Abstract
Transforming growth factor-β-activated kinase 1 (TAK1) plays an essential role in the tumor necrosis factor α (TNFα)- and interleukin-1β (IL-1β)-induced IκB kinase (IKK)/nuclear factor-κB (NF-κB) and c-Jun N-terminal kinase (JNK)/activator protein 1 (AP-1) activation. Here we report that TNFα and IL-1β induce Lys63-linked TAK1 polyubiquitination at the Lys158 residue within the kinase domain. Tumor necrosis factor receptor-associated factors 2 and 6 (TRAF2 and -6) act as the ubiquitin E3 ligases to mediate Lys63-linked TAK1 polyubiquitination at the Lys158 residue in vivo and in vitro. Lys63-linked TAK1 polyubiquitination at the Lys158 residue is required for TAK1-mediated IKK complex recruitment. Reconstitution of TAK1-deficient mouse embryo fibroblast cells with TAK1 wild type or a TAK1 mutant containing a K158R mutation revealed the importance of this site in TNFα and IL-1β-mediated IKK/NF-κB and JNK/AP-1 activation as well as IL-6 gene expression. Our findings demonstrate that Lys63-linked polyubiquitination of TAK1 at Lys158 is essential for its own kinase activation and its ability to mediate its downstream signal transduction pathways in response to TNFα and IL-1β stimulation.
Keywords: Cytokines/Interleukins, Cytokines/Tumor Necrosis Factor, Proteases/Ubiquitination, Protein/Post-translational Modification, Signal Transduction/Protein Kinases, Transcription/AP1, Transcription/NF-κB
Introduction
Tumor necrosis factor α (TNFα)3 and interleukin-1β (IL-1β) are two potent proinflammatory cytokines that play important roles in the regulation of immunity, inflammation, cell proliferation, differentiation, and apoptosis (1, 2). Cellular responses to TNFα and IL-1β are mediated by intracellular signaling pathways that control the activation of nuclear factor-κB (NF-κB) and activator protein 1 (AP-1) (3, 4).
Upon binding to its receptor, TNFα induces formation of a receptor-associated complex, including the adaptor proteins TRADD, TRAF2, TRAF5, and RIP1, which subsequently leads to Lys63-linked polyubiquitination of TRAF2 and RIP1 (5–7). In contrast, IL-1β binding to its receptor induces a receptor-associated complex formation, including MyD88, IRAK1, IRAK4, and TRAF6, which is followed by Lys63-linked polyubiquitination of TRAF6 and IRAKs (8–12). The formation of TRAF2-RIP1 and TRAF6-IRAK4 complexes as well as the Lys63-linked polyubiquitination of RIP1 and TRAF6 appear to enable the recruitment and activation of transforming growth factor-β-activated kinase 1 (TAK1) through binding of the TAK1 regulatory subunits TAB2 and TAB3 to the Lys63-polyubiquitinated RIP1 and TRAF6. The activated TAK1 then triggers the activation of the IκB kinase (IKK), c-Jun N-terminal kinase (JNK), and p38 MAPK (8, 13–17), which leads to activation of transcription factors NF-κB and AP-1 and up-regulation of many genes encoding proinflammatory cytokines, chemokines, adhesion molecules, and proteolytic enzymes (18).
The IKK complex consists of three subunits: two catalytic subunits, IKKα and IKKβ, and an essential regulatory subunit, IKKγ/NF-κB essential modulator (NEMO) (3, 19). Genetic studies have implicated that IKKβ and IKKγ/NEMO are essential for the TNFα- and IL-1β-mediated NF-κB activation (20–22). Phosphorylation of serine 177 and 181 residues in the activation loop is required for IKKβ activation (23). IKKγ/NEMO has been indicated to bind Lys63-linked polyubiquitin chains (7, 16, 24, 25). It is proposed that Lys63-polyubiquitin chains act as a scaffold to allow for assembly of a signaling complex that leads to IKKβ activation. Once activated, IKKβ phosphorylates IκB proteins and leads to IκB polyubiquitination with a Lys48-linked ubiquitin chain. Polyubiquitination-mediated degradation of IκBs allows NF-κB to translocate into the nucleus and activate NF-κB-dependent gene expression (26).
JNKs are members of three related mitogen-activated protein kinases (MAPKs), including the extracellular signal-regulated kinases (ERKs), JNKs, and p38 MAPKs (4, 27). JNKs and p38 MAPKs are involved in transmitting intracellular signals in response to proinflammatory cytokines, such as TNFα and IL-1β, and environmental stresses (28). Once activated, JNKs phosphorylate specific sites on the N-terminal transactivation domain of transcription factor c-Jun, an important component of transcriptional activator AP-1. Phosphorylation of these sites stimulates the transactivity of c-Jun to activate AP-1-dependent gene expression (29).
TAK1, a member of the MAPK kinase kinase family, was originally found to function in the transforming growth factor-β (TGF-β)-mediated MAPK activation (30). TAK1 has been demonstrated to be essential in TNFα- and IL-1β-mediated activation of NF-κB, JNK, and p38 (15, 31–33). Several regulatory subunits of TAK1, including TAB1, TAB2, TAB3, and TAB4, have been implicated to play a role in the regulation of TAK1 activity in response to TNFα and IL-1β stimulation (13, 34–37). TAB1 is a TAK1-interacting protein and induces TAK1 kinase activity through promoting autophosphorylation of key serine/threonine sites of the kinase activation loop (36). TAB1 is an inactive pseudophosphatase sharing homology with members of the PPM family of protein serine/threonine phosphatases (38). TAB2, TAB3, and TAB4 activate TAK1 through binding to polyubiquitinated proteins and promoting a larger complex formation during TNFα- and IL-1β-induced TAK1 activation (15, 37).
Currently, growing evidence suggests that protein phosphorylation and ubiquitination play an essential role in the regulation of TAK1 activation. X-linked inhibitor of apoptosis inhibits JNK1 activation by TGF-β1 through ubiquitin-mediated proteosomal degradation of TAK1 (39). Furthermore, several reports suggest TAK1 ubiquitination is involved in the regulation of TAK1-mediated signaling pathways (40–42). Recently, TRAF6-mediated Lys63-linked TAK1 polyubiquitination at the Lys34 residue has been shown to correlate with TAK1 activation in TGF-β signaling (43). Helicobacter pylori CagA activates NF-κB by targeting TAK1 for TRAF6-mediated Lys63-linked ubiquitination (44). However, the molecular regulation of TAK1 activation by TNFα- and IL-1β-induced protein ubiquitination remains poorly understood.
In this report, we have further investigated the molecular mechanism of TAK1 activation in the context of TNFα and IL-1β stimulation. Using biochemical and reporter assays, we identified the Lys158 residue within TAK1 kinase domain as a Lys63-linked polyubiquitination site required for TAK1-mediated IKK/NF-κB and JNK/AP-1 activation. Furthermore, we found that polyubiquitination of Lys158 residue can be induced by TNFα and IL-1β stimulation and is required for TNFα- and IL-1β-induced IKKγ/NEMO association with TAK1. In addition, we found that Lys63-linked TAK1 polyubiquitination at the Lys158 residue is essential for TNFα and IL-1β-mediated IKK/NF-κB and JNK/AP-1 activation as well as IL-6 gene expression. Together, our results provide biochemical and genetic evidence that the TNFα- and IL-1β-induced Lys63-linked TAK1 polyubiquitination at Lys158 is essential for TNFα and IL-1β-induced TAK1 activation and TAK1-mediated IKK/NF-κB and JNK/AP-1 activation.
EXPERIMENTAL PROCEDURES
Purification of GST-tagged Fusion Proteins
Recombinant GST-TRAF2, GST-TRAF6, GST-TAK1-WT-V5-(1–292), GST-TAK1-K63R-V5-(1–292), GST-TAK1-K150R-V5-(1–292), GST-TAK1-K158R-V5-(1–292), and GST-TAB1 proteins were generated and purified as described previously (45). Briefly, the plasmids encoding recombinant proteins were transformed into Escherichia coli BL-21 strain (Invitrogen), and then recombinant protein expression was inducted by 0.1 mm isopropyl β-d-thiogalactoside for 4 h at 30 °C. Bacteria were lysed with extraction buffer (50 mm Tris-HCl, pH 8.5, 100 mm NaCl, 1 mm EDTA, 1 mm dithiothreitol, 50 mg/ml lysozyme, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mm phenylmethylsulphonyl fluoride) for 45 min on ice and sonicated at 4 °C in 1% Sarkosyl (Sigma). After adding Triton X-100 (1%), 5 μg/ml DNase, and 5 μg/ml RNase (Roche Applied Science), the lysates were centrifuged at 15,000 × g, and the supernatants containing GST fusion protein were collected. Fusion proteins were purified from cell lysates using glutathione-Sepharose beads (Sigma) overnight at 4 °C. The beads were washed three times in extraction buffer containing 0.5% Triton X-100 and one time in extraction buffer containing 0.1% Triton X-100. Proteins were eluted in elution buffer (30.7% glutathione, 50 mm Tris-HCl, pH 8.0, 20% glycerol, 5 m NaCl) and dialyzed in 1× phosphate-buffered saline. The protein concentrations were then assessed with a Bradford protein assay (Bio-Rad). The proteins were resolved by 10% SDS-PAGE and visualized by Coomassie Blue staining of the gel.
