Abstract
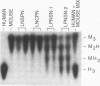
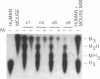
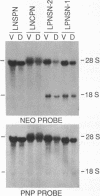
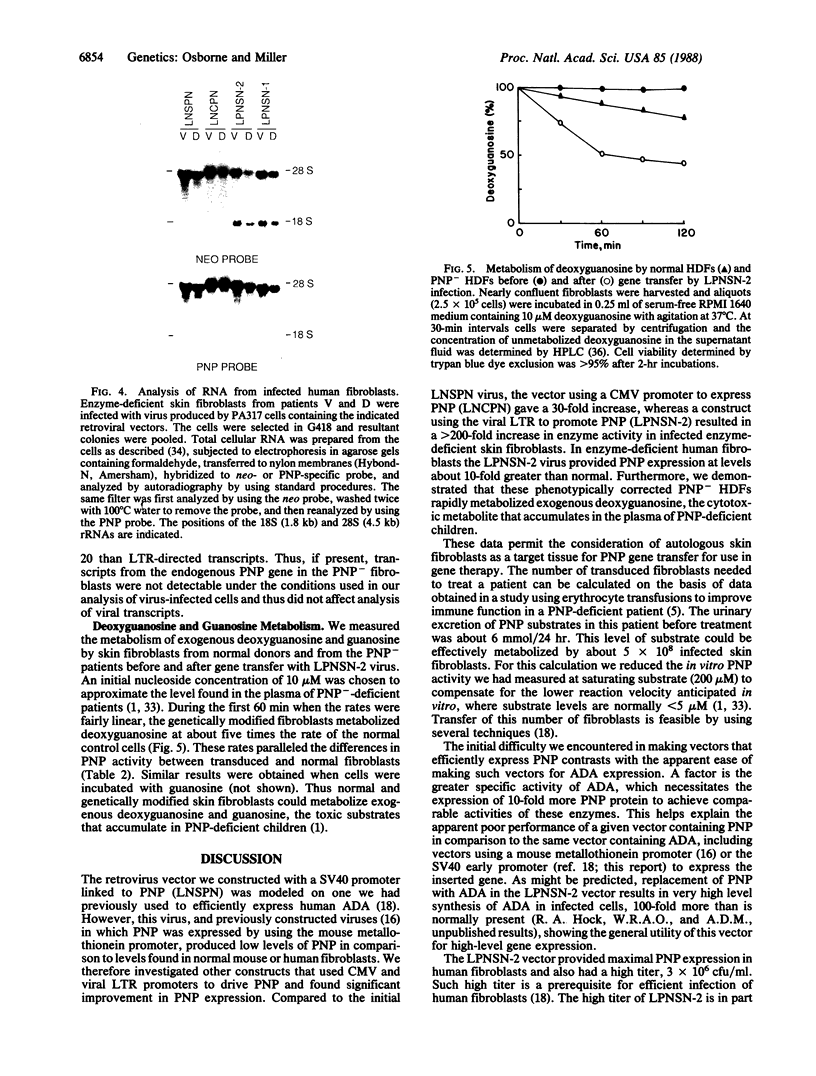
Purine nucleoside phosphorylase (PNP; purine-nucleoside orthophosphate ribosyltransferase, EC 2.4.2.1) deficiency is an inherited disorder associated with a severe immune defect that is fatal. Enzyme replacement therapy is an attractive approach to treatment of this disease. To this aim we constructed retroviral vectors containing a human PNP cDNA and a selectable gene encoding neomycin phosphotransferase. PNP expression was controlled by either the early promoter from simian virus 40, the immediate early promoter from human cytomegalovirus, or the retroviral promoter. Cultured skin fibroblasts from two unrelated PNP-deficient patients that were infected with these vectors expressed mean PNP activities of 0.03, 0.74, and 5.9 mumol/hr per mg of protein, respectively. The latter infectants had PNP activities eight times the level of 0.74 mumol/hr per mg of protein observed in normal skin fibroblasts, enabling rapid metabolism of exogenous deoxyguanosine, the cytotoxic metabolite that accumulates in the plasma of PNP-deficient patients. These experiments indicate that viral long terminal repeat was the strongest promoter for expression of PNP and suggest the potential of human skin fibroblasts as vehicles for therapeutic gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Bender M. A., Palmer T. D., Gelinas R. E., Miller A. D. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987 May;61(5):1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P., Gilboa E., Anderson W. F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Ammann A. J., Wara D. W., Sandman R., Diamond L. K. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975 May 3;1(7914):1010–1013. doi: 10.1016/s0140-6736(75)91950-9. [DOI] [PubMed] [Google Scholar]
- Goddard J. M., Caput D., Williams S. R., Martin D. W., Jr Cloning of human purine-nucleoside phosphorylase cDNA sequences by complementation in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4281–4285. doi: 10.1073/pnas.80.14.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock R. A., Miller A. D. Retrovirus-mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature. 1986 Mar 20;320(6059):275–277. doi: 10.1038/320275a0. [DOI] [PubMed] [Google Scholar]
- Hogge D. E., Humphries R. K. Gene transfer to primary normal and malignant human hemopoietic progenitors using recombinant retroviruses. Blood. 1987 Feb;69(2):611–617. [PubMed] [Google Scholar]
- Kantoff P. W., Gillio A. P., McLachlin J. R., Bordignon C., Eglitis M. A., Kernan N. A., Moen R. C., Kohn D. B., Yu S. F., Karson E. Expression of human adenosine deaminase in nonhuman primates after retrovirus-mediated gene transfer. J Exp Med. 1987 Jul 1;166(1):219–234. doi: 10.1084/jem.166.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Kwok W. W., Schuening F., Stead R. B., Miller A. D. Retroviral transfer of genes into canine hemopoietic progenitor cells in culture: a model for human gene therapy. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4552–4555. doi: 10.1073/pnas.83.12.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- McIvor R. S., Johnson M. J., Miller A. D., Pitts S., Williams S. R., Valerio D., Martin D. W., Jr, Verma I. M. Human purine nucleoside phosphorylase and adenosine deaminase: gene transfer into cultured cells and murine hematopoietic stem cells by using recombinant amphotropic retroviruses. Mol Cell Biol. 1987 Feb;7(2):838–846. doi: 10.1128/mcb.7.2.