Abstract
Atypical protein kinase C (PKC) ζ is an important regulator of inflammation through activation of the nuclear factor-κB (NF-κB) pathway. Chromatin remodeling on pro-inflammatory genes plays a pivotal role in cigarette smoke (CS)- and lipopolysaccharide (LPS)-induced abnormal lung inflammation. However, the signaling mechanism whereby chromatin remodeling occurs in CS- and LPS-induced lung inflammation is not known. We hypothesized that PKCζ is an important regulator of chromatin remodeling, and down-regulation of PKCζ ameliorates lung inflammation by CS and LPS exposures. We determined the role and molecular mechanism of PKCζ in abnormal lung inflammatory response to CS and LPS exposures in PKCζ-deficient (PKCζ−/−) and wild-type mice. Lung inflammatory response was decreased in PKCζ−/− mice compared with WT mice exposed to CS and LPS. Moreover, inhibition of PKCζ by a specific pharmacological PKCζ inhibitor attenuated CS extract-, reactive aldehydes (present in CS)-, and LPS-mediated pro-inflammatory mediator release from macrophages. The mechanism underlying these findings is associated with decreased RelA/p65 phosphorylation (Ser311) and translocation of the RelA/p65 subunit of NF-κB into the nucleus. Furthermore, CS/reactive aldehydes and LPS exposures led to activation and translocation of PKCζ into the nucleus where it forms a complex with CREB-binding protein (CBP) and acetylated RelA/p65 causing histone phosphorylation and acetylation on promoters of pro-inflammatory genes. Taken together, these data suggest that PKCζ plays an important role in CS/aldehyde- and LPS-induced lung inflammation through acetylation of RelA/p65 and histone modifications via CBP. These data provide new insights into the molecular mechanisms underlying the pathogenesis of chronic inflammatory lung diseases.
Keywords: Oxygen/Radicals, Chromatin, Histone Modification, Lung, NF-κB, Oxidative Stress, Aldehydes, Cigarette Smoke, Inflammation, Lipopolysaccharide, Histone Acetylation
Introduction
Cigarette smoke (CS)2 is the major etiological factor in the pathogenesis of chronic obstructive pulmonary diseases (COPD), which is characterized by chronic lung inflammation and accelerated decline in lung function. Previous studies showed that infiltration of immune inflammatory cells, such as macrophages, neutrophils, and lymphocytes, and release of nuclear factor-κB (NF-κB)-dependent pro-inflammatory mediators are increased in lungs of patients with COPD and in rodent lungs exposed to CS (1–6). It is well known that the transcription of pro-inflammatory genes is up-regulated by activation of NF-κB signaling pathway and modifications of histones, such as acetylation on lysine residues of histones, leading to increased accessibility for transcription factor NF-κB binding to coactivator complex (7, 8). Recently we have shown that the recruitment of the RelA/p65 subunit of NF-κB and the increased acetylation of histone proteins H3 and H4 on the promoters of pro-inflammatory genes in rodent lungs in response to CS exposure (1, 9, 10). This is corroborated by the findings that increased acetylation of histones occurs in lungs of smokers and patients with COPD (7, 11, 12). However, the molecular mechanism that underlies CS-mediated chromatin modifications (e.g. increased acetylation of histone proteins and subsequent NF-κB-dependent gene transcription) is not known.
It has been suggested that protein kinase C (PKC) ζ, an atypical PKC, participates in inflammatory response to diverse stimuli in vitro and in vivo (13–17). We have recently shown that PKCζ regulates NF-κB transcriptional activity by phosphorylating RelA/p65 at serine 311 and activating IκB kinase leading to translocation of RelA/p65 into the nucleus (18, 19). Separate evidence suggests that phosphorylation of RelA/p65 on serine 311 enhances its binding with CREB-binding protein (CBP), a transcriptional coactivator possessing intrinsic histone acetyltransferase (HAT) activity, leading to acetylation of RelA/p65 (20, 21). Hence, it is likely that PKCζ regulates the transcriptional activity of NF-κB through recruitment and association of CBP with RelA/p65 as well as CBP-mediated acetylation of histone proteins on promoters of pro-inflammatory genes.