In Vitro Kinase Assays
HEK-293T cells seeded onto 10-cm dishes were transfected with the TAK1-V5 and TAB1 expression plasmids. The TAK1-V5 proteins were immunoprecipitated from cell extracts with anti-V5 antibody and washed three times with the lysis buffer and twice with kinase reaction buffer (25 mm Tris, pH 7.5, 2 mm dithiothreitol, 0.1 mm Na3VO4, 10 mm MgCl2). Then the immunoprecipitates were resuspended in kinase buffer containing 100 mm ATP. His-MKK6 fusion protein was added as a substrate for TAK1. After a 30-min incubation at 30 °C, the samples were separated by SDS-PAGE, transferred to polyvinylidene difluoride membrane, and detected by anti-phospho-MKK6 antibodies.
In Vitro Ubiquitination Assays
For in vitro ubiquitination assays, recombinant GST proteins, including GST-TAK1-WT-V5-(1–292), GST-TAK1-K63R-V5-(1–292), GST-TAK1-K150R-V5-(1–292), GST-TAK1-K158R-V5-(1–292), GST-TAB1, GST-TRAF2, and GST-TRAF6, were added as indicated along with 50 nm UBE1, 875 nm E2 (Ubc13/Uev1a), 59 μm ubquitin (wild type, Lys63-only, and Lys48-only) and Mg-ATP (all from Boston Biochemicals) into a 20-μl total volume of ubiquitination buffer (20 mm HEPES, pH 7.4, 1 mm MgCl2, 1 mm dithiothreitol, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mm benzamidine, 20 mm disodium p-nitrophenyl phosphate, 1 mm phenylmethylsulfonyl fluoride). The mixture was incubated at 30 °C for 2 h with gentle agitation. After stopping the reaction, the mixture was added with SDS to 1% concentration and then boiled for 10 min. Subsequently, the sample was diluted 10-fold with 1× phosphate-buffered saline before immunoprecipitation with anti-V5 antibodies and immunoblotting with anti-ubiquitin (Ub) antibodies.
RESULTS
TNFα and IL-1β Induce Lys63-linked TAK1 Polyubiquitination
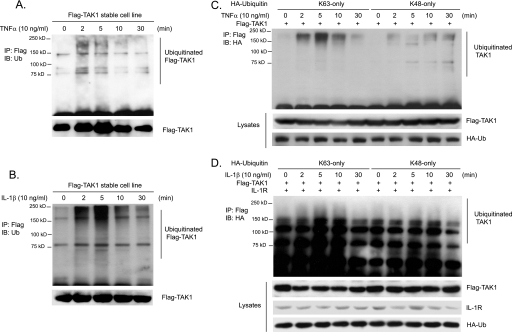
TAK1 plays an essential role in the TNFα and IL-1β-mediated IKKβ and JNK activation (15, 31–33). Recent studies suggest that TAK1 activity is regulated by ubiquitination (15, 40, 42). However, the molecular details of ubiquitination in TAK1 activation remain poorly understood. To further understand the role of ubiquitination in TAK1 activation and examine whether TNFα and IL-1β induce TAK1 ubiquitination, we generated a HeLa cell line with stable expression of human wild type TAK1 with an N-terminal FLAG tag in order to better detect the subtle change of TAK1 during TNFα- and IL-1β-induced TAK1 activation. Then we stimulated FLAG-TAK1 HeLa cells with TNFα and IL-1β for the time points indicated and lysed cells. FLAG-TAK1 proteins in the cell lysates were immunoprecipitated with anti-FLAG antibodies and immunoblotted with anti-ubiquitin antibodies. As shown in Fig. 1, A and B, TNFα and IL-1β rapidly induced the ubiquitination of TAK1 and the induced TAK1 ubiquitination was diminished at the later time points after stimulation. To determine whether TNFα and IL-1β induces Lys63- or Lys48-linked TAK1 polyubiquitination, we co-transfected FLAG-TAK1 into HEK-293T cells with HA-ubiquitin Lys63 or Lys48 only mutant and then stimulated cells with TNFα and IL-1β for the times indicated. FLAG-TAK1 proteins in the cells were immunoprecipitated with anti-FLAG antibodies and immunoblotted with anti-HA antibodies. In this assay, we found that TNFα and IL-1β induced a much stronger Lys63-linked TAK1 polyubiquitination within 5 min of stimulation compared with the Lys48-linked TAK1 polyubiquitination (Fig. 1, C and D). These results demonstrate that TNFα and IL-1β induce Lys63-linked TAK1 polyubiquitination.
FIGURE 1.
TNFα and IL-1β induce Lys63-linked TAK1 polyubiquitination. A and B, TNFα and IL-1β induce TAK1 polyubiquitination. HeLa cells with stable expression of FLAG-TAK1 were either untreated or treated with TNFα (10 ng/ml) (A) and IL-1β (10 ng/ml) (B) for the times indicated and subsequently lysed. FLAG-TAK1 proteins in the cell lysates were immunoprecipitated (IP) with anti-FLAG antibodies and immunoblotted (IB) with anti-ubiquitin antibodies to detect the presence of ubiquitinated FLAG-TAK1. C, TNFα induces Lys63-linked TAK1 polyubiquitination. Expression vectors encoding FLAG-TAK1 were co-transfected into HEK-293T cells with expression vectors encoding HA-ubiquitin-Lys63 only and Lys48 only, respectively. Then cells were either untreated or treated with TNFα (10 ng/ml) for the time points indicated and subsequently lysed. FLAG-TAK1 proteins in the cell lysates were immunoprecipitated with anti-FLAG antibodies and immunoblotted with anti-HA antibodies to detect the presence of ubiquitinated FLAG-TAK1. D, IL-1β induces Lys63-linked TAK1 polyubiquitination. Expression vectors encoding FLAG-TAK1 and IL-1R were co-transfected into HEK-293T cells with expression vectors encoding HA-ubiquitin-Lys63-only and Lys48-only, respectively. Then cells were either untreated or treated with IL-1β (10 ng/ml) for the times indicated and subsequently lysed. FLAG-TAK1 proteins in the cell lysates were immunoprecipitated with anti-FLAG antibodies and immunoblotted with anti-HA antibodies to detect the presence of ubiquitinated FLAG-TAK1.
Co-overexpression of TAK1/TAB1 Induces Lys63-linked TAK1 Polyubiquitination
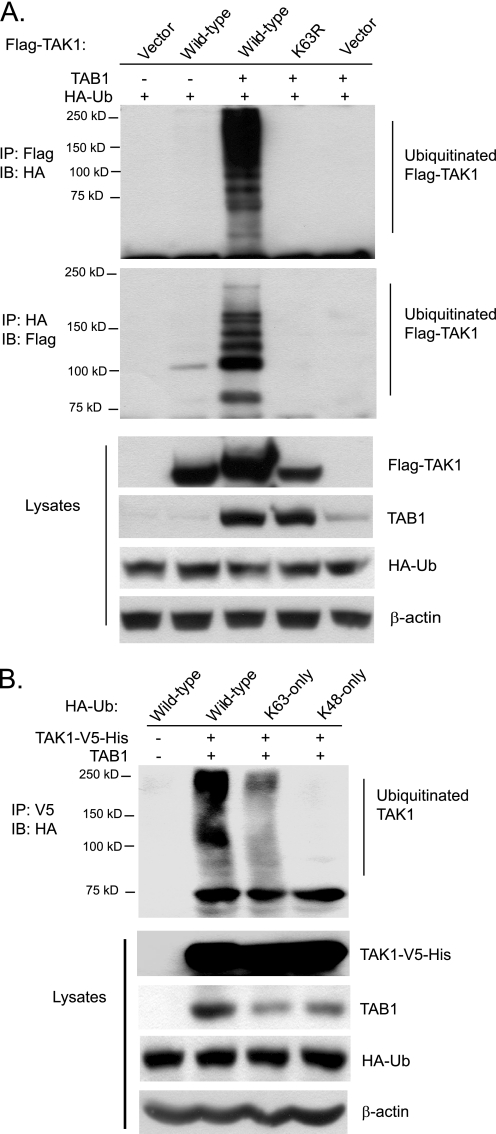
Co-overexpression of TAK1 with its regulatory subunit TAB1 leads to TAK1 autophosphorylation and activation in the cells (36). Therefore, we hypothesized that co-overexpression of TAK1 with its regulatory subunit TAB1 might also lead to TAK1 ubiquitination. To test this hypothesis, we co-transfected HA-Ub expression vectors with or without FLAG-TAK1 and TAB1 expression vectors into HEK-293T cells. The cell lysates from the transfected cells were heated in the presence of 1% SDS and diluted with lysis buffer in order to disrupt noncovalent protein-protein interactions. Then FLAG-TAK1 or HA-ubiquitin in the cell lysates were immunoprecipitated with anti-FLAG or anti-HA antibodies and immunoblotted with anti-HA or anti-FLAG antibodies for the detection of the ubiquitinated TAK1. In this assay, we found that co-overexpression of TAK1 wild type with TAB1 induced TAK1 polyubiquitination, whereas co-overexpression of a kinase-dead TAK1-K63R mutant (TAK1 kinase ATP binding site K63R mutation) with TAB1 and overexpression of TAK1 alone both failed to do so (Fig. 2A). This result indicates that co-overexpression of TAK1 with TAB1 induces TAK1 polyubiquitination and that TAK1 polyubiquitination is associated with its activation.
