Abstract
The C-terminal end of collagen XV, restin, has been the focus of several studies, but the functions of full-length collagen XV have remained unknown. We describe here studies on the production, purification, and function of collagen XV and the production of a monoclonal N-terminal antibody to it. Full-length human collagen XV was produced in insect cells using baculoviruses and purified from the cell culture medium. The yield was 15 mg/liter of cell culture medium. The collagen XV was shown to be trimeric, with disulfide bonds in the collagenous region. Rotary shadowing electron microscopy revealed rod-like molecules with a mean length of 241.8 nm and with a globular domain at one end. The globular domain was verified to be the N-terminal end by N-terminal antibody binding. The molecules show flexibility in their conformation, presumably due to the many interruptions in their collagenous domains. The ability of collagen XV to serve as a substrate for cells was tested in cell adhesion assays, and it was shown that cells did not bind to collagen XV-coated surfaces. When added to the culture medium of fibroblasts and fibrosarcoma cells, however, collagen XV rapidly bound to their fibronectin network. Solid phase assays showed that collagen XV binds to fibronectin, laminin, and vitronectin and that it binds to the collagen/gelatin-binding domain of fibronectin. No binding was detected to fibrillar collagens, fibril-associated collagens, or decorin. Interestingly, collagen XV was found to inhibit the adhesion and migration of fibrosarcoma cells when present in fibronectin-containing matrices.
Keywords: Cell/Stromal, Cell/Adhesion, Cell/Migration, Extracellular Matrix/Collagen, Extracellular Matrix/Fibronectin, Protein/Protein-Protein Interactions, Protein/Purification
Introduction
Lack of collagen XV in mice leads to the development of skeletal myopathy, collapsed capillaries, and degenerating endothelial cells in the heart and skeletal muscles, suggesting that collagen XV may be important for linking basement membranes to their surrounding tissues (1). Indeed, EM2 studies have indicated that collagen XV links banded collagen fibers to basement membranes (2).
It has been proposed that collagen XV may be involved in the invasiveness of tumors because it is lost in the malignant epithelial basement membranes of colonic adenocarcinomas and ductal carcinomas (3, 4) and because melanocytic nevi and malignant melanomas in situ were positive for collagen XV, whereas melanomas with dermal invasion were negative (5). Recent studies with collagen XV and XVIII double null mice have indicated that collagen XV may also have a regulatory role in inhibiting the migration of astrocytes (6).
Collagen XV is a non-fibrillar collagen widely distributed in various tissues, where it has been shown to localize to the basement membrane zones (7–9). Human collagen XV is a homotrimer with α chains composed of 1363 amino acid residues containing a highly interrupted collagenous domain of 577 amino acids flanked by large non-collagenous N- and C-terminal domains of 530 and 256 amino acids, respectively (10, 11). Collagen XV shares structural homology with collagen XVIII, and they together form a subgroup of collagens with their highest similarity in the C-terminal non-collagenous domain, the endostatin domain (12–14). Collagens XV and XVIII have some differences in their structural and functional properties and in their tissue distribution, indicating that their repertoires of biological interactions are overlapping but not identical. Although collagen XVIII is abundant in the liver and present only in small amounts in the skeletal muscle, collagen XV is absent from the liver, but high expression is seen in the skeletal muscle (9, 12, 15–19). Collagen XVIII-derived endostatin has been shown to possess antimigratory and antitumoral properties (20–27). Also, the corresponding fragment of collagen XV, restin, can be found in serum (28), and collagen XV-derived restin has been shown to inhibit endothelial cell migration in vitro, but the effect on proliferation is not definite (29, 30). Restin is less potent as a tumor suppressor than endostatin, has no posttranslational modifications, and has different binding partners in the extracellular matrix (28, 29, 31). To study the function of full-length human collagen XV, we produced recombinant collagen XV in a baculovirus expression system and used the purified protein to characterize it and its functions.
EXPERIMENTAL PROCEDURES
Construction of the Baculovirus Expressing Full-length Recombinant Human Collagen XV
A full-length human collagen XV cDNA clone was generated and cloned at EcoRV-EcoRI restriction sites in pBluescript as described earlier (9). A His6 tag was added to the 3′ end of the clone by removing a 1-kb StuI-EcoRI fragment and replacing it with a PCR product in which the His6 tag and an additional XbaI restriction site had been added before the EcoRI site. EcoRV/XbaI digestion was used to remove the insert from the pBluescript, and it was then ligated into the StuI/XbaI site of the pFastBac1 vector (Invitrogen). The recombinant bacmid DNA was produced and transfected into Spodoptera frugiperda Sf9 insect cells according to Ref. 43 (Invitrogen). The recombinant viruses were collected, amplified, and further plaque-purified and reamplified in Sf9 insect cells.
Production of the N-terminal Non-collagenous Fragment of Collagen XV (XVNTERM) and of Monoclonal Antibodies against XVNTERM
The 5′ region of the human collagen XV cDNA encoding the N-terminal non-collagenous domain (corresponding to amino acids 26–557) was amplified by PCR and ligated into the bacterial expression vector pQE-31 (Qiagen), which adds a His6 tag to the N terminus. The recombinant XVNTERM was expressed in Escherichia coli, and the protein was purified with Talon® metal affinity resin in urea-containing buffers according to the manufacturer's protocols (Clontech). The fragments containing XVNTERM were combined, and the urea was removed by stepwise dialysis. The renatured protein was further dialyzed against PBS. The expression and purification were analyzed by SDS-PAGE electrophoresis followed by Coomassie Blue staining or immunoblotting and detection with an antibody to the histidine tag, Penta-His (Qiagen).
Mouse monoclonal antibodies against the purified XVNTERM fragment were generated and prescreened commercially by enzyme-linked immunosorbent assay (DiaBor Oy, Oulu, Finland). One of the positive clones was purified (DB157.F1), and purified IgG was used in this study.
