Abstract
Acid phosphatase activity in the highly infectious intracellular pathogen Francisella tularensis is directly related with the ability of these bacteria to survive inside host cells. Pharmacological inactivation of acid phosphatases could potentially help in the treatment of tularemia or even be utilized to neutralize the infection. In the present work, we report inhibitory compounds for three of the four major acid phosphatases produced by F. tularensis SCHU4: AcpA, AcpB, and AcpC. The inhibitors were identified using a catalytic screen from a library of chemicals approved for use in humans. The best results were obtained against AcpA. The two compounds identified, ascorbate (Ki = 380 ± 160 μm) and 2-phosphoascorbate (Ki = 3.2 ± 0.85 μm) inhibit AcpA in a noncompetitive, nonreversible fashion. A potential ascorbylation site in the proximity of the catalytic pocket of AcpA was identified using site-directed mutagenesis. The effects of the inhibitors identified in vitro were evaluated using bioassays determining the ability of F. tularensis to survive inside infected cells. The presence of ascorbate or 2-phosphoascorbate impaired the intramacrophage survival of F. tularensis in an AcpA-dependent manner as it was probed using knockout strains. The evidence presented herein indicated that ascorbate could be a good alternative to be used clinically to improve treatments against tularemia.
Keywords: Enzyme Mechanisms, Enzyme Mutation, Enzyme Purification, Macrophage, Parasite, AcpA, AcpB, AcpC, Francisella, Intramacrophage Survival
Introduction
Francisella tularensis, the causative agent of human tularemia, is a highly infectious bacterium that could represent a serious bio-threat if genetically modified to resist antibiotic treatment (1–3). Four acid phosphatases are encoded by F. tularensis: AcpA, AcpB, AcpC, and Hap. A recent work indicated that a quadruple knockout mutant strain showed attenuated virulence and diminished macrophage vacuolar escape (4), thus the pharmacological inhibition of acid phosphatase activity is an excellent target to prevent the proliferation of these bacteria inside infected cells.
Acid phosphatases are produced by many intracellular pathogenic organisms. These enzymes are able to function inside host cell endosomes by hydrolyzing the phosphoester (P-O) bonds of phosphorylated molecules. Acid phosphatases have been identified in Coxiella (5), Bordetella (6), and Legionella (7, 8), and their activity has been directly linked with virulence in some of these organisms. Several of these enzymes have been purified, their biochemical characteristics described, and their structure solved. Despite evidence suggesting that these phosphatases suppress the respiratory burst of human neutrophils (7, 9, 10), their natural substrates, biological role, and molecular events involved during infection are still under study.
A combination of several factors makes it difficult to identify the intracellular substrate that these enzymes utilize to aid the bacteria during the infection process. For example, phosphate is the most abundant functional group present in the intracellular pool of cellular metabolites (11). In addition, most of the phosphatases previously studied have very low sequence homology and little substrate specificity, being able to release phosphate from a vast array of phosphorylated compounds in vitro (12). This biochemistry makes it challenging to rationally elaborate specific inhibitory compounds to be used in vivo. We should note, in addition to these biochemical problems, the further complexity of working with highly infectious intracellular microorganisms.
Taking advantage of two important characteristics of these enzymes, (i) their relaxed substrate specificity and (ii) their ability to work in a broad range of pH, we set up a rapid, simple, and reliable screen for F. tularensis phosphatase inhibitors. These characteristics allowed the use of p-nitrophenyl phosphate (pNPP)4 as a model substrate for our screening. Using this technique we performed an unbiased enzymatic screening looking for chemical compounds that inhibit the F. tularensis phosphatase activity in vitro. Envisioning a potential utilization in humans, we restricted our search to arrays of drugs approved by the Federal Drug Administration (FDA). Our hypothesis was that several natural/synthetic chemical scaffolds present in these libraries can interact with the purified acid phosphatases, blocking their specific activities. In the present work, we describe the biochemical study of the inhibitory compounds selected for three of the enzymes studied. We offer a molecular interpretation of the mechanism for the best inhibitory compound obtained for AcpA. The effects observed in vitro were validated using bioassays. We found that the ability of F. tularensis to proliferate inside macrophages was severely affected when treated with the compound selected in our screen.
EXPERIMENTAL PROCEDURES
Bacterial Strains
Francisella novicida U112 (JSG1819) and F. novicida ΔacpA JSG2660 (4) were routinely cultured at 37 °C on cysteine heart agar (CHA) (Hi-Media Laboratories, India) or in modified tryptic soy broth (TSB) (Difco Laboratories, Detroit, MI) containing 35 μg/ml ferric pyrophosphate and 0.1% cysteine hydrochloride. CHOC II plates (Difco Laboratories) were used for CFU enumeration. To obtain minimal inhibitory concentrations, F. novicida wild-type and mutant strains from 24-h CHA plates containing antibiotics were inoculated into modified TSB, as well as Chamberlain's medium, and the optical densities of the cultures were determined at 2-h intervals. All manipulations with F. novicida were performed in a class II biological safety laboratory. Escherichia coli DH5α cells were used to carry and propagate all vectors. When required for E. coli growth, Luria-Bertani medium (Difco Laboratories), was supplemented with ampicillin (100 μg/ml) as needed. All antibiotics and chemicals were purchased from Sigma-Aldrich.
