Abstract
Recent studies in rodent models suggest that liver X receptors (LXRs) may play an important role in the maintenance of glucose homeostasis and islet function. To date, however, no studies have comprehensively examined the role of LXRs in human islet biology. Human islets were isolated from non-diabetic donors and incubated in the presence or absence of two synthetic LXR agonists, TO-901317 and GW3965, under conditions of low and high glucose. LXR agonist treatment enhanced both basal and stimulated insulin secretion, which corresponded to an increase in the expression of genes involved in anaplerosis and reverse cholesterol transport. Furthermore, enzyme activity of pyruvate carboxylase, a key regulator of pyruvate cycling and anaplerotic flux, was also increased. Whereas LXR agonist treatment up-regulated known downstream targets involved in lipogenesis, we observed no increase in the accumulation of intra-islet triglyceride at the dose of agonist used in our study. Moreover, LXR activation increased expression of the genes encoding hormone-sensitive lipase and adipose triglyceride lipase, two enzymes involved in lipolysis and glycerolipid/free fatty acid cycling. Chronically, insulin gene expression was increased after treatment with TO-901317, and this was accompanied by increased Pdx-1 nuclear protein levels and enhanced Pdx-1 binding to the insulin promoter. In conclusion, our data suggest that LXR agonists have a direct effect on the islet to augment insulin secretion and expression, actions that should be considered either as therapeutic or unintended side effects, as these agents are developed for clinical use.
Keywords: Insulin, Metabolism, Nuclear Receptors, Pancreatic Islet, Transcription, Liver X Receptors, Pdx-1 Translocation, Human Islets, Insulin Pre-mRNA, Insulin Secretion
Introduction
The liver X receptors (LXRs),2 LXRα (NR1H3), and LXRβ (NR1H2) are members of the nuclear hormone receptor superfamily and function to integrate lipid metabolic and inflammatory signaling (1). Upon binding to oxysterol ligands and heterodimerization with retinoid X receptors, LXRs bind to conserved LXR-responsive elements in target genes to regulate their expression. LXRs augment lipogenesis through transcriptional up-regulation of the genes encoding sterol regulatory binding-protein 1c (SREBP-1c), fatty acid synthase (FAS), and stearoyl-CoA desaturase 1 (SCD). They also function to regulate reverse cholesterol transport through the induction of genes encoding the ATP binding cassette transporters (ABCs) (2, 3). Furthermore, LXRs contribute to the regulation of innate immunity and have anti-inflammatory effects that are mediated through repression of several downstream targets of NF-κB (nuclear factor κ light chain-enhancer of activated B cells) (4, 5).
In addition to these well described effects on lipid metabolism and inflammation, LXRs also appear to play a role in the maintenance of glucose homeostasis. Oral administration of synthetic LXR agonists to diabetic rodent models, including obese db/db mice and Zucker fatty rats, results in improved glucose tolerance (6, 7). These effects of LXR agonists appear to arise in part from actions on insulin-sensitive tissues, as LXRs have been shown to enhance insulin sensitivity in adipose tissue and suppress gluconeogenesis in the liver (6, 8, 9). Recent data also suggest that LXRs play a role in the augmentation of pancreatic islet function through enhanced insulin release (7, 10). However, the mechanisms underlying this augmentation are not completely understood. To date, all of the studies examining the role of LXRs in islet function have been performed in rodent models and tumorigenic islet-derived cells lines and/or using high concentrations of LXR agonists (10–14). In this regard important differences exist between rodent and human islets with respect to cytoarchitecture, gene regulation, expression profiles, and replication (15–19).
Given the important differences present between human and rodent islets as well as the potential development of these agents for clinical use in a number of disorders (3, 20), we sought to clarify the effects of LXR agonist treatment on human islet function and to comprehensively examine the mechanisms underlying these effects. Our results reveal that LXR agonists have a direct effect on the islet to enhance insulin release through a mechanism that involves increased flux through pyruvate and glycerolipid/free fatty acid cycling pathways, whereas insulin expression is increased chronically through enhancement of Pdx-1 nuclear levels and increased binding of Pdx-1 to the proximal insulin promoter. Importantly, these effects are independent of intra-islet lipid accumulation at the dose of agonist used in our study.
EXPERIMENTAL PROCEDURES
Materials
The LXR agonist, TO-901317, was purchased from Cayman Chemicals (Ann Arbor, MI). The LXR agonist GW3965 was kindly provided by Jon Collins and Timothy Willson (GlaxoSmithKline). Anti-LXRα/β rabbit antibody, anti-LXRβ rabbit antibody, LXRα/β blocking peptide, and LXRβ blocking peptide were purchased from Santa Cruz Biochemistry (Santa Cruz, CA). Anti-Pdx-1 rabbit antibody was purchased from Millipore (Billerica, MA). Anti-actin mouse antibody (C4 clone) was purchased from BD Biosciences. Horseradish peroxidase-coupled anti-mouse and anti-rabbit goat antibody were purchased from Jackson ImmunoResearch (West Grove, PA). Anti-acetyl-coenzyme A carboxylase and anti-FAS rabbit antibody were purchased from Abcam (Cambridge, MA). Anti-SCD rabbit antibody was purchased from Cell Signaling (Danvers, MA). Anti-isocitrate dehydrogenase 1 (IDH1) rabbit antibody was purchased from Proteintech Group Inc. (Chicago, IL). Oligonucleotides end-labeled with IRDyeTM 700 phosphoramidites were purchased from Eurofins MWG Operon (Huntsville, AL).
Primary Human Islet Preparations and Cell Culture
Human islets were isolated from 20 cadaver non-diabetic donors at facilities housed at the University of Washington, University of Miami, University of Alabama, Birmingham, City of Hope Hospital, Beta-Pro, LLC, and the National Disease Research Interchange. Comprehensive donor data were known for 19 of the islet preparations. Of these, 13 donors were male, and 7 were female. The average age was 40.3 ± 3.2 years (range 16–63). The average body mass index of donors was 26.5 ± 1.5 kg/m2 (range 17.9–43.9). The cold ischemia time ranged from 6 to 18 h (average 9.9 ± 0.9 h). Purity of the islet preparations ranged from 40 to 90% (average 76 ± 4%). On arrival, islets were immediately placed in Dulbecco's modified Eagle's medium culture media containing 5.5 mm glucose, 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) and incubated overnight at 37 °C with 5% CO2. INS-1 cells (832/13) were cultured in RPMI 1640 containing 11.1 mm glucose supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 10 mm HEPES, 2 mm l-glutamine, 1 mm sodium pyruvate, and 50 μm β-mercaptoethanol.
Glucose-stimulated Insulin Transcription and Quantitative RT (qRT)-PCR
Approximately 100 islets/well were preincubated for 2 h in 6-well plates containing RPMI 1640 with 2.5 mm glucose and 10% fetal bovine serum. Islets were then incubated in the presence or absence of 1 μm TO-901317 or 1 μm GW3965 for the designated times (0, 8, 24, and 48 h) at a glucose concentration of either 2.5 or 25 mm. Islets were washed and processed for total RNA using the Qiagen RNeasy kit (Valencia, CA) according to the manufacturer's instructions. For qRT-PCR experiments, total RNA (5 μg) from islets was reverse-transcribed at 37 °C for 1 h using 15 μg of random hexamers, 0.5 mm dNTPs, 5× first-strand buffer, 0.01 mm dithiothreitol, and 200 units of Moloney murine leukemia virus reverse transcriptase (Invitrogen) in a final reaction volume of 20 μl. Quantitative RT-PCR was performed for mature insulin transcript and insulin pre-mRNA as described previously (19). TaqMan® primer/probe combinations (Applied Biosystems, Foster City, CA) were used to measure the RNA levels of human pancreatic and duodenal homeobox factor-1 (PDX-1), glucokinase, glucose transporter 2 (GLUT2/SLC2A2), IDH1, pyruvate carboxylase, ATP-sensitive inward rectifier potassium channel 11 (KCNJ11), and acetyl-coenzyme A carboxylase (ACACA), hormone-sensitive lipase (HSL/LIPE), and adipose triglyceride lipase/patatin-like phospholipase domain containing 2 (PNPLA2) with cycling parameters described previously (21). SYBR Green chemistry was used to measure RNA levels of other genes of interest by qRT-PCR as previously described (22) using an ABI Prism 7900HT system. Gene-specific primers were validated by template titration curves and temperature dissociation analyses. Relative RNA levels were established against the invariate β-actin mRNA species using the comparative CT method as described previously (23, 24). Primer sequences and TaqMan® accession numbers are provided in supplemental Table 1.