Production of Recombinant Collagen XV in Insect Cells
Recombinant collagen XV protein expression was tested in High Five insect cells (Invitrogen) cultured in monolayers in TNM-FH medium (Sigma) supplemented with 10% fetal bovine serum (Bioclear) or in serum-free HyQ SFX (Hyclone) medium. Briefly, the insect cells were plated at a concentration of 15 million cells per 100-mm plate for 1 h at 27 °C prior to infection with baculoviruses. Cells were co-infected with viruses encoding the expression of collagen XV and prolyl-4-hydroxylase to ensure proper hydroxylation and stability of the collagen (32). Ascorbate (final concentration 80 μg/ml) was added to the cultures daily. Both cell lysates and culture medium were collected for the analysis of recombinant collagen XV protein expression, which was detected with Penta-His antibody recognizing the C-terminal His tag, a pan-collagen monoclonal antibody 95D1A (33), and the collagen XV antibody against XVNTERM (DB157.F1) generated for this purpose.
Purification of Recombinant Collagen XV from the Insect Cell Culture Medium
High Five cells were infected in the HyQ SFX medium as described above, and cell culture medium (100 ml) was collected 48 or 72 h after infection, chilled on ice, and centrifuged (1000 rpm, 5 min at 4 °C). The pH of the medium was adjusted to 7.6, and it was applied to a Talon® metal affinity resin column (Clontech) in 300 mm NaCl, 50 mm Tris, pH 7.6. The column was washed with the aforementioned buffer followed by a wash with 10 mm imidazole, 300 mm NaCl, 50 mm Tris, pH 7.6. Collagen XV was eluted from the column with 100 mm imidazole, 300 mm NaCl, 50 mm Tris, pH 7.6. When necessary, the elution fractions were concentrated using Centricon Ultracel YM-100 centrifugal filter devices (Millipore). The purity and concentration of collagen XV were evaluated using Coomassie Blue-stained SDS-PAGE gels. The purified recombinant collagen XV protein was subjected to N-terminal sequencing and MALDI-TOF mass spectrometry analysis. The N-terminal protein sequences were determined with a 492 ProciseTM protein sequencer (Applied Biosystems Inc.) from samples electroblotted onto a ProBlottTM membrane (Applied Biosystems Inc.), and the integrity of the recombinant protein was further confirmed from the peptide fingerprint obtained by MALDI-TOF mass spectrometry of the trypsinized protein as described earlier (34).
Proteolytic Digestion of Collagen XV
Purified collagen XV was digested with bacterial collagenase (Worthington Biochemical Corp., Freehold, NJ) 0.04 units/ml at 37 °C for 1 h in a buffer containing 300 mm NaCl, 1 mm CaCl2, 50 mm Tris, pH 7.6, with complete EDTA-free protease inhibitor mixture (Roche Applied Science GmbH). The digested samples were analyzed on SDS-PAGE gels. Immunoblotting, followed by staining with DB157.F1 or Penta-His antibody, was used to identify the N- and C-terminal domains of the protein.
Rotary Shadowing EM
50 μg/ml purified recombinant collagen XV were dialyzed against 0.2 m ammonium acetate in the presence or absence of 1 mm EDTA to prevent molecule aggregation and adjusted to 50% (v/v) glycerol. To identify the N-terminal extremity of the molecules, recombinant collagen XV molecules were incubated with antibodies directed against the N-terminal end of the molecule (DB157.F1) in 0.2 m ammonium bicarbonate. 5 μl of the different solutions were deposited onto freshly cleaved mica sheets, and the molecules were spread using the sandwich method. Samples were then placed on the holder of a MED 010 evaporator (Balzers) and subjected to rotary shadowing electron microscopy as described previously (35). Observations of replicas were performed with a Philips CM120 transmission electron microscope at the Centre Technique des Microstructures (Université Lyon 1, Villeurbanne, France) equiped with a GATAN Orius 200 2k × 2k digital camera. Measurements of molecule length were performed using a Bamboo graphic tablet (Wacom) and the ImageJ program.
Cell Proliferation Assay
HT1080 cell suspension (5 × 104 cells/ml) in a serum-containing medium were plated onto uncoated 96-well plates (100 μl/well), collagen XV or BSA was added to the medium to a final concentration of 20 μg/ml, and the cells were grown for 8, 24, 48, and 72 h. The medium containing collagen XV or BSA was changed daily. Cell proliferation was determined with a CyQuant cell proliferation kit (Invitrogen).
Cell Staining
To detect morphological changes, cells cultured on coverslips were fixed after 4 or 24 h and stained with phalloidin (Sigma) or vinculin (H-300, Santa Cruz Biotechnology).
Addition of Exogenous Soluble Collagen XV to Cells
Human fibroblasts were cultured on coverslips for 48 h in Dulbecco's modified Eagle's medium with 10% serum. Purified collagen XV was added to the cells to a final concentration of 10 μg/ml. After 30 min or 2 h, the cells were washed with PBS and fixed with 2% paraformaldehyde, PBS for 10 min at room temperature followed by washing with PBS. Blocking was performed with 1% BSA, PBS, and the coverslips were stained with DB157.F1 or Penta-His antibody and antibody to fibronectin (C-20, Santa Cruz Biotechnology). Cy3 anti-mouse and Cy2 anti-goat (Rockland, Gilbertsville, PA) were used as secondary antibodies.
Solid Phase Assay
The interactions of collagen XV with several ECM components were tested in solid phase assays. 96-well plates (MaxiSorp) were coated for 2 h at room temperature in triplicate with human plasma-derived vitronectin (VN), Engelbreth-Holm-Swarm mouse tumor-derived laminin-1 (Lam), and human plasma-derived fibronectin (FN) or human fibronectin fragments FN120, FN45, FN40 (BD Biosciences, Chemicon, or Sigma) at a concentration of 10 μg/ml in TBS with 1 mm CaCl2. FN120 contains most of the cell-binding domain and spans a small portion of the collagen-binding domain; FN45 comprises most of the collagen-binding domain; and FN40 contains a small segment of the cell-binding domain. (For a schematic drawing of the fibronectin fragments, please, see Ref. 36.) The other proteins tested were human collagens I, III, IV, and V (ColI, ColIII, ColIV, and ColV) from BD Biosciences, which were used for coating in 0.01 m acetic acid for 2 h at room temperature. In addition, recombinant human collagens XII and XIV (gifts from D. Zwolanek and M. Koch, University of Cologne, Germany) and recombinant human decorin (a gift from A. Oldberg, University of Lund, Sweden) (37) were tested; proteins were coated in TBS. After protein coating, the wells were washed thoroughly with TBS and blocked with 5% milk-TBS for 2 h at room temperature. The blocking solution was replaced with collagen XV diluted in TBS (10 μg/ml) and incubated overnight at 4 °C. Binding to fibronectin was tested with collagen XV concentrations from 0 to 100 μg/ml. The wells were washed with TBS, and Penta-His antibody at a dilution of 1:2000 was used to detect binding of collagen XV. For collagens XII and XIV and decorin, DB157.F1 antibody (1:500) was used to detect binding of collagen XV because the aforementioned collagens and decorin also contained a histidine tag. The secondary antibody was horseradish peroxidase-anti-mouse 1:2000, and tetramethylbenzidine (Sigma) was used as a substrate. The optic density was measured at a wavelength of 450 nm with a Victor multilabel counter and Wallac 1420 Manager software. The results of several experimental series performed in triplicate were combined, with all the series including FN and FN + collagen XV as a standard and all other ECM components included 2–5 times. The numerical value of the bound collagen XV as measured in terms of the optic density of FN alone was set as 1 for the purpose of constructing the graphs.
