5 KCl, 2 CaCl2, 1 MgCl2, 5 Hepes, pH 7.4) in the bath, and the potential was zeroed after the pipette was in contact with the cell. For perforated patch recording, the pipette and bath solutions (∼300 mosm) contained 130 mm tetramethylammonium methanesulfonate, 50 mm NH4+ in the form of 25 mm (NH4)2SO4, 2 mm MgCl2, 10 mm BES buffer, 1 mm EGTA and was titrated to pH 7.0 with tetramethylammonium hydroxide. The pipette solution included ∼500 μg/ml solubilized amphotericin B (∼45% purity; Sigma); the pipette tip was dipped in amphotericin-free solution. Experiments were done at 21 °C or at room temperature (20–25 °C).
The τact was obtained by fitting the rising current with a single exponential function, ignoring the initial sigmoid component. The steady-state H+ current (IH) was extrapolated using the fitted time constant. Deactivation was quantified by fitting tail currents with a single exponential to obtain the τtail. The values obtained for IH along with reversal potential measured in each cell were used to calculate the gH-V relationship. Shifts in the gH-V relationship were assessed using plots like that in Fig. 2D and determining the shift by interpolation at the smallest reliable gH values (where the voltage dependence is steepest). In some situations, the voltage at which time-dependent proton current was first detected, Vthreshold, was used as a rough indicator of the position of the gH-V relationship (12).
FIGURE 2.
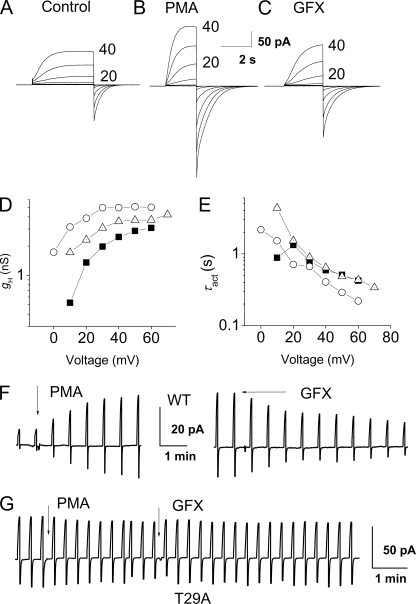
Enhanced proton channel gating elicited by PMA and reversed by GFX in an LK35.2 cell. A–C, families of proton currents during pulses in 10-mV increments up to +40 mV in an Hvcn1-transfected LK35.2 cell before (A) or after PMA stimulation (B) and after GFX (C). D, the steady-state gH, calculated from the currents in A (■), B (○), and C (▵) after fitting to a rising exponential, using reversal potentials measured in each solution. E, τact values in the same cell are faster after PMA, but the effect is reversed by GFX. F, time course of PMA and GFX responses in the cell in A–E, with test pulses to +10 mV repeated every 38 s from a holding potential of −40 mV. During the break in the time course, after the addition of PMA, the family of currents in B was recorded. G, proton currents during pulses to +20 mV repeated every 20 s from a holding potential of −40 mV in an LK35.2 cell transfected with the T29A mutant channel did not respond to PMA or GFX.
We routinely waited for stable recording conditions, added PMA, and later introduced GFX and considered the response genuine if PMA increased and GFX decreased the current. That the observed PMA responses were genuine was strongly corroborated by their reversal by GFX. Thus, although proton currents might increase due to improved access through the patch membrane or other spurious causes, it is unlikely that such increases would be reversed by GFX. Cells that were judged, based on access resistance, to have spontaneously entered whole-cell configuration were excluded from analysis. Responses to PMA or GFX are presented as ratios such that 1.0 means no response and a larger number means a larger response. Thus, for PMA responses, IH(PMA)/IH(control) and τact(control)/τact(PMA) are given. For GFX responses, IH(PMA)/IH(GFX) and τact(GFX)/τact(PMA) are given. Shifts in the gH-V relationship were determined by interpolation of gH values at voltages just above threshold.