Glucose-stimulated Insulin Secretion and Measurement of Insulin Content
Approximately 50 islets/well were preincubated overnight in 12-well plates containing RPMI 1640, 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin in the presence or absence of 1 μm TO-901317. The following day islets were transferred to Krebs-Ringer buffer, pH 7.4, containing 134 mm NaCl, 4.7 mm KCl, 1.2 mm KH2PO4, 1.2 mm MgSO4, 1.0 mm CaCl2, 5 mm NaHCO3, 10 mm HEPES, 0.1% BSA, and 2.5 mm glucose with or without 1 μm TO-901317 for 1 h. After 1 h, 100 μl of media was removed for insulin assay and replaced with an equal volume of Krebs-Ringer buffer and 1 m glucose to bring the final concentration of glucose to 25 mm. Islets were incubated an additional hour, and media were collected for insulin determination using the Mercodia enzyme-linked immunosorbent assay kit according to the manufacturer's instructions (American Laboratory Products Company, Windham, NH). Insulin content was measured after acid extraction as previously described and normalized to DNA concentration (25).
Immunoblot Analysis
Approximately 100 human islets (for measurement of total protein), 1000 human islets (for measurement of nuclear and cytosolic protein levels), or 1.5 × 105 INS-1 cells were preincubated in RPMI 1640 medium with 2.5 mm glucose for 2 h in the presence or absence of 1 μm TO-901317. The glucose concentration was increased to 25 mm for a specified time, after which total protein or nuclear and cytosolic fractions were isolated as previously described (26). Twenty μg of total protein, 20 μg of cytosolic fraction, or 10 μg of nuclear fraction were separated by SDS-PAGE, transferred to a polyvinylidene fluoride membrane, and incubated at 4 °C overnight with anti-Pdx-1 rabbit antibody (1:2,000 dilution), anti-actin mouse antibody (1:10,000 dilution), anti-acetyl-coenzyme A carboxylase rabbit antibody (1:1,000 dilution), anti-FAS rabbit antibody (1:1,000 dilution), anti-SCD rabbit antibody (1:1,000 dilution), anti-IDH1 antibody (1:500 dilution), or anti-actin mouse antibody (1:10,000 dilution). Bound primary antibodies were detected with anti-mouse goat antibody (1:10,000 dilution) or anti-rabbit goat antibody (1:10,000 dilution). Immunoreactivity was visualized using either the ECL-Plus® system (Amersham Biosciences) using ImageQuant Version 5.2 to calculate blot density (Amersham Biosciences) or after fluorometric scanning on an Odyssey imaging system (Li-cor Biosciences, Lincoln, NE).
Islet Triglyceride Measurement and Lipolysis Assay
For measurement of triglyceride accumulation, ∼100 human islets were incubated in Dulbecco's modified Eagle's medium media containing 5.5 mm glucose and 1 μm TO-901317 for 72 h. After lipid extraction as previously described, islet triglyceride content was measured using the GPO-Trinder Kit (Sigma) according to the manufacturer's instructions (27). To measure lipolysis, islets were incubated with or without 1 μm TO-901317 in media containing 25 mm glucose for 72 h. Free glycerol release into the supernatant was measured using a quantitative colorimetric enzymatic assay (Sigma). Results for both assays were normalized to total protein content.
Pyruvate Carboxylase Enzyme Activity
The measurement of pyruvate carboxylase enzyme activity was performed according to the method of Liu et al. (28). Approximately 300 human islets were homogenized in 10 mm HEPES, pH 7.4, 250 mm sucrose, 2.5 mm EDTA, 2 mm l-cysteine, and 0.02% bovine serum albumin. Fifty μl of extract was added to 0.95 ml of reaction buffer (80 mm Tris/HCl, pH 8.2, 2 mm ATP, 8 mm sodium pyruvate, 21 mm KHCO3, 9 mm MgSO4, 0.16 mm acetyl-CoA, 0.16 mm NADH, and 5 units/ml malate dehydrogenase). Absorbance was measured at 340 nm for 10 min at 30 °C.
Statistical Analysis
Differences between groups were examined for significance using either the Student's t test, one-way ANOVA followed by the Tukey-Kramer post-test, or two-way ANOVA (factors: glucose, TO-901317) using GraphPad Prism statistics software. A p value < 0.05 was taken to indicate the presence of a significant difference.
RESULTS
LXRs Are Expressed in Human Islets
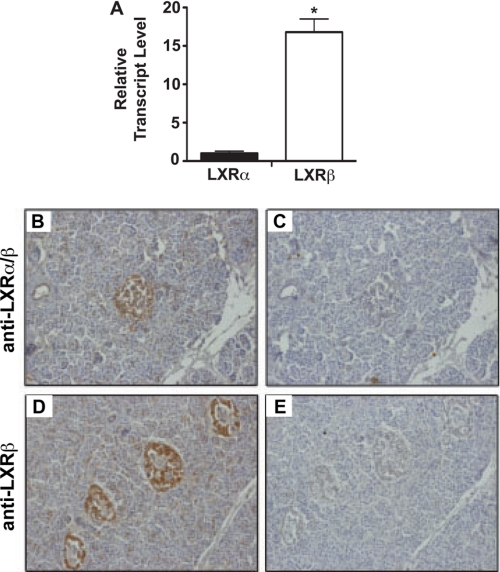
Whereas previous studies have shown the presence of LXRs in rodent islets, we sought to determine the expression characteristics of LXRs in human pancreata by qRT-PCR and immunohistochemistry. qRT-PCR results confirmed that LXRβ was the dominant subtype in human islets, consistent with previous data from both rodent and human studies (Fig. 1A) (14, 29). The immunostaining pattern of LXRα and -β protein showed enrichment in islets relative to pancreatic acinar and ductal tissue (Fig. 1, B and D). Immunoreactivity was blocked upon the addition of peptide competitors for both LXR antibodies, demonstrating the specificity of immunohistochemical staining for LXRs (Fig. 1, C and E).
FIGURE 1.
Distribution of LXRs in human islets. A, qRT-PCR was performed using total mRNA isolated from primary human islets maintained in 11 mm glucose. LXR mRNA levels were normalized to β-actin mRNA levels. All data are shown as the mean ± S.E. (n = 3). *, data indicate statistical difference (p < 0.05) compared with LXRα mRNA level. Panels B–E, human pancreas sections were immunostained with anti-LXRα/β antibody (B) or anti-LXRα/β antibody plus blocking peptide for LXRα/β (C). Human pancreas sections were immunostained with anti-LXRβ antibody (D) or anti-LXRβ antibody plus blocking peptide for LXRβ (E). Antibody binding was revealed using peroxidase-conjugated secondary IgG and diaminobenzidine tetrahydrochloride substrate to yield a brown signal.
