Abstract
The regulation of NFATc1 expression is important for osteoclast differentiation and function. Herein, we demonstrate that macrophage-colony-stimulating factor induces NFATc1 degradation via Cbl proteins in a Src kinase-dependent manner. NFATc1 proteins are ubiquitinated and rapidly degraded during late stage osteoclastogenesis, and this degradation is mediated by Cbl-b and c-Cbl ubiquitin ligases in a Src-dependent manner. In addition, NFATc1 interacts endogenously with c-Src, c-Cbl, and Cbl-b in osteoclasts. Overexpression of c-Src induces down-regulation of NFATc1, and depletion of Cbl proteins blocks NFATc1 degradation during late stage osteoclastogenesis. Taken together, our data provide a negative regulatory mechanism by which macrophage-colony-stimulating factor activates Src family kinases and Cbl proteins, and subsequently, induces NFATc1 degradation during osteoclast differentiation.
Keywords: Cell/Differentiation, Cytokines, Gene/Regulation, Proteases/Ubiquitination, Protein/Degradation, Cbl, NFATc1, Osteoclast Differentiation
Introduction
Bone remodeling continuously renews the skeleton and maintains its structure through a spatially coordinated balance between bone formation by osteoblasts and bone resorption by osteoclasts. Multinucleated mature osteoclasts differentiate from hematopoietic cells following a sequential process that includes proliferation, differentiation, fusion, and activation. Two essential factors, macrophage-colony-stimulating factor (M-CSF)2 and receptor activator of nuclear factor κB ligand (RANKL), are produced by osteoblasts and can support osteoclast formation from monocyte/macrophage precursors of hematopoietic origin (1, 2).
Binding of RANKL to its receptor, receptor activator of nuclear factor κB (RANK), activates NF-κB, c-Jun N-terminal kinase (JNK), p38, extracellular signal-regulated kinase (ERK), and Akt, which are important for osteoclast differentiation, activation, and survival (3). RANKL regulates various transcription factors including NF-κB, c-Fos, and nuclear factor of activated T cells (NFAT) c1, which act as positive modulators of osteoclast differentiation (4, 5). During osteoclastogenesis, RANKL binding to its receptor up-regulates c-Fos expression. The binding of c-Fos to the NFATc1 promoter region induces NFATc1 gene expression (6), and NFATc1 can autoamplify its expression by binding to its own promoter, which leads to robust induction of NFATc1 during RANKL-induced osteoclast differentiation (7).
Ectopic expression of NFATc1 causes osteoclast precursors to undergo efficient differentiation in the absence of RANKL, and NFATc1-deficient embryonic stem cells fail to differentiate into osteoclasts in response to RANKL, suggesting that NFATc1 acts as a key modulator of osteoclastogenesis (6, 8). NFATc1 induces the expression of target genes by binding to the NFAT-binding sites in the promoter regions of genes such as: ACP5, CTSK, and osteoclast-associated receptor (OSCAR) (6, 9, 10), which are key regulators of osteoclast differentiation and/or function. Recently, we have demonstrated that NFATc1 plays a role in the osteoclast multinucleation process via up-regulation of the d2 isoform of vacuolar ATPase V0 domain (Atp6v0d2) and the dendritic cell-specific transmembrane protein (DC-STAMP) (11). Therefore, NFATc1 is thought be a key regulator of RANKL-induced osteoclast differentiation, fusion, and activation.
Cbl family proteins are cytoplasmic adaptor molecules that have been implicated in the regulation of signaling from a variety of receptor tyrosine kinases, including antigen receptors in lymphocytes and growth factor receptors (12). Cbl-b and c-Cbl are two members of the mammalian Cbl family, and like other Cbl family members, these proteins consist of several functional domains, including an SH2-like phosphotyrosine-binding domain, a RING finger that interacts with the E2 ubiquitin-conjugating enzyme, a proline-rich domain, and a leucine zipper (12). One mechanism by which Cbl-b and c-Cbl may negatively regulate signaling is by acting as E3 ubiquitin ligases, which results in the degradation of activated molecules by way of the proteasome (12, 13). This E3 ubiquitin ligase activity has been localized to the RING finger of Cbl proteins, and the RING finger has been associated with the negative regulation of a number of tyrosine kinases, including epidermal growth factor receptor and Syk (13). Cbl-mediated ubiquitination of Src, epidermal growth factor receptor, and other target proteins is required for the Src-catalyzed phosphorylation of Cbl (14, 15). The c-Cbl-dependent ubiquitination of Src and c-Cbl requires the c-Cbl RING finger, Src kinase activity, and the tyrosine phosphorylation of c-Cbl on Tyr-371 (15). Inhibition of Src family kinases blocks epidermal growth factor-induced phosphorylation of c-Cbl and ubiquitination of epidermal growth factor receptor (14).
In this study, we report that NFATc1 is degraded at the late stage of osteoclast differentiation by means of Src kinase-dependent Cbl protein-mediated ubiquitination. NFATc1 interacts with c-Src, Cbl-b, and c-Cbl in osteoclasts, and Src kinases mediate M-CSF-induced phosphorylation of Cbl proteins, and subsequently, NFATc1 degradation. This study reveals a novel negative regulation of osteoclast differentiation via ubiquitination of NFATc1 by Cbl proteins.
EXPERIMENTAL PROCEDURES
Mice
Cbl-b-deficient mice were described previously (16). Mice deficient in IFNAR1 were purchased from The Jackson Laboratory (17). Breeding and genotyping were performed as described previously (17, 18).