RESULTS
In Vitro Kinase Assay for Phosphorylation
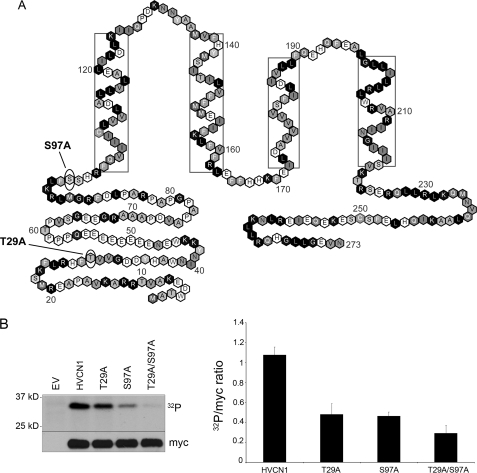
Fig. 1A shows the positions of two candidates for PKC phosphorylation sites tested here, Thr29 and Ser97. These were identified by criteria determined by Fujii et al. (13). Both occur in the N terminus on intracellular regions of the human voltage-gated proton channel (HV1) protein. It should be noted that because the structure of the proton channel is not known, the proximity of these sites to the membrane or to other parts of the channel is not established. Phosphorylation of these sites upon PMA stimulation was assessed in an in vitro kinase assay with recombinant PKC-δ. Fig. 1B shows that phosphorylation was reduced significantly when compared with WT HV1 for either single mutant S97A or T29A. Phosphorylation was further reduced in the double mutant T29A/S97A, which supports the conclusion that both sites are phosphorylated by PKC-δ.
FIGURE 1.
Identification of candidate phosphorylation sites in the human proton channel. A, location of the two putative PKC-δ phosphorylation sites in the sequence of HV1. Both predicted PKC-δ sites are in the N terminus, with Ser97 near the membrane boundary. Boxed residues indicate helical transmembrane regions (37). B, phosphorylation of HV1 WT, T29A, S97A, and T29A/S97A mutants in the presence of recombinant PKC-δ and [γ-32P]ATP in an in vitro kinase assay. PKC-δ was activated with 1 μm PMA. The myc immunoblot indicates loading. EV indicates empty vector control. The graph on the right-hand side represents densitometric analysis of the 32P band versus loading control of 3–4 separate experiments (p < 0.05 for each mutant versus WT by Student's t test). Error bars indicate S.E.
Electrophysiological Measurements of Enhanced Gating
Identifying sites that might contribute to enhanced gating requires an expression system in which expressed proton channels respond to PMA. Although proton channels in human neutrophils and eosinophils respond dramatically to PMA (7, 14), HEK-293 cells transfected with the proton channel, Hvcn1, do not respond (12). However, we found that when Hvcn1 is expressed in the B lymphocytic cell line, LK35.2, the resulting proton current responds to PMA in a manner qualitatively like the response of human phagocytes (Fig. 2F). An advantage of the LK35.2 B lymphocytic line is that cells that were not transfected or transfected with empty vector had no detectable proton currents (n = 8), whereas LK35.2 cells transfected with Hvcn1 expressed large proton currents (Fig. 2A). The experimental approach is illustrated in Fig. 2F. Test pulses were applied, and then PMA was introduced, after which the proton current amplitude IH increased progressively over several minutes. On average, IH increased 2.4-fold during test pulses (Table 1). After application of a voltage clamp family and measurement of the reversal potential, Vrev, test pulses were again applied. Then GFX was added to the bath, and the enhanced proton current was progressively reduced toward its amplitude before stimulation.
TABLE 1.
Effects of PMA and GFX on proton channel parameters (mean ± S.E. (n))
Relative values of proton channel parameters in each cell before (c = control) or after PMA stimulation and after GFX reversal are shown. All ratios are presented so that a larger number means a larger effect. The gH-V relationship shift by PMA is relative to control, and GFX is relative to PMA (see “Experimental Procedures”). The T29A, T29D, and double mutant T29A/S97A did not respond. Significance by one-sample t test, *, p < 0.05, **, p < 0.01, compared with no effect (1.0 for ratios, 0 for gH-V shift). None of the differences between WT and S97D or S97A are significant (Student's t test). For comparison, average parameter values from human neutrophils from CGD patients and normal subjects from a previous study (36) are given in columns 4 and 5, respectively.