Recently, a role of PKCζ in allergic airway inflammation is shown by the finding that disruption of PKCζ attenuated ovalbumin-induced airway inflammation in mice (15). Importantly, the expression of PKCζ is elevated in lungs of patients with COPD implicating the critical role of PKCζ in chronic airway diseases (22). However, the role of endogenous PKCζ in regulation of lung inflammatory response to CS exposure or any inflammatory agents is not known. We hypothesized that down-regulation of PKCζ protects lungs against inflammatory response and chromatin modifications by CS, its reactive aldehydes as well as other pro-inflammatory agents, such as lipopolysaccharide (LPS), because endotoxin present in tobacco smoke is an important etiological factor for the pathogenesis of COPD/emphysema and exacerbates asthma from smoking (23–25). To test this hypothesis, PKCζ-deficient (PKCζ−/−) and wild-type (WT) mice were exposed to CS and LPS, and the role of PKCζ in lung inflammatory response, NF-κB signaling, and histone modifications was determined in lungs. Furthermore, human monocyte-macrophage cells (MonoMac6 cells) and primary mouse alveolar macrophages were used to determine the role and molecular mechanism of PKCζ in CS-, aldehyde-, and LPS-mediated inflammatory response.
EXPERIMENTAL PROCEDURES
Mice and CS Exposure
The generation of PKCζ−/− mice were described previously with their background WT mice being the 129/SVJ strain (18). The mice were housed in the Vivarium Facility of the University of Rochester with a 12-h light/dark cycle (light on at 6:00 a.m.). All animal procedures described in this study were approved by the University Committee on Animal Research at the University of Rochester. Ten-week-old male PKCζ−/− and WT mice were used for CS exposure as described previously (3, 4). In brief, research grade cigarettes (2R4F; Kentucky Tobacco Research and Development Center, University of Kentucky, Lexington) were used to generate smoke, and mice were exposed to CS according to the Federal Trade Commission protocol (1 puff/min of 2 s duration and 35-ml volume) using a Baumgartner-Jaeger CSM2072i automatic CS generating machine (CH Technologies, Westwood, NJ). Mainstream CS was diluted with filtered air and directed into the exposure chamber. The smoke exposure (total particulate matter in per cubic meter of air) was monitored in real time with a MicroDust Pro-aerosol monitor (Casella CEL, Bedford, UK) and verified daily by gravimetric sampling (2, 3, 9, 26, 27). The smoke concentration was set at a nominal value of ∼300 mg/m3 total particulate matter (corresponding to human consumption of 1–1.5 packs per day (28)), by adjusting the flow rate of the diluted medical air, and the level of carbon monoxide in the chamber was 350 ppm (3, 9, 26, 27, 29). The level of carboxyhemoglobin in blood was 17%. Mice received two 1-h exposures (1 h apart) daily for 3 consecutive days and were sacrificed at 2 and 24 h post-last exposure. Control mice were exposed to filtered air in an identical chamber according to the same protocol described for CS exposure.
LPS Aerosolization
Age-matched adult male WT and PKCζ-deficient mice (8–10 weeks age) were exposed to an aerosol of saline alone or saline containing Escherichia coli LPS (1 mg/ml; Sigma) for 8 min as described previously (4, 26). Two and 24 h after LPS aerosolization, mice were anesthetized and sacrificed.
Bronchoalveolar Lavage (BAL)
BAL in mouse was performed as described previously (3, 4, 30). The total cell numbers in BAL fluid were determined by counting on a hemocytometer. Differential cell counts were performed on cytospin-prepared slides (Thermo Shandon, Pittsburgh, PA) stained with Diff-Quik (Dade Behring, Newark, DE). The remaining BAL cells were kept at −80 °C for later analysis.