LXRs Regulate Lipid-associated Genes in Human Islets
To investigate the role of LXRs in human islets, islets were incubated in the presence or absence of the LXR agonist TO-901317 (1 μm, 24 h) under low (2.5 mm) or high (25 mm) glucose culture conditions. Global gene expression was evaluated by microarray, which included assessment of known LXR target genes as well as glucose-responsive genes. Fifty-three genes were found to be differentially regulated by glucose and/or TO-901317 as shown in the heat map (supplemental Fig. S1). LXR agonist treatment led to increased expression of a number of LXR target genes previously characterized in other tissues, including ABCA1, ABCG1, SREBP-1, FAS, and SCD.
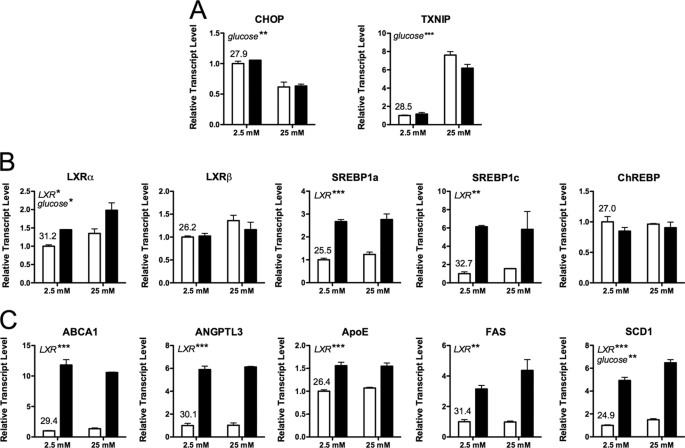
Quantitative RT-PCR using additional islet preparations was subsequently performed to verify our microarray results. We observed a decrease in DDIT3 (CHOP) mRNA and an increase TXNIP mRNA levels, demonstrating the efficacy of our glucose treatment (Fig. 2A) (30, 31). Results from the microarray were confirmed, as we again observed an increase in the expression of known LXR target genes (Fig. 2, B–C).
FIGURE 2.
Gene expression changes following islet exposure to LXR agonist. Human islets (∼100/well) were preincubated for 2 h in 2.5 mm glucose, then switched to either low glucose (2.5 mm) or high glucose (25 mm) in the presence or absence of 1 μm TO-901317 for 24 h. RNA was measured by qRT-PCR using an ABI Prism system and SYBR chemistry (primers are provided in supplemental Table 1). A, the efficacy of glucose exposure was revealed by changes in CHOP and TXNIP mRNA levels. B, shown is expression of relevant transcription factors. C, shown is expression of LXR target genes involved in lipid metabolism. mRNA levels were normalized to β-actin, and the results represent the mean ± S.E. of duplicate biologic samples. The qRT-PCR cycle number at the threshold for each gene is provided for the vehicle-treated, low-glucose group. Two-way ANOVA was performed, and significant effects of glucose and LXR agonist are indicated in italics (*p < 0.05; **p < 0.01; ***p < 0.001); no significant interaction of glucose and ligand was observed for these RNA species. Similar results were obtained with a second independent experiment and were observed with an alternate LXR agonist, GW3965 (Fig. 3).
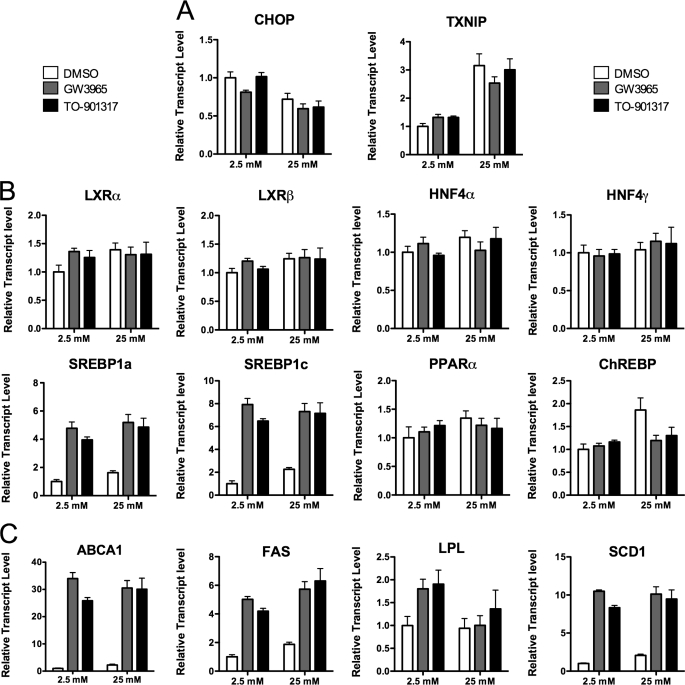
TO-901317 has been reported to also function as a potent ligand for human pregnane X receptor (PXR) (32). As such, we evaluated LXR-induced changes in gene expression with an alternative agonist, GW3965, a compound with no reported PXR activity (32). We observed similar changes in gene expression with both compounds (Fig. 3, A–C). Furthermore, our microarray results did not indicate any change in expression of PXR target genes including CYP3A4, CYP2B6, UGT1A1, UGT1A6, MDR1, or ALAS1 (data not shown) (33). These observations are consistent with our previous report that PXR mRNA is virtually undetectable in human islets (29). In total, these data demonstrate that changes attributed to TO-901317 in human islets are, in fact, mediated through LXR and not PXR activation.
FIGURE 3.
Similar gene expression changes are observed with two different LXR agonists. Human islets (∼100/well) were preincubated for 2 h in 2.5 mm glucose, then switched to either low glucose (2.5 mm) or high glucose (25 mm) with 1 μm GW3965 (gray bars), 1 μm TO-901317 (black bars), or vehicle (DMSO, white bars). RNA was measured by qRT-PCR using an ABI Prism system and SYBR chemistry (primers are provided in supplemental Table 1). RNA levels are depicted relative to the housekeeping gene β-actin and depict the average of triplicate analyses of a single RNA sample from ∼100-pooled human islets. A, the efficacy of glucose exposure was revealed by changes in CHOP and TXNIP RNA levels. B, shown is expression of relevant transcription factors. C, shown is expression of LXR target genes involved in lipid metabolism. PPAR, peroxisome proliferator-activated receptor.
Treatment of Human Islets with an LXR Agonist Enhances Insulin Secretion
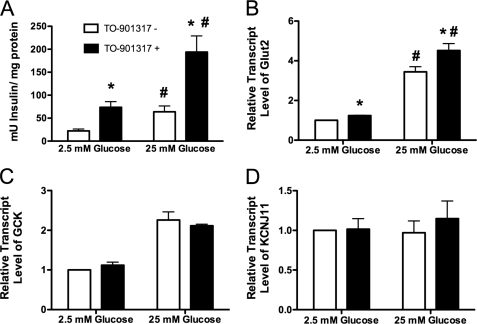
To characterize the effect of LXR activation on insulin secretion, we measured glucose-stimulated insulin secretion in human islets after pretreatment with the LXR agonist TO-901317. As shown Fig. 4A, 16 h of treatment with 1 μm TO-901317 augmented insulin secretion at both low (2.5 mm) and high (25 mm) glucose concentrations. Insulin secretion after stimulation with high glucose was increased 2.6-fold in islets pretreated with the agonist.
FIGURE 4.