| WT | S97A or S97D | CGD neutrophils | Neutrophils | |
|---|---|---|---|---|
| IH PMA/c | 2.39* ± 0.51 (10) | 1.42** ± 0.13 (12) | 2.55 | 3.3 |
| IH PMA/GFX | 2.19** ± 0.37 (12) | 1.22 ± 0.11 (8) | ||
| τact c/PMA | 2.12 ± 0.53 (10) | 1.62** ± 0.15 (12) | 1.94 | 3.7 |
| τact GFX/PMA | 1.87** ± 0.22 (12) | 1.51* ± 0.21 (8) | ||
| τtail PMA/c | 1.22 ± 0.11 (10) | 1.13 ± 0.067 (12) | 1.15 | 5.5 |
| τtail PMA/GFX | 1.54** ± 0.17 (13) | 1.14 ± 0.06 (8) | ||
| gH-V shift PMA (mV) | −10.7** ± 1.9 (7) | −7.8** ± 1.6 (8) | −13.3 | −38.8 |
| gH-V shift GFX (mV) | 9.4** ± 1.6 (8) | 6.7** ± 1.2 (7) |
In each experimental condition, we applied families of depolarizing pulses, as illustrated in Fig. 2, A–C, to characterize the response in greater detail. Fig. 2D shows that PMA increased gH and shifted the gH-V relationship negatively. The mean shift of the gH-V relationship was −11 mV (Table 1). GFX reversed this shift substantially. The rate of channel opening increased after PMA stimulation; the time constant of H+ current activation, τact, became twice faster. Fig. 2E shows that the proportional change in τact was similar at all voltages. GFX reversed the change in τact (Fig. 2E). The effects of PMA and GFX on proton channel parameters are summarized in Table 1. In contrast with the responses of proton currents in human phagocytes, there was little slowing of deactivation (τtail). The differences in the PMA responses of LK35.2 cells when compared with human neutrophils may reflect the lack of NADPH oxidase activity in LK35.2 cells (15) (see “Discussion”).
Responses of Mutant Proton Channels
The two candidate phosphorylation sites were mutated to Ala individually (S97A and T29A) and together. We also studied mutants S97D and T29D designed as phosphorylation mimics. LK35.2 cells transfected with the double mutant proton channel (S97A/T29A) never responded detectably to PMA or GFX (n = 15). Similarly, no response was detected in the T29A (Fig. 2G) or T29D mutants, with the exception of a possible small response in 1 of 12 cells studied. However, the S97A and S97D mutants did respond (Table 1). Combined, these results strongly implicate Thr29 as the key phosphorylation site on the human proton channel.
If Thr29 is the primary phosphorylation site, then the designed phosphorylation mimic T29D might already appear “activated,” in comparison with WT channels. In this scenario, the expectation would be that the threshold voltage, Vthreshold, would be more negative than WT. Examination of Vthreshold for such an effect revealed that the WT channel was first distinctly activated at +2.1 ± 2.2 mV (mean ± S.E., n = 17). Vthreshold in T29D expressing cells was −0.7 ± 4.4 mV (n = 7), a small shift in the expected direction but not significantly different. The effects of PMA and GFX on individual cells are clearly evident because each cell serves as its own control. In contrast, detecting a small difference in properties between populations of unstimulated cells may not be feasible. Alternatively, phosphorylation mimics (X → Glu or X → Asp) are not always distinguishable from simple alanine mutants (X → Ala) (16).
The lack of response of Thr29 mutants suggests that the entire phosphorylation response is ascribable to phosphorylation of Thr29. However, we compared the responses in Ser97 mutants statistically to test the possibility that Ser97 might contribute measurably to the response (Table 1). Although the enhancement of proton channel gating by PMA and its reversal by GFX tended to be larger in WT controls than in Ser97 mutants, the difference failed to reach statistical significance for any parameter (whether S97A and S97D were compared with WT separately or together). However, the probability that all eight parameters would exhibit smaller responses in S97X than WT is <0.01. Thus, we cannot rule out that Ser97 may play a minor role or affect other functions of the channel. However, it is clear that phosphorylation of Thr29 is the major contributor to the enhanced gating of proton channels.