Reagents and Plasmids
MG132 and SU6656 were purchased from Calbiochem. ALLM and E-64 were purchased from Biomol-Enzo Life Sciences (Plymouth Meeting, PA). Expression constructs encoding FLAG-tagged NFATc1, chicken c-Src, a kinase-inactive mutant of c-Src (c-Src-KD), hemagglutinin (HA)-tagged Cbl-b, c-Cbl, an N-terminal deletion mutant of Cbl-b (Cbl-b-ΔN), and a ubiquitin ligase-deficient RING finger mutant of c-Cbl (C3AHN) were described previously (9, 19–22). For c-Cbl knockdown experiments, oligonucleotides for siRNA were generated by targeting a 19-base sequence (5′-GGAGACACTTTCCGGATTA-3′) of the mouse c-Cbl gene. Sense and antisense oligonucleotides were annealed and ligated into the pSuper-retro vector (Oligoengine, Seattle, WA).
Osteoclast Formation
Murine osteoclasts were prepared from bone marrow cells as described previously (11). In brief, bone marrow cells were cultured in α-minimal essential medium containing 10% fetal bovine serum with M-CSF (5 ng/ml) for 16 h. Non-adherent cells were harvested and cultured for 3 days in the presence of M-CSF (30 ng/ml). Floating cells were removed, and adherent cells (bone marrow-derived macrophages (BMMs)) were used as osteoclast precursors. To generate osteoclasts, BMMs were cultured with M-CSF (30 ng/ml) and RANKL (100 ng/ml) for 3–4 days.
Retroviral Infection
To generate retroviral stock, retroviral vectors were transfected into packaging cell line Plat E (a gift from T. Kitamura) using FuGENE 6 (Roche Applied Sciences). Viral supernatant was collected from cultured media 24–48 h after transfection. BMMs were incubated with viral supernatant for 8 h in the presence of Polybrene (10 μg/ml). After removing the viral supernatant, BMMs were used to generate osteoclasts.
Semiquantitative Reverse Transcription-PCR and Real-time PCR
Semiquantitative reverse transcription-PCR and real-time PCR analyses were performed as described previously (23, 24)
Northern Blot Analysis
Total RNA samples were electrophoresed and transferred to nylon membranes as described (25). Hybridization was performed at 42 °C for 16 h with a [32P]dCTP DNA probe prepared using the Ready-to-Go labeling kit (Amersham Biosciences) and ProbeQuant G-50 purification kit (Amersham Biosciences). After washing with 0.1× SSC and 0.1% SDS at 56 °C for 1 h, the membrane was exposed to x-ray film (Eastman Kodak).
Fractionation, Immunoprecipitation, and Western Blot Analysis
Cells from transfected HEK 293T, BMMs, or osteoclasts were harvested after washing with ice-cold phosphate-buffered saline and then lysed in extraction buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1 mm EDTA, 0.5% Nonidet P-40, 0.01% protease inhibitor mixture). Cultured cells were fractionated using the NE-PER nuclear and cytoplasmic extraction reagents (Pierce) according to the manufacturer's protocol. Samples were immunoprecipitated with the indicated antibodies, or whole cell lysates were subjected to SDS-PAGE and Western blotting. Primary antibodies included c-Fos (Calbiochem), 4G10 (Upstate Biotech Millipore), OSCAR (26), actin, FLAG (Sigma-Aldrich), HA (Roche Applied Sciences), NFATc1, retinoblastoma-associated protein, ubiquitin, Cbl-b, c-Src, TRAP (Santa Cruz Biotechnology, Santa Cruz, CA), and c-Cbl (BD Biosciences). Horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences) were probed and developed with ECL solution (Amersham Biosciences). Signals were detected and analyzed by an LAS3000 luminescent image analyzer (Fuji Photo Film Co., Tokyo, Japan).
RESULTS
NFATc1 Proteins Are Down-regulated at the Late Stage of Osteoclastogenesis
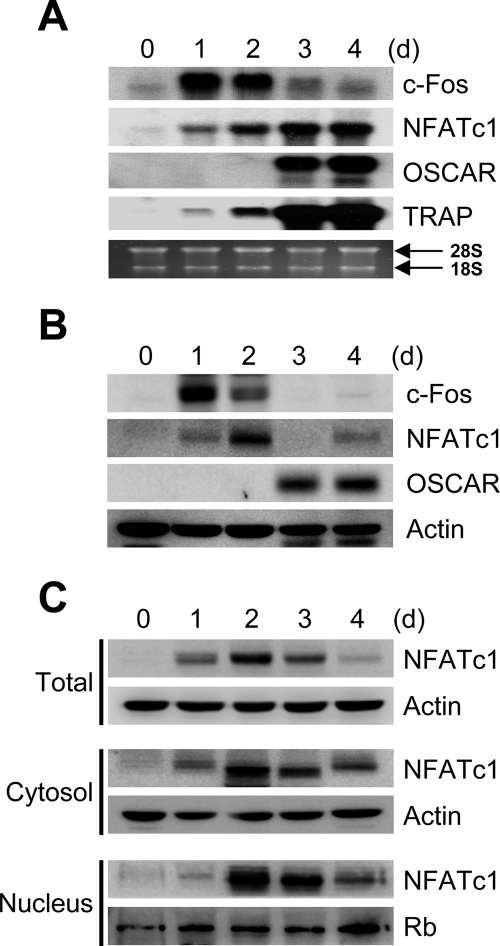
We first investigated the expression patterns of various genes during the osteoclast differentiation. When BMMs were cultured with M-CSF and RANKL, c-Fos mRNA expression levels reached a peak at day 1 and gradually decreased during osteoclastogenesis (Fig. 1A). The induction of c-Fos gene expression was followed by the expression of NFATc1. In addition, osteoclast-specific genes such as ACP5 and OSCAR were also expressed at later time points than NFATc1. When we examined the expression pattern of those genes by Western blot analysis, similar results were obtained (Fig. 1B). Interestingly, inconsistent with the expression pattern of NFATc1 mRNA expression, which gradually increased in the presence of RANKL until day 4, NFATc1 protein levels peaked at day 2 and dramatically decreased to basal level by day 4. Because expression levels of NFATc1 proteins were greatly decreased during late stage osteoclastogenesis, we hypothesized that the down-regulation of NFATc1 could be due to nuclear import of these proteins in the presence of RANKL. This possibility prompted us to investigate the localization of NFATc1 proteins during RANKL-induced osteoclastogenesis. When the amounts of total NFATc1 proteins in nuclear and cytoplasmic extracts were assessed, the rate of disappearance of NFATc1 proteins in both fractions was comparable with that in total extracts (Fig. 1C). These results suggest that NFATc1 proteins are down-regulated during late stage osteoclastogenesis but that NFATc1 continues to be transcribed until the end stage of osteoclast differentiation as indicated by its high representation in nuclear extracts.
