Abstract
Elevated blood concentrations of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric-oxide (NO) synthase, are found in association with diabetes, hypertension, congestive heart failure, and atherosclerosis. ADMA levels are controlled by dimethylarginine dimethylaminohydrolases (DDAHs), cytosolic enzymes that hydrolyze ADMA to citrulline and dimethylamine. ADMA also has been proposed to be regulated through an alternative pathway by alanine-glyoxylate aminotransferase 2 (AGXT2), a mitochondrial aminotransferase expressed primarily in the kidney. The goal of this study was to define the subcellular localization of human AGXT2 and test the hypothesis that overexpression of human AGXT2 protects from ADMA-induced inhibition in nitric oxide (NO) production. AGXT2 was cloned from human kidney cDNA and overexpressed in COS-7 cells and human umbilical vein endothelial cells with a C-terminal FLAG epitope tag. Mitochondrial localization of human AGXT2 was demonstrated by confocal microscopy and a 41-amino acid N-terminal mitochondrial cleavage sequence was delineated by N-terminal sequencing of the mature protein. Overexpression of human AGXT2 in the liver of C57BL/6 mice using an adenoviral expression vector produced significant decreases in ADMA levels in plasma and liver. Overexpression of human AGXT2 also protected endothelial cells from ADMA-mediated inhibition of NO production. We conclude that mitochondrially localized human AGXT2 is able to effectively metabolize ADMA in vivo resulting in decreased ADMA levels and improved endothelial NO production.
Keywords: Amino Acid, Endothelium, Mitochondria, Nitric Oxide, Nitric-Oxide Synthase, ADMA
Introduction
NG,NG-Asymmetric dimethylarginine (ADMA)2 is an endogenous inhibitor of nitric-oxide synthase (NOS) (1). Elevated blood levels of ADMA are associated with increased cardiovascular morbidity and mortality, suggesting that ADMA may be an independent cardiovascular risk factor (2). Elevation of ADMA levels in animal models or human subjects leads to endothelial dysfunction, decreased renal blood flow, increased renovascular resistance, renal sodium retention, and elevated systemic blood pressure (3–5).
There are two known metabolic pathways for the removal of ADMA in mammals: 1) the hydrolysis of ADMA to citrulline and dimethylamine in the cytoplasm by the dimethylarginine dimethylaminohydrolases (DDAH-1 and DDAH-2) (6–8) and 2) the transamination of ADMA to α-keto-δ-(N,N-dimethylguanidino)valeric acid (DMGV) by alanine-glyoxylate aminotransferase 2 (AGXT2) (6). The DDAH pathway of ADMA metabolism has been shown to contribute to vascular homeostasis in vivo. Heterozygous deficiency of DDAH-1 in gene-targeted mice leads to accumulation of ADMA, impairment in nitric oxide (NO)-dependent endothelial function, and elevated systemic and pulmonary blood pressure (3). In distinction, overexpression of DDAH-1 in transgenic mice results in decreased levels of ADMA in plasma and protection from endothelial dysfunction and myocardial reperfusion injury (9–11). Overexpression of DDAH-1 or DDAH-2 in transgenic mice also protects from angiotensin II-induced vascular injury and organ damage (12, 13).
Much less is known about the physiological role of AGXT2 in ADMA metabolism. AGXT2 is a pyridoxal phosphate-dependent aminotransferase that, in the rat, is expressed at high levels in the kidney (14, 15). AGXT2 is one of two mammalian alanine-glyoxylate aminotransferases, along with alanine-glyoxylate aminotransferase 1 (AGXT1), which catalyzes the conversion of glyoxylate to glycine using alanine as the amino donor (16). AGXT2, but not AGXT1, can also utilize ADMA as an amino donor, leading to the formation of DMGV (17). This pathway of ADMA metabolism is likely to occur in vivo, because ADMA-derived DMGV and a related metabolite, α-keto-δ-(N,N-dimethylguanidino)butyric acid (DMGB) have been observed to accumulate in the urine after rats are injected with radiolabeled ADMA (18).
Interestingly, the subcellular localization of AGXT1 varies in different mammalian species. In humans, rabbits, and guinea pigs, AGXT1 is a peroxisomal enzyme (16). In contrast, AGXT1 has been found to be localized to mitochondria in dogs and cats and to both mitochondria and peroxisomes in rats and mice (16). Mutations that result in the mistargeting of human AGXT1 from peroxisomes to mitochondria can cause primary hyperoxaluria type 1 (19), an autosomal recessive disorder of oxalate metabolism (20, 21). This observation suggests that altered subcellular localization of AGXT1 can significantly affect its function. AGXT2 is localized in mitochondria in the liver of cats, rats, mice, and some other species (16). The intracellular localization of human AGXT2 has not been reported, and it is not known whether mitochondrial AGXT2 can metabolize ADMA in vivo.
The goals of this study were to determine the intracellular localization of human AGXT2 and test the hypothesis that overexpression of AGXT2 lowers ADMA and protects from ADMA-induced inhibition of NO production. Our results demonstrate that human AGXT2 is localized in mitochondria and contains a 41-amino acid N-terminal mitochondrial cleavage sequence. Ectopic expression of human AGXT2 in mice produces a decrease in ADMA levels in the liver and blood plasma. Finally, overexpression of AGXT2 increased basal NO generation and protected from ADMA-mediated impairment of NO production in endothelial cells.