DISCUSSION
Voltage-gated proton channels are necessary for a normal phagocyte respiratory burst; NADPH oxidase activity by neutrophils, eosinophils, monocytes, macrophages, B lymphocytes and PLB-985 cells is greatly reduced when proton currents are inhibited by Zn2+ or other polyvalent metal cations (4, 9, 17–22) or when the proton channel is removed genetically (23–25). Proton channels and NADPH oxidase are both activated during phagocytosis, but their interaction is complex, and the mechanism remains controversial (15). The idea that the proton channel is part of the NADPH oxidase complex (26) and is thus active de facto during NADPH oxidase activity (3) appears untenable in light of the demonstration of NADPH oxidase-generated electron current in phagocytes from Hvcn1-deficient mice (6). Another view is that these two molecules are activated independently but by similar pathways (10).
The suspicion that voltage-gated proton channels in leukocytes are activated by PKC phosphorylation (10, 27, 28) is strongly supported by the present results. Furthermore, our data indicate that the channel protein itself is phosphorylated. The in vitro kinase assay detected phosphorylation of both Ser97 and Thr29. However, in our experimental system, Thr29 appeared to be responsible for the entire measurable response of proton channels. T29A or T29D mutants did not respond to PMA, nor did the double mutant T29A/S97A. In contrast, S97A or S97D mutants did respond to PMA, although their response may have been slightly weaker than that of WT channels (Table 1). These results do not resolve the physiological significance of phosphorylation of Ser97. That Ser97 was phosphorylated, yet S97A and S97D exhibited no deficit, is not without precedent. Many proteins have multiple phosphorylation sites of which only a fraction exert specific functional effects. For example, cardiac troponin T has four phosphorylation sites, of which only one exhibited functional defects upon mutation (16). Another protein with multiple phosphorylation sites is p47phox, whose phosphorylation is an absolute prerequisite for NADPH oxidase assembly and function. Individual mutation of 10 phosphorylated serines revealed just one mutation, S379A, that abolished activation, five Ser → Ala mutations that inhibited activation by ∼50%, and three that had no effect (29).
In future studies, we will attempt to identify the PKC isoform that phosphorylates the proton channel. By criteria determined by Fujii et al. (13), Thr29 is a likely PKC-δ site, but ScanSite identifies Thr29 as a possible target for cAMP-dependent protein kinase, PKC-μ, Akt kinase, or calmodulin kinase. Ser97 is also identified by criteria determined by Fujii et al. (13) as a likely PKC-δ site, but ScanSite identifies Ser97 as a possible target for PKC-α, PKC-β, or PKC-γ. PKC-δ appears to be important in responses of human phagocytes (9, 30–32) and murine B lymphocytes (33, 34).
Proton channels studied in perforated-patch conditions in human neutrophils, eosinophils, monocytes, and to a lesser extent, in basophils, respond dramatically to PMA (7, 12, 14, 35). In contrast, proton channels in other cells, such as rat alveolar epithelial cells (7) or HEK-293 or COS-7 cells transfected with HV1 (12), do not respond at all. Hypothetical explanations for this variable response in different tissues include (a) different proton channel isoforms, (b) different signaling pathways, and (c) regulation of the channel by a separate molecule whose phosphorylation modulates proton channel behavior (15). Our results do not support the existence of a separate regulatory molecule (other than NADPH oxidase; see next paragraph).