LXR activation enhances insulin secretion in human islets. A, 50 human islets were exposed to 2.5 or 25 mm glucose for 1 h after 16 h of preincubation with or without 1 μm TO-901317. Insulin secretion was measured by enzyme-linked immunosorbent assay and normalized to total protein content. All data are shown as the mean ± S.E. (n = 3). Groups were compared by two-way ANOVA. *, data indicate statistical difference (p < 0.05) compared with islets incubated in the absence of TO-901317. #, data indicate statistical difference (p < 0.05) compared with islets incubated in 2.5 mm glucose. Panels B–D, human islets (∼100/well) were preincubated for 2 h in 2.5 mm glucose and then switched to either low glucose (2.5 mm) or high glucose (25 mm) in the presence or absence of 1 μm TO-901317 for 24 h. RNA was measured by qRT-PCR using TaqMan primer/probe combinations (primers are provided in supplemental Table 1). All data are shown as the mean ± S.E. (n = at least 3) and are displayed as relative expression compared with islets incubated in 2.5 mm glucose in the absence of agonist. Groups were compared using two-way ANOVA. *, data indicate statistical difference (p < 0.05) versus human islets incubated without TO-901317. #, data indicate statistical difference (p < 0.0001) compared with islets incubated in 2.5 mm glucose. GCK, glucokinase; KCNJ11, potassium channel 11.
To determine the mechanism of this increase in insulin secretion, qRT-PCR was performed to measure expression of several key genes involved in the classically described KATP-mediated pathway of glucose-stimulated insulin secretion (34). Our results show that in response to treatment with TO-901317, expression of the gene encoding the glucose transporter 2 (SLC2A2 or GLUT2) was significantly increased (Fig. 4B), whereas no effect on the expression of the genes encoding glucokinase (GCK) or the potassium inwardly rectifying channel, subfamily J, member 11 (KCNJ11) was observed (Fig. 4, C and D).
Emerging data have demonstrated an equally important role for additional processes including anaplerosis and pyruvate cycling, lipid metabolism, and NADH shuttling systems to effect sustained release of insulin in response to elevated glucose (35–40). Of the enzymes involved in pyruvate cycling, we observed a small but significant increase in the expression of the gene encoding IDH1 (Fig. 5A), and we observed an increase in protein levels of isocitrate dehydrogenase after treatment with 1 μm TO-901317 (Fig. 5B). Although expression of the gene encoding pyruvate carboxylase was not affected, we did observe a significant increase in enzyme activity of pyruvate carboxylase (Fig. 5, C and D). These results suggest that activation of anaplerotic pathways contributes in part to LXR-induced increases in glucose-stimulated insulin secretion in human islets.
FIGURE 5.
Treatment with LXR agonist increases pyruvate carboxylase enzyme activity in human islets. A and C, human islets (∼100/well) were preincubated for 2 h in 2.5 mm glucose and then switched to either low glucose (2.5 mm) or high glucose (25 mm) in the presence or absence of 1 μm TO-901317 for 24 h. RNA was measured by qRT-PCR using TaqMan primer/probe combinations (primers provided in supplemental Table 1). All data are shown as the mean ± S.E. (n = at least 3) and are displayed as relative expression compared with islets incubated in 2.5 mm glucose in the absence of agonist. Groups were compared using two-way ANOVA. *, data indicate statistical difference (p < 0.05) versus human islets incubated without TO-901317. B, total protein was isolated from ∼100 human islets incubated in 25 mm glucose in the presence or absence of 1 μm TO-901317 for 24 h. IDH1 protein levels on immunoblot were normalized to actin protein levels. All data are shown as the mean ± S.E. (n = 4). Groups were compared using a t test. *, data indicate statistical difference (p < 0.05) versus human islets incubated without TO-901317. D, human islets (∼300/well) were incubated in 25 mm glucose in the presence or absence of 1 μm TO-901317 for 24 h. Pyruvate carboxylase (PC) enzyme activity was measured in total cell lysate and normalized to total protein content. All data are shown as the mean ± S.E. (n = 4). Groups were compared using a t test. *, data indicate statistical difference (p < 0.05) versus human islets incubated without TO-901317.
Treatment with 1 μm TO-901317 Does Not Result in Excessive Intra-islet Lipid Accumulation in Human Islets
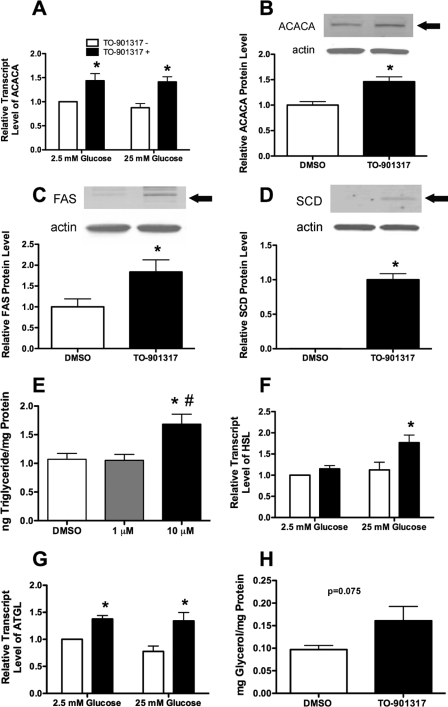
Previous studies have demonstrated a number of detrimental effects after the incubation of rodent islets and islet-derived cell lines with higher doses (>1 μm) of LXR agonist, including pathologic accumulation of lipids leading to increased apoptosis and decreased proliferation (11–13). Whereas we observed an increase in the expression of several known LXR-targets as well as other genes known to be involved in lipogenesis, we sought to measure protein levels of key enzymes involved in lipogenesis under identical treatment conditions. After incubation with 1 μm TO-901317, we observed an increase in expression of the gene encoding acetyl-coenzyme A carboxylase and increased protein levels of ACACA, FAS, and SCD (Fig. 6, A–D). To test the effect of lower doses of TO-901317 on intra-islet triglyceride accumulation, we measured triglyceride levels after treatment with 1 or 10 μm TO-901317 for 72 h at 5.5 mm glucose. Interestingly, there was no difference in triglyceride levels between human islets treated with 1 μm TO-901317 and islets incubated with the drug delivery vehicle, DMSO. However, we did observe an increase in triglyceride accumulation in islets treated with 10 μm TO-901317, consistent with previous results in cell lines (Fig. 6E) (11, 13).
FIGURE 6.
Treatment with 1 μm TO-901317 stimulates lipogenesis and lipolysis and does not lead to increased triglyceride accumulation in human islets. A, F, and G, human islets (∼100/well) were preincubated for 2 h in 2.5 mm glucose and then switched to either low glucose (2.5 mm) or high glucose (25 mm) in the presence or absence of 1 μm TO-901317 for 24 h. mRNA levels were measured by qRT-PCR using TaqMan primer/probe combinations (primers are provided in supplemental Table 1). All data are shown as the mean ± S.E. (n = at least 3) and are displayed as relative expression compared with islets incubated in 2.5 mm glucose in the absence of agonist. Groups were compared using two-way ANOVA. *, data indicate statistical difference (p < 0.05) versus human islets incubated without TO-901317. HSL, hormone-sensitive lipase. B–D, total protein was isolated from ∼100 human islets incubated in 25 mm glucose in the presence or absence of 1 μm TO-901317 for 24 h. ACACA, FAS, and SCD protein levels on immunoblot were normalized to actin protein levels. All data are shown as the mean ± S.E. (n = 4). Groups were compared using a t test. *, data indicate statistical difference (p < 0.05) versus human islets incubated without TO-901317. E, human islets (∼100/well) were incubated with DMSO and 1 μm or 10 μm TO-901317 for 72 h in media containing 5.5 mm glucose. Triglyceride concentration was measured and normalized to total protein content. Data shown are the mean ± S.E. (n = 4). Groups were compared using one-way ANOVA. *, data indicate statistical difference (p < 0.05) versus human islets incubated without TO-901317. #, data indicate statistical difference (p < 0.05) versus human islets incubated with 1 μm TO-901317. H, human islets (∼100/well) were incubated with DMSO or 1 μm TO-901317 for 72 h in media containing 25 mm glucose. Glycerol release into the supernatant was measured and normalized to total protein content. Data shown are the mean ± S.E. (n = 4), and results were compared using a t test (p = 0.075 for the comparison).