FIGURE 1.
NFATc1 protein expression is down-regulated during late stage osteoclastogenesis. A–C, BMMs were cultured with M-CSF and RANKL for the indicated times. A, total RNA was collected from each time point and analyzed by Northern blot using probes for c-Fos, NFATc1, OSCAR, and TRAP. B, whole cell extracts were subjected to Western blot analysis with c-Fos, NFATc1, OSCAR, and actin. C, whole cell extract (upper), cytoplasmic (middle), or nuclear (lower) fractions were harvested from cultured cells and analyzed by Western blot for detection of NFATc1. Actin and retinoblastoma-associated protein (Rb) were used as loading controls.
NFATc1 Is Ubiquitinated in the Cytoplasm
Because IFN-β signaling plays a negative role in RANKL-induced osteoclastogenesis through down-regulation of c-Fos proteins (27), we examined whether IFN-β signaling is involved in the down-regulation of NFATc1 proteins during osteoclast differentiation using IFNAR1 knock-out mice deficient in one of the IFN-α/β receptor components. Total cell extracts from BMMs of wild type (WT) and IFNAR1 knock-out mice cultured with M-CSF and RANKL for the indicated times were analyzed by Western blotting (supplemental Fig. 1). Although the protein expression levels of c-Fos, NFATc1, and OSCAR were slightly increased in IFNAR1 knock-out as compared with WT, down-regulation of NFATc1 proteins during late stage osteoclastogenesis was observed in both WT and IFNAR1 knock-out cultures (supplemental Fig. 1), suggesting that IFN-β signaling does not affect the down-regulation of NFATc1 proteins during RANKL-mediated osteoclastogenesis.
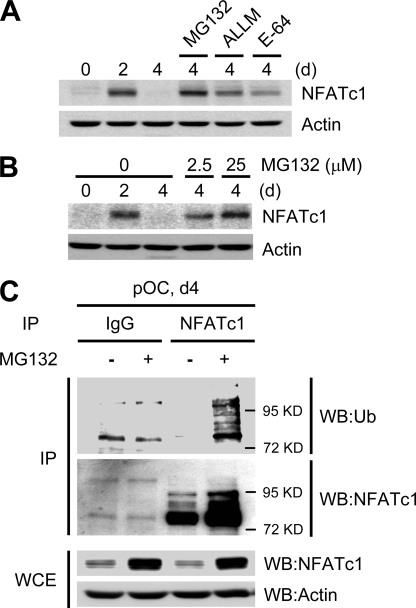
To determine the major degradation pathways for NFATc1 during late stage osteoclastogenesis, we compared NFATc1 protein expression levels in the presence of various protease inhibitors, including MG132, an inhibitor of the ubiquitin-proteasome system; ALLM a calpain inhibitor; E-64; and a cysteine protease inhibitor. BMMs were cultured for the indicated times in the presence of M-CSF and RANKL and were treated with different protease inhibitors for 6 h on day 4. MG132 strongly increased the expression of NFATc1 proteins, whereas ALLM and E-64 treatment did not noticeably affect NFATc1 expression (Fig. 2A). MG132 increased the expression level of NFATc1 proteins in a dose-dependent manner (Fig. 2B), suggesting that degradation of NFATc1 proteins during late stage osteoclastogenesis is chiefly mediated by the ubiquitin-proteasome pathway.
FIGURE 2.
Ubiquitin-mediated NFATc1 degradation. A–C, BMMs were cultured with M-CSF and RANKL for the indicated times. A, the proteasome inhibitor MG132 (25 μm), the calpain inhibitor ALLM (50 μm), or the cysteine inhibitor E-64 (50 μm) were added 6 h before cell lysis on day 4 as indicated. B, various concentrations of MG132 were added 6 h prior to cell lysis on day 4 as indicated. In A and B, lysates were immunoblotted with NFATc1 and actin antibodies. C, BMMs were cultured with M-CSF and RANKL for 4 days. MG132 (25 μm) was added 4 h before cell lysis on day 4 as indicated. Whole cell lysates (WCE) from mature osteoclasts were immunoprecipitated with anti-NFATc1 or IgG control antibodies; immunoprecipitates (IP) were probed with anti-ubiquitin (Ub, upper panel) or anti-NFATc1 antibodies (middle panel). Whole cell extracts were probed with anti-NFATc1 or anti-actin (control) antibodies (lower panel). WB, Western blot.