Lung Tissue Preparation, Hematoxylin and Eosin, and Macrophage Immunohistochemical Staining
Mouse lungs (which had not been lavaged) were inflated with 1% low melting agarose at a pressure of 25 cm of H2O and then fixed with 4% neutral buffered paraformaldehyde. The fixed lung tissues were dehydrated and embedded in paraffin and sectioned using a rotary microtome (MICROM International GmbH, Walldorf, Germany) into 4-μm sections. Hematoxylin and eosin staining was performed on lung midsagittal sections to investigate the influx of inflammatory cells in lungs (30). For immunohistochemical staining of macrophages, the deparaffinized and rehydrated lung sections were exposed to 3% H2O2 in methanol so as to quench endogenous peroxidase activity. Rat anti-mouse Mac-3 monoclonal antibody (Pharmingen) at a titer of 1:50 was used to detect macrophages. The number of Mac-3-positive cells in the lung sections (five random microscopic fields per lung section in three different sections) was counted manually under a microscope with ×200 magnification and averaged (30).
Myeloperoxidase Assay
Myeloperoxidase activity was assayed in lung homogenates as described previously (4). The lung tissues were homogenized in 10 volumes of 100 mm phosphate buffer (pH 7.4) with protease inhibitors. Myeloperoxidase activity in enzymatic extract was determined using tetramethylbenzidine (Sigma) as substrate. The activity was expressed in the samples as units/mg protein.
Preparation of Aqueous Cigarette Smoke Extract (CSE)
CSE (10%) was prepared by bubbling smoke from one research grade cigarette 3R4F (University of Kentucky) into 10 ml of culture media at a rate of one cigarette/min as described previously (4, 30). The pH of the CSE was adjusted to 7.4, and was sterile-filtered through a 0.45-μm filter (25 mm Acrodisc; Pall Corp., Ann Arbor, MI). CSE preparation was standardized by measuring the absorbance (0.85 ± 0.05) at a wavelength of 320 nm. CSE (10%), containing up to 394 μm acrolein (31) equivalent to 39.4 μm in 1% of CSE, was freshly prepared and diluted with culture media (RPMI 1640 medium) to indicated concentrations used for all experiments within 15 min of preparation. Control medium was prepared by bubbling air through 10 ml of serum-free RPMI 1640 medium, adjusting pH to 7.4, and sterile-filtering as described above.
Cell Culture, Transfection, and Treatments
MonoMac6 cells were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT) at 37 °C in humidified atmosphere under 5% CO2 (2, 30). Cells were seeded at a density of 2.0 × 106 cells in 100-mm culture plates in a total volume of 5 ml of RPMI 1640 medium containing 0.5% fetal bovine serum for 12 h. Cells were pretreated with a myristoylated PKCζ pseudosubstrate peptide inhibitor (PS-PKCζ, 0.3, 1, and 3 μm) (Invitrogen) for 2 h prior to incubation with or without CSE (0.25, 0.5, and 1%), LPS (1 and 10 ng/ml) (Sigma),or acrolein (10 μm, Sigma) for 12 h. In certain experiments, MonoMac6 cells were transfected with WT CBP-HAT plasmid or mutated CBP without HAT activity. The plasmids for WT and mutated CBP (Leu1690/Cys1691 mutated into Lys1690/Leu1691) were the kind gifts from Dr. Sankar Ghosh (Columbia University Medical Center) (20, 32). Transient transfection was performed in MonoMac6 cells with 8 μg of plasmids in the presence of Lipofectamine 2000 transfection reagent (Invitrogen) for 24 h before the aforementioned PKCζ inhibitor pretreatment and subsequent CSE, acrolein, and LPS exposures in a 6-well plate. Transfection efficiency in the case of both plasmid transfections was more than 80%. After treatment, cells were washed with ice-cold sterile Ca2+/Mg2+-free phosphate-buffered saline, and whole cell and nuclear proteins were extracted as described below.