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Law M. F., Verma I. M. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985 Mar;5(3):431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Trauber D. R., Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- Osborne W. R., Barton R. W. A rat model of purine nucleoside phosphorylase deficiency. Immunology. 1986 Sep;59(1):63–67. [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Chen S. H., Giblett E. R., Biggar W. D., Ammann A. A., Scott C. R. Purine nucleoside phosphorylase deficiency. Evidence for molecular heterogeneity in two families with enzyme-deficient members. J Clin Invest. 1977 Sep;60(3):741–746. doi: 10.1172/JCI108826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Deeg H. J., Slichter S. J. A canine model of induced purine nucleoside phosphorylase deficiency. Clin Exp Immunol. 1986 Oct;66(1):166–172. [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Hammond W. P., Dale D. C. Canine cyclic hematopoiesis is associated with abnormal purine and pyrimidine metabolism. J Clin Invest. 1983 May;71(5):1348–1355. doi: 10.1172/JCI110887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R. Human red cell purine nucleoside phosphorylase. Purification by biospecific affinity chromatography and physical properties. J Biol Chem. 1980 Aug 10;255(15):7089–7092. [PubMed] [Google Scholar]
- Osborne W. R., Spencer N. Partial purification and properties of the common inherited forms of adenosine deaminase from human erythrocytes. Biochem J. 1973 May;133(1):117–123. doi: 10.1042/bj1330117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T. D., Hock R. A., Osborne W. R., Miller A. D. Efficient retrovirus-mediated transfer and expression of a human adenosine deaminase gene in diploid skin fibroblasts from an adenosine deaminase-deficient human. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1055–1059. doi: 10.1073/pnas.84.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rich K. C., Majias E., Fox I. H. Purine nucleoside phosphorylase deficiency: improved metabolic and immunologic function with erythrocyte transfusions. N Engl J Med. 1980 Oct 23;303(17):973–977. doi: 10.1056/NEJM198010233031705. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staal G. E., Stoop J. W., Zegers B. J., Siegenbeek van Heukelom L. H., van der Vlist M. J., Wadman S. K., Martin D. W. Erythrocyte metabolism in purine nucleoside phosphorylase deficiency after enzyme replacement therapy by infusion of erythrocytes. J Clin Invest. 1980 Jan;65(1):103–108. doi: 10.1172/JCI109639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead R. B., Kwok W. W., Storb R., Miller A. D. Canine model for gene therapy: inefficient gene expression in dogs reconstituted with autologous marrow infected with retroviral vectors. Blood. 1988 Mar;71(3):742–747. [PubMed] [Google Scholar]
- Williams D. A., Orkin S. H., Mulligan R. C. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2566–2570. doi: 10.1073/pnas.83.8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. R., Goddard J. M., Martin D. W., Jr Human purine nucleoside phosphorylase cDNA sequence and genomic clone characterization. Nucleic Acids Res. 1984 Jul 25;12(14):5779–5787. doi: 10.1093/nar/12.14.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]