DNA Manipulations and Gene Cloning
Standard methods were used for site-directed mutagenesis, chromosomal DNA isolation, restriction enzyme digestion, agarose gel electrophoresis, ligation, and transformation (13). Plasmids were isolated using spin miniprep kits (Qiagen), and PCR products were purified using QIAquick purification kits (Qiagen). Mutagenesis was performed using the QuikChange site-directed mutagenesis kit (Stratagene). For protein expression and purification, the selected gene was amplified from F. tularensis tularensis SCHU4 chromosomal DNA (kindly provided by Dr. Wehrly, National Institutes of Health) by PCR. The primers used were: for AcpA (FTT0221): acpAFw, 5′-atgaagctcaataaaattactttagg-3′ and acpARv, 5′-ttagtttaatttatccactactaatcctg-3′; for AcpB (FTT0156): acpBFw, 5′-atgacgcaacaacaagttattagc-3′ and acpBRv, 5′-ctaaaatcttgagttctcac-3′; for AcpC (FTT0620): acpCFw, 5′-atgagacaaataatattaatctttgtg-3′ and acpCRv, 5′-ttattgccagctgccataca-3′; and Hap (FTT1064): hapFw, 5′-atgagaaaaatattcactatcg-3′ and hapRv, 5′-ctatgttggtgtagaaactt-3′. The extra sequences 5′-ttgtatttccagggc-3′ and 5′-caagcttcgtcatca-3′ were added to the 5′-end of the forward and reverse primers, respectively, for the ligation-independent cloning using a BD-infusionTM CF Dry-Down PCR cloning kit (BD Biosciences). The plasmid p15TV-L (GenBankTM accession number EF456736) obtained from the Structural Genomics Consortium (Toronto) was used as a vector for all clones (13). This construct provides for an N-terminal hexahistidine tag separated from the protein by a tobacco etch virus protease recognition site (ENLYFQ↓GS). To achieve better protein recovery the p15TV-L vector was further engineered using inverse PCR by adding the sequence MPLGKNVKKK upstream of the His tag (14) to improve the solubility of the protein.
Protein Purification
The His-tagged fusion proteins were overexpressed in E. coli BL21-Star(DE3) cells (Stratagene) harboring an extra plasmid encoding three rare tRNAs (AGG and AGA for Arg and ATA for Ile). The cells were grown in LB with 1 m sorbitol and 2.5 mm betaine at 23 °C to an A600 of ∼0.6, and expression was induced with 0.4 mm isopropyl 1-thio-β-d-galactopyranoside. After addition of isopropyl 1-thio-β-d-galactopyranoside, the cells were incubated with shaking at (17 °C) overnight as described (15). The cells were harvested, resuspended in binding buffer (500 mm NaCl, 5% glycerol, 20 mm Tris-HCl, pH 8.2, 15 mm imidazole), flash-frozen in liquid N2, and stored at −70 °C. The thawed cells were disrupted by passage through a French press, and the lysate was clarified by centrifugation (30 min at 17,000 rpm) and passed through a metal chelate affinity-column charged with Ni2+. After the column was washed, the protein was eluted from the column in elution buffer (binding buffer with 250 mm imidazole). The purified proteins were dialyzed against 20 mm Tris-HCl, pH 7.9, 500 mm NaCl, 1 mm dithiothreitol, and concentrated using a Vivaspin 20 concentrator (Sartorius Biolab Products, Germany). Protein concentration was estimated using the Bio-Rad protein assay kit (Bio-Rad).
Enzymatic Assays
Phosphatase activity was determined by continuous reading at 412 nm for a period of 40 min at 37 °C using pNPP as substrate. For AcpA the standard 150-μl assay mixture contained 25 mm (MES), pH 6.2, 1 mm pNPP, 1 μg/ml AcpA (unless stated otherwise). Under these conditions, enzyme activity was linear with time for at least 60 min. The amount of p-nitrophenol released was quantified at A412 using the extinction coefficient ϵ = 16,300 m−1 cm−1 as described previously (16). Enzymatic activity was also determined using 2-phosphoascorbate as a substrate using malachite green ammonium molybdate detection reagent (17).
The pH optimum was determined using overlapping buffers at 25 mm: MES (pH 6.0–6.6), MOPS (pH 6.6–7.0), HEPES (pH 6.8–8.0), Tris (pH 8.2–8.8), CHES (pH 8.6–9.6), Maleate (pH 5.6–6.4), citrate (pH 5.6–6.4), and acetate (pH 6.0–6.6). The effects of cations on the hydrolysis of pNPP were determined using 1 mm each of Fe2+, Cd2+, Co2+, Mn2+, Ca2+, Fe3+, Mg2+, Ni2+, Cu2+, and Zn2+ in the form of chloride salts. Saturation kinetics was assessed over a range of substrate concentrations (0.05–15 mm). Kinetic parameters (Km and Vmax) were determined by nonlinear curve fitting using Origin 8 software (Northampton, MA).