Alveolar macrophages from C57BL/6J mice were isolated as described previously (26). Briefly, the lungs were lavaged with sterile Ca2+/Mg2+-free phosphate-buffered saline. BAL cells were seeded in a 6-well plate with RPMI 1640 medium supplemented with 10% fetal bovine serum at 37 °C in a humidified atmosphere containing 5% CO2. After 2 h of incubation, wells were gently pipetted to remove nonadherent cells, and adherent cells were cultured in medium containing 0.5% fetal bovine serum for 4 h. After starvation, the cells were treated with CSE (0.25% equivalent to 10 μm of reactive aldehydes; see Refs. 31–35), LPS (10 ng/ml), acrolein (5 and 10 μm, Sigma), and acetaldehyde (10 and 30 μm, Sigma) for 12 h followed by incubation with or without PS-PKCζ (1 μm) for 2 h at 37 °C with 5% CO2. Both acrolein and acetaldehyde were used in this study because they are the main aldehyde components present in CS, and one cigarette yields ∼500 μg of acrolein and ∼1000 μg of acetaldehyde depending on cigarette type and determination methods (31, 33–35). Culture media from these adherent alveolar macrophages were collected and stored at −80 °C for KC assay. The cells were washed with cold sterile Ca2+/Mg2+-free phosphate-buffered saline and lysed for the determination of NF-κB activation.
Preparation of Whole Cell Lysate and Isolation of Nuclear Proteins from Lung Tissues, BAL Cells, and Cultured Macrophages
The preparation of whole cell lysate and isolation of nuclear proteins from lung tissues and cells were described previously (3, 4, 30). Briefly, 100 mg of lung tissue was mechanically homogenized with 0.5 ml of RIPA buffer, and the tissue homogenates were kept on ice for 45 min to allow total cell lysis. Likewise, MonoMac6 and BAL cells were resuspended with RIPA buffer, kept on ice for 30 min, and then were vortexed for 15 s. Following centrifugation at 13,000 × g for 5 min, the supernatant was collected as a whole cell lysate. As for isolation of nuclear proteins, 100 mg of lung tissue was mechanically homogenized in 0.5 ml of ice-cold buffer A. The homogenate was centrifuged at 2000 × g for 30 s at 4 °C to remove cellular debris. The supernatant was further centrifuged for 30 s at 13,000 × g at 4 °C. Similarly, cultured macrophages, including MonoMac6 cells and primary mouse alveolar macrophages, were lysed with 0.4 ml of buffer A for 15 min, and lysates were centrifuged for 30 s at 13,000 × g at 4 °C. The pellet was resuspended in 50–100 μl of buffer C and placed on the rotating rocker in the cold room for 30 min. Following centrifugation at 13,000 × g for 5 min, the supernatant was collected as the nuclear extract, and stored at −80 °C until it was used.
Acid Extraction of Histone Proteins from Lung Tissues
Acid extraction of histone protein was performed as described previously (1, 10). In brief, pellets from the aforementioned nuclear fraction were resuspended in 150 μl of deionized water containing 0.2 n HCl and 0.36 n H2SO4. The histone proteins were precipitated from the supernatant, agitated overnight at 4 °C using a rotating rocker, and then centrifuged at 13,000 × g for 10 min. The supernatants were decanted into a fresh tube. Ice-cold acetone precipitation samples were incubated overnight at −80 °C and centrifuged, and the air-dried pellets were resuspended in 50 μl of deionized water.
Assay of Pro-inflammatory Mediators
The levels of pro-inflammatory mediators (i.e. granulocyte-macrophage colony-stimulating factor, interferon-γ, IL-10, IL-13, IL-17, IL-18, IL-1β, and IL-6, KC, MCP-1, MIP-2, and tumor necrosis factor-α) in lung homogenates (50 μl) were measured by LuminexTM 100 using the Beadlyte Mouse Multi-Cytokine BeadmasterTM kit (Bio-Rad) according to the manufacturer's instructions (3). The levels of IL-8 and KC in culture media of MonoMac6 cells and primary mouse alveolar macrophages were determined, respectively, using corresponding enzyme-linked immunosorbent assay kits (IL-8 kit, Invitrogen; KC kit, R & D Systems, Minneapolis, MN).