As triglyceride accumulation is the balance of both lipogenesis and lipolysis, we measured expression of two key genes involved in lipolysis and glycerolipid/free fatty acid cycling. In response to treatment with TO-901317, we observed an increase in the expression of genes encoding hormone-sensitive lipase (HSL) and adipose triglyceride lipase (Fig. 6, F and G). Furthermore, to assess actual lipolysis, we measured glycerol release from islets cultured with and without 1 μm TO-901317 and observed a strong trend toward increased lipolysis in islets incubated in the presence of LXR agonist (Fig. 6H; p = 0.075).
LXR Activation Induces Insulin Expression in Human Islets
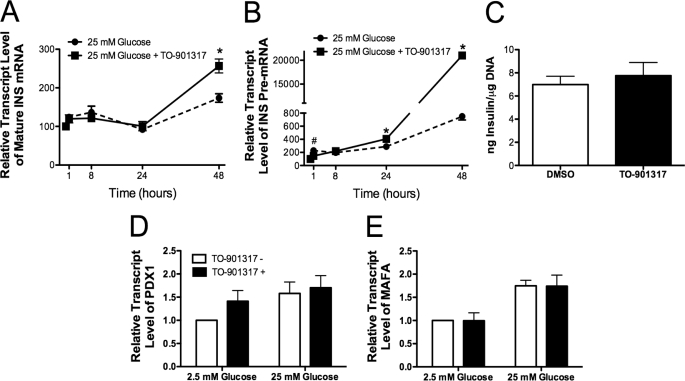
Insulin secretion and transcription are tightly coupled in the β cell (41); thus, we sought to measure the effect of LXR activation on insulin (INS) gene expression. Human islets were incubated under conditions of low (2.5 mm) and high (25 mm) glucose in the presence or absence of the LXR agonist TO-901317. Insulin mRNA was measured at 0, 8, 24, and 48 h after agonist addition (Fig. 7A). Interestingly, an increase of mature INS mRNA was not observed until 48 h after LXR agonist treatment. Whereas mature INS transcript is a highly abundant species with a half-life of >36–48 h, measurement of INS pre-mRNA (i.e. unspliced mRNA) allows for a more accurate assessment of acute changes in insulin gene transcriptional rates (19, 21). A 2-fold increase in INS pre-mRNA was observed within 1 h of exposure to high glucose, consistent with previously published results (19). There was no additive effect of LXR activation on pre-mRNA levels at the 1- or 8-h time points. Importantly, however, at 24 and 48 h, INS pre-mRNA was significantly increased (4- and 200-fold) in islets treated with high glucose and TO-901317, suggesting an augmentation of INS expression with chronic exposure to LXR agonists (Fig. 7B).
FIGURE 7.
LXR activation induces insulin transcription. Approximately 100 human islets were incubated with 25 mm glucose in the presence (indicated by a solid line) or absence (indicated by a dashed line) of 1 μm TO-901317 for 1, 8, and 24 h (panels A and B). mRNA levels of insulin (mature RNA, panel A; pre-mRNA, panel B) were determined by qRT-PCR and normalized to β-actin mRNA levels. All data are shown as the mean ± S.E. (n = at least 3) and are displayed as relative expression compared with islets incubated in 2.5 mm glucose in the absence of agonist. Groups were compared using one-way ANOVA. #, data indicate statistical difference (p < 0.05) versus human islets treated with TO-901317. *, data indicate statistical difference (p < 0.05) versus human islets incubated without TO-901317. Panel C, human islets (∼100/well) were incubated for 48 h in 25 mm glucose in the presence or absence of 1 μm TO-901317. Total islet insulin content was measured and normalized to DNA content. Panels D and E, human islets (∼100/well) were preincubated for 2 h in 2.5 mm glucose, then switched to either low glucose (2.5 mm) or high glucose (25 mm) in the presence or absence of 1 μm TO-901317 for 24 h. mRNA levels were measured by qRT-PCR using TaqMan primer/probe combinations or Syber chemistry (primers are provided in supplemental Table 1). All data are shown as the mean ± S.E. (n = at least 3) and are displayed as relative expression compared with islets incubated in 2.5 mm glucose in the absence of agonist. Groups were compared using two-way ANOVA.
To determine whether this increase in INS expression led to changes in islet insulin content, human islets were treated with 1 μm TO-901317 and 25 mm glucose for 48 h, and total islet insulin content was measured. There was no difference in insulin content between islets treated with LXR agonist and islets treated with the drug delivery vehicle, DMSO (Fig. 7C). To assess whether treatment with TO-901317 impacted insulin mRNA stability, we incubated islets treated with and without TO-901317 with actinomycin (to stop transcription) for a period of 2 h. Results from these short term experiments did not reveal any gross changes in mature INS mRNA or INS pre-mRNA stability (data not shown).
LXR Increases INS Expression through an Indirect Mechanism
Previous studies have shown that insulin gene transcription is regulated by a region within a few hundred base pairs upstream of the transcriptional start site (16). The A3 element of the INS promoter is a region known to regulate glucose-stimulated insulin transcription. LXR-responsive elements are direct repeats of the core sequence AGGTCA separated by 4 nucleotides (3). Scanning of the human INS promoter did not reveal a complete consensus LXR-responsive element, although several half-sites were present, including one that was located within the A3 element (42). To determine whether LXRs directly bind the insulin promoter, we performed both quantitative chromatin immunoprecipitation assays and electrophoretic mobility shift assays (EMSAs). However, no binding was observed by LXRs in either assay (data not shown), suggesting that LXRs activate INS expression in an indirect manner not involving binding to the promoter.
We next investigated the possibility that LXRs may indirectly activate the insulin gene via known activators. The homeodomain-containing transcription factor Pdx-1 and basic leucine zipper transcription factor MafA are known to directly bind to the INS promoter and play an important role in the regulation of glucose-stimulated INS gene transcription (17, 42, 43). We measured expression of the genes encoding these transcription factors in response to LXR activation and did not observe an increase in either PDX-1 or MAFA mRNA levels after treatment with TO-901317 (Fig. 7, D and E).
Pdx-1 is known to contain a nuclear targeting sequence and has been shown to translocate into the nucleus under high glucose conditions and into the cytoplasm by oxidative stress or lipotoxicity (44–46). To further characterize the mechanism of LXR-induced insulin expression, we determined the effect of LXR activation on Pdx-1 cellular localization. Human islets were treated with TO-901317 and 25 mm glucose, and Pdx-1 protein content was evaluated in the nuclear fraction by immunoblot and EMSA. After treatment with LXR agonist, we observed a small increase in nuclear Pdx-1 protein levels at 25 mm glucose conditions (Fig. 8A) without a change in total Pdx-1 protein levels (Fig. 8B). Furthermore, EMSA experiments using the A3 element of the INS promoter showed enhanced binding of Pdx-1 to the A3 element after treatment with the LXR agonist, TO-901317 (Fig. 8C).
FIGURE 8.