Inhibitor Screening Assays
The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical, France) at a final concentration of 1.3 μg/ml. The dosage dependence of potential inhibitors was tested in different buffers at concentrations ranging from 15 mm to 50 nm. The equation used for calculation of the inhibition constant for competitive inhibition was Km′ = (Km*(1 + [I]/Ki), and Vmax′ = Vmax/(1 + [I]/Ki) for noncompetitive inhibitions. All measurements were made in triplicate and in at least two separate experiments.
Construction of the LVS acpA Mutant
The acpA (FTL_158) gene deletion in live vaccine strain (LVS) was constructed as described with other genes (18). In brief, a 1100-bp upstream fragment was amplified from LVS chromosomal DNA (forward primer, 5′-acgcgtcgacGGA GTT AGT GAT TTA GTT GCA ATA GGT GTT GC-3′; reverse primer, 5′-cgcggatccGCT TCA TAT GAT ACC TTT AGT TGT TAG ATT CAA AGG AAA TAT TAA TAA C-3′), digested with SalI and BamHI and inserted into SalI/BamHI-digested pJC84 plasmid. The resulting plasmid was digested with BamHI and XmaI and ligated into this was a 1100-bp downstream fragment of acpA (forward primer, 5′-cgcggatccAAA TAT TTA CTC GGT AAG TTG CTT TAA TCT AGT ATT TTC GC-3′; reverse primer, 5′-tccccccgggGCT AAA GAT AAG GGC ATA AAG ACT ATC AAA GAG AG-3′). The final construct named pJC84-AcpA was transformed into LVS strain by electroporation and was processed as described earlier (18). The LVS-acpA mutant was confirmed by PCR and Southern blot analysis.
In Vitro Minimal Inhibitory Concentration Assay
The minimal inhibitory concentration assay was performed in 96-well format. l-Ascorbic acid and 2-phospho-l-ascorbic acid (Sigma) were prepared fresh and diluted in recommended diluents to a final concentration of 500 mm. Overnight cultures grown in TSB with 0.1% cysteine hydrochloride were diluted to 2 × 107 CFU/ml in TSB. Diluted cultures (50 μl) were placed in 96-well microtiter dishes and mixed with various amounts of inhibitors (50 μl, 2× concentration) diluted in TSB. Cells and peptides were incubated at 37 °C overnight in a humid environment. Wells were evaluated for growth visually to assess bacterial viability. Controls contained TSB in place of inhibitors.
Intramacrophage Survival Assay
Intramacrophage survival assay was performed in J774.1 and phorbol 12-myristate 13-acetate (10 nm/ml) induced THP-1 macrophages. In brief, 2 × 105 macrophages were seeded in 24-well tissue culture plates. Monolayers were infected with F. novicida, LVS, and their acpA mutants at multiplicity of infection of 50:1 in the presence or absence of the AcpA inhibitors (10, 30, or 100 mm). After 2 h of infection monolayers were washed and treated with fresh media containing gentamicin (50 μg/ml) for 30 min to remove extracellular bacteria. Cells were washed and replenished with fresh media containing gentamicin 10 μg/ml and the AcpA inhibitors. The cells were lysed at different time points with 0.05% SDS, diluted in phosphate-buffered saline, and plated on CHOC-II plates to enumerate the CFU.
Macrophages viability was evaluated by trypan blue dye exclusion assay, and their ability to produce interleukin 1β in the presence of ascorbate and 2-phosphoascorbate was assessed by using a DuoSet ELISA kit after induction with 10 ng of purified Salmonella lipopolysaccharide. Results are shown in supplemental Fig. S2.
RESULTS
Protein Purification
The four main acid phosphatases of F. tularensis were successfully cloned, overexpressed, and purified. AcpB produced the best yield; 1 mg/liter of culture media was centrifuged, whereas AcpA, AcpC, and Hap were obtained at low yields (50–100 μg/liter of culture centrifuged). After purification the enzymes were individually dialyzed against saline Tris-HCl buffer, pH 7.0, prior to enzymatic assays. Tris-HCl buffer, pH 8.00, was the best choice to purify AcpB as a low amount of protein was recovered when using HEPES buffer. Additionally, AcpB lost activity quickly when dialyzed against Tris-HCl buffer, pH 7.0. To overcome this problem we set up a systematic dialysis protocol using a battery of buffers at different pH values (range 5–7.5), combined with salts and reducing compounds. Unexpectedly, it was possible to recover active protein only after 18–24 h dialysis against 25 mm HEPES, pH 7.5, containing 500 mm NaCl and 1 mm dithiothreitol. The procedure was systematically repeated, and the results were consistent and reproducible. All enzymes were preserved in dialysis buffer with 5% glycerol at −80 °C. Thawed aliquots demonstrated good activity after a year of preservation in these conditions.