Proton channels expressed in the B lymphocytic cell line LK35.2 were found to respond qualitatively like those in primary leukocytes, but the response was substantially less profound (Table 1). Each of the four main responses is weaker in LK35.2 cells than in neutrophils, but the most obvious differences are the lack of profound slowing of channel closing (τtail) and the smaller hyperpolarizing shift of the gH-V relationship. The requirements for a full phagocyte-like proton channel response are unclear. Circumstantial evidence suggests that a crucial factor is the presence or absence of NADPH oxidase activity. Neutrophils from chronic granulomatous disease (CGD) patients lack functional NADPH oxidase and also exhibit a reduced PMA response with no slowing of τtail and only an ∼10-mV hyperpolarization of the gH-V relationship (36). The responses of proton channels in LK35.2 cells were essentially identical to the responses in CGD cells (Table 1) and in PLB-985 cells lacking gp91phox (36). We did not detect electron current in PMA-stimulated LK35.2 cells, consistent with low levels of NADPH oxidase activity (23). Human basophils also lack NADPH oxidase and exhibit PMA and GFX responses smaller than those of neutrophils or eosinophils and specifically lack a slowing of τtail (35). Thus, it is clear that a proton channel response is possible without NADPH oxidase, but the response differs in characteristic ways from that in cells with NADPH oxidase activity. The alternative postulate, that proton channels are preferentially phosphorylated by specific PKC isoforms that are more active in phagocytes, does not explain the meager response of CGD neutrophils (Table 1).
In summary, enhanced gating of HV1 is primarily the result of phosphorylation at Thr29 by PKC. The magnitude and specific characteristics of the PMA response in LK35.2 cells closely resembled that in other cells lacking NADPH oxidase activity, suggesting that a crucial interaction between these molecules occurs in phagocytes. The identification of the location of the key phosphorylation site that activates phagocyte proton channels provides a rational target for modulation of innate immune responses.
This work was supported, in whole or in part, by National Institutes of Health Research Grant HL61437 through the NHLBI. This work was also supported by grants from Prof. Dr. Adolf Schmidtmann-Stiftung, Philip Morris USA Inc. and Philip Morris International, and the MRC, United Kingdom.
- τact
- activation time constant
- τtail
- deactivation (tail current) time constant
- gH
- proton conductance
- gH-V
- proton conductance-voltage relationship
- IH
- steady-state proton current amplitude
- CGD
- chronic granulomatous disease
- GFX
- GF109203X
- HV1
- the human Hvcn1 voltage-gated proton channel gene product
- PKC
- protein kinase C
- PMA
- phorbol myristate acetate
- WT
- wild type
- BES
- 2-[bis(2-hydroxyethyl)amino]ethanesulfonic acid.
REFERENCES
- 1.Henderson L. M., Chappell J. B., Jones O. T. (1987) Biochem. J. 246, 325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeCoursey T. E., Cherny V. V. (1993) Biophys. J. 65, 1590–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bánfi B., Schrenzel J., Nüsse O., Lew D. P., Ligeti E., Krause K. H., Demaurex N. (1999) J. Exp. Med. 190, 183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeCoursey T. E., Morgan D., Cherny V. V. (2003) Nature 422, 531–534 [DOI] [PubMed] [Google Scholar]
- 5.Murphy R., DeCoursey T. E. (2006) Biochim. Biophys. Acta 1757, 996–1011 [DOI] [PubMed] [Google Scholar]
- 6.Morgan D., Capasso M., Musset B., Cherny V. V., Ríos E., Dyer M. J., DeCoursey T. E. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 18022–18027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeCoursey T. E., Cherny V. V., Zhou W., Thomas L. L. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 6885–6889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petheo G. L., Demaurex N. (2005) Biochem. J. 388, 485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bankers-Fulbright J. L., Kita H., Gleich G. J., O'Grady S. M. (2001) J. Cell. Physiol. 189, 306–315 [DOI] [PubMed] [Google Scholar]