LXR activation increases Pdx-1 protein content in the nucleus of human islets. Nuclear (A) or total protein (B) was isolated from ∼1000 human islets incubated in media containing 25 mm glucose in the presence or absence of 1 μm TO-901317 for 18 h. Pdx-1 protein levels on immunoblot were normalized to actin protein levels. All data are shown as the mean ± S.E. (n = 4). Groups were compared using a t test. *, data indicate statistical difference (p < 0.05) versus human islets incubated without TO-901317. C, 2 μg of human islet nuclear extract was used for each EMSA reaction with or without 5 μl of preimmune serum or anti-Pdx-1 antiserum and separated by 5% polyacrylamide gel. EMSA was performed using the A3 element of the human INS promoter containing the sequence CTG GTT AAG ACT CTA ATG ACC CGC TGG TCC TG. For competition experiments, an unlabeled probe of the same sequence was added at 100 or 200 times the concentration of labeled probe. Data shown are representative of three independent experiments.
To provide further characterization of Pdx-1 translocation in response to LXR activation, glucose-responsive rat insulinoma cells (INS-1 832/13) were treated with TO-901317, and Pdx-1 protein levels were quantitated in the nuclear and cytoplasmic fractions. Treatment with LXR agonist increased Pdx-1 protein in the nuclear fraction of INS-1 cells regardless of glucose concentration, whereas cytosolic Pdx-1 levels decreased, with no change in total Pdx-1 protein levels (Fig. 9, A–C). EMSA experiments performed using INS-1 nuclear lysate demonstrated an increase in Pdx-1 binding to the A3 element of the insulin promoter in response to LXR activation (Fig. 9D). To confirm these findings, quantitative chromatin immunoprecipitation experiments were performed in INS-1 cells, and a 3-fold increase in Pdx-1 binding to the insulin promoter was observed after treatment with 1 μm TO-901317 (Fig. 9E).
FIGURE 9.
LXR activation increases Pdx-1 nuclear translocation and Pdx-1 binding to the insulin promoter in INS-1 cells. Nuclear fraction (A), cytosolic fraction (B), and total cell lysate (C) were isolated from INS-1 cells cultured in the presence or absence of 1 μm TO-901317 for 16 h in media containing 2.5 mm or 25 mm glucose and immunoblotted with anti-Pdx-1 antibody or anti-actin antibody. Pdx-1 protein levels were normalized to actin protein levels. All data are shown as the mean ± S.E. (n = 4). Groups were compared using two-way ANOVA. *, data indicate statistical difference (p < 0.05) versus INS-1 cells treated without TO-901317. #, data indicate statistical difference (p < 0.05) versus INS-1 cells treated with 2.5 mm glucose. D, 1 μg of INS-1 nuclear extract was used for each EMSA reaction with or without 5 μl of preimmune serum or anti-Pdx-1 antiserum and separated by 5% polyacrylamide gel. EMSA was performed using the A3 element of the human insulin promoter. Data shown are representative of three independent experiments. E, INS-1 cells were harvested and subjected to chromatin immunoprecipitation analysis as detailed under supplemental “Experimental Procedures.” Quantitative PCR was used to measure recovery of the proximal insulin promoter using antibody to Pdx-1 or normal rabbit serum (NRS). Results are expressed as percent recovery of the gene fragment relative to input chromatin. Results represent the mean ± S.E. for three independent experiments and were analyzed using two-way ANOVA (A–C) or one-way ANOVA (E). *, data indicate statistical difference (p < 0.005) compared with INS-1 cells incubated without TO-901317.
DISCUSSION
The nuclear hormone receptors LXRα and -β play important roles in a variety of cellular processes, including those that regulate pathways of lipid metabolism and inflammation (3). Recent studies in rodents and rodent-derived tumorigenic β cell lines have suggested an additional role for LXRs in glucose homeostasis and islet function (7, 10, 47). Genetic deletion of LXRβ or the LXR-associated coactivator ASC2 (activating signal co-integrator-2) in the mouse results in impaired islet glucose-stimulated insulin secretion, whereas acute administration of an LXR agonist to rodent islets and insulinoma cell lines results in enhanced insulin secretion (7, 10, 47). The goal of our study was to comprehensively evaluate the effects of LXR agonists on human islet function and to investigate mechanisms underlying these effects.
Consistent with observations in rodent models, we observed an increase in insulin secretion and expression after treatment with TO-901317 in human islets (10, 14). An interesting component of this increase in insulin secretion is that it occurred independent of glucose concentration. Classically, insulin secretion in the pancreatic β cell has been ascribed to a KATP-dependent mechanism (48). Recent analyses have demonstrated that this canonical pathway is responsible for only a fraction of insulin secretory response. In a series of elegant studies, a role for pyruvate carboxylase-mediated anaplerotic pathways in the regulation of insulin secretion has emerged. These pathways involve exchange of pyruvate with several tricarboxylic acid cycle intermediates and subsequent activation of mitochondrial metabolic cycles (35–40).
Given this existing context, we sought to further examine the mechanism of LXR-induced insulin secretion in human islets. Consistent with a role for pyruvate cycling in insulin secretion, expression of the gene encoding IDH1 and protein levels of IDH1 were both modestly increased. In further support of LXR-mediated activation of anaplerotic pathways, we observed an increase in pyruvate carboxylase enzyme activity in human islets treated with 1 μm TO-901317. Previous studies have shown that siRNA-mediated knockdown of IDH1 or pyruvate carboxylase leads to a significant decrease in glucose-stimulated insulin secretion in INS-1 cells and rat islets, with decreased flux through these alternative NADPH producing pathways (36, 49).
An important limitation to the development of LXR agonists for human use has been the observed side effect of hepatic steatosis that results from up-regulation of lipid biosynthesis. Three previous reports suggest that chronic activation of LXR in INS-1 cells and rat islets results in intra-islet lipid accumulation and eventual apoptosis (11–13). However, these adverse effects were observed with very high agonist concentrations (10 μm) and only in the presence of high 9-cis-retinoic acid levels or prolonged exposure to high glucose. It is noteworthy in our studies that incubation of human islets with 1 μm TO-901317 did not result in excessive accumulation of triglyceride at physiologic glucose levels. A recent study in human islets using 1 μm GW3965 similarly reported no change in islet lipid content but, in contrast to our findings, did not detect any alteration in glucose-stimulated insulin secretion (5).
The relationship between lipids and β cell function is complicated. In the short term, exposure to increased free fatty acids stimulates insulin release from the β cell, whereas chronic exposure to free fatty acids and triglycerides diminish insulin secretion and gene expression (50, 51). Thus, excess LXR activation could contribute to increased fatty acid/triacylglycerol-associated lipotoxicity and islet dysfunction.
Actual triglyceride accumulation, however, involves a balance between lipogenesis and lipolysis. Several groups have demonstrated an important role for lipolysis and glycerolipid/free fatty acid cycling in the islet, and products of this pathway have been shown to stimulate insulin secretion (52, 53). As such, we examined the effect of LXR activation on lipolysis in the islet and found the expression of two key enzymes involved in glycerolipid/free fatty acid cycling in the islet, hormone-sensitive lipase, and adipose triglyceride lipase (54, 55) to be increased. Furthermore, our data showed a strong trend toward increased lipolysis in LXR-treated human islets.
Recent studies also suggest that a diminished efflux of cholesterol in islets could contribute to impaired islet function. Moreover, mice with a β cell-specific deletion of the reverse cholesterol transporter ABCA1 manifest defective insulin secretion in the context of normal peripheral insulin sensitivity (56). Thus, LXR activation might also be predicted to relieve an intracellular cholesterol burden to protect islets through activation of ABC transporters.