Biochemical Properties of the Francisella Acid Phosphatases, Establishment of Enzymatic Screen Conditions
Although the biochemical characteristics of F. tularensis AcpA were previously described by Reilly et al. (10, 19), we carried out a set of preliminary assays to establish the conditions that would allow the screening of a large number of small molecules in a high throughput manner. In all cases, the enzyme activity was dependent on the chemical composition of the buffer utilized. We determine the best buffers for each enzyme: MES or MOPS, pH 6.2, for AcpA, MOPS, pH 6.4, for AcpC, and HEPES, pH 7.0, for AcpB and Hap. Buffers were used at a 25 mm concentration. No differences in activity were detected in the presence or absence of the His6 tag in the purified protein (data not shown). AcpB, AcpC, and Hap activities were enhanced by the presence of Mg2+ or Co2+ (∼2- to 2.5-fold). The presence of Fe2+ stimulated AcpC (40% increase) and Hap (20% increase), but the activity of AcpA and AcpB was diminished by 60% to 80%. Nevertheless, the cations with a positive effect were not necessary to visualize the enzyme activity using colorimetric methods or to perform classic saturation assays. Therefore, no cofactors were added to perform the saturation assays with AcpA, AcpB, and AcpC. On the contrary, Hap required 0.1 mm of Mg2+ in the reaction mixture to use pNPP efficiently and reach full saturation. All the enzymes displayed a characteristic Michaelis-Menten saturation curve. The kinetic parameters of the purified enzymes are summarized in Table 1. All proteins showed similarly low catalytic efficiency. AcpB displayed the lowest affinity (3.11 mm) toward the model substrate, whereas the other three phosphatases have Km values in a narrow comparable range (0.68–0.88 mm).
TABLE 1.
Kinetic parameters of purified F. tularensis acid phosphatases
| Vmaxa | Kma | kcatb | kcat/Km | |
|---|---|---|---|---|
| μmol min−1mg−1 | mm | s−1 | m−1s−1 | |
| AcpA | 0.25 ± 0.05 | 0.78 ± 0.18 | 0.24 | 3.0 × 102 |
| AcpB | 11.84 ± 0.87 | 3.11 ± 0.28 | 4.48 | 1.44 × 103 |
| AcpC | 2.03 ± 0.08 | 0.88 ± 0.13 | 0.97 | 1.10 × 103 |
| Hap | 0.30 ± 0.00 | 0.68 ± 0.01 | 0.23 | 0.34 × 103 |
a Estimated by nonlinear regression analysis using MicroCal Origin 8.
b kcat was determined based on the monomeric molecular weight of each protein.
Screening methodology was optimized to follow enzyme activity in a continuous fashion for 30–40 min. The best conditions to measure AcpA activity was obtained with 1 μg/ml protein in 25 mm MES, pH 6.2, at 37 °C. Similar activity was obtained in MOPS and acetate buffer as described before (10). AcpB was used at 0.5 μg/ml and AcpC at 1 μg/ml. The substrate (pNPP) was used at the same concentration (1 mm) for all proteins. This concentration is slightly above the Km of AcpA and AcpC (0.78 ± 0.18 and 0.88 ± 0.13 mm, respectively). Keeping the substrate concentration respect to Km in a range of 1.1–1.3 was considered optimal to maximize the sensitivity of the method. AcpB, on the contrary, showed low affinity toward pNPP. In this particular case we kept the substrate concentration lower than the Km due to the high velocity of the enzyme (Table 1). A lower substrate concentration with respect to Km minimized the noise generated by the product released after hydrolysis, improving the potential to identify enzyme inhibitors. Due to low Hap recovery, low hydrolysis rate, and purity degree, this enzyme could not be screened.
High Throughput Screening for AcpA, AcpB, and AcpC Inhibitors
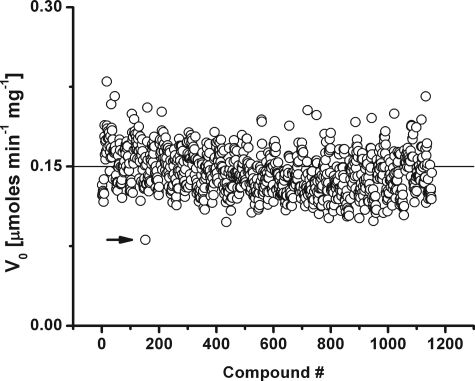
We performed an unbiased screen of AcpA, AcpB, and AcpC against a library of 1152 small molecules by following the time course of pNPP hydrolysis. All compounds that decreased the enzyme velocity by >40% were selected and individually analyzed. Once the best hits for each enzyme were identified, the inhibition constants from saturation kinetics were estimated. The results obtained are summarized in Table 2. A typical chart with the results of the screening assay obtained for AcpA is depicted in Fig. 1. When available, compounds with related chemical scaffold were assessed, and their kinetic parameters were determined.
TABLE 2.