Proteins targeted for proteasome-mediated degradation usually undergo ubiquitination. To investigate whether NFATc1 down-regulation occurs directly via the ubiquitin-proteasome pathway, we examined the ubiquitination of NFATc1 during late stage osteoclastogenesis. BMMs were cultured with M-CSF and RANKL for 4 days in the absence or presence of MG132. When total cell extracts were harvested and immunoprecipitated with anti-NFATc1 and probed with anti-ubiquitin or anti-NFATc1, ubiquitinated NFATc1 proteins were detected only in MG132-treated day 4 samples (Fig. 2C). Ubiquitination was not observed in samples immunoprecipitated with IgG control antibodies (Fig. 2C). Ubiquitinated NFATc1 proteins were detected in the cytoplasmic extracts derived from BMMs treated with M-CSF and RANKL in the presence of MG132, whereas ubiquitinated NFATc1 proteins were not detected in nuclear extracts even in the presence of MG132 (data not shown). Taken together, these results indicate that NFATc1 is directly ubiquitinated in the cytoplasm during late stage osteoclastogenesis.
Cbl Proteins Mediate NFATc1 Ubiquitination
Cbl proteins are well known E3 ubiquitin ligases that target activated receptor tyrosine kinases as well as other non-receptor protein tyrosine kinases (12, 15, 28–30). Src and Cbl proteins are known to be key players in the regulation of signaling cascades in osteoclasts (31). Therefore, we examined the expression patterns of Src and Cbl proteins during osteoclastogenesis. As shown in Fig. 3A, NFATc1 protein expression was up-regulated and subsequently degraded after day 2 in culture, whereas Cbl-b, c-Cbl, and Src expression levels gradually increased during osteoclast differentiation. These data suggest that Cbl proteins might mediate NFATc1 ubiquitination. To determine whether Cbl proteins are involved in NFATc1 degradation, we transiently transfected HEK 293T cells with various constructs driving the expression of Cbl-b, c-Cbl, or NFATc1. As a means of activating Cbl proteins, c-Src was co-expressed (20). When co-expressed with c-Src, both Cbl-b and c-Cbl caused a marked reduction in NFATc1 protein expression levels (Fig. 3, B and C, lane 2). NFATc1 degradation by Cbl-b/c-Cbl was dependent on c-Src kinase activity as no reductions in NFATc1 expression levels were seen when a kinase-dead form of c-Src was co-expressed in culture (Fig. 3, B and C, lane 3). In addition, NFATc1 degradation was dependent on the presence of a functional E3 ligase domain of Cbl proteins as evidenced by the lack of NFATc1 degradation in cultures in which a truncated form of Cbl-b (Cbl-DN, a deletion of N-terminal SH2 domain) (21) or a ubiquitin ligase-deficient RING finger mutant of c-Cbl (C3AHN) (22) was co-expressed (Fig. 3, D and E). Furthermore, degradation of NFATc1 in the presence of Cbl and c-Src proteins was attenuated by the addition of MG132 (Fig. 3, F and G, lanes 2 and 4). We observed that Cbl proteins were also down-regulated by overexpression of Src (Fig. 3, B–G). Taken together, these data suggest that Cbl proteins in conjunction with ubiquitin ligases likely play a key role in NFATc1 down-regulation during osteoclastogenesis.
FIGURE 3.
Cbl proteins mediate NFATc1 ubiquitination in a Src kinase-dependent manner. A, BMMs were cultured with M-CSF and RANKL for the indicated times. Lysates were immunoblotted with NFATc1, Cbl-b, c-Cbl, c-Src, TRAP, and actin antibodies. B, HEK 293T cells were transfected with FLAG-NFATc1, HA-Cbl-b, and either HA-c-Src-WT or a kinase-dead (KD) mutant form of c-Src (K295M) as indicated. C, HEK 293T cells were transfected with FLAG-NFATc1, HA-c-Cbl, and either c-Src-WT or c-Src-KD as indicated. D, HEK 293T cells were transfected with FLAG-NFATc1, HA-c-Src, and either HA-Cbl-b-WT or a truncation mutant in which the N-terminal SH2 domain of Cbl-b is deleted (ΔN) as indicated. E, HEK 293T cells were transfected with FLAG-NFATc1, c-Src, and either HA-c-Cbl-WT or a ubiquitin ligase-deficient RING finger mutant of c-Cbl (C3AHN) as indicated. F, HEK 293T cells were transfected with FLAG-NFATc1, HA-Cbl-b, and HA-c-Src. MG132 was added 4 h before cell lysis at a concentration of 25 μm as indicated. G, HEK 293T cells were transfected with FLAG-NFATc1, HA-c-Cbl, and c-Src. MG132 was added 8 h before cell lysis at a concentration of 10 μm as indicated. B–G, lysates were immunoblotted with FLAG, HA, and actin antibodies. Results presented are representative of three independent sets of similar experiments.