EXPERIMENTAL PROCEDURES
Construction of Adenoviral Vectors
Human AGXT2 cDNA was cloned from human kidney PCR-ready cDNA (Ambion, Austin, TX) using the following primers: 5′-ATGACTCTAATCTGGAGAC-3′ (forward) and 5′-CTGACAATGTTACTTAGCTC-3′ (reverse). The AGXT2 cDNA sequence was identical to the GenBankTM sequence NM_031900 with the exception of four base pairs that have been described previously as single nucleotide polymorphisms (SNPs) (rs37370, rs2279651, rs180749, and rs466067). The AGXT2 cDNA contained bases T, A, and G, respectively at SNPs rs37370, rs180749, and rs466067 (forward strand in the Entrez SNP data base), which are the most prevalent alleles at those SNPs in the Entrez SNP data base. The AGXT2 cDNA contained a G at SNP rs2279651, which has a similar population frequency as A at this SNP (Entrez SNP data base). A FLAG epitope (5′-GATTACAAGGATGACGACGATAAG-3′) was inserted at the C terminus of the AGXT2 cDNA using PCR. The resulting FLAG-tagged AGXT2 cDNA was inserted in the direct orientation into the XhoI site of the pacAd5 CMV internal ribosome entry site (IRES)-enhanced green fluorescent protein (GFP) pA shuttle vector (University of Iowa Gene Transfer Vector Core).
Human DDAH1 cDNA was cloned from HepG2 cells (kindly provided by Dr. Michael Welsh, University of Iowa) using the following primers: 5′-CTAAGCCTCCCGAAGCCATG-3′ (forward) and 5′-GGACTCTGCAGCTCAGGAG-3′ (reverse). The DDAH1 cDNA sequence was identical to GenBankTM sequence NM_012137. The DDAH1 cDNA was inserted in direct orientation into the XhoI site of the pacAd5 CMV IRES eGFP pA shuttle vector.
Replication-deficient adenoviral vectors encoding either FLAG-tagged human AGXT2 cDNA (AdAGXT2FLAG) or human DDAH1 cDNA (AdDDAH1) under the control of the early CMV promoter were generated from the shuttle vectors using the RAPAdTM system as described previously (22). Additional replication-deficient adenoviral vectors, AdGFP, AdCre, or AdEmpty, which encoded the enhanced green fluorescent protein gene, the Cre recombinase gene, or no gene, respectively, under control of the CMV early promoter, were used as control vectors (23).
Cell Culture
The COS-7 cell line, an SV40-transformed African green monkey kidney fibroblast cell line, was obtained from the American Type Culture Collection (ATCC, Manassas, VA). COS-7 cells were cultured to 80% confluency in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Cells were then washed with PBS and infected with either AdAGXT2FLAG or a control vector (AdGFP or AdCre) at 20 MOI in serum-free DMEM. The medium was replaced with DMEM containing 10% FBS and 1% penicillin-streptomycin 24 h after infection. Cells were studied 48 h after infection.
Primary human umbilical vein endothelial cells (HUVEC) were purchased from Lonza (Walkersville, MD) and cultured to 80% confluency in EGM-2 medium (Lonza) containing 2% FBS. Cells were washed with PBS and infected with AdAGXT2FLAG or AdCre at 100 MOI in EGM-2 medium. After 5 h, the medium was replaced with EGM-2 medium lacking the adenovirus vector. Cells were studied 72 h after infection.
The MS-1 murine pancreatic islet endothelial cell line was obtained from the ATCC. MS-1 cells were cultured to 80% confluency in DMEM containing 5% FBS and 1% penicillin-streptomycin. Cells were then washed with PBS and infected with AdAGXT2FLAG, AdDDAH1, or the control vector AdEmpty at 2000 MOI in serum-free DMEM. After 24 h, the medium was replaced with DMEM containing 2% FBS and 1% penicillin-streptomycin in the absence or presence of 100 μm ADMA (EMD Chemicals, Inc, San Diego, CA). Cells were incubated for 48 h, with one change of medium after 24 h, and then collected for measurement of total protein (Bradford Protein Assay, Bio-Rad) and immunoblotting. The conditioned medium was collected for measurement of nitrite.
Immunoblotting
Washed cells or freshly isolated tissue samples were homogenized in ice-cold HEMGN buffer (25 mmol/liter HEPES, pH 7.6, 0.1 mmol/liter EDTA, 12.5 mmol/liter MgCl2, 100 mmol/liter KCl, 10% glycerol (v/v), 0.1% Nonidet P-40 (v/v)) containing a protease inhibitor mixture (CompleteTM Mini EDTA-free, Roche Applied Science, Indianapolis, IN). Homogenates were centrifuged at 14,000 × g for 30 min at 4 °C. Protein concentrations of supernatant fractions were determined using the Bradford protein assay (Bio-Rad). Samples (1–20 μg of protein) were separated by SDS-PAGE under reducing conditions on 10% polyacrylamide gels and transferred to PVDF membranes (Immobilon-P Transfer Membrane, Millipore, Billerica, MA). Membranes were probed with 1 μg/ml monoclonal antibody raised against rat DDAH1 (kindly provided by Dr. Masumi Kimoto, Okayama Prefectural University) (24) or 2 μg/ml monoclonal anti-FLAG antibody (Sigma-Aldrich) for 2 h at room temperature. To control for sample loading, the membranes were reprobed with 0.5 μg/ml anti-β-actin monoclonal antibody (Abcam, Cambridge, MA) for 2 h at room temperature. Horseradish peroxidase-conjugated goat-anti-mouse antibody (Pierce) was used as the secondary antibody. Immunoreactive bands were visualized using SuperSignal West Femto (Pierce) detection system.