Biochemical characteristics of F. tularensis acid phosphatases inhibitors
| Enzyme | Small molecule | Kia | Interaction |
|---|---|---|---|
| μm | |||
| AcpA | |||
| Ascorbic acid | 380.0 ± 160 | NCb | |
| (+)-5,6-O-Isopropylidene-l-ascorbic acid | 3.27 ± 0.85 | NC | |
| 2-Phospho-l-ascorbic acid | 0.32 ± 0.16 | NC | |
| 2-Sulfate-l-ascorbic | NDc | ||
| 6-Palmitate-l-ascorbic | ND | ||
| AcpB | Thonzonium bromide | 0.59 ± 0.23 | Cd |
| Procainamide hydrochloride | 14.8 ± 3.4 | C | |
| AcpC | Atracurium besylate | 342.0 ± 50 | C |
| Myricetin | 154.0 ± 89 | C | |
| Kaempferol | 190.0 ± 30 | C |
a Calculated from at least three independent determinations using different concentration of inhibitors.
b NC, no competitive interaction.
c ND, inhibition was not detected.
d C, competitive interaction.
FIGURE 1.
Effect of small molecules on AcpA phosphatase activity. Enzyme activity was measured using 1 mm pNPP as substrate in 25 mm MES, pH 6.2, buffer. A control assay without inhibitor was always included in the plates as positive internal standard. The development of yellow color was quantified by continuous reading at 412 nm, and the p-nitrophenol released by the action of the enzyme was calculated using ϵ = 17.7 mm−1 cm−1. Screening was performed in 96-well format in a final volume of 300 μl with 1 μg/ml of reaction mixture of purified enzyme. The enzyme activity was assayed against 1152 small molecules dispensed in the Prestwick Chemical Library. The effects of ascorbate on AcpA activity, indicated by the arrowhead, inhibited >40% the release of p-nitrophenol and was selected for further studies.
Two inhibitors, thonzonium bromide and procainamide, were identified in the primary screen for AcpB. Further characterization of their kinetic parameters showed they are competitive inhibitors with Ki in the low micromolar range (0.59 ± 0.23 μm and 14.8 ± 3.4 μm, respectively, Table 2). Similarly, two competitive inhibitors were identified for AcpC, atracurium besylate and myricetin, with Ki of 342 ± 50 μm and 154 ± 89 μm, respectively (Table 2). Kaempherol, a flavonoid similar to myricetin, also showed competitive inhibition with similar Ki (Table 2).
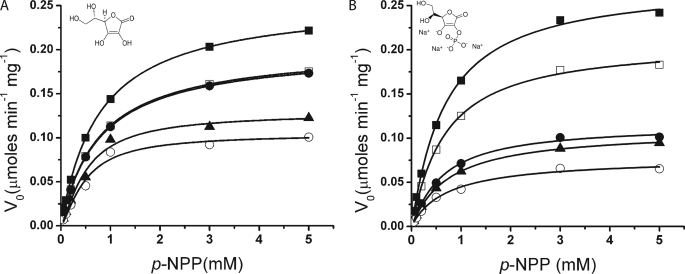
The results obtained in the primary screen of AcpA showed that ascorbate decreased the enzyme V0 by 53% (Fig. 2). The derived kinetic parameters showed that ascorbate behaves as a noncompetitive inhibitor with apparent Ki of 380 ± 160 μm. Once AcpA reacted with ascorbate, the activity was not recovered even after exhaustive dialysis. This loss of activity may indicate that the enzyme modification was irreversible and occurs by covalent modification.
FIGURE 2.
AcpA enzyme kinetics in presence of inhibitors. Assays were performed in triplicate in 96-well plates using the model substrate pNPP in 25 mm MES buffer, pH 6.2, with 1 μg/ml purified enzyme. Enzyme activity was monitored continuously, and the initial velocity (V0) was calculated from the linear range. The kinetic parameters were estimated by nonlinear regression analysis using MicroCal Origin 8. Symbols: The control assay (no inhibitor added) is indicated by ■ in both panels. The symbols used to identify the concentrations of ascorbate are: A, 0.05 mm (□), 0.25 mm (●), 0.5 mm (▴), and 0.75 mm (○). 2-Phosphorascorbate results are depicted in B and the concentrations used were 0.05 μm (□), 0.25 μm (●), 0.5 μm (▴), and 0.75 μm (○). The values used in each figure are the average of three independent determinations. The chemical scaffolds of the inhibitors used in each case are shown as sodium salts.
Using the ascorbate scaffold as a guide, the effect of four other compounds with different functional groups on the ascorbate skeleton was assayed. The inhibition constants obtained are shown in Table 2. We found that 2-phospho-l-ascorbic acid and 5,6-isopropylidene-l-ascorbic acid were stronger inhibitors than l-ascorbic acid, whereas 2-sulfate-l-ascorbic acid and 6-palmitate-l-ascorbic acid had no effect on AcpA activity. The highest inhibition was achieved with 2-phosphoascorbate (apparent Ki of 0.32 ± 0.16 μm). Saturation kinetics determined that 2-phosphoascorbate as well as 5,6-isopropylidene ascorbate Ki of 3.27 ± 0.85 μm) are noncompetitive inhibitors of AcpA (Fig. 2). Because the former compound has a phosphate group, we used malachite green (17) to determine if 2-phosphoascorbate could serve as an enzymatic substrate for AcpA. After incubating a reaction mix with AcpA (1–5 μg/ml) for more than 4 h using 2-phospho-l-ascorbic acid as a substrate, no pyrophosphate was released (data not shown). These results demonstrated that 2-phosphoascorbate is not an enzymatic substrate for AcpA.