FIGURE 2.
Co-overexpression of TAK1/TAB1 induces Lys63-linked TAK1 polyubiquitination. A, co-overexpression of TAK1/TAB1 induces TAK1 polyubiquitination. Expression vectors encoding FLAG-TAK1 and HA-ubiquitin were co-transfected into HEK-293T cells with control vector and expression vectors encoding TAB1, respectively. FLAG-TAK1 proteins in the transfected cells were immunoprecipitated (IP) with anti-FLAG antibodies (or anti-HA antibodies) and immunoblotted (IB) with anti-HA antibodies (or anti-FLAG antibodies) to detect the presence of ubiquitinated FLAG-TAK1. B, co-overexpression of TAK1/TAB1 induces Lys63-linked TAK1 polyubiquitination. Expression vectors encoding TAK1-V5-His and TAB1 were co-transfected into HEK-293T cells with control vector and expression vectors encoding HA-ubiquitin wild type, Lys63-only, and Lys48-only, respectively. TAK1-V5-His proteins in the transfected cells were immunoprecipitated with anti-V5 antibodies and immunoblotted with anti-HA antibodies to detect the presence of ubiquitinated TAK1-V5-His.
To determine the linkage type of polyubiquitin chains on TAK1, we co-transfected TAK1-V5-His and TAB1 into HEK-293T cells along with HA-Ub wild type or mutant containing one lysine only at position 63 (Lys63-only) or 48 (Lys48-only). As shown in Fig. 2B, TAK1 polyubiquitination was observed with the Ub wild type and Lys63-only mutant but not with the Lys48-only mutant. This result suggests that polyubiquitin chains on TAK1 were linked primarily through Lys63 of Ub. Together, these results indicate that co-overexpression of TAK1 with TAB1 induces Lys63-linked TAK1 polyubiquitination and that Lys63-linked TAK1 polyubiquitination is associated with its activation.
Co-overexpression of TAK1/TAB1 Induces Lys63-linked TAK1 Polyubiquitination at the Lys158 Residue within the Kinase Domain
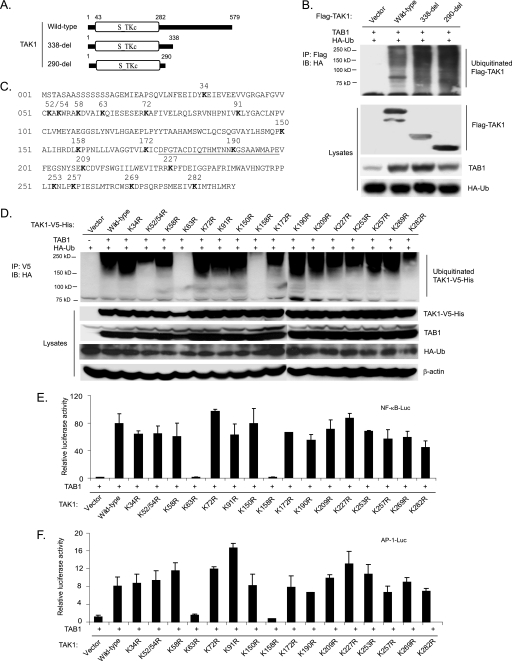
To address the role of the TAK1 Lys63-linked polyubiquitination in its activation and TAK1-mediated downstream signaling events, it is essential to map the Ub lysine acceptor site(s) of TAK1 and characterize the functional effects of eliminating the site(s). To determine the location of the Ub lysine acceptor site(s) of TAK1, we first generated two TAK1 C-terminal deletion mutants that sequentially deleted the TAK1 regulatory domain at amino acid 338 and 290, respectively (Fig. 3A). Then we co-transfected HA-Ub and TAB1 into HEK-293T cells along with TAK1 wild type or two TAK1 C-terminal deletion mutants (338-del and 290-del). As shown in Fig. 3B, TAK1 wild type and two TAK1 C-terminal deletion mutants were polyubiquitinated at similar levels. Therefore, we hypothesized that one or more Lys residues within the TAK1 N-terminal kinase domain (1–290 amino acids) may serve as the Ub lysine acceptor site(s).
FIGURE 3.
Co-overexpression of TAK1/TAB1 induces Lys63-linked TAK1 polyubiquitination at the Lys158 residue within the kinase domain. A, schematic representation of TAK1 wild type and deletion mutants with the kinase domain indicated. B, co-overexpression of the TAK1/TAB1-induced TAK1 polyubiquitination site is located within the kinase domain. Expression vectors encoding HA-ubiquitin and TAB1 were co-transfected into HEK-293T cells with control vector and expression vectors encoding FLAG-TAK1 wild type and two deletion mutants (338-del and 290-del), respectively. FLAG-TAK1 proteins in the transfected cells were immunoprecipitated (IP) with anti-FLAG antibodies and immunoblotted (IB) with anti-HA antibodies to detect the presence of ubiquitinated FLAG-TAK1. C, TAK1 primary sequence with the lysine residues within its N-terminal 290 amino acids indicated. The kinase activation loop is underlined. D, co-overexpression of TAK1/TAB1 induces TAK1 polyubiquitination at Lys158 within the kinase domain. Expression vectors encoding HA-ubiquitin and TAB1 were co-transfected into HEK-293T cells with control vector and expression vectors encoding TAK1-V5-His wild type and 16 lysine to arginine mutants, respectively. TAK1-V5-His proteins in the transfected cells were immunoprecipitated with anti-V5 antibodies and immunoblotted with anti-HA antibodies to detect the presence of ubiquitinated TAK1-V5-His. E and F, the effect of overexpression of TAK1 lysine to arginine mutants with TAB1 on TAK1/TAB1-induced NF-κB (E) and AP-1 (F) activation. TAB1 expression vectors, NF-κB luciferase reporter, and control Renilla luciferase reporter vectors were co-transfected into TAK1-deficient MEF cells with empty vector or expression vectors encoding TAK1 wild type and lysine to arginine mutants, respectively. The relative luciferase activity was measured 48 h later and normalized with the Renilla activity. Error bars, ±S.D. in triplicate experiments.
TAK1 contains 17 lysine residues within the first 290 amino acids, including its kinase domain (Fig. 3C). To identify the likely Ub lysine acceptor site(s) in TAK1, we systematically replaced each of these Lys residues with an Arg residue, which maintains the positive charge but does not serve as an acceptor site for Ub modification. The expression vectors encoding the C-terminal V5-tagged TAK1 wild type and mutants with different Lys to Arg mutations were co-transfected with TAB1 into HEK-293T cells along with HA-Ub. The cell lysates were boiled in the presence of 1% SDS and immunoprecipitated with anti-V5, followed by SDS-PAGE and immunoblotting with anti-HA antibodies. Of the 17 Lys to Arg mutants examined, only K63R (a kinase ATP binding site mutant) and K158R mutants failed to induce TAK1 polyubiquitination compared with the other mutants (Fig. 3D).
Consistent with these results, luciferase analysis with NF-κB and AP-1-responsive reporters showed that TAK1 K63R and K158R mutants failed to induce the luciferase reporter gene expression, whereas TAK1 wild type and other mutants induced a high level of reporter gene activity when co-overexpressed with TAB1 in TAK1-deficient MEF cells (Fig. 3, E and F). Because the TAK1 Lys63 residue is a conserved lysine residue in the ATP pocket essential for coordinating kinase ATP binding and activation, it is likely that Lys158 is the predominant Ub acceptor site for TAK1 polyubiquitination. Taken together, these results suggest that polyubiquitination of TAK1 at Lys158 is required for the TAK1/TAB1 co-overexpression-induced NF-κB and AP-1 activation.