Confocal Microscopy
COS-7 cells or HUVEC grown on coverslips or Lab-Tek 4-well glass chamber slides (Nunc, Rochester, NY), respectively, were infected with AdAGXT2FLAG or AdCre, washed with DMEM, and incubated with 100 nmol/liter MitoTracker Orange CMTMRos (Sigma-Aldrich), in DMEM for 45 min at 37 °C. Cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.1% Triton X-100 in PBS for 10 min. Cells were then washed with PBS, blocked with 10% normal goat serum and 3% BSA in PBS for 1 h, and incubated with 30 μg/ml anti-FLAG M2 (Sigma-Aldrich) overnight at 4 °C. Cells were then washed and incubated with a 1:200 dilution of HiLyte Fluor 647-labeled goat anti-mouse IgG (Invitrogen) for 1 h in the dark.
To define the staining pattern of the endoplasmic reticulum and Golgi, HUVEC were fixed, permeabilized, blocked, and then incubated with either a mouse monoclonal antibody to glucose-regulated protein 78 (GRP78) (BD Transduction Laboratories, Franklin Lakes, NJ) or a mouse monoclonal antibody to giantin (Alexis Biochemicals, San Diego, CA) for 2 h at room temperature followed by incubation with Alexa Fluor 568 goat anti-mouse IgG (Invitrogen) for 30 min at room temperature. A separate set of adenovirus-infected HUVEC were incubated with a rabbit polyclonal antibody to peroxisomal membrane protein 70 (PMP70) (Abcam, Cambridge, MA) for 2 h at room temperature followed by a 30-min incubation with Alexa Fluor 568 goat anti-rabbit IgG (Invitrogen). The cells were then washed with PBS and incubated with 30 μg/ml anti-FLAG M2 (Sigma-Aldrich) overnight at 4 °C followed by HiLyte Fluor 647-labeled goat anti-mouse IgG (Invitrogen) for 1 h in the dark. Cells were then washed and mounted in Vecta-Shield (Vector). Images were obtained using a Bio-Rad Radience 2100MP confocal microscope in the University of Iowa Central Microscopy Research Facility.
Purification and N-terminal Sequencing of AGXT2FLAG
Mitochondria from COS-7 cells infected with AdAGXT2FLAG were isolated as described previously (25). AGXT2FLAG was purified from mitochondrial lysates using FLAG affinity chromatography (FLAG® M Purification kit, Sigma). Purification products were eluted using FLAG peptide, separated on SDS-PAGE under reducing conditions, transferred to PVDF membrane, and stained with Coomassie Blue. A 52-kDa band was cut from the membrane and subjected to partial N-terminal sequencing. N-terminal sequencing was performed in the University of Iowa Molecular Analysis Facility using a Procise 492 N-Terminal Sequencer (Applied Biosystems, Foster City, CA).
Measurement of ADMA
ADMA in plasma or tissue lysates was measured by liquid chromatography/mass spectrometry as described previously (26).
Measurement of Nitrite
Nitrite concentrations in conditioned medium were determined in the University of Iowa Electron Spin Resonance Core Facility by the reductive generation of NO from nitrite as described previously (26). The resulting NO was detected by the gas-phase chemiluminescent reaction of NO with ozone (O3) using a Sievers 280 Nitric Oxide Analyzer (Sievers Instrument Inc., Boulder, CO).
Adenoviral Gene Transfer to Mice
Animal protocols were approved by the University of Iowa Animal Care and Use Committee. Adenoviral vectors (1 × 1011 particles in 170 μl of PBS containing 3% sucrose) were injected retro-orbitally into C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) at 2–3 months of age. Four days after injection of adenovirus, blood was collected by cardiac puncture into EDTA (final concentration, 5 mm). The plasma was separated by centrifugation, flash frozen, and stored at −80 °C for measurement of ADMA. Samples of liver were collected for immunoblotting and measurement of AGXT activity and ADMA levels.
AGXT Activity
Alanine-glyoxylate aminotransferase activity was assayed using a modification of a method described previously (27). A 50-μl reaction mixture containing 40 mm l-alanine, 10 mm sodium glyoxylate, 10 μm pyridoxal phosphate, 5 mm dithiothreitol, 100 mm potassium phosphate buffer, pH 8.0, and tissue lysate containing 40 μg of total protein was incubated at 37 °C for 30 min. Samples were boiled to stop the reaction, and 100 μl of 1.0 m Tris/HCl, pH 8.0 was added to each sample, and the mixture was incubated for 30 min at room temperature. To measure the reaction product pyruvate, 50 μl of 10.8 units/ml lactate dehydrogenase (LDH) and 1 mm NADH in 1.0 m Tris/HCl, pH 8.0 were added, and the reaction mixtures were further incubated for 10 min. Changes in the concentration of NADH, which is consumed during the conversion of pyruvate to lactate by LDH, were assessed by measuring absorbance at 340 nm. Under these reaction conditions, Tris forms an adduct with glyoxylate, preventing the use of glyoxylate as a substrate by LDH (28, 29).
Statistical Analysis
Comparisons of ADMA levels, nitrite levels, and AGXT activity were performed using the 2-tailed Student's t test. Statistical significance was defined as a p value <0.05. Values are reported as mean ± S.E.