Cell Adhesion to Collagen XV and Mixed Substrates of Collagen XV and Other ECM Proteins
96-well cell culture plates were coated in triplicate with (a) 10 μg/ml BSA; (b) 10 μg/ml collagen XV; (c) 10 μg/ml FN or its fragments FN120, FN45, or FN40; (d) 5 μg/ml VN (in 1 mm CaCl2, 1 mm MgCl2, PBS; or (e) 5 μg/ml ColI in 0.01 n acetic acid for 2 h at room temperature followed by washing with PBS and blocking with 1% heat-inactivated BSA. To test the effect of mixed substrates of collagen XV and other ECM components, additional coating with 10 μg/ml purified human collagen XV in 1 mm CaCl2, 1 mm MgCl2, PBS was performed overnight after blocking. The wells were washed briefly with PBS, after which HT1080 fibrosarcoma cells (ATCC) in serum-free medium supplemented with 0.2% BSA were plated onto the wells. The cells were allowed to adhere for 30 min, after which the wells were washed with PBS. The relative quantity of cells in each well was determined using the CyQuant cell proliferation assay kit according to the manufacturer's instructions (Invitrogen). The resulting emissions were counted using a Victor multilabel counter and Wallac 1420 Manager software, and the results were analyzed with Microsoft® Excel 2002 SP3 and/or SPSS 13.0. Results of four experiments were combined by comparing the proportions of adhering cells, with adherence to FN set as 100. Adhesion to the collagen XV-coated matrix was also tested with EAhy926 endothelial cells and fibroblasts.
Migration Assays
HT1080 cells were used in these experiments because they have good migratory properties, and they responded well in the XV collagen adhesion assay. HTS Transwell-24 system polyester (Corning Inc.) inserts with 3.0-μm pore size were coated with (a) 25 μg/ml FN; (b) 25 μg/ml FN, 5 μg/ml BSA; (c) 25 μg/ml FN, 5 μg/ml collagen XV; (d) 25 μg/ml FN, 25 μg/ml collagen XV; or (e) 5 μg/ml collagen XV or 25 μg/ml collagen XV overnight at 4 °C and washed with PBS. HT1080 cells in serum-free medium with 0.2% BSA were placed in the upper chamber, with the lower chamber containing serum-free Dulbecco's modified Eagle's medium with 0.2% BSA and 0.02 μg/ml insulin. The cells were allowed to migrate for 4 h, after which the inserts were rinsed with PBS and fixed with methanol at −20 °C for 5 min. The cells from the upper chamber were removed by wiping with a cotton stick, and the cells that had migrated through the membrane were stained with hematoxylin. The stained membranes were cut out and mounted with Shandon Immu-Mount (Thermo Fisher Scientific Inc.) and photographed, and the area covered by cells was measured with MetaMorph 6.1 (Universal Imaging Corp.). Data from three separate experiments each performed in duplicate were combined, and the number of migrating cells on the 25 μg/ml FN, 5 μg/ml BSA-coated membranes was set as 100%.
RESULTS
Purification and Characterization of Recombinant Collagen XV
Human recombinant collagen XV with a C-terminal His tag was produced in insect cells with the baculovirus expression system by co-infection with viruses encoding collagen XV and prolyl-4-hydroxylase. The recombinant collagen was detected both in the cell lysates and in the culture medium as a secreted protein. Co-infection of the cells with viruses encoding prolyl-4-hydroxylase resulted in a more stable collagen XV with less degradation in the recombinant protein found in the culture medium (not shown).
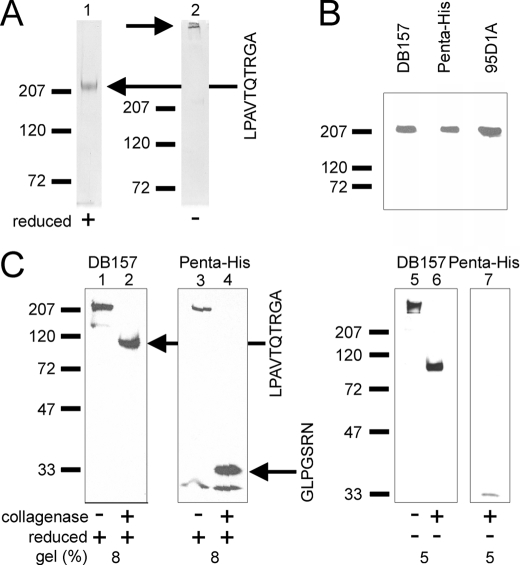
A protocol for purifying collagen XV from serum-free cell culture medium using metal affinity was developed. The estimated recovery was 15 mg/liter of cell culture medium. The purity of the recombinant collagen XV was determined by Coomassie Blue staining. Under reducing conditions, collagen XV migrated at 220 kDa, and larger collagen XV molecules corresponding to disulfide-linked trimers were detected under non-reducing conditions (Fig. 1A). MALDI-TOF and N-terminal sequencing confirmed that collagen XV was present on the SDS-PAGE gels as a 220-kDa protein. The N terminus of the recombinant protein was determined by N-terminal sequencing (LPAVTQTRGA), revealing that the signal peptide was cleaved between amino acids Pro-22–Lys-23, which is three amino acids earlier than predicted previously (10, 11).