The Lys158 Residue Is Required for TNFα- and IL-1β-induced TAK1 Polyubiquitination and NF-κB Activation
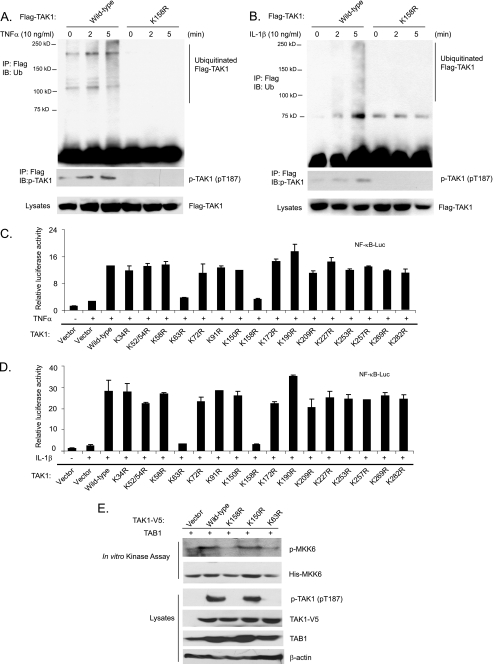
To determine whether the Lys158 residue is an acceptor site for Ub modification of TAK1 in response to TNFα and IL-1β stimulation, we stimulated the stable HeLa cell lines harboring FLAG-TAK1 wild type and FLAG-TAK1 K158R mutant with TNFα and IL-1β. As shown in Fig. 4, A and B, FLAG-TAK1 polyubiquitination and phosphorylation were rapidly induced by TNFα and IL-1β in the cells expressing FLAG-TAK1 wild type but not K158R mutant. This result suggests that the Lys158 residue is an acceptor site for Ub modification of TAK1 in response to TNFα and IL-1β stimulation.
FIGURE 4.
TNFα and IL-1β induce TAK1 polyubiquitination at the Lys158 residue. A and B, TNFα and IL-1β induce TAK1 polyubiquitination at Lys158. HeLa cells with stable expression of FLAG-TAK1 wild type and K158R mutant were either untreated or treated with TNFα (10 ng/ml) (A) and IL-1β (10 ng/ml) (B) for the time points indicated and subsequently lysed. FLAG-TAK1 proteins in the cell lysates were immunoprecipitated (IP) with anti-FLAG antibodies and immunoblotted (IB) with anti-ubiquitin antibodies to detect the presence of ubiquitinated FLAG-TAK1. C and D, the effect of overexpression of TAK1 lysine to arginine mutants on TNFα-induced (C) and IL-1β-induced (D) NF-κB activation. NF-κB luciferase reporter and control Renilla luciferase reporter vectors were co-transfected into TAK1-deficient MEF cells with empty vector or expression vectors encoding TAK1 wild type and lysine to arginine mutants for 48 h, respectively. Cells were then either untreated or treated with TNFα (1 ng/ml) and IL-1β (1 ng/ml) for 6 h. The relative luciferase activity was measured and normalized with the Renilla activity. The error bars indicate ±S.D. in triplicate experiments. E, TAK1 Lys158 is required for TAK1 activation. TAB1 were transfected into HEK-293T cells with TAK1-V5 wild type, K158R, K150R, and K63R, respectively. TAK1-V5 proteins were immunoprecipitated from cell extracts with anti-V5 antibodies for an in vitro kinase assay using recombinant His-MKK6 as a substrate.
To determine whether the TAK1 Lys158 residue is required for TNFα- and IL-1β-induced NF-κB activation, we performed luciferase analysis in TAK1-deficient MEF cells transfected with TAK1 wild type and various lysine to arginine mutants with NF-κB-responsive reporter. In these assays, TAK1 K63R and K158R mutants blocked the TNFα and IL-1β-induced NF-κB-dependent luciferase activities, whereas TAK1 wild type and other lysine to arginine mutants mediated a high level of reporter gene activity (Fig. 4, C and D). To further confirm the role of Lys63-linked polyubiquitination at Lys158 in TAK1 activation, we co-transfected TAB1 into HEK-293T cells with vector control, TAK1-V5 wild type, K158R, K150R, and K63R, respectively. TAK1 wild type and mutant proteins were immunoprecipitated with anti-V5 antibodies for an in vitro kinase assay using recombinant MKK6 as a substrate. In this assay, we found that TAK1 K158R and K63R mutants failed to phosphorylate MKK6 in vitro compared with TAK1 wild type and K150R mutant (Fig. 4E). Taken together, these results suggest that polyubiquitination of TAK1 at Lys158 residue is required for TNFα- and IL-1β-induced NF-κB activation.
TRAF2 and TRAF6 Mediate Lys63-linked TAK1 Polyubiquitination at the Lys158 Residue in Vivo and in Vitro
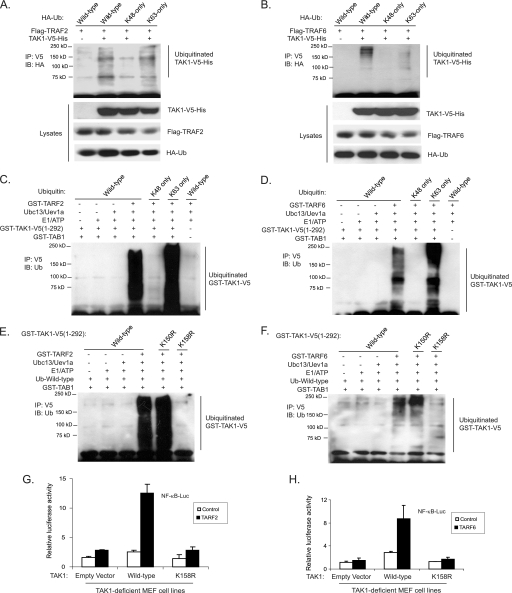
TRAF2 and TRAF6 have been identified to act as the Ub E3 ligases in the TNFα- and IL-1β-mediated NF-κB signal transduction pathway to facilitate the synthesis of unique Lys63-linked poly-Ub chains rather than the conventional Lys48-linked poly-Ub chains that target proteins for degradation (46). To determine whether TRAF2 and TRAF6 mediate Lys63-linked TAK1 polyubiquitination, we co-transfected TAK1-V5-His and TRAF2/6 into HEK-293T cells along with HA-Ub wild type, Lys48-only, and Lys63-only, respectively. As shown in Fig. 5, A and B, overexpression of TRAF2 and TRAF6 induced a strong TAK1 polyubiquitination with Ub wild type and Lys63-only but not Lys48-only chains. This result suggests that TRAF2 and TRAF6 are the Ub E3 ligases that mediate Lys63-linked polyubiquitination of TAK1 in the cells.
FIGURE 5.
TRAF2 and TRAF6 mediate Lys63-linked TAK1 polyubiquitination at the Lys158 residue in vivo and in vitro. A and B, overexpression of TRAF2 (A) and TRAF6 (B) induces Lys63-linked TAK1 polyubiquitination. Expression vectors encoding TAK1-V5-His and TRAF2 (A) or TRAF6 (B) were co-transfected into HEK-293T cells with control vector and expression vectors encoding HA-ubiquitin wild type, Lys63-only and Lys48-only, respectively. TAK1-V5-His proteins in the transfected cells were immunoprecipitated (IP) with anti-V5 antibodies and immunoblotted (IB) with anti-HA antibodies to detect the presence of ubiquitinated TAK1-V5-His. C and D, TRAF2 (C) and TRAF6 (D) mediate Lys63-linked TAK1 polyubiquitination in vitro. Recombinant GST-TAK1-V5-(1–292), GST-TAB1, E1, E2, (Ubc13/Uev1a), and GST-TRAF2 (C) or GST-TRAF6 (D) were co-incubated in the ubiquitination buffer with recombinant ubiquitin wild type, Lys48-only, and Lys63-only, respectively. After 2 h, GST-TAK1-V5-(1–292) proteins were immunoprecipitated with anti-V5 antibodies and immunoblotted with anti-ubiquitin antibodies to detect the presence of ubiquitinated TAK1-V5-(1–292). E and F, TRAF2 (E) and TRAF6 (F) mediate Lys63-linked TAK1 polyubiquitination at Lys158 in vitro. Recombinant ubiquitin wild type, GST-TAB1, E1, E2 (Ubc13/Uev1a), and GST-TRAF2 (E) or GST-TRAF6 (F) were co-incubated in the ubiquitination buffer with GST-TAK1-V5-(1–292) wild type, K158R, or K150R, respectively. After 2 h, GST-TAK1-V5-(1–292) wild type and mutant proteins were immunoprecipitated with anti-V5 antibodies and immunoblotted with anti-ubiquitin antibodies to detect the presence of ubiquitinated TAK1-V5-(1–292). G and H, TAK1 lysine 158 to arginine mutant inhibits TRAF2-induced (G) and TRAF6-induced (H) NF-κB activation. TRAF2 or TRAF6 expression vectors, NF-κB luciferase reporter, and control Renilla luciferase reporter vectors were co-transfected into TAK1-deficient MEF cells with empty vector or expression vectors encoding TAK1 wild type and lysine 158 to arginine (K158R) mutant. The relative luciferase activity was measured 48 h later and normalized with the Renilla activity. Error bars, ±S.D. in triplicate experiments.