Analysis of AcpA Histidine Ascorbylation
The modification of the kinetic parameters by ascorbate, 2-phospho-l-ascorbate, and 5,6-isopropylidene ascorbate on AcpA activity indicated that the inhibition is noncompetitive and irreversible. Although the crystal structure of AcpA is available (20), it was not possible to identify the specific sites of ligand-protein interaction by in silico modeling due to the kind of inhibition identified. However, it is well known that histidine, lysine, and arginine residues are the main targets of the Maillard reaction and that these residues can be modified by glycation or ascorbylation (21). A previous analysis of the protein structure indicates that the catalytic site has four histidine residues, His-106, His-287, His-288, and His-350 (20). These observations, together with the higher inhibition effects of 2-phosphoascorbate on AcpA activity, suggested potential ascorbylation sites. Consequently, we conducted site-directed mutagenesis on the four His residues of the active site (His-106 → Ala, His-287 → Ala, His-288 → Ala, and His-350 → Ala), and the mutant proteins were successfully purified. Replacement of His with Ala at positions 106, 288, and 350 rendered inactive enzymes (data not shown). In the His-287 → Ala mutant the enzyme activity decreased ∼10-fold related to the wild type (0.025 ± 0.006 μmol min−1 mg−1), however 2-phosphoascorbate no longer inhibited the enzyme activity (supplemental Fig. S1).
Intramacrophage Survival Assays
F. tularensis acid phosphatase A contributes to 90% of the total phosphatase activity displayed by this bacterium. Its biological role is still obscure, but this enzyme has been indicated as a major F. tularensis virulence factor (4, 22). Since we successfully identified a compound that specifically inhibits AcpA activity, a set of bioassays were performed to validate the biological relevance of the biochemical results herein described. The assays were carried out using two different wild-type strains, F. tularensis novicida U112 and F tularensis LVS, as well as with their respective ΔacpA mutant strains.
To determine if the AcpA inhibitors affected the growth/survival of Francisella, growth inhibition assays were performed in liquid culture in presence of the chemical compounds selected. None of the AcpA inhibitors affected the viability of the strains when used at concentrations lower than 40 mm. The minimal inhibitory concentrations for the compounds assayed (ascorbate and 2-phosphoascorbate) were similar and higher than 100 mm.
Because AcpA has been related to the ability of F. tularensis to survive inside the host phagocytic cells (4), we evaluated the effect of the inhibitors using macrophage cell lines. The effect of ascorbate and 2-phosphoascorbate on the intracellular growth of F. novicida was tested at 10, 30, and 100 mm throughout the assay against each of the strains described. We found that the highest concentration was toxic to the macrophages as evidenced by vital staining with Trypan blue, whereas the addition of either 30 mm ascorbate or 2-phosphoascorbate to macrophages caused no deleterious effect on viability and interleukin production (Fig. S2). At 10 mm a 2 log decrease in the intramacrophage survival of F. novicida was observed while the strongest effect was obtained at 30 mm (Fig. 3A). Similar results were observed for F. tularensis LVS (Fig. 3C). The specificity of the inhibition was assayed by testing the effect of the inhibitors on the survival of the respective isogenic F. tularensis novicida ΔacpA (Fig. 3B) and F. tularensis LVS ΔacpA (Fig. 3D) under the same conditions. Although the ΔacpA mutant survives less than the wild-type strain within macrophages, no effect was observed for any of the inhibitors (Fig. 3, B and D). This result indicates that the chemicals specifically targeted AcpA. Similar results were obtained using either JT774.1 or THP-1 macrophage cell lines.
FIGURE 3.
F. tularensis intramacrophage survival assays performed in the presence of AcpA-specific inhibitors. The assay was carried out using J774.1 murine macrophage cell line infected with F. tularensis novicida (A) and F. tularensis novicida ΔacpA (B). The second assays was carried out with phorbol 12-myristate 13-acetate-induced THP-1 macrophage cell line infected with F. tularensis LVS (C) and F. tularensis LVS ΔacpA (D). Mutant strains were used to demonstrate the specific action of the inhibitors selected. Survival is expressed in CFU/ml counted in agar plates after macrophages were lysed by 0.05% SDS treatment. Inhibitors were added to the cell culture at 30 mm final concentration and kept throughout the experiment until the cells were collected. Symbols: Control assay (no inhibitor added) (■); ascorbate 10 mm (□); 2-phosphoascorbate 10 mm (▾); ascorbate 30 mm (●); and 2-phosphoascorbate (▵) 30 mm. The assays were performed in triplicate.