RESULTS
Expression of AGXT2FLAG in COS-7 Cells
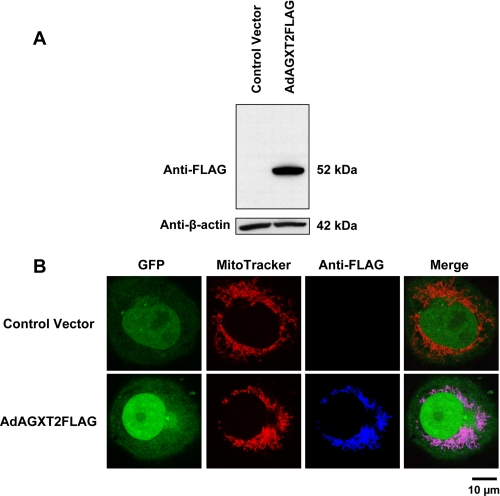
COS-7 cells were infected with AdAGXT2FLAG or a control virus, AdGFP. Expression of the human AGXT2FLAG was confirmed by immunoblotting with anti-FLAG (Fig. 1A). Under reducing conditions, human AGXT2FLAG migrated on SDS-PAGE as a single band of ∼52 kDa.
FIGURE 1.
Expression and localization of human AGXT2 in COS-7 cells. A, immunoblotting of COS-7 cells infected with AdGFP (Control Vector) or AdAGXT2FLAG. Cell lysates were probed with anti-FLAG (upper panel) or anti-β-actin (lower panel) antibodies. B, intracellular localization of human AGXT2FLAG in COS-7 cells. COS-7 cells were infected with either the AdCre control vector or AdAGXT2FLAG, stained with MitoTracker and anti-FLAG antibodies. Fluorescence confocal microscopy was performed to detect GFP (green), MitoTracker (red), FLAG (blue), or a merged image of all three markers (Merge).
Intracellular Localization of AGXT2
The intracellular localization of FLAG-tagged human AGXT2 expressed in COS-7 cells was determined using immunofluorescence confocal microscopy (Fig. 1B). COS-7 cells infected with the AdCre vector were chosen as the control condition for this experiment, because both AdCre and AdAGXT2FLAG contain the GFP sequence located after an internal ribosome entry site and therefore give comparable levels of expression of GFP. Green GFP fluorescence was localized predominantly in the nucleus of COS-7 cells infected with either AdCre or AdAGXT2FLAG. Immunofluorescent staining of AdAGXT2FLAG-infected COS-7 cells with anti-FLAG antibodies demonstrated perinuclear localization of AGXT2FLAG fluorescence that co-localized with MitoTracker staining for mitochondria. No fluorescence was detected with anti-FLAG in AdCre-infected COS-7 cells.
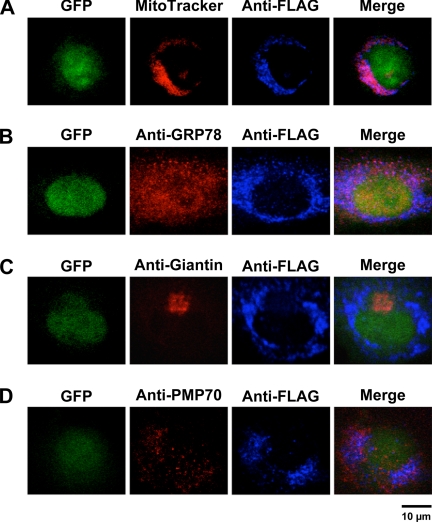
To determine if AGXT2 also localizes to mitochondria in endothelial cells, HUVEC were infected with AdAGXT2FLAG and immunofluorescence confocal microscopy was performed with anti-FLAG antibodies. Strong co-localization of anti-FLAG with MitoTracker was observed in HUVEC (Fig. 2A). No co-localization of anti-FLAG was seen with anti-GRP78 (a marker of endoplasmic reticulum, Fig. 2B), anti-giantin (a marker of the Golgi, Fig. 2C), or anti-PMP70 (a marker of peroxisomes, Fig. 2D). No staining for FLAG was observed in HUVEC infected with the control virus, AdCre (data not shown).
FIGURE 2.
Intracellular localization of human AGXT2 in HUVEC. HUVEC were infected with AdAGXT2FLAG and stained with anti-FLAG antibodies and either: A, MitoTracker; B, anti-GRP78 (a marker of endoplasmic reticulum); C, anti-giantin (a marker of the Golgi); or D, anti-PMP70 (a marker of peroxisomes). Fluorescence confocal microscopy was performed to detect GFP (green), MitoTracker (red), GRP78 (red), giantin (red), PMP70 (red), FLAG (blue), or a merged image of multiple markers (Merge).
Identification of the Mitochondrial Cleavage Sequence
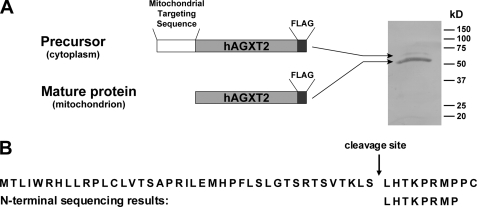
Mitochondrial proteins expressed from nuclear genes are typically imported into mitochondria as precursors with subsequent cleavage of an N-terminal mitochondrial-targeting sequence (30). To determine the mitochondrial cleavage site of human AGXT2, COS-7 cells were infected with AdAGXT2FLAG and FLAG-tagged human AGXT2 was purified using FLAG immunoaffinity chromatography. The purification products were separated by SDS-PAGE under reducing conditions, transferred to a PVDF membrane, and stained with Coomassie Blue. Two bands were observed: a major band of 52 kDa corresponding to the predicted mature mitochondrial protein and a minor band of 60 kDa corresponding to the predicted precursor protein (Fig. 3A). The major band was cut from the membrane and subjected to N-terminal sequencing to determine the mitochondrial cleavage site. The results demonstrated that the mature human AGXT2 protein has an N-terminal sequence of LHTKPRMP. Alignment of this sequence with the predicted amino acid sequence of human AGXT2 indicated that the N-terminal 41 amino acids of human AGXT2 constitute its mitochondrial cleavage sequence (Fig. 3B).