Protein Assay
Protein level in samples was measured with a BCA kit (Pierce). Linear regression was used to determine the actual protein concentration of the samples.
Immunoblot
Immunoblotting was performed with specific anti-PKCζ, anti-phospho-PKCζ (Thr410), anti-RelA/p65, anti-CBP (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-RelA/p65 (Ser311), anti-phospho-RelA/p65 (Ser276), anti-acetyl-RelA/p65 (Lys310), anti-histone H3, anti-histone H4, anti-phosphorylated/acetylated histone H3 (Ser10/Lys9), and anti-acetylated histone H4 antibodies (Cell Signaling Technology, MA) to determine the respective proteins. Equal loading of the samples was determined by quantitation of proteins as well as by reprobing membranes for β-actin. Antibodies against lamin B and α-tubulin were used for checking the purity of nuclear proteins.
CBP Immunoprecipitation and Its Interaction with PKCζ or RelA/p65
Whole cell lysate were used for CBP immunoprecipitation with an antibody against CBP (1:40 dilution; Santa Cruz Biotechnology), which was added to 100 μg of sample proteins in a final volume of 400 μl, and incubated for 1 h. Protein-A/G-agarose beads (20 μl) (Santa Cruz Biotechnology) were added to each sample and kept overnight at 4 °C on a rotating rocker. The beads were washed three times and then resuspended in 40 μl of RIPA buffer. For immunoblot, the immunoprecipitated CBP-agarose bead suspension was resolved by SDS-PAGE. Negative alone (beads only) was used as a negative control. To assess the interaction of CBP protein with PKCζ or RelA/p65, the membranes of immunoprecipitated CBP were blotted against PKCζ or RelA/p65 (Santa Cruz Biotechnology), respectively.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP was performed as described previously (1, 10) to investigate the role of PKCζ in recruiting transcription factor RelA/p65 and others coactivators on the promoters of pro-inflammatory genes in response to CS and LPS exposures. Lung tissue was homogenized in phosphate-buffered saline containing 1 mg/ml bovine serum albumin and protease inhibitor (Roche Applied Science) and cross-linked with 1% formaldehyde for 10 min, and then 0.5 ml of 2.5 m glycine was added. After a brief centrifugation, cell pellets were resuspended with SDS-lysis buffer (50 mm Tris-HCl, 1% SDS, 5 mm EDTA, 5 mm sodium butyrate, protease inhibitors). Sonication of the nuclear pellet containing chromatin was performed four times for 30 s and one time for 15 s at a maximum speed using Misonix-3000 Sonicator (Misonix Inc, Farmingdale, NY). Supernatants were collected and diluted (1:10 dilution) with buffer (1% Triton X-100, 2 mm EDTA, 150 mm NaCl, 20 mm Tris-HCl (pH 8.0), 5 mm sodium butyrate, and protease inhibitor) followed by preclearing the extract with 60 μl of protein A-agarose/salmon sperm DNA (catalog no. 16-157, Upstate Biotechnology, Inc.) for 3 h at 4 °C (1, 10, 36). Immunoprecipitation was mixed with 1 μg of specific antibodies at 4 °C overnight. After immunoprecipitation, 40 μl of protein A agarose/salmon sperm DNA was added and incubated for 2 h, followed by brief centrifugation. Precipitates were washed sequentially with Paro buffer I, Paro buffer II, and Paro buffer III for 5 min at 4 °C. Precipitates were again washed twice with Tris buffer for 5 min each. The antigen-antibody complexes were extracted two times with 50 μl of elution buffer (0.2 μg/μl proteinase K, 1% SDS, and 0.1 m NaHCO3). The eluted samples were incubated at 65 °C overnight to reverse formaldehyde cross-linking. The recovered DNA was purified with a QIAquick PCR purification kit (Qiagen, Valencia, CA) (10, 36). Samples of input DNA were also prepared in the same way as described above. PCR amplification was performed under the following conditions: 94 °C for 180 s; 30–38 cycles at 94 °C for 45 s, 60 °C for 60 s, and 72 °C for 60 s; and final elongation at 72 °C for 10 min. PCR for the input reaction was performed using 100 ng of genomic DNA. The following primers were used in PCR: mip-2, 5′-CAA CAG TGT ACT TAC GCA GAC G-3′ (sense) and 5′-CTA GCT GCC TGC CTC ATT CTA C-3′ (antisense); il-6, 5′-GAC ATG CTC AAG TGC TGA GTC AC-3′ (sense) and 5′-AGA TTG CAC AAT GTG ACG TCG-3′ (antisense). PCR products were analyzed on a 1.5–2.0% agarose gel.