M-CSF Induces Cbl Protein Phosphorylation and NFATc1 Degradation
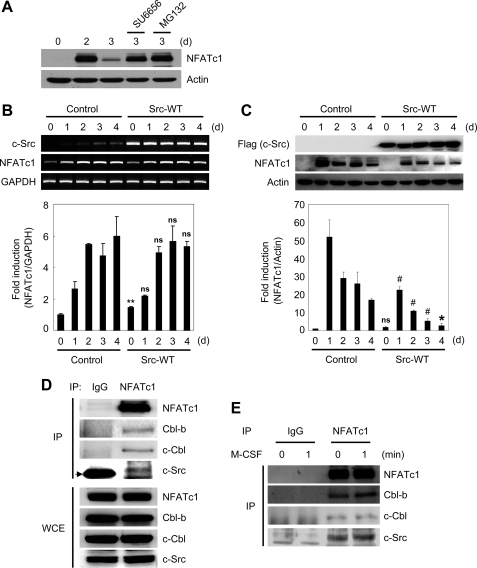
Given our observation that Cbl proteins down-regulate NFATc1 expression when co-expressed with c-Src in HEK 293T cells, we tested whether Src kinase activity is requisite for NFATc1 degradation during osteoclast differentiation. BMMs were cultured with M-CSF and RANKL for 3 days. On day 3, cultures were treated with Src kinase inhibitor SU6656 or MG132 for 4 h prior to cell lysis. NFATc1 protein levels peaked at day 2 and then dramatically decreased at day 3; however, NFATc1 degradation was strongly attenuated in the presence of MG132 or SU6656 (Fig. 4A). In addition, overexpression of c-Src-WT, but not kinase-inactive c-Src-K295M, strongly attenuated RANKL-induced NFATc1 protein expression but did not significantly affect NFATc1 mRNA levels (Fig. 4, B and C, and supplemental Fig. 2). These data suggest that Src kinase activity plays an important role in NFATc1 degradation during osteoclastogenesis and that blockade of the proteasome pathway by MG132 can override Src kinase activity and sustain NFATc1 protein levels.
FIGURE 4.
Src-Cbl proteins are involved in down-regulation of NFATc1 protein. A, BMMs were cultured with M-CSF and RANKL for the indicated times. The Src kinase inhibitor SU6656 (2 μm) or MG132 (25 μm) was added 8 h before cell lysis. Lysates were probed with NFATc1 and actin antibodies. B and C, BMMs were transduced with pMX-IRES-EGFP (Control) or c-Src-WT retrovirus (Src-WT) and cultured with M-CSF and RANKL for the indicated times. B, reverse transcription-PCR (upper panel) and real-time PCR (lower panel) were performed to detect c-Src, NFATc1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). C, lysates were immunoblotted with FLAG (for overexpressed c-Src), NFATc1, and actin antibodies (upper panel). The relative amounts of NFATc1 are shown in the lower panel. B and C, ns, not significant, #, p < 0.05, *, p < 0.01, **, p < 0.001 versus positive control. Data are expressed as mean ± S.D. of triplicate samples. Results presented are representative of three independent sets of similar experiments. D, lysates from preosteoclasts were immunoprecipitated (IP) with anti-NFATc1 or control IgG antibodies. Immunoprecipitated samples (upper panel) or whole cell lysates (WCE; lower panel) were subjected to Western blotting for detection of NFATc1, Cbl-b, c-Cbl, and c-Src. The arrow indicates the IgG band. E, preosteoclasts were stimulated with M-CSF for the indicated times. Lysates from preosteoclasts were immunoprecipitated with anti-NFATc1 or control IgG antibodies. Immunoprecipitated samples were subjected to Western blot analysis for detection of NFATc1, Cbl-b, c-Cbl, and c-Src.
To examine the interaction of NFATc1 with c-Src and Cbl proteins in osteoclasts, preosteoclast cell lysates were immunoprecipitated with anti-NFATc1 followed by Western blot analysis. Our data indicate that NFATc1 can interact with c-Src and Cbl proteins in osteoclasts in the presence or absence of M-CSF (Fig. 4, D and E).
Next, we determined which cytokine is involved in activation of Cbl proteins during osteoclast differentiation. Preosteoclasts were starved and stimulated with M-CSF or RANKL. Cell lysates were immunoprecipitated with anti-Cbl-b or anti-c-Cbl antibodies followed by Western blot with anti-phosphotyrosine antibody 4G10. M-CSF induced phosphorylation of Cbl-b and c-Cbl, and this phosphorylation was completely blocked by treatment with the Src kinase inhibitor SU6656 (Fig. 5A). Of note, RANKL treatment did not lead to detectable Cbl protein phosphorylation in preosteoclasts (data not shown).
FIGURE 5.
M-CSF induces Cbl phosphorylation and NFATc1 degradation. A, preosteoclasts were starved and pretreated with vehicle or SU6656 (2 μm) for 30 min before stimulation with M-CSF as indicated. Lysates were immunoprecipitated (IP) with anti-Cbl-b or anti-c-Cbl antibodies. Immunoprecipitated samples were probed with anti-phosphotyrosine 4G10, anti-Cbl-b, or anti-c-Cbl antibodies. B, BMMs were cultured for 2 days with M-CSF and RANKL. Cultured cells were washed and further incubated with M-CSF alone or M-CSF and RANKL. MG132 (25 μm) was added 3 h before cell lysis. Lysates were probed with anti-NFATc1 and control anti-actin antibodies.