FIGURE 3.
Determination of the mitochondrial cleavage sequence of human AGXT2. A, human AGXT2FLAG was purified from a mitochondrial preparation of AdAGXT2FLAG-infected COS-7 cells by FLAG affinity chromatography, separated by SDS-PAGE, transferred to PVDF membranes, and stained with Coomassie Blue to identify a major mature protein of 52 kDa and a minor precursor protein of 60 kDa. The 52-kDa band was excised from the membrane and subjected to partial N-terminal sequencing. B, N-terminal sequencing results aligned with the predicted N-terminal amino acid sequence of full-length human AGXT2.
Overexpression of AGXT2 in Mice
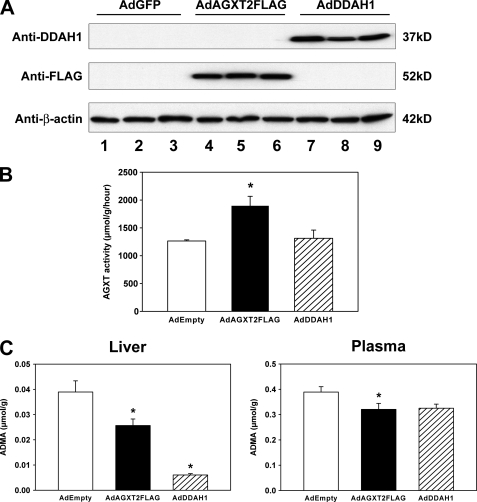
To determine whether overexpression of AGXT2 can lower ADMA in vivo, C57BL/6 mice were injected retro-orbitally with AdAGXT2FLAG. As a positive control, some mice were injected with AdDDAH1, because overexpression of DDAH1 in transgenic mice has been shown to decrease ADMA levels in vivo (9, 11). Negative control mice were injected with AdEmpty. Four days after injection, expression of AGXT2FLAG or DDAH1 was detected in liver lysates by immunoblotting (Fig. 4A). Mice infected with AdAGXT2FLAG had a 50% increase in total AGXT activity in the liver (p < 0.01), which suggested that the AGXT2FLAG protein was active in catalyzing the transamination of alanine with glyoxylate in vivo (Fig. 4B). Mice infected with AdDDAH1 did not have any change in total AGXT activity in the liver. Both AdAGXT2FLAG and AdDDAH1 caused statistically significant decreases in ADMA levels in liver lysates (by 34 and 84%, respectively, p < 0.05) (Fig. 4C). Plasma ADMA levels were decreased by ∼30% in mice infected with AdAGXT2FLAG compared with mice infected with the control adenovirus (p < 0.05) (Fig. 4C). Mice infected with AdDDAH1 had similar levels of plasma ADMA as mice infected with AdAGXT2FLAG, but the difference in plasma ADMA between AdDDAH1 and AdEmpty did not reach statistical significance (p > 0.05).
FIGURE 4.
Expression of human AGXT2 in mice. A, C57BL/6 mice were injected retro-orbitally with AdGFP (lanes 1–3), AdAGXT2FLAG (lanes 4–6), or AdDDAH1 (lanes 7–9). Four days after injection, the livers were harvested and subjected to immunoblotting with antibodies to DDAH1, FLAG, or β-actin. Three separate mice were injected with each adenovirus. B, total AGXT activity of liver lysates prepared 4 days after injection of AdEmpty (n = 4), AdAGXT2FLAG (n = 4), or AdDDAH1 (n = 4). C, levels of ADMA in the liver and plasma 4 days after injection of AdEmpty (n = 10 for liver and n = 25 for plasma), AdAGXT2FLAG (n = 10 for liver and n = 25 for plasma), or AdDDAH1 (n = 5 for liver and n = 5 for plasma). Values are mean ± S.E. *, p < 0.05 compared with mice infected with AdEmpty.
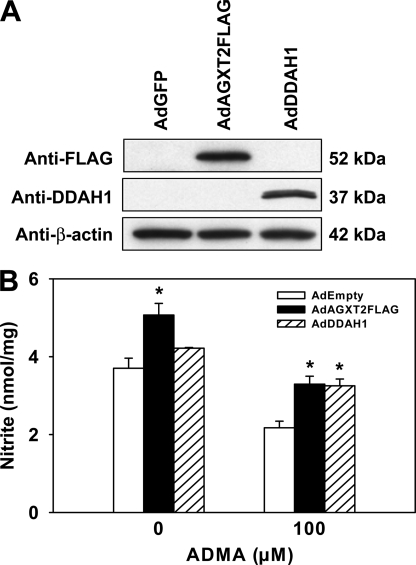
Effect of AGXT2 Overexpression on NO Production
To determine whether overexpression of AGXT2 increases NO production, MS-1 endothelial cells were infected with AdAGXT2FLAG. As with the previous experiments in mice, infections with AdDDAH1, AdGFP, or AdEmpty were performed as control conditions. Expression of AGXT2FLAG or DDAH1 in infected MS-1 cells was confirmed by immunoblotting (Fig. 5A). NO production was assessed by measuring nitrite in MS-1 cell-conditioned medium. Cells infected with AdAGXT2FLAG had 37% higher levels of NO production than cells infected with AdEmpty (p < 0.05) (Fig. 5B). Incubation with 100 μm ADMA caused a 40% decrease in NO production in control MS-1 cells infected with AdEmpty (Fig. 5B). This ADMA-induced decrease in NO production was blocked by infection with either AdAGXT2FLAG or AdDDAH1 (p < 0.05).