To further determine whether TRAF2 and TRAF6 are the Ub E3 ligases that mediate Lys63-linked polyubiquitination of TAK1, we set up an in vitro ubiquitination assay with purified ubiquitin wild type, Lys48-only and Lys63-only mutants as well as GST-TRAF2, GST-TRAF6, Ubc13/Uev1A, E1/ATP, GST-TAB1, GST-TAK1-(1–292) wild type, and K150R and K158R mutants, respectively. As shown in Fig. 5, C and D, both TRAF2 and TRAF6 catalyze the Ub wild type and Lys63-linked but not Lys48-linked TAK1 polyubiquitination. Notably, recombinant TRAF2 and TRAF6 mediated a strong polyubiquitination of TAK1 wild type and K150R mutant, whereas they failed to mediate polyubiquitination of TAK1 K158R mutant completely (Fig. 5, E and F).
To further confirm that TAK1 K158R is the predominant Ub acceptor site, we performed in vitro ubiquitination assays with GST-TAK1-(1–292) wild type and K63R and K158R mutants, respectively. In these assays, we found that TRAF2 and TRAF6 catalyze a strong polyubiquitination of TAK1 wild type and K63R mutant, whereas they failed to mediate polyubiquitination of the TAK1 K158R mutant (supplemental Fig. S1). These results suggest that TRAF2 and TRAF6 act as the Ub E3 ligases to mediate Lys63-linked TAK1 polyubiquitination at the Lys158 residue.
Consistent with these results, luciferase analysis with NF-κB- and AP-1-responsive reporters showed that TAK1 K158R mutant but not wild type blocked TRAF2 or TRAF6 overexpression-induced luciferase reporter gene activity (Fig. 5, G and H). This result indicates that Lys63-linked TAK1 polyubiquitination at the Lys158 residue is required for TRAF2- and TRAF6-mediated NF-κB activation.
UBC13 has been shown to play an important role in TRAF6-mediated Lys63-linked polyubiquitination (8, 15). To further confirm the role of the Lys63-linked TAK1 polyubiquitination in TAK1-mediated NF-κB activation, we cotransfected NF-κB reporter and TAK1/TAB1 into HEK-293T cells along with sh-Control or shUBC13 plasmids. In this reporter assay, we found that suppression of UBC13 expression inhibited TAK1/TAB1-induced NF-κB activation (supplemental Fig. S2). Taken together, these results demonstrate that TRAF2 and TRAF6 are the Ub E3 ligases that specifically catalyze Lys63-linked TAK1 polyubiquitination at the Lys158 residue in the cells.
IKKγ/NEMO Binds to Polyubiquitinated TAK1
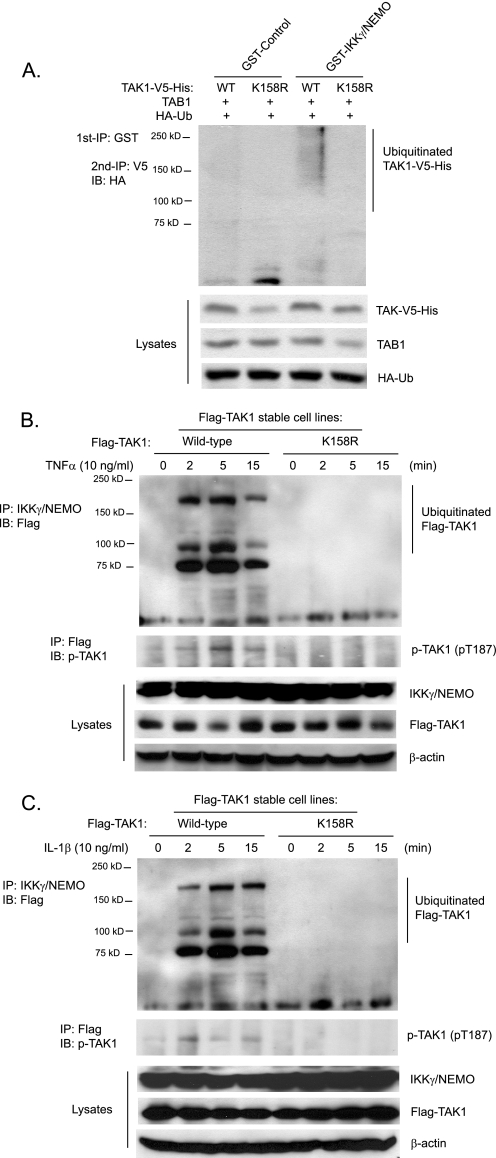
The regulatory subunit IKKγ/NEMO of the IKK complex binds polyubiquitin chains and mediates TNFα- and IL-1β-induced IKKβ-NF-κB activation (7, 25). Previous studies demonstrate that TAK1 is the major kinase mediating TNFα and IL-1β-induced IKK/NF-κB activation (15, 31–33). Therefore, we hypothesized that IKKγ/NEMO binds to Lys63-linked polyubiquitinated TAK1 for IKK activation. To test this hypothesis, we co-transfected TAK1 wild type or K158R mutant with HA-Ub and TAB1 into HEK-293T cells and subsequently lysed the cells. Then we incubated recombinant GST and GST-IKKγ/NEMO bound to glutathione-agarose beads with the above cell lysates for the detection of the amount of TAK1 that was pulled down. As shown in Fig. 6A, GST-IKKγ/NEMO but not GST control pulled down a significant amount of polyubiquitinated TAK1 wild type but not polyubiquitination-deficient K158R mutant proteins. This result suggests that IKKγ/NEMO binds to the polyubiquitinated TAK1.
FIGURE 6.
IKKγ/NEMO binds to polyubiquitinated TAK1. A, recombinant IKKγ/NEMO pulls down polyubiquitinated TAK1. Expression vectors encoding TAB1 and HA-ubiquitin were co-transfected into HEK-293T cells with expression vectors encoding TAK1-V5-His wild type and K158R mutant, respectively. The cell lysates were co-incubated with recombinant GST control and GST-IKKγ/NEMO proteins bound to glutathione-agarose beads for 2 h, respectively. GST control and GST-IKKγ/NEMO proteins bound to glutathione-agarose beads were first precipitated with centrifugation and boiled. Then co-precipitated TAK1-V5-His proteins were immunoprecipitated (IP) with anti-V5 antibodies and immunoblotted (IB) with anti-HA antibodies to detect the presence of polyubiquitinated TAK1-V5-His proteins. B and C, TNFα (B) and IL-1β (C) induce association of IKKγ/NEMO with TAK1 wild type but not K158R mutant proteins. HeLa cells with stable expression of FLAG-TAK1 wild type and K158R mutant were either untreated or treated with TNFα (10 ng/ml) (B) and IL-1β (10 ng/ml) (C) for the times indicated and subsequently lysed. Endogenous IKKγ/NEMO proteins in the cell lysates were immunoprecipitated with anti-NEMO antibodies and immunoblotted with anti-FLAG antibodies to detect the co-immunoprecipitation of FLAG-TAK1.
To determine whether IKKγ/NEMO binds to polyubiquitinated TAK1 in the cells in response to TNFα and IL-1β stimulation, we stimulated HeLa cell lines expressing FLAG-TAK1 wild type and K158R mutant with TNFα and IL-1β for the times indicated, and endogenous NEMO was immunoprecipitated from the cell lysates and immunoblotted with anti-FLAG antibodies to detect the presence of FLAG-TAK1. As shown in Fig. 6, B and C, the ubiquitinated FLAG-TAK1 wild type but not ubiquitination-deficient K158R was rapidly pulled down by immunoprecipitation of IKKγ/NEMO in response to TNFα and IL-1β stimulation.
To further determine the role of TAK1 Lys158 in TNFα- and IL-1β-induced complex formation, including TRAFs, TAK1/TAB1, and IKKs, we transfected expression vectors encoding wild type and K158R TAK1-V5-His into HEK-293T cells and stimulated cells with TNFα and IL-1β (IL-1β receptor expression vectors were co-transfected in the IL-1β-stimulated cells) at the time points indicated. TAK1-V5-His proteins in the cell lysates were pulled down with nickel affinity beads and immunoblotted with anti-TRAF2/6 and anti-IKKβ antibodies. In this assay, we found that TNFα and IL-1β induced association of TRAF2 and -6 with both TAK1 wild type and K158R mutant equally well, whereas TNFα and IL-1β failed to induce significant interaction of TAK1 K158R mutant with IKKβ compared with TAK1 wild type (supplemental Fig. S3). Taken together, these results demonstrate that TNFα- and IL-1β-induced polyubiquitination of TAK1 is required for the association of TAK1 with IKKγ/NEMO and IKKβ.