FIGURE 1.
Recombinant collagen XV is a disulfide-bonded trimer. A, Coomassie Blue staining of the purified recombinant collagen XV under reduced (+) and non-reduced (−) conditions shows that it is trimeric. Collagen XV is indicated with black arrows, and the N-terminal sequence of the protein is shown on the right. Molecular mass markers (kDa) are shown on the left. B, recombinant collagen XV is detected both with specific N- and C-terminal antibodies (DB157 and Penta-His, respectively) and with the pan-collagen antibody 95D1A. C, collagenase treatment of collagen XV. Western blots of the recombinant collagen XV with the N-terminal collagen specific antibody DB157 and an antibody to the C-terminal histidine tag (Penta-His) with (+) or without (−) collagenase treatment are shown under reduced (+) and non-reduced (−) conditions. The collagenase treatments show that the non-collagenous domains of 100 (N terminus) and 31 kDa (C terminus) do not form disulfide-bonded complexes so that the existing interchain disulfide bonds must be in the collagenous region of the recombinant collagen XV. The lower band detected with the Penta-His antibody is a C-terminal degradation product. The N-terminal sequences of the fragments in lanes 2 and 4 (black arrows) are shown on the right of lane 4. Molecular mass markers are shown on the left.
Antibodies against the N and C terminus recognized the recombinant protein visible in Coomassie Blue staining (Fig. 1B). The collagen XV protein was detected with an antibody against the C-terminal histidine tag. A monoclonal antibody DB157.F1, generated here against the N-terminal domain of collagen XV expressed in E. coli, recognized the recombinant collagen XV protein both in Western blotting (Fig. 1B) and by immunostaining of cultured cells (see Fig. 5). Collagen XV was also detected by the pan-collagen antibody 95D1A.
FIGURE 5.
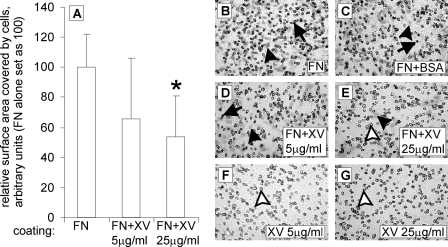
Collagen XV inhibits the migration of HT1080 cells in a dose-dependent manner. In A, the coating material is presented on the x axis, and the relative surface area covered by cells that have migrated onto the coated membrane is presented on the y axis, where the value for fibronectin alone is set at 100. FN+XV, simultaneous coating with FN and collagen XV (with the given concentrations 5 and 25 μg/ml) and coating with collagen XV (XV, with the given concentrations). A, collagen XV used as a 5 μg/ml coating with FN resulted in a 34% reduction in migration, and collagen XV used as a 25 μg/ml coating with FN resulted in a 46% reduction in migration. An asterisk marks a p value of 0.009. Error bars indicate S.D. B–G, migratory cells are seen either spread on the membrane (black arrowheads) or as round cells still in the membrane pores (arrows). With a 25 μg/ml concentration of collagen XV together with FN (E), mostly empty pores are seen (white arrowhead), with occasional spread cells (black arrowhead). Membranes coated with collagen XV alone (F and G) contained virtually no cells, and the pores were mainly empty (white arrowheads).
Collagenase Digestion of Collagen XV
Bacterial collagenase was used to digest the collagenous domain of recombinant collagen XV. The N- and C-terminal antibodies detected non-collagenous undigested material with molecular masses of 100 and 31 kDa, respectively (Fig. 1C). The N-terminal sequence LPAVTQTRGA of the N-terminal collagenase fragment (recognized by DB157.F1) is the same as in the untreated mature collagen XV. The other non-collagenous fragment (recognized by anti-Penta-His), with the sequence GLPGSRN, starts at position 1127 and is located at the end of COL7, the extreme C-terminal collagenous domain of collagen XV. The N-terminal non-collagenous fragment of recombinant human collagen XV produced in insect cells was shown to migrate with a molecular mass larger than that predicted from the sequence (100 versus 57.7 kDa, respectively), and the N-terminal non-collagenous domain expressed in E. coli for antibody production also exhibited slower migration. This has also been seen with collagen XV isolated from umbilical cords (38).
Detection of the similar sized unreduced and reduced collagenase-treated degradation products with N- and C-terminal antibodies demonstrated that there are no interchain disulfide bonds located in the non-collagenous N- and C-terminal domains (Fig. 1C). This is indirect evidence that they are to be found in the collagenous domain because the full-length collagen XV migrates under reducing conditions differently from in reducing conditions. Similarly, collagen XV extracted from human placenta was shown to contain no S–S bonds in the N- and C-terminal domains (39).
Molecular Structure of Recombinant Collagen XV
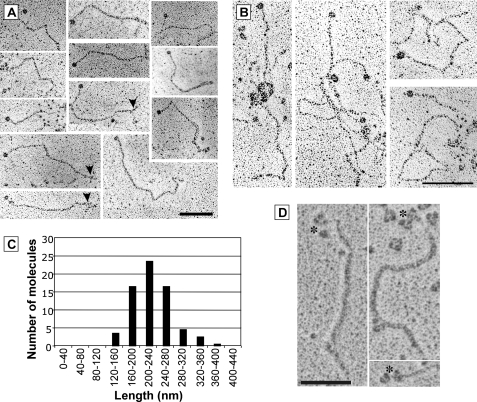
In rotary shadowing EM, the purified recombinant collagen XV appeared as an extended protein with numerous kinks (Fig. 2A). The collagen XV molecules exhibited a globular domain in one end that was identified as the N-terminal domain after incubation with antibodies against the N-terminal end of the collagen XV (Fig. 2D). Molecule length distribution showed that values ranged from 160 to 360 nm with a mean length of 241.8 ± 9.9 nm (n = 71) (Fig. 2C). A trident structure at the other end of the molecule was observed (Fig. 2A, arrowheads) in rare cases. Most of the collagen XV molecules aggregated into large complexes (Fig. 2B). The N-terminal globules were very frequently observed free, emerging from the molecular complexes, suggesting that interactions between the different molecules are mediated by the collagenous domains. The addition of EDTA improved individual molecule observation but did not prevent molecule multimer formation.