FIGURE 5.
Effect of AGXT2 on production in endothelial cells. A, immunoblotting of lysates of MS-1 cells infected with AdGFP, AdAGXT2FLAG, or AdDDAH1. Membranes were probed with antibodies to DDAH1 (Anti-DDAH1), FLAG (Anti-FLAG), or β-actin (Anti-β-actin). B, total nitrite levels in the conditioned medium from MS-1 cells infected with AdEmpty, AdAGXT2FLAG, or AdDDAH1 and incubated with or without 100 μm ADMA for 24 h (n = 3, representative of five separate experiments). Values are mean ± S.E. *, p < 0.05 compared with cells infected with AdEmpty.
DISCUSSION
AGXT2 was identified 20 years ago as a functional ADMA-metabolizing activity in rat kidney (17). Despite widespread interest in ADMA as a cardiovascular risk factor and a large amount of recent work on the regulation of ADMA by DDAH (1, 31), relatively little is known about the role of human AGXT2 in ADMA metabolism. In the current work, we expressed recombinant human AGXT2 in cultured cells and mice to test the hypothesis that human AGXT2 can lower ADMA levels and protect from ADMA-induced impairment in NO production in vivo.
The main findings of this study are: 1) unlike human AGXT1, human AGXT2 is localized in mitochondria, 2) the mitochondrial cleavage site of human AGXT2 is at the Ser41-Leu42 bond, suggesting that the mitochondrial targeting sequence is contained within the N-terminal 41 amino acids; 3) adenoviral-mediated ectopic expression of human AGXT2 lowers ADMA levels in the liver and plasma of mice; and 4) overexpression of human AGXT2 protects from ADMA-induced impairment in NO production in endothelial cells. These findings suggest that mitochondrially localized human AGXT2 can effectively metabolize ADMA and regulate NO production in vivo.
The results presented here represent the first report of the cloning and characterization of human AGXT2. Previous work on AGXT2 has been limited mainly to the characterization of rat AGXT2 and was focused primarily on its effects on aminotransferase reactions in vitro (14, 32). In contrast to human AGXT1, which is localized to peroxisomes, human AGXT2 was demonstrated to be localized to mitochondria (Figs. 1B and 2A). This finding is consistent with the observation by Takada and Noguchi (16) that AGXT2 is a mitochondrial enzyme in several other mammalian species, including cats, rats, mice, and pigs. The mitochondrial cleavage site of human AGXT2 (the Ser41-Leu42 bond) determined in the current study is homologous to the previously determined mitochondrial cleavage site of rat AGXT2 (14) despite the fact that the N-terminal sequences share less than 50% amino acid identity between rat and human AGXT2 (GenBankTM NM_031835 and NM_031900, respectively).
To determine whether human AGXT2 affects ADMA levels in vivo, we utilized adenoviral gene transfer to express human AGXT2 in the liver of C57BL/6 mice (Fig. 4). Gene transfer of human AGXT2FLAG resulted in a 50% increase in hepatic AGXT activity and significant decreases in ADMA levels in the liver and plasma. Together with the mitochondrial localization experiments, these findings indicate that ADMA is transported into mitochondria and is metabolized by human AGXT2 in vivo. These findings also suggest that the presence of the C-terminal FLAG epitope, which was used to facilitate immunolocalization and protein purification, did not disrupt the function of human AGXT2.
We also found that adenoviral-mediated expression of human AGXT2FLAG decreased basal NO production and protected from ADMA-induced impairment in NO production in cultured endothelial cells (Fig. 5). The magnitude of the effects of AGXT2FLAG overexpression on ADMA levels in vivo and NO production in endothelial cells was comparable to that of DDAH1, which is a major physiological regulator of ADMA effects on vascular homeostasis (3, 9). Interestingly, although similar decreases in ADMA were observed in plasma after gene transfer of AGXT2FLAG and DDAH1, a greater decrease in ADMA in the liver was seen with DDAH1 than with AGXT2FLAG (Fig. 3C). One potential explanation for this observation could be an effect of AGXT2 on ADMA transport between the mitochondrial, cytoplasmic, and extracellular pools. Alternatively, there could be differences in the extrahepatic metabolism or clearance of ADMA between AGXT2FLAG- and DDAH1-infected animals.
Although our findings clearly demonstrate that overexpression of AGXT2 can affect ADMA levels in vivo, the normal physiological role of AGXT2 in ADMA metabolism remains poorly defined. Ogawa et al. (18) have detected the products of AGXT2-mediated transamination of ADMA (DMGV and DMGB) in the urine of rats that were injected with radiolabeled ADMA. Most of the radioactivity, however, was found in the tissues in the form of the metabolites of DDAH-mediated hydrolysis of ADMA (citrulline and its analogs), which suggests that ADMA is metabolized primarily by DDAH1 and/or DDAH2 under normal physiological conditions. DDAH1 and DDAH2 may be down-regulated in several pathological conditions, such as hyperhomocysteinemia (26, 33), chronic kidney disease (34), pulmonary hypertension (35), and diabetes (36). Down-regulation of DDAH could result in an increased contribution of AGXT2 to the metabolism of ADMA in these pathophysiological disorders. In this regard, it is of interest that AGXT2 is primarily expressed in the kidney, because kidney function is known to be a key determinant of plasma ADMA levels in humans, and elevated ADMA may be a mediator of endothelial dysfunction and cardiovascular disease in renal disease (37).