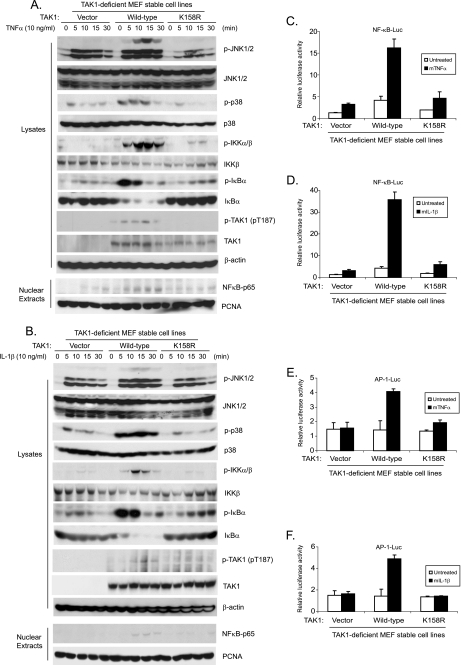
TAK1 Polyubiquitination at the Lys158 Residue Is Required for TNFα- and IL-1β-induced IKK/NF-κB and JNK/AP-1 Activation
To further explore the role of polyubiquitination of TAK1 at the Lys158 residue in TNFα and IL-1β-induced IKK/NF-κB and JNK/AP-1 activation, the TAK1 wild type and K158R mutant expression vectors were stably introduced back into the TAK1-deficient MEF cells by a retroviral transduction system. Then TAK1-deficient, TAK1 wild type, and K158R mutant reconstituted MEF cells were treated with TNFα or IL-1β at different time points, and then the cell lysates were immunoblotted with the indicated antibodies to examine TNFα- and IL-1β-induced JNK, p38 MAPK, IKK, and IκBα phosphorylation as well as IκBα degradation. In this assay, TNFα- and IL-1β-induced-JNK2 and p38 phosphorylation were completely blocked in the TAK1-deficient and K158R mutant MEF cells, whereas TNFα and IL-1β-induced-JNK1 activation was only partially inhibited in the TAK1-deficient and K158R mutant MEF cells compared with the TAK1 wild type reconstituted cells (Fig. 7, A and B). Similarly, TNFα and IL-1β-induced IKK and IκBα phosphorylation as well as IκBα degradation were impaired in the MEF cells with control vector and TAK1 K158R mutant compared with the cells with TAK1 wild type (Fig. 7, A and B). We also isolated nuclear extracts from the above cells treated with TNFα and IL-1β at the indicated time points and found that TNFα- and IL-1β-induced NF-κB nuclear translocation was also significantly impaired in the MEF cells with control vector and TAK1 K158R mutant compared with cells with TAK1 wild type (Fig. 7, A and B). These results suggest that the TAK1 Lys158 residue is required for TNFα and IL-1β-mediated IKK/NF-κB and JNK/AP-1 activation.
FIGURE 7.
Polyubiquitination of TAK1 at Lys158 residue is required for TNFα- and IL-1β-induced optimal IKK/NF-κB and JNK/AP-1 activation. A and B, expression of the TAK1 K158R mutant inhibits TNFα-induced (A) and IL-1β-induced (B) JNK, p38, IKK, and IκBα phosphorylation as well as IκBα degradation and NF-κB nuclear translocation. TAK1-deficient MEF cells were transduced with the retrovirus encoding the vector control, TAK1 wild type, or TAK1 K158R mutant and subsequently selected with puromycin (2 μg/ml) to establish the TAK1-deficient MEF cell lines with the stable expression of either TAK1 wild type or K158R mutant. TAK1-deficient, TAK1 wild type, and K158R mutant reconstituted MEF cells were untreated or treated with TNFα (10 ng/ml) (A) and IL-1β (10 ng/ml) (B) for the times indicated and subsequently harvested. Whole cell extracts and nuclear extracts were subjected to SDS-PAGE and immunoblotted (IB) with antibodies indicated. β-Actin was detected as a loading control for whole cell extracts, and PCNA was used as a loading control for nuclear extracts. C–F, expression of the TAK1 K158R mutant inhibits TNFα (C and E) and IL-1β (D and F)-induced NF-κB (C and D) and AP-1 (E and F) reporter activities. NF-κB (C and D) and AP-1 (E and F) reporter and 20 ng of Renilla-Luc plasmids were cotransfected into TAK1-deficient MEF control, TAK1 wild type, and K158R reconstituted cells for 48 h. Cells were then either untreated or treated with TNFα (1 ng/ml) and IL-1β (1 ng/ml) for 6 h. The relative luciferase activity was measured and normalized with the Renilla activity. Error bars, ±S.D. in triplicate experiments.
Consistent with the above results, TNFα and IL-1β induced much higher NF-κB- and AP-1-dependent luciferase activities in MEF cells expressing the TAK1 wild type, compared with the TAK1-deficient and K158R mutant cells (Fig. 7, C–F).
Consistent with earlier reports that TAK1 is required for lipopolysaccharide-induced but not lysophosphatidic acid and protein kinase C-induced IKK/NF-κB activation (15, 32, 33, 47), we found that TAK1 K158R mutant inhibited lipopolysaccharide-mediated but not lysophosphatidic acid- and protein kinase C-mediated IKK/NF-κB activation (supplemental Figs. S4 and S5).
Taken together, these results indicate that polyubiquitination of TAK1 at the Lys158 site is required for the TNFα- and IL-1β-induced optimal IKK/NF-κB and JNK/AP-1 activation.
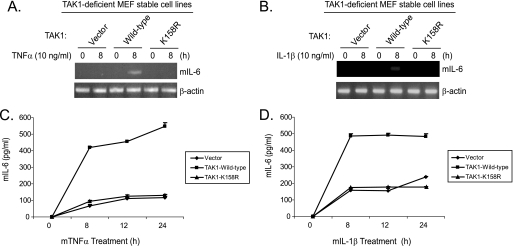
TAK1 Polyubiquitination at the Lys158 Residue Is Required for TNFα- and IL-1β-induced IL-6 Gene Expression
TAK1 mediates TNFα- and IL-1β-induced NF-κB and AP-1 activation and IL-6 gene expression (48, 49). To determine the role of TAK1 polyubiquitination at the Lys158 residue in TNFα- and IL-1β-induced IL-6 gene expression, the TAK1-deficient stable MEF cell lines with vector control, TAK1 wild type, and K158R mutant were treated with TNFα or IL-1β. Then we examined the TNFα- and IL-1β-induced IL-6 gene expression at the mRNA level in the cells. As shown in Fig. 8, A and B, TNFα- and IL-1β-induced IL-6 expression was blocked in the TAK1-deficient and K158R mutant cells compared with the TAK1 wild type cells. Consistent with this result, TNFα- and IL-1β-induced IL-6 production in the cell medium was significantly inhibited in the TAK1-deficient and K158R mutant cells compared with the TAK1 wild type cells (Fig. 8, C and D). These results demonstrate that polyubiquitination at the Lys158 residue is required for TNFα- and IL-1β-induced IL-6 expression and production in the cells.
FIGURE 8.
Polyubiquitination of TAK1 at Lys158 residue is required for TNFα and IL-1β-induced IL-6 gene expression. A and B, expression of the TAK1 K158R mutant inhibits IL-1-induced IL-6 gene transcription. TAK1-deficient, TAK1 wild type, and K158R mutant reconstituted MEF cells were untreated or treated with TNFα (10 ng/ml) (A) and IL-1β (10 ng/ml) (B) for 8 h and subsequently harvested for extraction of total RNA using TRIzol reagent. 1 μg of total RNA was used to synthesize first-strand cDNA using a reverse transcription kit according to the manufacturer's instructions. These synthesized cDNAs were used as templates for mouse IL-6 PCR amplification. The PCR products were resolved in 2% agarose gel. C and D, expression of the TAK1 K158R mutant inhibits IL-1-induced IL-6 production. TAK1-deficient, TAK1 wild type, and K158R mutant reconstituted MEF cells were untreated or treated with TNFα (2 ng/ml) (C) and IL-1β (2 ng/ml) (D) for the times indicated. The supernatants from the cell cultures were collected and subjected to the mouse IL-6 ELISA analysis according to the manufacturer's instruction.
DISCUSSION
In this report, we further examined the mechanism of ubiquitination in TNFα- and IL-1β-mediated IKK/NF-κB and JNK/AP-1 activation and gained novel insights into the regulatory role of Lys63-linked polyubiquitination in the TAK1-mediated NF-κB and AP-1 activation. Here we show that TAK1 is polyubiquitinated with a Lys63-linked ubiquitin chain at the Lys158 residue within its kinase domain in response to TNFα and IL-1β stimulation and that Lys63-linked polyubiquitination of TAK1 at the Lys158 residue binds to IKKγ/NEMO, resulting in the recruitment of IKK complexes to the activated TAK1. Furthermore, we show that TRAF2 and TRAF6 are the Ub E3 ligases that mediate Lys63-linked polyubiquitination of TAK1. Interestingly, a lysine to arginine mutation at Lys158 of TAK1 blocks TNFα- and IL-1β-induced TAK1 polyubiquitination and downstream IKK/NF-κB and JNK/AP-1 activation. Taken together, our results indicate that Lys63-linked polyubiquitination of TAK1 at Lys158 and binding of polyubiquitinated TAK1 by IKKγ/NEMO are required for the TNFα- and IL-1-mediated IKK activation. In addition to the polyubiquitin chains on RIP1, TRAF2/6 and IRAKs, the polyubiquitin chains on TAK1 also serve as a part of a platform to recruit the IKK complex, JNK, and p38 MAPKs, allowing TAK1 to activate IKK, JNK, and p38-mediated signal transductions. We propose that Lys63-linked polyubiquitination of TAK1 at Lys158 within the kinase domain is a general mechanism for TAK1-mediated IKK/NF-κB and JNK/AP-1 activation.