FIGURE 2.
Collagen XV molecules examined by rotary shadowing transmission electron microscopy appear as extended proteins with kinks. A, gallery of individual collagen XV molecules. Numerous kinks are present in the triple helix domain, corresponding to interruptions in the Gly-X-Y repeats. A trident structure can be observed at the other end of some molecules (arrowheads). B, collagen XV molecules have a tendency to form aggregates. Scale bar = 100 nm. C, histogram of the collagen XV length distribution (n = 71). D, collagen XV molecules incubated with antibodies against the N terminus; asterisks indicate the globular domain complexed with antibodies that exhibit a characteristic three-lobed shape. Scale bar = 50 nm.
Binding of Collagen XV to Cell Surfaces
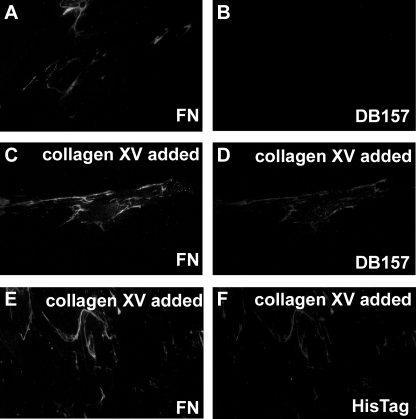
To study the putative influence of collagen XV on cells, purified collagen XV was added to the culture medium of cultured human fibroblasts and fibrosarcoma HT1080 cells. Immunostaining of human fibroblasts with the collagen XV antibody DB157.F1 or the His tag antibody Penta-His gave a strong staining in the extracellular space, not seen in untreated cells, within 30 min of the addition of collagen XV. This rapid binding of collagen XV may be taken to indicate that the structures it was binding to already existed on the cell surfaces. Because the staining resembled a fibronectin matrix staining pattern, the cells were double-stained with an antibody to fibronectin (Fig. 3). In untreated cultures, where no added collagen XV was present, there was no staining with the collagen XV antibody in the double-stained cells (Fig. 3, A and B), whereas the stainings for added collagen XV and fibronectin overlapped, demonstrating that the exogenous collagen XV presumably bound to the already formed fibronectin matrix (Fig. 3, C–F). For good visualization of the overlapping staining, areas of low fibronectin density were chosen for the figures. The fibroblasts used in this study were of late passage and therefore produced moderate amounts of fibronectin. HT1080 cells produced even lower amounts of fibronectin in culture, and hence only a weak staining of collagen XV was seen on their surfaces after the addition of collagen XV to the culture medium (not shown).
FIGURE 3.
Exogenous recombinant collagen XV added to the culture medium of human fibroblasts binds rapidly to the fibronectin network formed by the fibroblasts. A and B, no added collagen XV; C–F, collagen XV added to the culture medium. A, C, and E are stained with antibody to fibronectin; B and D are stained with antibody to collagen XV; and F is stained with an antibody to the histidine tag of collagen XV. Cultured human fibroblasts did not express endogenous collagen XV, but they did form a fibronectin matrix (A and B). When collagen XV was added to the culture medium, it rapidly bound to the fibronectin network, as shown in panel pairs C and D and E and F, and this was visualized with both the collagen XV-specific antibody and the antibody to the histidine tag in collagen XV (D and F). Original magnification: ×40.
Proliferation of Cells
The C-terminal fragment of collagen XV, restin, inhibits the migration of endothelial cells in vitro, but the effect on the proliferation of these cells is controversial (29, 30). To test whether the full-length protein has an effect on proliferation, soluble collagen XV was added to HT1080 cells, endothelial cells (EAhy926), and fibroblasts, and their proliferation was estimated over 72 h. Full-length recombinant collagen XV was shown to have no effect on the proliferation of these cells (not shown).
Effect of Collagen XV on Cell Adhesion
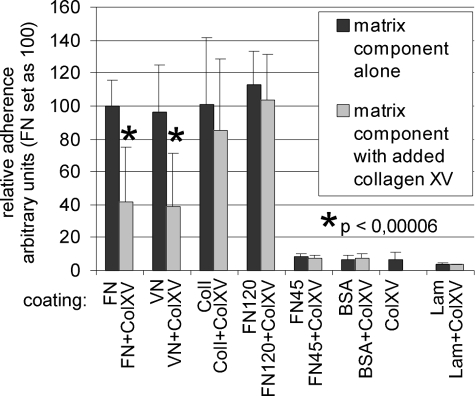
The collagen XV in the cell culture experiments was presumably interacting with fibronectin, which is involved in many cellular events such as adhesion. We therefore tested the ability of collagen XV to influence cell adhesion. In our experiments, HT1080 cells (Fig. 4), endothelial cells or fibroblasts did not adhere to a collagen XV substrate. The HT1080 cells readily adhered to FN, VN, and ColI but not to BSA or Lam (Fig. 4). When collagen XV was present in the substrate together with fibronectin or vitronectin, it reduced the adhesion of HT1080 cells but had no effect on cell binding to ColI. Pretreatment of cells with collagen XV had no effect on the binding of HT1080 cells to FN (not shown). Of the various fragments of fibronectin, HT1080 cells adhered readily to FN120, which contains the cell-binding domain but not to FN45, which lacks the cell-binding RGD sequence (Fig. 4). Interestingly, collagen XV did not interfere with the adhesion of HT1080 cells to FN120 (Fig. 4), indicating that other regions of fibronectin are required to mediate the effect of collagen XV.
FIGURE 4.
Collagen XV inhibits the adhesion of fibrosarcoma HT1080 cells to fibronectin and vitronectin. The coating material is presented on the x axis, and the relative adhesion of cells is presented on the y axis, where the number of cells adhering to fibronectin alone is set at 100. FN120 and FN45 are fragments of fibronectin, and ColXV indicates collagen XV coating alone. The results obtained after an additional coating with collagen XV are presented in the lighter gray series. Collagen XV inhibits the adhesion of HT1080 cells when present together with FN and VN. The inhibition is statistically significant (p < 0.00006). Collagen XV does not affect the adhesion of HT1080 cells to ColI or Lam. Inhibition of the adhesion of cells to FN requires the presence of a collagen-binding domain in FN as collagen XV was unable to inhibit the adhesion of cells to FN120 containing the cell-binding domain but lacking the collagen-binding domain. Error bars indicate S.D.