Experimental models of altered AGXT2 expression could be used in future studies to better define the role of AGXT2 in the cardiovascular system. The adenoviral gene transfer approach used in the current study has several limitations. Human AXGT2FLAG was expressed primarily in the liver, with no expression detected in the kidney by immunoblotting or AGXT activity (data not shown). Adenoviral-mediated expression of AGXT2FLAG was transient (peaking at 3–4 days after injection), which precluded using this approach to examine longer term cardiovascular endpoints in chronic disease models. Additional animal models in which AGXT2 expression is altered in transgenic or gene-targeted mice are needed to better address the pathophysiological effects of AGXT2 on ADMA metabolism, vascular reactivity, and cardiovascular disease progression. Such animal models also would provide a DDAH-independent approach to modulate ADMA levels in vivo, which would facilitate studies to determine the relative importance of the ADMA-dependent and ADMA-independent cardiovascular effects seen in animal models of altered DDAH expression (3, 9, 11).
Pharmacological approaches to increase the activity of AGXT2 could have potential therapeutic value in the treatment of cardiovascular disorders and other conditions in which ADMA may be a pathophysiological mediator. The therapeutic up-regulation of AGXT2 may have advantages compared with the up-regulation of DDAH1 or DDAH2, because these enzymes may have cancer-promoting effects that are independent of ADMA (38).
Acknowledgments
We thank Brett Wagner and Katherine Walters for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants HL63943 and NS24621. This work was also supported by the Office of Research and Development, United States Department of Veterans Affairs.
- ADMA
- asymmetric dimethylarginine
- AGXT
- alanine-glyoxylate aminotransferase
- DDAH
- dimethylarginine dimethylaminohydrolase
- DMEM
- Dulbecco's modified Eagle's medium
- DMGB
- α-keto-δ-(N,N-dimethylguanidino)butyric acid
- DMGV
- α-keto-δ-(N,N-dimethylguanidino)valeric acid
- GRP78
- glucose-regulated protein 78
- HUVEC
- human umbilical vein endothelial cells
- IRES
- internal ribosome entry site
- LDH
- lactate dehydrogenase
- NOS
- nitric-oxide synthase
- PMP70
- peroxisomal membrane protein 70
- SNP
- single nucleotide polymorphism
- FBS
- fetal bovine serum
- PBS
- phosphate-buffered saline
- MOI
- multiplicity of infection
- PVDF
- polyvinylidene difluoride
- GFP
- green fluorescent protein.
REFERENCES
- 1.Vallance P., Leiper J. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 1023–1030 [DOI] [PubMed] [Google Scholar]
- 2.Böger R. H., Cooke J. P., Vallance P. (2005) Vasc. Med. 10, Suppl. 1, S1–S2 [DOI] [PubMed] [Google Scholar]
- 3.Leiper J., Nandi M., Torondel B., Murray-Rust J., Malaki M., O'Hara B., Rossiter S., Anthony S., Madhani M., Selwood D., Smith C., Wojciak-Stothard B., Rudiger A., Stidwill R., McDonald N. Q., Vallance P. (2007) Nat. Med. 13, 198–203 [DOI] [PubMed] [Google Scholar]
- 4.Wang D., Gill P. S., Chabrashvili T., Onozato M. L., Raggio J., Mendonca M., Dennehy K., Li M., Modlinger P., Leiper J., Vallance P., Adler O., Leone A., Tojo A., Welch W. J., Wilcox C. S. (2007) Circ. Res. 101, 627–635 [DOI] [PubMed] [Google Scholar]
- 5.Kielstein J. T., Impraim B., Simmel S., Bode-Böger S. M., Tsikas D., Frölich J. C., Hoeper M. M., Haller H., Fliser D. (2004) Circulation 109, 172–177 [DOI] [PubMed] [Google Scholar]
- 6.Ogawa T., Kimoto M., Sasaoka K. (1989) J. Biol. Chem. 264, 10205–10209 [PubMed] [Google Scholar]
- 7.MacAllister R. J., Parry H., Kimoto M., Ogawa T., Russell R. J., Hodson H., Whitley G. S., Vallance P. (1996) Br. J. Pharmacol. 119, 1533–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leiper J. M., Santa Maria J., Chubb A., MacAllister R. J., Charles I. G., Whitley G. S., Vallance P. (1999) Biochem. J. 343, 209–214 [PMC free article] [PubMed] [Google Scholar]
- 9.Dayoub H., Achan V., Adimoolam S., Jacobi J., Stuehlinger M. C., Wang B. Y., Tsao P. S., Kimoto M., Vallance P., Patterson A. J., Cooke J. P. (2003) Circulation 108, 3042–3047 [DOI] [PubMed] [Google Scholar]
- 10.Stühlinger M. C., Conci E., Haubner B. J., Stocker E. M., Schwaighofer J., Cooke J. P., Tsao P. S., Pachinger O., Metzler B. (2007) Cardiovasc. Res. 75, 417–425 [DOI] [PubMed] [Google Scholar]