The processes of insulin secretion and transcription are tightly coupled within the β cell. Glucose stimulates both insulin release and transcription, whereas insulin protein is thought to act in an autocrine manner to stimulate expression of the INS gene (19, 57). As such, we investigated the possibility that LXRs might function as a link between extracellular glucose and INS transcription within the β cell. Through measurement of INS pre-mRNA, our results show that LXR activation does not affect INS gene transcription in the short term, but rather, an increase in expression was observed after 24 h. Whereas we saw a concomitant increase in glucose-stimulated insulin secretion and INS expression, no increase in islet insulin content was observed. Together, these data suggest that INS transcription may be increased to maintain islet insulin content in the face of enhanced insulin release. We cannot, however, rule out the possibility that LXR activation impacts insulin mRNA stability such that the effects of augmented INS transcription, which might otherwise produce an increase in insulin content, are masked by accelerated mRNA degradation. To partially address this issue, actinomycin experiments were performed, and no gross differences in mature INS mRNA or INS pre-mRNA degradation were observed. However, given the long half-life of mature INS message, we cannot exclude a change outside the time frame of our study.
Nevertheless, the effects on insulin expression do not appear to be the result of a direct effect of LXR on the INS promoter. Rather, these changes were coincident with enhanced Pdx-1 binding to the insulin promoter and modest increases in nuclear Pdx-1 levels. This increase in Pdx-1 nuclear protein levels may also serve to explain in part our observed increase in GLUT2 transcription, as Pdx-1 has been shown to be a transactivator of the GLUT2 gene (58).
Fig. 10 depicts a model of our experimental findings. Our results suggest that LXRs have a direct effect on the islet to enhance insulin release through a number of pathways including those that involve pyruvate cycling, glycerolipid/free fatty acid cycling, and reverse cholesterol transport. Insulin expression is increased chronically through a mechanism that involves increased Pdx-1 binding to the INS promoter. Although these effects may prove beneficial in the treatment of islet dysfunction and diabetes, they should also be considered as unintended side effects of LXR agonist therapy. Further studies are needed to clarify the dose-dependent effects of LXR agonists in human and model systems, as these agents are developed for clinical use.
FIGURE 10.
Summary model; LXR activation has multiple effects in human islets. Treatment of human islets with the LXR agonist, TO-901317, increases insulin secretion through a mechanism that involves increased pyruvate carboxylase enzyme activity and increased expression of several genes known to influence islet function including those involved in reverse cholesterol transport and glycerolipid/free fatty acid cycling (GL/FFA). After chronic exposure to LXR agonist, insulin transcription is also increased and corresponds to increased Pdx-1 binding to the proximal INS promoter. HSL, hormone-sensitive lipase; ATGL, adipose triglyceride lipase. * denotes genes known to be direct transcriptional targets of LXRs.
Acknowledgments
We gratefully acknowledge Dr. Robert Harris for assistance in establishing our pyruvate carboxylase assay and Shari Upchurch for editorial assistance in preparing this manuscript. We also acknowledge the generous support of the Touchstone Center for Diabetes Research at UT Southwestern for assistance with the microarray analyses.
This work was supported, in whole or in part, by National Institutes of Health Grants R01DK60581 (to R. G. M.) and K08 DK080225 (to C. E.-M.). This work was also supported by a Manpei Suzuki Diabetes Foundation Grant (to T. O.), an American Diabetes Association mentor-based post-doctoral fellowship (to R. G. M.), and an American Diabetes Association Research Award (to J. J. R.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Fig. S1.
- LXR
- liver X receptor
- ABC
- ATP binding cassette
- Pdx-1
- pancreatic and duodenal homeobox factor-1
- PXR
- human pregnane X receptor
- qRT
- quantitative reverse transcriptase
- EMSA
- electrophoretic mobility shift assay
- FAS
- fatty acid synthase
- SCD1
- stearoyl-CoA desaturase 1
- SREBP
- sterol regulatory-binding protein
- IDH1
- isocitrate dehydrogenase
- ACACA
- acetyl-coenzyme A carboxylase
- ANOVA
- analysis of variance
- INS
- insulin
- GLUT2
- glucose transporter 2.
REFERENCES
- 1.Edwards P. A., Kennedy M. A., Mak P. A. (2002) Vascul. Pharmacol. 38, 249–256 [DOI] [PubMed] [Google Scholar]
- 2.Varga G., Su C. (2007) BioDrugs 21, 117–124 [DOI] [PubMed] [Google Scholar]
- 3.Zelcer N., Tontonoz P. (2006) J. Clin. Invest. 116, 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joseph S. B., Castrillo A., Laffitte B. A., Mangelsdorf D. J., Tontonoz P. (2003) Nat. Med. 9, 213–219 [DOI] [PubMed] [Google Scholar]
- 5.Scholz H., Lund T., Dahle M. K., Collins J. L., Korsgren O., Wang J. E., Foss A. (2009) Diabetologia 52, 1352–1362 [DOI] [PubMed] [Google Scholar]
- 6.Cao G., Liang Y., Broderick C. L., Oldham B. A., Beyer T. P., Schmidt R. J., Zhang Y., Stayrook K. R., Suen C., Otto K. A., Miller A. R., Dai J., Foxworthy P., Gao H., Ryan T. P., Jiang X. C., Burris T. P., Eacho P. I., Etgen G. J. (2003) J. Biol. Chem. 278, 1131–1136 [DOI] [PubMed] [Google Scholar]
- 7.Gerin I., Dolinsky V. W., Shackman J. G., Kennedy R. T., Chiang S. H., Burant C. F., Steffensen K. R., Gustafsson J. A., MacDougald O. A. (2005) J. Biol. Chem. 280, 23024–23031 [DOI] [PubMed] [Google Scholar]
- 8.Commerford S. R., Vargas L., Dorfman S. E., Mitro N., Rocheford E. C., Mak P. A., Li X., Kennedy P., Mullarkey T. L., Saez E. (2007) Mol. Endocrinol. 21, 3002–3012 [DOI] [PubMed] [Google Scholar]
- 9.Laffitte B. A., Chao L. C., Li J., Walczak R., Hummasti S., Joseph S. B., Castrillo A., Wilpitz D. C., Mangelsdorf D. J., Collins J. L., Saez E., Tontonoz P. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5419–5424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Efanov A. M., Sewing S., Bokvist K., Gromada J. (2004) Diabetes 53, S75–S78 [DOI] [PubMed] [Google Scholar]
- 11.Choe S. S., Choi A. H., Lee J. W., Kim K. H., Chung J. J., Park J., Lee K. M., Park K. G., Lee I. K., Kim J. B. (2007) Diabetes 56, 1534–1543 [DOI] [PubMed] [Google Scholar]
- 12.Wente W., Brenner M. B., Zitzer H., Gromada J., Efanov A. M. (2007) Endocrinology 148, 1843–1849 [DOI] [PubMed] [Google Scholar]
- 13.Green C. D., Jump D. B., Olson L. K. (2009) Endocrinology 150, 2637–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zitzer H., Wente W., Brenner M. B., Sewing S., Buschard K., Gromada J., Efanov A. M. (2006) Endocrinology 147, 3898–3905 [DOI] [PubMed] [Google Scholar]