TRAF2/RIP1 and TRAF6/IRAKs are proximal signaling components in TNFα and IL-1β receptor-mediated IKK/NF-κB and JNK/AP-1 activation, respectively (6, 7). Both TRAF2 and TRAF6 contain a RING domain that confers their E3 activity (46). Our studies demonstrate that TRAF2 and TRAF6 specifically catalyze TAK1 ubiquitination at the Lys158 residue in vivo and in vitro. Considering there are 34 lysine residues in TAK1 protein sequence, it is very unusual that TRAF2- and TRAF6-mediated TAK1 polyubiquitination is so site-specific and consistent in both in vivo and in vitro ubiquitination assays. In the case of RIP1, none of the lysine mutations prevented RIP1 ubiquitination or NF-κB activation once overexpressed in HEK-293T cells (7).
Previous studies suggest that TAK1 as a serine/threonine kinase is recruited to receptor associated TRAF2/RIP1 and TRAF6/IRAKs through binding of TAB2 and TAB3 to polyubiquitinated RIP1 and IRAKs in response to TNFα and IL-1β stimulation, whereas the IKK complex is recruited via binding of IKKγ/NEMO to the polyubiquitin chain on RIP1 and IRAKs (8–12). Our current results demonstrate that TAK1 is polyubiquitinated at the Lys158 residue, and this site-specific Lys63-linked polyubiquitination is required for binding of NEMO to TAK1 in response to TNFα and IL-1β stimulation. Previous structure studies indicate that TAK1 Lys158 residue is on the surface of the protein structure and exposed to solvent (50). Therefore, the TAK1 K158R mutation should not induce any significant structural perturbation (supplemental Fig. S6). Consistent with this study, we found that both TRAF2 and TRAF6 successfully catalyzed TAK1 K63R polyubiquitination in an in vitro ubiquitination assay (supplemental Fig. S1). Together, these results suggest that TAK1 polyubiquitination at the Lys158 residue contributes to the formation of TNFα- and IL-1β-induced intracellular signaling complex that is essential for optimal downstream IKK/NF-κB and JNK/AP-1 activation.
Interestingly, as two serine/threonine kinases, RIP1 and IRAK1 are polyubiquitinated with a Lys63 linkage type at lysine residues outside of their kinase domain in response to TNFα and IL-1β stimulation, respectively (8–12). In addition, Lys63-linked polyubiquitination of these two kinases are not reported to be involved in the regulation of their kinase activities. Furthermore, site-specific monoubiquitination of IKKβ within its kinase domain regulates its persistent activation (51). In contrast, TNFα- and IL-1β-induced Lys63-linked TAK1 polyubiquitination within the kinase domain is required for TNFα- and IL-1β-induced activation of TAK1 and the downstream signal transduction pathway.
Using a HeLa cell line stably expressing FLAG-TAK1 wild type and K158R mutant, we found that TNFα and IL-1β induced binding of IKKγ/NEMO to TAK1 wild type but not K158R mutant. This result strongly indicates that TAK1 polyubiquitination at the Lys158 residue is essential for TNFα- and IL-1β-induced association of TAK1 with IKKγ/NEMO. It is highly likely that only polyubiquitinated TAK1 is able to phosphorylate and activate the IKKγ/NEMO-associated IKKβ in a complex containing the ubiquitinated TAK1, IKKγ/NEMO, and IKKβ. Further study is required to dissect the mechanism by which polyubiquitinated TAK1 is recruited to the receptor-associated protein complex and activates IKK/NF-κB and JNK/AP-1 in response to TNFα and IL-1β stimulation.
Our data show that polyubiquitination of TAK1 is not only required for TAK-1-mediated IKKβ activation but also for JNK and p38 activation. Importantly, we also failed to observe the phosphorylation at the Thr178, Thr184, and Thr187 residues in the activation loop of TAK1 upon co-overexpression of TAK1 K158R with TAB1 in HEK-293T cells (data not shown). These results suggest that polyubiquitination of TAK1 at the Lys158 residue is not only required for maintaining the receptor-associated complex formation but also essential for TAK1 phosphorylation and activation. Further studies are needed to determine the molecular mechanism of Lys63-linked TAK1 polyubiquitination in the regulation of TAK1 activation.
During preparation of this paper, Sorrentino et al. (43) reported that TRAF6-mediated Lys63-linked TAK1 polyubiquitination at the Lys34 residue correlates with TGF-β-induced TAK1 activation. TAK1 Lys34 is located at the N terminus of the TAK1 protein sequence, outside of its kinase domain. Surprisingly, we did not find any role of the Lys34 residue in TRAF2- and TRAF6-mediated Lys63-linked TAK1 polyubiquitination and NF-κB activation. Therefore, more work is needed to determine whether Lys34 residue is an actual Ub lysine acceptor site on TAK1 in TGF-β signaling. However, it is possible that TRAF6 mediates TAK1 polyubiquitination at different sites in different signaling pathways.
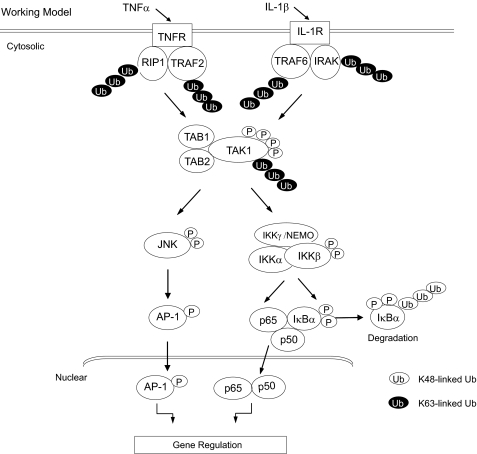
In summary, our study revealed that polyubiquitination of TAK1 at the Lys158 residue within the kinase activation loop is required for TNFα and IL-1β-mediated IKK/NF-κB and JNK/AP-1 activation. Importantly, our finding in this study is the first report that Lys63-linked polyubiquitination of a serine/threonine kinase within the kinase activation loop is required for kinase activation and proinflammatory cytokine TNFα- and IL-1β-mediated signal transduction. In view of the data presented here and in previous reports, we propose a working model (Fig. 9) in which, upon TNFα and IL-1β binding to their cognate receptors, the receptor-mediated signaling events induce TRAF2/6-mediated TAK1 polyubiquitination at the Lys158 and phosphorylation at the Thr178/Thr184/Thr187/Ser192 residues within the kinase domain, which in turn trigger the activation of TAK1-mediated IKK/NF-κB and JNK/AP-1 as well as NF-κB- and AP-1-dependent IL-6 gene expression in the cells.
FIGURE 9.
A working model for the role of Lys63-linked TAK1 polyubiquitination in TNFα- and IL-1β-mediated IKK/NF-κB and JNK/AP-1 activation. TNFα and IL-1β induces TAK1 polyubiquitination at Lys158 residue and phosphorylation at the Thr178, Thr184, Thr187, and Ser192 residues for TAK1 activation. In turn, activated TAK1 mediates IKK/NF-κB and JNK/AP-1 activation as well as NF-κB- and AP-1-dependent IL-6 gene expression in the cells.
Acknowledgments
We thank Dr. Xinhua Feng for HA-ubiquitin expression construct, Dr. Michael Karin for sh-UBC13 construct, and Dr. Zhijian J. Chen for His-MKK6 construct.
This work was supported, in whole or in part, by National Institutes of Health, NCI, Grant 1R21CA106513-01A2 (to J. Y.). This work was also supported by American Cancer Society Grant RSG-06-070-01-TBE (to J. Y.) and a grant from the Virginia and L. E. Simmons Family Foundation Collaborative Research Fund (to J. Y.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- TNFα
- tumor necrosis factor-α
- NF-κB
- nuclear factor-κB
- IKK
- IκB kinase
- IL
- interleukin
- NEMO
- NF-κB essential modulator
- JNK
- c-Jun N-terminal kinase
- MAPK
- mitogen-activated protein kinase
- TGF-β
- transforming growth factor-β
- GFP
- green fluorescent protein
- GST
- glutathione S-transferase
- Ub
- ubiquitin
- MEF
- mouse embryonic fibroblast.