- 11.Dayoub H., Rodionov R. N., Lynch C., Cooke J. P., Arning E., Bottiglieri T., Lentz S. R., Faraci F. M. (2008) Stroke 39, 180–184 [DOI] [PubMed] [Google Scholar]
- 12.Jacobi J., Maas R., Cordasic N., Koch K., Schmieder R. E., Böger R. H., Hilgers K. F. (2008) Am. J. Physiol. Heart Circ. Physiol 294, H1058–H1066 [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa K., Wakino S., Tatematsu S., Yoshioka K., Homma K., Sugano N., Kimoto M., Hayashi K., Itoh H. (2007) Circ. Res. 101, e2–10 [DOI] [PubMed] [Google Scholar]
- 14.Lee I. S., Muragaki Y., Ideguchi T., Hase T., Tsuji M., Ooshima A., Okuno E., Kido R. (1995) J. Biochem. 117, 856–862 [DOI] [PubMed] [Google Scholar]
- 15.Lee I. S., Nishikimi M., Inoue M., Muragaki Y., Ooshima A. (1999) Nephron 83, 184–185 [DOI] [PubMed] [Google Scholar]
- 16.Takada Y., Noguchi T. (1982) Comp. Biochem. Physiol. B 72, 597–604 [DOI] [PubMed] [Google Scholar]
- 17.Ogawa T., Kimoto M., Sasaoka K. (1990) J. Biol. Chem. 265, 20938–20945 [PubMed] [Google Scholar]
- 18.Ogawa T., Kimoto M., Watanabe H., Sasaoka K. (1987) Arch. Biochem. Biophys. 252, 526–537 [DOI] [PubMed] [Google Scholar]
- 19.Danpure C. J., Cooper P. J., Wise P. J., Jennings P. R. (1989) J. Cell Biol. 108, 1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., Roe S. M., Hou Y., Bartlam M., Rao Z., Pearl L. H., Danpure C. J. (2003) J. Mol. Biol. 331, 643–652 [DOI] [PubMed] [Google Scholar]
- 21.Danpure C. J. (2005) Am. J. Nephrol. 25, 303–310 [DOI] [PubMed] [Google Scholar]
- 22.Anderson R. D., Haskell R. E., Xia H., Roessler B. J., Davidson B. L. (2000) Gene Ther. 7, 1034–1038 [DOI] [PubMed] [Google Scholar]
- 23.Vasquez E. C., Johnson R. F., Beltz T. G., Haskell R. E., Davidson B. L., Johnson A. K. (1998) Exp. Neurol. 154, 353–365 [DOI] [PubMed] [Google Scholar]
- 24.Kimoto M., Whitley G. S., Tsuji H., Ogawa T. (1995) J. Biochem. 117, 237–238 [DOI] [PubMed] [Google Scholar]
- 25.Kaschnitz R. M., Hatefi Y., Pedersen P. L., Morris H. P. (1979) Methods Enzymol. 55, 79–88 [DOI] [PubMed] [Google Scholar]
- 26.Dayal S., Rodionov R. N., Arning E., Bottiglieri T., Kimoto M., Murry D. J., Cooke J. P., Faraci F. M., Lentz S. R. (2008) Am. J. Physiol. Heart Circ. Physiol. 295, H816–H825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper A. J., Krasnikov B. F., Okuno E., Jeitner T. M. (2003) Biochem. J. 376, 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noguchi T., Okuno E., Takada Y., Minatogawa Y., Okai K., Kido R. (1978) Biochem. J. 169, 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowsell E. V., Snell K., Carnie J. A., Rowsell K. V. (1972) Biochem. J. 127, 155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neupert W., Herrmann J. M. (2007) Annu. Rev. Biochem. 76, 723–749 [DOI] [PubMed] [Google Scholar]
- 31.Palm F., Onozato M. L., Luo Z., Wilcox C. S. (2007) Am. J. Physiol. Heart Circ. Physiol 293, H3227–H3245 [DOI] [PubMed] [Google Scholar]
- 32.Kontani Y., Kaneko M., Kikugawa M., Fujimoto S., Tamaki N. (1993) Biochim. Biophys. Acta 1156, 161–166 [DOI] [PubMed] [Google Scholar]
- 33.Stühlinger M. C., Tsao P. S., Her J. H., Kimoto M., Balint R. F., Cooke J. P. (2001) Circulation 104, 2569–2575 [DOI] [PubMed] [Google Scholar]
- 34.Matsuguma K., Ueda S., Yamagishi S., Matsumoto Y., Kaneyuki U., Shibata R., Fujimura T., Matsuoka H., Kimoto M., Kato S., Imaizumi T., Okuda S. (2006) J. Am. Soc. Nephrol. 17, 2176–2183 [DOI] [PubMed] [Google Scholar]
- 35.Pullamsetti S., Kiss L., Ghofrani H. A., Voswinckel R., Haredza P., Klepetko W., Aigner C., Fink L., Muyal J. P., Weissmann N., Grimminger F., Seeger W., Schermuly R. T. (2005) FASEB J. 19, 1175–1177 [DOI] [PubMed] [Google Scholar]
- 36.Lin K. Y., Ito A., Asagami T., Tsao P. S., Adimoolam S., Kimoto M., Tsuji H., Reaven G. M., Cooke J. P. (2002) Circulation 106, 987–992 [DOI] [PubMed] [Google Scholar]
- 37.Rodionov R. N., Lentz S. R. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1031–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartzoulakis B., Rossiter S., Gill H., O'Hara B., Steinke E., Gane P. J., Hurtado-Guerrero R., Leiper J. M., Vallance P., Rust J. M., Selwood D. L. (2007) Bioorg. Med. Chem. Lett. 17, 3953–3956 [DOI] [PubMed] [Google Scholar]