Next, we tried to determine which cytokines induce NFATc1 degradation during osteoclast differentiation. BMMs were cultured in the presence of M-CSF and RANKL for 2 days. Cultured cells were washed and incubated with M-CSF alone or M-CSF and RANKL. MG132 was added for 3 h prior to cell lysis. As shown in Fig. 5B, NFATc1 degradation occurred in the presence of M-CSF, and this degradation was blocked by the addition of MG132, indicating that NFATc1 degradation in the presence of M-CSF occurs via the proteasome. Treatment with M-CSF and RANKL did not lead to marked NFATc1 degradation, indicating that RANKL somehow sustains NFATc1 expression. We could not analyze the RANKL-treated samples because of the rapid cell death in the absence of M-CSF. Taken together, these data indicate that M-CSF activates Cbl proteins via c-Src kinases, which leads to NFATc1 degradation during osteoclast differentiation.
Cbl Proteins Mediate NFATc1 Degradation in Osteoclasts
Given our observation that overexpression of Cbl ubiquitin ligases can cause NFATc1 degradation in HEK 293T cells, we compared the NFATc1 protein expression levels during osteoclastogenesis from Cbl-b-deficient mice and WT control littermates. We found that the rapid NFATc1 degradation, which we observed during late stage osteoclastogenesis, was comparable between WT and Cbl-b-deficient samples (Fig. 6A), suggesting that other Cbl protein(s) such as c-Cbl might compensate the deficiency of Cbl-b in these knock-out mice. Because Cbl-b/c-Cbl double knockouts are embryonic lethal (32), we used a c-Cbl siRNA retroviral construct to mimic c-Cbl deficiency in vitro. We transduced BMMs from Cbl-b-deficient mice and control littermates with c-Cbl-siRNA-expressing and control vectors, respectively. Infected cells were cultured with M-CSF and RANKL, and samples were analyzed by Western blot analysis and real-time PCR. As shown in Fig. 6B, NFATc1 protein levels in control vector-infected WT samples were greatly diminished by day 3; however, NFATc1 degradation was attenuated in c-Cbl-siRNA transduced Cbl-b-deficient samples. In contrast to NFATc1 protein level, NFATc1 mRNA expression level was comparable in both samples (Fig. 6C). These data suggest that Cbl-b as well as c-Cbl may mediate NFATc1 degradation during osteoclast differentiation.
FIGURE 6.
Cbl proteins mediate NFATc1 degradation. A, BMMs from WT and Cbl-b-deficient mice were cultured with M-CSF and RANKL for the indicated times. Lysates were immunoblotted with NFATc1 and actin antibodies. KO, knock-out. B and C, BMMs from WT and Cbl-b-deficient mice were transduced with pSuper-Retro (control) or a c-Cbl siRNA-expressing (c-Cbl-si) retrovirus, respectively. Cells were cultured with M-CSF and RANKL for the indicated times. B, lysates were probed with NFATc1, Cbl-b, c-Cbl, and actin antibodies (upper panel). The relative amounts of NFATc1 are shown in the lower panel. C, real-time PCR was performed for detection of NFATc1. B and C, ns, not significant, *, p < 0.01 versus positive control. Data are expressed as mean ± S.D. of triplicate samples. Results presented are representative of three independent sets of similar experiments.
DISCUSSION
Although two essential cytokines, M-CSF and RANKL, are required for osteoclastogenesis, RANKL, not M-CSF, induces the expression of various transcription factors such as c-Fos and NFATc1. NFATc1, a master regulator of osteoclastogenesis, is strongly induced by stimulation with RANKL, and its mRNA expression is maintained until the end of the osteoclastogenesis (6). NF-κB and AP-1 complexes containing c-Fos are required for the initial induction and autoamplification of NFATc1 gene expression (7), enabling the robust induction of NFATc1. NFATc1, cooperating with other transcription factors such as PU.1 and Mitf, activates osteoclast-specific genes, including ACP5, CTSK, CALCR, and OSCAR (2, 9, 33, 34). Co-stimulatory signals mediated by immunoreceptor tyrosine-based activation motif-harboring adaptors, including tyrosine kinase-binding protein and Fc receptor common γ chain, cooperate with RANKL during osteoclastogenesis, and their activation enhances the induction of NFATc1 via calcium signaling (35). OSCAR, a co-stimulatory receptor, activates NFATc1 via association with the Fc receptor common γ chain (35). Thus, a positive feedback loop comprised of the immunoreceptor-NFATc1 pathway further enhances NFATc1 gene expression. Our data indicate that M-CSF induces NFATc1 ubiquitination and degradation via Cbl proteins in a Src kinase-dependent manner during late stage osteoclastogenesis. Based on our data, it appears that NFATc1 degradation is primarily induced by M-CSF as opposed to RANKL, suggesting that M-CSF and RANKL play distinct roles in the regulation of NFATc1 expression; M-CSF down-regulates NFATc1 at the protein levels, whereas RANKL activates NFATc1, and subsequently, up-regulates NFATc1 mRNA expression. However, it has been reported that RANK associates with TRAF6, c-Src, and Cbl proteins upon ligand engagement (19, 20). Therefore, we cannot rule out the possibility that RANKL also induces degradation of NFATc1 protein.