REFERENCES
- 1.Chen G., Goeddel D. V. (2002) Science 296, 1634–1635 [DOI] [PubMed] [Google Scholar]
- 2.Dunne A., O'Neill L. A. (2003) Sci. STKE 2003, re3. [DOI] [PubMed] [Google Scholar]
- 3.Häcker H., Karin M. (2006) Sci. STKE 2006, re13. [DOI] [PubMed] [Google Scholar]
- 4.Karin M., Gallagher E. (2005) IUBMB Life 57, 283–295 [DOI] [PubMed] [Google Scholar]
- 5.Li S., Wang L., Dorf M. E. (2009) Mol. Cell 33, 30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song H. Y., Régnier C. H., Kirschning C. J., Goeddel D. V., Rothe M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 9792–9796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ea C. K., Deng L., Xia Z. P., Pineda G., Chen Z. J. (2006) Mol. Cell 22, 245–257 [DOI] [PubMed] [Google Scholar]
- 8.Deng L., Wang C., Spencer E., Yang L., Braun A., You J., Slaughter C., Pickart C., Chen Z. J. (2000) Cell 103, 351–361 [DOI] [PubMed] [Google Scholar]
- 9.Lamothe B., Besse A., Campos A. D., Webster W. K., Wu H., Darnay B. G. (2007) J. Biol. Chem. 282, 4102–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conze D. B., Wu C. J., Thomas J. A., Landstrom A., Ashwell J. D. (2008) Mol. Cell Biol. 28, 3538–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Windheim M., Stafford M., Peggie M., Cohen P. (2008) Mol. Cell Biol. 28, 1783–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ordureau A., Smith H., Windheim M., Peggie M., Carrick E., Morrice N., Cohen P. (2008) Biochem. J. 409, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takaesu G., Kishida S., Hiyama A., Yamaguchi K., Shibuya H., Irie K., Ninomiya-Tsuji J., Matsumoto K. (2000) Mol. Cell 5, 649–658 [DOI] [PubMed] [Google Scholar]
- 14.Jiang Z., Ninomiya-Tsuji J., Qian Y., Matsumoto K., Li X. (2002) Mol. Cell Biol. 22, 7158–7167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C., Deng L., Hong M., Akkaraju G. R., Inoue J., Chen Z. J. (2001) Nature 412, 346–351 [DOI] [PubMed] [Google Scholar]
- 16.Li H., Kobayashi M., Blonska M., You Y., Lin X. (2006) J. Biol. Chem. 281, 13636–13643 [DOI] [PubMed] [Google Scholar]
- 17.Xia Z. P., Sun L., Chen X., Pineda G., Jiang X., Adhikari A., Zeng W., Chen Z. J. (2009) Nature 461, 114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayden M. S., Ghosh S. (2004) Genes Dev. 18, 2195–2224 [DOI] [PubMed] [Google Scholar]
- 19.Rothwarf D. M., Karin M. (1999) Sci. STKE 1999, RE1. [DOI] [PubMed] [Google Scholar]
- 20.Li Z. W., Chu W., Hu Y., Delhase M., Deerinck T., Ellisman M., Johnson R., Karin M. (1999) J. Exp. Med. 189, 1839–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka M., Fuentes M. E., Yamaguchi K., Durnin M. H., Dalrymple S. A., Hardy K. L., Goeddel D. V. (1999) Immunity 10, 421–429 [DOI] [PubMed] [Google Scholar]
- 22.Rudolph D., Yeh W. C., Wakeham A., Rudolph B., Nallainathan D., Potter J., Elia A. J., Mak T. W. (2000) Genes Dev. 14, 854–862 [PMC free article] [PubMed] [Google Scholar]
- 23.Delhase M., Hayakawa M., Chen Y., Karin M. (1999) Science 284, 309–313 [DOI] [PubMed] [Google Scholar]
- 24.Tang E. D., Wang C. Y., Xiong Y., Guan K. L. (2003) J. Biol. Chem. 278, 37297–37305 [DOI] [PubMed] [Google Scholar]
- 25.Wu C. J., Conze D. B., Li T., Srinivasula S. M., Ashwell J. D. (2006) Nat. Cell Biol. 8, 398–406 [DOI] [PubMed] [Google Scholar]
- 26.Karin M., Ben-Neriah Y. (2000) Annu. Rev. Immunol. 18, 621–663 [DOI] [PubMed] [Google Scholar]
- 27.Morrison D. K., Davis R. J. (2003) Annu. Rev. Cell Dev. Biol. 19, 91–118 [DOI] [PubMed] [Google Scholar]
- 28.Davis R. J. (1999) Biochem. Soc. Symp. 64, 1–12 [DOI] [PubMed] [Google Scholar]
- 29.Karin M., Liu Z., Zandi E. (1997) Curr. Opin. Cell Biol. 9, 240–246 [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi K., Shirakabe K., Shibuya H., Irie K., Oishi I., Ueno N., Taniguchi T., Nishida E., Matsumoto K. (1995) Science 270, 2008–2011 [DOI] [PubMed] [Google Scholar]
- 31.Ninomiya-Tsuji J., Kishimoto K., Hiyama A., Inoue J., Cao Z., Matsumoto K. (1999) Nature 398, 252–256 [DOI] [PubMed] [Google Scholar]
- 32.Sato S., Sanjo H., Takeda K., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Matsumoto K., Takeuchi O., Akira S. (2005) Nat. Immunol. 6, 1087–1095 [DOI] [PubMed] [Google Scholar]
- 33.Shim J. H., Xiao C., Paschal A. E., Bailey S. T., Rao P., Hayden M. S., Lee K. Y., Bussey C., Steckel M., Tanaka N., Yamada G., Akira S., Matsumoto K., Ghosh S. (2005) Genes Dev. 19, 2668–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung P. C., Nebreda A. R., Cohen P. (2004) Biochem. J. 378, 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishitani T., Takaesu G., Ninomiya-Tsuji J., Shibuya H., Gaynor R. B., Matsumoto K. (2003) EMBO J. 22, 6277–6288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibuya H., Yamaguchi K., Shirakabe K., Tonegawa A., Gotoh Y., Ueno N., Irie K., Nishida E., Matsumoto K. (1996) Science 272, 1179–1182 [DOI] [PubMed] [Google Scholar]
- 37.Prickett T. D., Ninomiya-Tsuji J., Broglie P., Muratore-Schroeder T. L., Shabanowitz J., Hunt D. F., Brautigan D. L. (2008) J. Biol. Chem. 283, 19245–19254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conner S. H., Kular G., Peggie M., Shepherd S., Schüttelkopf A. W., Cohen P., Van Aalten D. M. (2006) Biochem. J. 399, 427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaur S., Wang F., Venkatraman M., Arsura M. (2005) J. Biol. Chem. 280, 38599–38608 [DOI] [PubMed] [Google Scholar]
- 40.Thiefes A., Wolf A., Doerrie A., Grassl G. A., Matsumoto K., Autenrieth I., Bohn E., Sakurai H., Niedenthal R., Resch K., Kracht M. (2006) EMBO Rep. 7, 838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koga T., Lim J. H., Jono H., Ha U. H., Xu H., Ishinaga H., Morino S., Xu X., Yan C., Kai H., Li J. D. (2008) J. Biol. Chem. 283, 12546–12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiley W. W., Jin W., Lee A. J., Wright A., Wu X., Tewalt E. F., Leonard T. O., Norbury C. C., Fitzpatrick L., Zhang M., Sun S. C. (2007) J. Exp. Med. 204, 1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorrentino A., Thakur N., Grimsby S., Marcusson A., von Bulow V., Schuster N., Zhang S., Heldin C. H., Landström M. (2008) Nat. Cell Biol. 10, 1199–1207 [DOI] [PubMed] [Google Scholar]
- 44.Lamb A, Yang X. D., Tsang Y. H., Li J. D., Higashi H., Hatakeyama M., Peek R. M., Blanke S. R., Chen L. F. (2009) EMBO Rep. 10, 1242–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun W., Yu Y., Dotti G., Shen T., Tan X., Savoldo B., Pass A. K., Chu M., Zhang D., Lu X., Fu S., Lin X., Yang J. (2009) Cell. Signal. 21, 95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adhikari A., Xu M., Chen Z. J. (2007) Oncogene 26, 3214–3226 [DOI] [PubMed] [Google Scholar]
- 47.Sun W., Li H., Yu Y., Fan Y., Grabiner B. C., Mao R., Ge N., Zhang H., Fu S., Lin X., Yang J. (2009) Cell. Signal. 21, 1488–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dinarello C. A. (1997) Cytokine Growth Factor Rev. 8, 253–265 [DOI] [PubMed] [Google Scholar]
- 49.Arend W. P. (2002) Cytokine Growth Factor Rev. 13, 323–340 [DOI] [PubMed] [Google Scholar]
- 50.Brown K., Vial S. C., Dedi N., Long J. M., Dunster N. J., Cheetham G. M. (2005) J. Mol. Biol. 354, 1013–1020 [DOI] [PubMed] [Google Scholar]
- 51.Carter R. S., Pennington K. N., Arrate P., Oltz E. M., Ballard D. W. (2005) J. Biol. Chem. 280, 43272–43279 [DOI] [PubMed] [Google Scholar]