Inhibition of Cell Migration by Collagen XV
The effect of collagen XV on cell migration was tested in Transwell assays. The presence of collagen XV in the FN substrate clearly inhibited the migration of HT1080 cells in that the addition of collagen XV at a concentration of 5 μg/ml reduced their migration by 34%, and its addition at 25 μg/ml reduced their migration by 46% (Fig. 5A). The reduction seen in the migration assay in which the surface area of the migrating cells was counted was not due to non-spreading of the cells (Fig. 5, B–E). Coating with collagen XV alone totally blocked the migration of HT1080 cells onto the membrane (Fig. 5, F and G).
Binding of Collagen XV to Other ECM Proteins
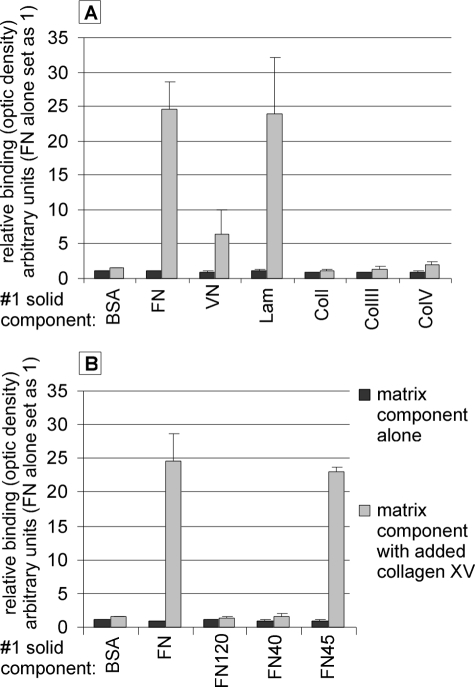
Solid-phase assays were used to study protein-protein interactions between collagen XV and other ECM components. Various proteins were used as a solid substrate, and the binding of collagen XV to them was determined. We discovered that collagen XV could bind to fibronectin and laminin, and to a lesser extent, to vitronectin (Fig. 6A). (Supplemental Fig. 1 shows the binding of different concentrations of collagen XV to FN.) In addition, the binding of collagen XV to fibrillar collagens was tested because it has been postulated that it may link collagen fibers to basement membrane (2). The binding of collagen XV to collagens I, III, V, and IV was not significant, however (Fig. 6A, not shown for ColIV). In addition, binding of collagen XV to fibril-associated collagens XII and XIV as well as to the small fibril-associated proteoglycan, decorin, was tested. However, no interaction was detected in solid-phase assays (data not shown).
FIGURE 6.
Collagen XV (XV) interacts with FN, Lam, and VN and binds to the collagen-binding region of FN. Solid-phase assay of the binding of collagen XV to various matrix proteins. The first solid components are shown on the x axis, and the proportion of bound collagen XV is shown on the y axis, where the optic density of fibronectin alone is set at 1. FN120, FN40, and FN45 are fragments of fibronectin. Collagen XV was shown to bind to FN and Lam and to a lesser extent to VN (A). Of the various fragments of fibronectin, collagen XV was shown to bind to FN45, which contains the collagen-binding region, but not to FN120 or FN40, which lack the collagen-binding region (B). p values for the binding of collagen XV to other matrix molecules were evaluated but are not presented here as the difference seen in the binding of collagen XV to FN, FN45, laminin, and VN relative to binding to BSA is evident, with no overlapping of the standard deviations. The original optical density values were: FN alone, 0.044; BSA + XV (collagen XV added), 0.067; FN + XV, 1.084; VN + XV, 0.279; Lam + XV, 1.044; and FN45 + XV, 1.013. Error bars indicate S.D.
Of the various fragments of fibronectin, collagen XV was found to bind to FN45 (Fig. 6B), which comprised most of the collagen-binding domain. When compared with control, collagen XV binding to FN120, the central cell-binding domain of FN, or to FN40 was not significant.
DISCUSSION
The molecular characteristics and biological properties of collagen XV are not well understood. We produced recombinant human collagen XV in insect cells to study its properties. Rotary shadowing EM of the purified protein showed that it often forms aggregates. Individual collagen XV molecules appeared in an elongated configuration with kinky regions, although some molecules with a more bent shape were seen. Often a globular domain was seen at one end, and this was verified by antibody incubation to represent the N terminus of collagen XV. Earlier EM rotary shadowing of collagen XV extracted from human umbilical cords had shown collagen XV mostly in a curled/pretzel-like configuration (38). Occasionally there were three globular nodules in the N terminus of collagen XV, whereas the other end of the molecule lacked any globular domain or contained only a small knob (38). The recombinant full-length collagen XV used in our EM rotary shadowing studies had a mean length of 241.8 ± 9.9 nm, which is more than the length of 190 nm reported for the pretzel-shaped collagen XV isolated from tissues (38). Rotary shadowing EM has been used previously to determine the structure of collagen XVIII, and except for the globular structure (which was not observed in collagen XVIII), the recombinant full-length human collagen XV produced here resembles the recombinant chicken collagen XVIII produced in HEK293-EBNA cells (40). Collagen XVIII purified from chicken vitreous body also seemed to be in a more bent conformation than the recombinant collagen XVIII (38), and we are inclined to conclude that tissue-derived collagen XV is more compressed than recombinant collagen XV. Both the recombinant collagen XV and the collagen XV isolated from tissues form multimeric structures. The recombinant collagen XV used in this study presumably does not carry chondroitin sulfate side chains (because no smear typical for proteoglycans was seen) and has a C-terminal histidine tag. These may affect the ability of recombinant collagen XV to assemble into exactly similar multimeric structures seen with protein isolated from tissues.
Fibronectin is known to bind collagens, and we showed here that collagen XV binds to fibronectin and also to laminin and vitronectin in a solid-phase assay. In addition, it binds to the fibronectin network produced by cultured cells. As collagen XV is present in the vicinity of basement membranes in blood vessels, muscles, and skin, it can be speculated that it serves a function in the organization of the basement membrane and the surrounding tissue. On the EM level, collagen XV in tissues has been shown to associate with structures linking banded collagen fibers near basement membranes (2). Although we found the in vitro binding of collagen XV to collagens I, III, V, and IV, fibril-associated collagens XII and XIV, and a fibril-associated proteoglycan, decorin, to be poor, the possibility still remains that collagen XV may interact with the collagen fibril-fiber environment in vivo. It could also be speculated that collagen XV may bind in vivo to fibronectin fibrils linking banded collagen fibers.