M-CSF binds to its receptor tyrosine kinase c-Fms and induces receptor dimerization and autophosphorylation. This activation leads to rapid tyrosine phosphorylation of various proteins and the recruitment of signaling molecules to the receptor (36). M-CSF induces cytoplasmic spreading in osteoclasts, which is accompanied by rapid reorganization of the actin cytoskeleton. Insogna et al. (37) reported that M-CSF induces tyrosine phosphorylation of several cellular proteins, including c-Src and a Grb2-binding protein in osteoclasts, and that c-Src kinase activity is also increased after osteoclasts are treated with M-CSF. These data indicate that c-Src plays a role in M-CSF-induced downstream signaling cascades.
c-Src is highly expressed in terminally differentiated mature osteoclasts and is responsible for bone resorption (18, 38). In this study, we show strong evidence that Src kinases are involved in NFATc1 degradation during late stage osteoclastogenesis, indicating that Src kinases play dual roles as both positive regulators of osteoclast activation and negative regulators of osteoclast differentiation. Fully differentiated, mature osteoclasts are short-lived, which could be a way that the bone system prevents excessive resorption. Therefore, negative regulation of osteoclastogenesis by Src/Cbl signaling pathways might be important for tight regulation of bone remodeling in microenvironments.
Cbl-b and c-Cbl ubiquitin ligases negatively regulate various signaling responses through down-regulation of receptor tyrosine kinases as well as non-receptor protein tyrosine kinases such as Syk and Src family kinases (12, 13, 15, 28–30). Herein, we demonstrate that Cbl-b and c-Cbl interact endogenously with NFATc1 in osteoclasts and that Cbl proteins down-regulate NFATc1 proteins in a Src kinase-dependent manner. These data imply that NFATc1 is a novel substrate of Cbl family proteins. Based on our results, it appears that Cbl-b and c-Cbl proteins play a key role in NFATc1 degradation in osteoclasts. However, we found that there was detectable degradation of NFATc1 in Cbl-b-deficient cultures (either derived from Cbl-b-deficient mice or derived from cultures in which siRNA against c-Cbl was used), indicating that other ubiquitin ligase(s) may be involved in NFATc1 degradation during osteoclastogenesis. To address this issue, osteoclast-specific Cbl-b/c-Cbl conditional double knock-out mice will be useful to determine the exact mechanism of NFATc1 degradation in osteoclasts.
In this study, our data reveal that M-CSF induces NFATc1 degradation during late stage osteoclastogenesis through ubiquitination of NFATc1 by Cbl proteins in a Src kinase-dependent manner. Therefore, we demonstrate that M-CSF/Src kinases/Cbl signaling play a novel negative role in osteoclastogenesis by regulation of NFATc1 proteins. Further elucidation of the detailed mechanisms underlying NFATc1 regulation will provide additional therapeutic approaches to various bone diseases.
Acknowledgments
We thank T. Kitamura (University of Tokyo) for Plat E cells and A. Ko (Chonnam National University Medical School) for assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants AR053843 and DE19381 (to Y. C.). This work was also supported in part by the Korea Science and Engineering Foundation National Research Laboratory Program grant funded by the Korean government Grant R0A-2007-000-20025-0 and Grant R13-2002-013-03001-0 from the Korea Science and Engineering Foundation through the Medical Research Center for Gene Regulation at Chonnam National University and by a grant from the Brain Korea 21 Project (to J. H. K. and K. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- M-CSF
- macrophage-colony-stimulating factor
- RANK
- receptor activator of nuclear factor κB
- RANKL
- RANK ligand
- TRAP
- tartrate-resistant acid phosphatase
- OSCAR
- osteoclast-associated receptor
- NFAT
- nuclear factor of activated T-cells
- siRNA
- small interfering RNA
- BMM
- bone marrow-derived macrophages
- HA
- hemagglutinin
- IFN
- interferon
- WT
- wild type.
REFERENCES
- 1.Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M. T., Martin T. J. (1999) Endocr. Rev. 20, 345–357 [DOI] [PubMed] [Google Scholar]
- 2.Walsh M. C., Kim N., Kadono Y., Rho J., Lee S. Y., Lorenzo J., Choi Y. (2006) Annu. Rev. Immunol. 24, 33–63 [DOI] [PubMed] [Google Scholar]
- 3.Lee Z. H., Kim H. H. (2003) Biochem. Biophys. Res. Commun. 305, 211–214 [DOI] [PubMed] [Google Scholar]
- 4.Boyle W. J., Simonet W. S., Lacey D. L. (2003) Nature 423, 337–342 [DOI] [PubMed] [Google Scholar]
- 5.Teitelbaum S. L., Ross F. P. (2003) Nat Rev Genet 4, 638–649 [DOI] [PubMed] [Google Scholar]
- 6.Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H., Saiura A., Isobe M., Yokochi T., Inoue J., Wagner E. F., Mak T. W., Kodama T., Taniguchi T. (2002) Dev. Cell 3, 889–901 [DOI] [PubMed] [Google Scholar]