CONCLUSIONS
Using a systematic enzyme screening approach we were able to select chemical compounds with the ability to inhibit three of the four primary F. tularensis acid phosphatases. Exhaustive analysis using enzymatic inhibition kinetics indicated that AcpB and AcpC were competitively inhibited. On the other hand, ascorbate and ascorbate derivatives inhibited AcpA in a nonreversible, noncompetitive fashion. Several lines of evidence suggest that AcpA activity is critical for F. tularensis survival inside infected cells and that purified AcpA could inhibit the respiratory burst of porcine neutrophils (10) High expression of the AcpA gene was detected in pathogenic F. tularensis when it was compared with nonvirulent strains (23), and ΔacpA mutants were less virulent than the isogenic wild-type strain (4, 22, 24). Because of its importance in the pathogenesis of this organism, we focused our work on evaluating the mechanisms of AcpA inhibition and validating the biochemical results in intramacrophage survival. Ascorbate, as well as sugars, react nonenzymatically with proteins by forming covalent adducts with amino acids. This biochemical process is known as the Maillard reaction (21). The amino acids lysine, arginine, and histidine were previously identified as the preferred targets of protein ascorbylation (21). Any of these residues present in AcpA can be equally ascorbylated, and consequently the mechanism affecting activity is difficult to elucidate. However, when 2-phosphoascorbate was tested, the Ki values achieved were three orders of magnitude lower than those obtained with ascorbate. Although this interaction is noncompetitive, this observation directed our research to analyze the residues in close proximity of the catalytic pocket. Previous analysis of the structure of AcpA revealed a patch of four His residues on the solvent side of the enzyme, contiguous to the active residue (Ser-175). It was proposed that these histidines work as the general base to activate a water molecule during catalysis (20). Our hypothesis was that the electrostatic interaction between the positively charged His patch and the negatively charged phosphate group of 2-phosphoascorbate can enhance the interaction of this compound with this precise location on the protein surface. The results herein obtained with Ala replacement assays are in agreement with the importance previously predicted for the histidine patch (20). The replacement of any His residues (106, 287, 288, and 350) drastically affected the catalytic abilities of this protein. Notwithstanding, the low residual activity displayed by the His-287 → Ala mutant was useful to identify these residues as putative ascorbylation targets, because the activity of this mutant protein was not inhibited by the presence of 2-phosphoascorbate.
Chemical reactions between ascorbate, or the products of oxidative ascorbate degradation with proteins, can occur in vitro under physiological conditions (25). Some evidence suggests that these reactions also occur in the same way in vivo (26). The question arises as to whether or not the biological function of AcpA can be hampered by this modification. Can ascorbate be used to inhibit AcpA activity and directly affect the ability of F. tularensis to survive inside macrophages? Our results indicate that F. tularensis does not replicate and is killed inside macrophages in the presence of ascorbate.
The clinical importance of ascorbate has been extensively studied and analyzed (27–29). However, a biochemical mechanism by which ascorbate inhibits enzymatic activities with a clear in vivo impact has not been described. The discussion of the clinical importance of vitamin C is well known and out of the scope of this report. Dr. Robert F. Klenner has published hundreds of clinical cases of viral and bacterial infections treated successfully with massive intravenous doses of vitamin C (30). He described toxin neutralization, antibiotic effects, and stimulation of the immune system. Perhaps the results herein discussed can offer some insight at the molecular level to help further understanding of the positive effects of ascorbate in clinical cases described more than 50 years ago.
Acknowledgments
We thank Dr. Max Teplitski for his valuable comments, Beverly Driver for her technical assistance, and Ricardo Valladares for the proofreading of the manuscript.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- pNPP
- p-nitrophenyl phosphate
- CHA
- on cysteine heart agar
- TSB
- tryptic soy broth
- MES
- 4-morpholineethanesulfonic acid
- MOPS
- 4-morpholinepropanesulfonic acid
- CHES
- 2-(cyclohexylamino)ethanesulfonic acid
- LVS
- live vaccine strain.