- 10.Morgan D., Cherny V. V., Finnegan A., Bollinger J., Gelb M. H., DeCoursey T. E. (2007) J. Physiol. 579, 327–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan D., Cherny V. V., Murphy R., Xu W., Thomas L. L., DeCoursey T. E. (2003) J. Physiol. 550, 447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musset B., Cherny V. V., Morgan D., Okamura Y., Ramsey I. S., Clapham D. E., DeCoursey T. E. (2008) J. Physiol. 586, 2477–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii K., Zhu G., Liu Y., Hallam J., Chen L., Herrero J., Shaw S. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 13744–13749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeCoursey T. E., Cherny V. V., DeCoursey A. G., Xu W., Thomas L. L. (2001) J. Physiol. 535, 767–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musset B., Cherny V. V., Morgan D., DeCoursey T. E. (2009) FEBS Lett. 583, 7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumandea M. P., Pyle W. G., Kobayashi T., de Tombe P. P., Solaro R. J. (2003) J. Biol. Chem. 278, 35135–35144 [DOI] [PubMed] [Google Scholar]
- 17.Chvapil M., Stankova L., Bernhard D. S., Weldy P. L., Carlson E. C., Campbell J. B. (1977) Infect. Immun. 16, 367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson L. M., Chappell J. B., Jones O. T. (1988) Biochem. J. 255, 285–290 [PMC free article] [PubMed] [Google Scholar]
- 19.Simchowitz L., Foy M. A., Cragoe E. J., Jr. (1990) J. Biol. Chem. 265, 13449–13456 [PubMed] [Google Scholar]
- 20.Lowenthal A., Levy R. (1999) J. Biol. Chem. 274, 21603–21608 [DOI] [PubMed] [Google Scholar]
- 21.Femling J. K., Cherny V. V., Morgan D., Rada B., Davis A. P., Czirják G., Enyedi P., England S. K., Moreland J. G., Ligeti E., Nauseef W. M., DeCoursey T. E. (2006) J. Gen. Physiol. 127, 659–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musset B., Cherny V., DeCoursey T. E. (2009) Biophys. J. 96, 667a–668a [Google Scholar]
- 23.Capasso M., Bhamrah M. K., Henley T., Boyd R. S., Langlais C., Cain K., Dinsdale D., Pulford K., Khan M., Musset B., Cherny V. V., Morgan D., Gascoyne R. D., Vigorito E., DeCoursey T. E., MacLennan I. C., Dyer M. J. (2010) Nat. Immunol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsey I. S., Ruchti E., Kaczmarek J. S., Clapham D. E. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 7642–7647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okochi Y., Sasaki M., Iwasaki H., Okamura Y. (2009) Biochem. Biophys. Res. Commun. 382, 274–279 [DOI] [PubMed] [Google Scholar]
- 26.Henderson L. M., Banting G., Chappell J. B. (1995) J. Biol. Chem. 270, 5909–5916 [PubMed] [Google Scholar]
- 27.Nanda A., Grinstein S. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 10816–10820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapus A., Szászi K., Ligeti E. (1992) Biochem. J. 281, 697–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Benna J., Dang P. M., Gougerot-Pocidalo M. A., Marie J. C., Braut-Boucher F. (2009) Exp. Mol. Med. 41, 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He R., Nanamori M., Sang H., Yin H., Dinauer M. C., Ye R. D. (2004) J. Immunol. 173, 7462–7470 [DOI] [PubMed] [Google Scholar]
- 31.Zhao X., Xu B., Bhattacharjee A., Oldfield C. M., Wientjes F. B., Feldman G. M., Cathcart M. K. (2005) J. Leukoc. Biol. 77, 414–420 [DOI] [PubMed] [Google Scholar]
- 32.Cheng N., He R., Tian J., Dinauer M. C., Ye R. D. (2007) J. Immunol. 179, 7720–7728 [DOI] [PubMed] [Google Scholar]
- 33.Mecklenbräuker I., Saijo K., Zheng N. Y., Leitges M., Tarakhovsky A. (2002) Nature 416, 860–865 [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto A., Nakayama K., Imaki H., Hirose S., Jiang Y., Abe M., Tsukiyama T., Nagahama H., Ohno S., Hatakeyama S., Nakayama K. I. (2002) Nature. 416, 865–869 [DOI] [PubMed] [Google Scholar]
- 35.Musset B., Morgan D., Cherny V. V., MacGlashan D. W., Jr., Thomas L. L., Ríos E., DeCoursey T. E. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 11020–11025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeCoursey T. E., Cherny V. V., Morgan D., Katz B. Z., Dinauer M. C. (2001) J. Biol. Chem. 276, 36063–36066 [DOI] [PubMed] [Google Scholar]
- 37.Ramsey I. S., Moran M. M., Chong J. A., Clapham D. E. (2006) Nature 440, 1213–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]