Collagen XV was shown here to be a poor substrate for cells. Using various FN fragments, we showed that it binds to the collagen-binding domain of FN. Fibronectin has pro-adhesive, pro-spreading, and pro-migratory effects, which are exerted by providing a matrix for the cells with different integrins to bind to. Interestingly, when collagen XV was used as a coating material, it was able to inhibit the adhesion of cells to FN (and VN) and also the migration of cells on FN. Collagen XV expression in human cervical carcinoma cells has previously been shown to reduce their tumorigenicity, probably by creating an altered extracellular matrix that interacts differently with the tumor cells (41). In the light of the findings reported here, this could be due to the antiadhesive and antimigratory properties of collagen XV.
Another ECM antiadhesive family of proteins of a different type is that of the tenascins, where tenascin-C in particular shows some functions similar to those of collagen XV; it binds to fibronectin, it interferes with the fibronectin-mediated spreading of fibroblasts when added to the medium or mixed with fibronectin used for coating, and it disturbs the adhesion of cells to fibronectin (42). The proposed mechanism for the inhibition of spreading involves tenascin binding to fibronectin, thus preventing the fibronectin from binding to syndecan-4, resulting in the disassembly of actin stress fibers (42). A similar mechanism for collagen XV as for tenascin could be proposed because binding of collagen XV to the collagen-binding region of FN was required to prevent the adhesion of cells to FN. It could also be speculated that upon binding to the collagen-binding region of FN located in the N-terminal part of FN, collagen XV prevents the binding of integrin(s) to the RGD sequence of FN by making the binding site inaccessible through involvement of a third, yet unidentified, molecule or by affecting the tertiary structure of the fibronectin matrix and thereby preventing adhesion and migration. Thus with spatial and timely expression of collagen XV, it could be possible to modify the FN matrix and its cell binding properties. It is also interesting that mice lacking tenascin-C have an enhanced migration rate of oligodendrocyte precursors (42) because the migration of retinal astrocytes was induced in mice lacking collagen XV and XVIII (6). By modulating the expression of collagen XV, cells may change the structure and function of the surrounding ECM. The results presented here may help to identify the significant networks in which collagen XV participates in vivo.
Acknowledgments
We thank Daniela Zwolanek and Manuel Koch for the gift of recombinant collagens XII and XIV and Åke Oldberg for providing recombinant decorin. We are grateful to Marilyne Malbouyres for expert assistance with rotary shadowing. We also thank Sirkka Vilmi, Päivi Tuomaala, and Aila White for expert technical assistance, as well as Ulrich Bergmann and Hongmin Tu for performing the protein analysis of collagen XV and Risto Bloigu for statistical help. Transmission EM observations were performed at the Centre Technique des Microstructures (Université Lyon 1, Villeurbanne, France).
This work was supported by European Commission Grant 504 743, Research Council for Health of the Academy of Finland Grants 115237 and 114784, and a grant from the Sigrid Jusélius Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- EM
- electron microscopy
- MALDI-TOF
- matrix-assisted laser desorption/ionization-time of flight
- VN
- vitronectin
- FN
- fibronectin
- Lam
- laminin-1
- Col
- collagen
- PBS
- phosphate-buffered saline
- TBS
- Tris-buffered saline
- BSA
- bovine serum albumin
- ECM
- extracellular matrix.
REFERENCES
- 1.Eklund L., Piuhola J., Komulainen J., Sormunen R., Ongvarrasopone C., Fássler R., Muona A., Ilves M., Ruskoaho H., Takala T. E., Pihlajaniemi T. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 1194–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amenta P. S., Scivoletti N. A., Newman M. D., Sciancalepore J. P., Li D., Myers J. C. (2005) J. Histochem. Cytochem. 53, 165–176 [DOI] [PubMed] [Google Scholar]
- 3.Amenta P. S., Briggs K., Xu K., Gamboa E., Jukkola A. F., Li D., Myers J. C. (2000) Hum. Pathol. 31, 359–366 [DOI] [PubMed] [Google Scholar]
- 4.Amenta P. S., Hadad S., Lee M. T., Barnard N., Li D., Myers J. C. (2003) J. Pathol. 199, 298–308 [DOI] [PubMed] [Google Scholar]
- 5.Fukushige T., Kanekura T., Ohuchi E., Shinya T., Kanzaki T. (2005) J. Dermatol. 32, 74–83 [PubMed] [Google Scholar]
- 6.Hurskainen M., Eklund L., Hägg P. O., Fruttiger M., Sormunen R., Ilves M., Pihlajaniemi T. (2005) FASEB J. 19, 1564–1566 [DOI] [PubMed] [Google Scholar]
- 7.Kivirikko S., Saarela J., Myers J. C., Autio-Harmainen H., Pihlajaniemi T. (1995) Am. J. Pathol. 147, 1500–1509 [PMC free article] [PubMed] [Google Scholar]
- 8.Myers J. C., Dion A. S., Abraham V., Amenta P. S. (1996) Cell Tissue Res. 286, 493–505 [DOI] [PubMed] [Google Scholar]
- 9.Hägg P. M., Hägg P. O., Peltonen S., Autio-Harmainen H., Pihlajaniemi T. (1997) Am. J. Pathol. 150, 2075–2086 [PMC free article] [PubMed] [Google Scholar]