- 15.Cabrera O., Berman D. M., Kenyon N. S., Ricordi C., Berggren P. O., Caicedo A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melloul D., Marshak S., Cerasi E. (2002) Diabetologia 45, 309–326 [DOI] [PubMed] [Google Scholar]
- 17.Hay C. W., Docherty K. (2006) Diabetes 55, 3201–3213 [DOI] [PubMed] [Google Scholar]
- 18.Brissova M., Fowler M. J., Nicholson W. E., Chu A., Hirshberg B., Harlan D. M., Powers A. C. (2005) J. Histochem. Cytochem. 53, 1087–1097 [DOI] [PubMed] [Google Scholar]
- 19.Evans-Molina C., Garmey J. C., Ketchum R., Brayman K. L., Deng S., Mirmira R. G. (2007) Diabetes 56, 827–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malini N., Rajesh H., Berwal P., Phukan S., Balaji V. N. (2008) Chem. Biol. Drug Des. 71, 140–154 [DOI] [PubMed] [Google Scholar]
- 21.Iype T., Francis J., Garmey J. C., Schisler J. C., Nesher R., Weir G. C., Becker T. C., Newgard C. B., Griffen S. C., Mirmira R. G. (2005) J. Biol. Chem. 280, 16798–16807 [DOI] [PubMed] [Google Scholar]
- 22.Kurrasch D. M., Huang J., Wilkie T. M., Repa J. J. (2004) Methods Enzymol. 389, 3–15 [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarti S. K., James J. C., Mirmira R. G. (2002) J. Biol. Chem. 277, 13286–13293 [DOI] [PubMed] [Google Scholar]
- 24.Schmittgen T. D., Livak K. J. (2008) Nat. Protoc. 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- 25.Amrani A., Durant S., Throsby M., Coulaud J., Dardenne M., Homo-Delarche F. (1998) Endocrinology 139, 1115–1124 [DOI] [PubMed] [Google Scholar]
- 26.Ogihara T., Watada H., Kanno R., Ikeda F., Nomiyama T., Tanaka Y., Nakao A., German M. S., Kojima I., Kawamori R. (2003) J. Biol. Chem. 278, 21693–21700 [DOI] [PubMed] [Google Scholar]
- 27.Shimabukuro M., Koyama K., Chen G., Wang M. Y., Trieu F., Lee Y., Newgard C. B., Unger R. H. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 4637–4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y. Q., Tornheim K., Leahy J. L. (1999) Diabetes 48, 1747–1753 [DOI] [PubMed] [Google Scholar]
- 29.Chuang J. C., Cha J. Y., Garmey J. C., Mirmira R. G., Repa J. J. (2008) Mol. Endocrinol. 22, 2353–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson S. G., Fawcett T. W., Bartlett J. D., Bernier M., Holbrook N. J. (1993) Mol. Cell. Biol. 13, 4736–4744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minn A. H., Hafele C., Shalev A. (2005) Endocrinology 146, 2397–2405 [DOI] [PubMed] [Google Scholar]
- 32.Mitro N., Vargas L., Romeo R., Koder A., Saez E. (2007) FEBS Lett. 581, 1721–1726 [DOI] [PubMed] [Google Scholar]
- 33.Maglich J. M., Stoltz C. M., Goodwin B., Hawkins-Brown D., Moore J. T., Kliewer S. A. (2002) Mol. Pharmacol. 62, 638–646 [DOI] [PubMed] [Google Scholar]
- 34.Tarasov A., Dusonchet J., Ashcroft F. (2004) Diabetes 53, S113–S122 [DOI] [PubMed] [Google Scholar]
- 35.Jensen M. V., Joseph J. W., Ronnebaum S. M., Burgess S. C., Sherry A. D., Newgard C. B. (2008) Am. J. Physiol. Endocrinol. Metab. 295, E1287–E1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronnebaum S. M., Ilkayeva O., Burgess S. C., Joseph J. W., Lu D., Stevens R. D., Becker T. C., Sherry A. D., Newgard C. B., Jensen M. V. (2006) J. Biol. Chem. 281, 30593–30602 [DOI] [PubMed] [Google Scholar]
- 37.Ronnebaum S. M., Jensen M. V., Hohmeier H. E., Burgess S. C., Zhou Y. P., Qian S., MacNeil D., Howard A., Thornberry N., Ilkayeva O., Lu D., Sherry A. D., Newgard C. B. (2008) J. Biol. Chem. 283, 28909–28917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ronnebaum S. M., Joseph J. W., Ilkayeva O., Burgess S. C., Lu D., Becker T. C., Sherry A. D., Newgard C. B. (2008) J. Biol. Chem. 283, 14248–14256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farfari S., Schulz V., Corkey B., Prentki M. (2000) Diabetes 49, 718–726 [DOI] [PubMed] [Google Scholar]
- 40.MacDonald M. J. (1995) J. Biol. Chem. 270, 20051–20058 [PubMed] [Google Scholar]
- 41.Leibiger B., Wahlander K., Berggren P. O., Leibiger I. B. (2000) J. Biol. Chem. 275, 30153–30156 [DOI] [PubMed] [Google Scholar]
- 42.Melloul D., Ben-Neriah Y., Cerasi E. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 3865–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H., Brun T., Kataoka K., Sharma A. J., Wollheim C. B. (2007) Diabetologia 50, 348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macfarlane W. M., McKinnon C. M., Felton-Edkins Z. A., Cragg H., James R. F., Docherty K. (1999) J. Biol. Chem. 274, 1011–1016 [DOI] [PubMed] [Google Scholar]
- 45.Elrick L. J., Docherty K. (2001) Diabetes 50, 2244–2252 [DOI] [PubMed] [Google Scholar]
- 46.Hagman D. K., Hays L. B., Parazzoli S. D., Poitout V. (2005) J. Biol. Chem. 280, 32413–32418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeom S. Y., Kim G. H., Kim C. H., Jung H. D., Kim S. Y., Park J. Y., Pak Y. K., Rhee D. K., Kuang S. Q., Xu J., Han D. J., Song D. K., Lee J. W., Lee K. U., Kim S. W. (2006) Mol. Cell. Biol. 26, 4553–4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarasov A., Dusonchet J., Ashcroft F. (2004) Diabetes 53, S113–S122 [DOI] [PubMed] [Google Scholar]
- 49.Hasan N. M., Longacre M. J., Stoker S. W., Boonsaen T., Jitrapakdee S., Kendrick M. A., Wallace J. C., MacDonald M. J. (2008) J. Biol. Chem. 283, 28048–28059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gravena C., Mathias P. C., Ashcroft S. J. (2002) J. Endocrinol. 173, 73–80 [DOI] [PubMed] [Google Scholar]
- 51.Zhou Y. P., Grill V. (1995) J. Clin. Endocrinol. Metab. 80, 1584–1590 [DOI] [PubMed] [Google Scholar]
- 52.Nolan C. J., Leahy J. L., Delghingaro-Augusto V., Moibi J., Soni K., Peyot M. L., Fortier M., Guay C., Lamontagne J., Barbeau A., Przybytkowski E., Joly E., Masiello P., Wang S., Mitchell G. A., Prentki M. (2006) Diabetologia 49, 2120–2130 [DOI] [PubMed] [Google Scholar]
- 53.Prentki M., Madiraju S. R. (2008) Endocr. Rev. 29, 647–676 [DOI] [PubMed] [Google Scholar]
- 54.Peyot M. L., Guay C., Latour M. G., Lamontagne J., Lussier R., Pineda M., Ruderman N. B., Haemmerle G., Zechner R., Joly E., Madiraju S. R., Poitout V., Prentki M. (2009) J. Biol. Chem. 284, 16848–16859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peyot M. L., Nolan C. J., Soni K., Joly E., Lussier R., Corkey B. E., Wang S. P., Mitchell G. A., Prentki M. (2004) Diabetes 53, 1733–1742 [DOI] [PubMed] [Google Scholar]
- 56.Brunham L. R., Kruit J. K., Pape T. D., Timmins J. M., Reuwer A. Q., Vasanji Z., Marsh B. J., Rodrigues B., Johnson J. D., Parks J. S., Verchere C. B., Hayden M. R. (2007) Nat. Med. 13, 340–347 [DOI] [PubMed] [Google Scholar]
- 57.Leibiger I. B., Leibiger B., Moede T., Berggren P. O. (1998) Mol. Cell 1, 933–938 [DOI] [PubMed] [Google Scholar]
- 58.Waeber G., Thompson N., Nicod P., Bonny C. (1996) Mol. Endocrinol. 10, 1327–1334 [DOI] [PubMed] [Google Scholar]