REFERENCES
- 1.Dennis D. T., Inglesby T. V., Henderson D. A., Bartlett J. G., Ascher M. S., Eitzen E., Fine A. D., Friedlander A. M., Hauer J., Layton M., Lillibridge S. R., McDade J. E., Osterholm M. T., O'Toole T., Parker G., Perl T. M., Russell P. K., Tonat K. (2001) JAMA 285, 2763–2773 [DOI] [PubMed] [Google Scholar]
- 2.Khan A. S., Morse S., Lillibridge S. (2000) Lancet 356, 1179–1182 [DOI] [PubMed] [Google Scholar]
- 3.Oyston P. C., Sjostedt A., Titball R. W. (2004) Nat. Rev. Microbiol. 2, 967–978 [DOI] [PubMed] [Google Scholar]
- 4.Mohapatra N. P., Soni S., Reilly T. J., Liu J., Klose K. E., Gunn J. S. (2008) Infect. Immun. 76, 3690–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baca O. G., Roman M. J., Glew R. H., Christnet R. F., Buhler J. E., Aragon A. S. (1993) Infect. Immun. 61, 4232–4239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chhatwal G. S., Walker M. J., Yan H., Timmis K. H., Guzmán C. A. (1997) Microb. Pathog. 22, 257–264 [DOI] [PubMed] [Google Scholar]
- 7.Saha A. K., Dowling J. N., LaMarco K. L., Das S., Remaley A. T., Olomu N., Pope M. T., Glew R. H. (1985) Arch. Biochem. Biophys. 243, 150–160 [DOI] [PubMed] [Google Scholar]
- 8.Aragon V., Kurtz S., Cianciotto N. P. (2001) Infect. Immun. 69, 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron G. S., Reilly T. J., Nano F. E. (1999) FEMS Microbiol. Lett. 176, 85–90 [DOI] [PubMed] [Google Scholar]
- 10.Reilly T. J., Baron G. S., Nano F. E., Kuhlenschmidt M. S. (1996) J. Biol. Chem. 271, 10973–10983 [DOI] [PubMed] [Google Scholar]
- 11.Nobeli I., Ponstingl H., Krissinel E. B., Thornton J. M. (2003) J. Mol. Biol. 334, 697–719 [DOI] [PubMed] [Google Scholar]
- 12.Kuznetsova E., Proudfoot M., Gonzalez C. F., Brown G., Omelchenko M. V., Borozan I., Carmel L., Wolf Y. I., Mori H., Savchenko A. V., Arrowsmith C. H., Koonin E. V., Edwards A. M., Yakunin A. F. (2006) J. Biol. Chem. 281, 36149–36161 [DOI] [PubMed] [Google Scholar]
- 13.Lorca G. L., Ezersky A., Lunin V. V., Walker J. R., Altamentova S., Evdokimova E., Vedadi M., Bochkarev A., Savchenko A. (2007) J. Biol. Chem. 282, 16476–16491 [DOI] [PubMed] [Google Scholar]
- 14.Shoju H., Sueyoshi N., Kameshita I. (2006) Anal. Biochem. 353, 290–292 [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez C. F., Ackerley D. F., Lynch S. V., Matin A. (2005) J. Biol. Chem. 280, 22590–22595 [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez C. F., Proudfoot M., Brown G., Korniyenko Y., Mori H., Savchenko A. V., Yakunin A. F. (2006) J. Biol. Chem. 281, 14514–14522 [DOI] [PubMed] [Google Scholar]
- 17.Baykov A. A., Evtushenko O. A., Avaeva S. M. (1988) Anal. Biochem. 171, 266–270 [DOI] [PubMed] [Google Scholar]
- 18.Wehrly T. D., Chong A., Virtaneva K., Sturdevant D. E., Child R., Edwards J. A., Brouwer D., Nair V., Fischer E. R., Wicke L., Curda A. J., Kupko J. J., 3rd, Martens C., Crane D. D., Bosio C. M., Porcella S. F., Celli J. (2009) Cell Microbiol. 11, 1128–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reilly T. J., Felts R. L., Henzl M. T., Calcutt M. J., Tanner J. J. (2006) Protein Expr. Purif. 45, 132–141 [DOI] [PubMed] [Google Scholar]
- 20.Felts R. L., Reilly T. J., Tanner J. J. (2006) J. Biol. Chem. 281, 30289–30298 [DOI] [PubMed] [Google Scholar]
- 21.Hasenkopf K., Rönner B., Hiller H., Pischetsrieder M. (2002) J. Agric. Food. Chem. 50, 5697–5703 [DOI] [PubMed] [Google Scholar]
- 22.Mohapatra N. P., Balagopal A., Soni S., Schlesinger L. S., Gunn J. S. (2007) Infect. Immun. 75, 390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernychová L., Stulík J., Halada P., Macela A., Kroca M., Johansson T., Malina M. (2001) Proteomics 1, 508–515 [DOI] [PubMed] [Google Scholar]
- 24.Sjöstedt A. (2003) Curr. Opin. Microbiol. 6, 66–71 [DOI] [PubMed] [Google Scholar]
- 25.Ortwerth B. J., Olesen P. R. (1980) Biochim. Biophys. Acta 956, 10–22 [DOI] [PubMed] [Google Scholar]
- 26.Simpson G. L., Ortwerth B. J. (2000) Biochim. Biophys. Acta 1501, 12–24 [DOI] [PubMed] [Google Scholar]
- 27.Pauling L. (1971) Am. J. Clin. Nutr. 24, 1294–1299 [DOI] [PubMed] [Google Scholar]
- 28.Pauling L. (1989) Science 24, 1535. [DOI] [PubMed] [Google Scholar]
- 29.Mandl J., Szarka A., Bánhegyi G. (2009) Br. J. Pharmacol. 157, 1097–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klenner F. R. (1951) South Med. J. 113, 101–107 [PubMed] [Google Scholar]