- 10.Muragaki Y., Abe N., Ninomiya Y., Olsen B. R., Ooshima A. (1994) J. Biol. Chem. 269, 4042–4046 [PubMed] [Google Scholar]
- 11.Kivirikko S., Heinämäki P., Rehn M., Honkanen N., Myers J. C., Pihlajaniemi T. (1994) J. Biol. Chem. 269, 4773–4779 [PubMed] [Google Scholar]
- 12.Rehn M., Pihlajaniemi T. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 4234–4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rehn M., Hintikka E., Pihlajaniemi T. (1994) J. Biol. Chem. 269, 13929–13935 [PubMed] [Google Scholar]
- 14.Hägg P. M., Muona A., Liétard J., Kivirikko S., Pihlajaniemi T. (1998) J. Biol. Chem. 273, 17824–17831 [DOI] [PubMed] [Google Scholar]
- 15.Tomono Y., Naito I., Ando K., Yonezawa T., Sado Y., Hirakawa S., Arata J., Okigaki T., Ninomiya Y. (2002) Cell Struct. Funct. 27, 9–20 [DOI] [PubMed] [Google Scholar]
- 16.Musso O., Rehn M., Saarela J., Théret N., Liétard J., Hintikka Lotrian D., Campion J. P., Pihlajaniemi T., Clément B. (1998) Hepatology 28, 98–107 [DOI] [PubMed] [Google Scholar]
- 17.Saarela J., Rehn M., Oikarinen A., Autio-Harmainen H., Pihlajaniemi T. (1998) Am. J. Pathol. 153, 611–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muragaki Y., Timmons S., Griffith C. M., Oh S. P., Fadel B., Quertermous T., Olsen B. R. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 8763–8767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halfter W., Dong S., Schurer B., Cole G. J. (1998) J. Biol. Chem. 273, 25404–25412 [DOI] [PubMed] [Google Scholar]
- 20.Wickström S. A., Alitalo K., Keski-Oja J. (2004) J. Biol. Chem. 279, 20178–20185 [DOI] [PubMed] [Google Scholar]
- 21.Wilson R. F., Morse M. A., Pei P., Renner R. J., Schuller D. E., Robertson F. M., Mallery S. R. (2003) Anticancer Res. 23, 1289–1295 [PubMed] [Google Scholar]
- 22.Yamaguchi N., Anand-Apte B., Lee M., Sasaki T., Fukai N., Shapiro R., Que I., Lowik C., Timpl R., Olsen B. R. (1999) EMBO J. 18, 4414–4423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin X., Bookstein R., Wills K., Avanzini J., Tsai V., LaFace D., Terracina G., Shi B., Nielsen L. L. (2001) Cancer Gene Ther. 8, 982–989 [DOI] [PubMed] [Google Scholar]
- 24.Eriksson K., Magnusson P., Dixelius J., Claesson-Welsh L., Cross M. J. (2003) FEBS Lett. 536, 19–24 [DOI] [PubMed] [Google Scholar]
- 25.Olsson A. K., Johansson I., Akerud H., Einarsson B., Christofferson R., Sasaki T., Timpl R., Claesson-Welsh L. (2004) Cancer Res. 64, 9012–9017 [DOI] [PubMed] [Google Scholar]
- 26.Ackley B. D., Crew J. R., Elamaa H., Pihlajaniemi T., Kuo C. J., Kramer J. M. (2001) J. Cell Biol. 152, 1219–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyberg P., Heikkilä P., Sorsa T., Luostarinen J., Heljasvaara R., Stenman U. H., Pihlajaniemi T., Salo T. (2003) J. Biol. Chem. 278, 22404–22411 [DOI] [PubMed] [Google Scholar]
- 28.John H., Radtke K., Ständker L., Forssmann W. G. (2005) Biochim. Biophys. Acta 1747, 161–170 [DOI] [PubMed] [Google Scholar]
- 29.Ramchandran R., Dhanabal M., Volk R., Waterman M. J., Segal M., Lu H., Knebelmann B., Sukhatme V. P. (1999) Biochem. Biophys. Res. Commun. 255, 735–739 [DOI] [PubMed] [Google Scholar]
- 30.Xu R., Xin L., Fan Y., Meng H. R., Li Z. P., Gan R. B. (2002) Sheng Wu Hua. Xue Yu Sheng Wu Wu Li Xue Bao 34, 138–142 [PubMed] [Google Scholar]
- 31.Sasaki T., Larsson H., Tisi D., Claesson-Welsh L., Hohenester E., Timpl R. (2000) J. Mol. Biol. 301, 1179–1190 [DOI] [PubMed] [Google Scholar]
- 32.Vuorela A., Myllyharju J., Nissi R., Pihlajaniemi T., Kivirikko K. I. (1997) EMBO J. 16, 6702–6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snellman A., Keränen M. R., Hägg P. O., Lamberg A., Hiltunen J. K., Kivirikko K. I., Pihlajaniemi T. (2000) J. Biol. Chem. 275, 8936–8944 [DOI] [PubMed] [Google Scholar]
- 34.Ohlmeier S., Kastaniotis A. J., Hiltunen J. K., Bergmann U. (2004) J. Biol. Chem. 279, 3956–3979 [DOI] [PubMed] [Google Scholar]
- 35.Fichard A., Tillet E., Delacoux F., Garrone R., Ruggiero F. (1997) J. Biol. Chem. 272, 30083–30087 [DOI] [PubMed] [Google Scholar]
- 36.Sarret Y., Stamm C., Jullien D., Schmitt D. (1992) J. Invest. Dermatol. 99, 656–659 [DOI] [PubMed] [Google Scholar]
- 37.Kalamajski S., Aspberg A., Oldberg A. (2007) J. Biol. Chem. 282, 16062–16067 [DOI] [PubMed] [Google Scholar]
- 38.Myers J. C., Amenta P. S., Dion A. S., Sciancalepore J. P., Nagaswami C., Weisel J. W., Yurchenco P. D. (2007) Biochem. J. 404, 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li D., Clark C. C., Myers J. C. (2000) J. Biol. Chem. 275, 22339–22347 [DOI] [PubMed] [Google Scholar]
- 40.Marneros A. G., Keene D. R., Hansen U., Fukai N., Moulton K., Goletz P. L., Moiseyev G., Pawlyk B. S., Halfter W., Dong S., Shibata M., Li T., Crouch R. K., Bruckner P., Olsen B. R. (2004) EMBO J. 23, 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris A., Harris H., Hollingsworth M. A. (2007) Mol. Cancer Res. 5, 1241–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiquet-Ehrismann R., Chiquet M. (2003) J. Pathol. 200, 488–499 [DOI] [PubMed] [Google Scholar]
- 43.Invitrogen Corp. (2008) BAC-TO-BAC Baculovirus Expression System Instruction Manual, Invitrogen, Carlsbad, CA [Google Scholar]