- 7.Asagiri M., Sato K., Usami T., Ochi S., Nishina H., Yoshida H., Morita I., Wagner E. F., Mak T. W., Serfling E., Takayanagi H. (2005) J. Exp. Med. 202, 1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirotani H., Tuohy N. A., Woo J. T., Stern P. H., Clipstone N. A. (2004) J. Biol. Chem. 279, 13984–13992 [DOI] [PubMed] [Google Scholar]
- 9.Kim K., Kim J. H., Lee J., Jin H. M., Lee S. H., Fisher D. E., Kook H., Kim K. K., Choi Y., Kim N. (2005) J. Biol. Chem. 280, 35209–35216 [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto M., Kogawa M., Wada S., Takayanagi H., Tsujimoto M., Katayama S., Hisatake K., Nogi Y. (2004) J. Biol. Chem. 279, 45969–45979 [DOI] [PubMed] [Google Scholar]
- 11.Kim K., Lee S. H., Ha Kim J., Choi Y., Kim N. (2008) Mol. Endocrinol. 22, 176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thien C. B., Langdon W. Y. (2005) Growth Factors 23, 161–167 [DOI] [PubMed] [Google Scholar]
- 13.Thien C. B., Langdon W. Y. (2005) Biochem. J. 391, 153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kassenbrock C. K., Hunter S., Garl P., Johnson G. L., Anderson S. M. (2002) J. Biol. Chem. 277, 24967–24975 [DOI] [PubMed] [Google Scholar]
- 15.Yokouchi M., Kondo T., Sanjay A., Houghton A., Yoshimura A., Komiya S., Zhang H., Baron R. (2001) J. Biol. Chem. 276, 35185–35193 [DOI] [PubMed] [Google Scholar]
- 16.Rangachari M., Penninger J. M. (2004) Curr. Opin. Pharmacol. 4, 415–422 [DOI] [PubMed] [Google Scholar]
- 17.Müller U., Steinhoff U., Reis L. F., Hemmi S., Pavlovic J., Zinkernagel R. M., Aguet M. (1994) Science 264, 1918–1921 [DOI] [PubMed] [Google Scholar]
- 18.Soriano P., Montgomery C., Geske R., Bradley A. (1991) Cell 64, 693–702 [DOI] [PubMed] [Google Scholar]
- 19.Arron J. R., Vologodskaia M., Wong B. R., Naramura M., Kim N., Gu H., Choi Y. (2001) J. Biol. Chem. 276, 30011–30017 [DOI] [PubMed] [Google Scholar]
- 20.Wong B. R., Besser D., Kim N., Arron J. R., Vologodskaia M., Hanafusa H., Choi Y. (1999) Mol. Cell 4, 1041–1049 [DOI] [PubMed] [Google Scholar]
- 21.Elly C., Witte S., Zhang Z., Rosnet O., Lipkowitz S., Altman A., Liu Y. C. (1999) Oncogene 18, 1147–1156 [DOI] [PubMed] [Google Scholar]
- 22.Ota S., Hazeki K., Rao N., Lupher M. L., Jr., Andoniou C. E., Druker B., Band H. (2000) J. Biol. Chem. 275, 414–422 [DOI] [PubMed] [Google Scholar]
- 23.Kim K., Kim J. H., Lee J., Jin H. M., Kook H., Kim K. K., Lee S. Y., Kim N. (2007) Blood 109, 3253–3259 [DOI] [PubMed] [Google Scholar]
- 24.Kim N., Kadono Y., Takami M., Lee J., Lee S. H., Okada F., Kim J. H., Kobayashi T., Odgren P. R., Nakano H., Yeh W. C., Lee S. K., Lorenzo J. A., Choi Y. (2005) J. Exp. Med. 202, 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J., Kim K., Kim J. H., Jin H. M., Choi H. K., Lee S. H., Kook H., Kim K. K., Yokota Y., Lee S. Y., Choi Y., Kim N. (2006) Blood 107, 2686–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim N., Takami M., Rho J., Josien R., Choi Y. (2002) J. Exp. Med. 195, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takayanagi H., Kim S., Matsuo K., Suzuki H., Suzuki T., Sato K., Yokochi T., Oda H., Nakamura K., Ida N., Wagner E. F., Taniguchi T. (2002) Nature 416, 744–749 [DOI] [PubMed] [Google Scholar]
- 28.Lupher M. L., Jr., Rao N., Lill N. L., Andoniou C. E., Miyake S., Clark E. A., Druker B., Band H. (1998) J. Biol. Chem. 273, 35273–35281 [DOI] [PubMed] [Google Scholar]
- 29.Rao N., Miyake S., Reddi A. L., Douillard P., Ghosh A. K., Dodge I. L., Zhou P., Fernandes N. D., Band H. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 3794–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hakak Y., Martin G. S. (1999) Curr. Biol. 9, 1039–1042 [DOI] [PubMed] [Google Scholar]
- 31.Horne W. C., Sanjay A., Bruzzaniti A., Baron R. (2005) Immunol. Rev. 208, 106–125 [DOI] [PubMed] [Google Scholar]
- 32.Naramura M., Jang I. K., Kole H., Huang F., Haines D., Gu H. (2002) Nat. Immunol. 3, 1192–1199 [DOI] [PubMed] [Google Scholar]
- 33.Asagiri M., Takayanagi H. (2007) Bone 40, 251–264 [DOI] [PubMed] [Google Scholar]
- 34.Kim Y., Sato K., Asagiri M., Morita I., Soma K., Takayanagi H. (2005) J. Biol. Chem. 280, 32905–32913 [DOI] [PubMed] [Google Scholar]
- 35.Koga T., Inui M., Inoue K., Kim S., Suematsu A., Kobayashi E., Iwata T., Ohnishi H., Matozaki T., Kodama T., Taniguchi T., Takayanagi H., Takai T. (2004) Nature 428, 758–763 [DOI] [PubMed] [Google Scholar]
- 36.Pixley F. J., Stanley E. R. (2004) Trends Cell Biol. 14, 628–638 [DOI] [PubMed] [Google Scholar]
- 37.Insogna K. L., Sahni M., Grey A. B., Tanaka S., Horne W. C., Neff L., Mitnick M., Levy J. B., Baron R. (1997) J. Clin. Invest. 100, 2476–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyce B. F., Yoneda T., Lowe C., Soriano P., Mundy G. R. (1992) J. Clin. Invest. 90, 1622–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]