Statistical Analysis
Statistical analysis of significance was calculated using one-way analysis of variance followed by Tukey's post hoc test for multigroup comparisons using STATVIEW. The results are shown as the mean ± S.E. The p < 0.05 is considered as statistically significant.
RESULTS
PKCζ Is Activated and Translocated into Nucleus in Lungs of WT Mice Exposed to CS and LPS
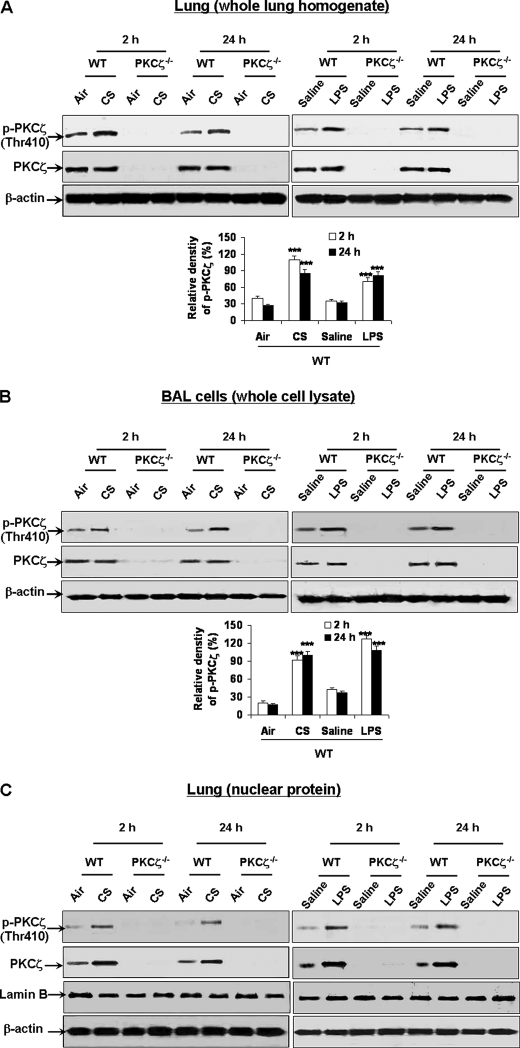
We determined whether PKCζ was activated in mouse lung exposed to CS and LPS. Immunoblot was performed to determine the phosphorylation of PKCζ on Thr410 in lung tissues and BAL cells by exposure to CS or LPS. We found that the levels of phosphorylated PKCζ (Thr410), which is shown to be required for activation of PKCζ (37), were significantly increased in lungs and BAL cells of WT mice exposed to CS and LPS (Fig. 1, A and B). As expected, there was no expression of phosphorylated and total PKCζ in lungs and BAL cells of PKCζ-deficient mice (Fig. 1, A and B). Nuclear translocation and activation of PKCζ are shown to regulate cell function in response to diverse stimuli (38–41). Therefore, it is possible that CS and LPS exposures can induce the translocation and phosphorylation of PKCζ in the nucleus leading to increased transcription of pro-inflammatory mediators in lungs. We determined the levels of phosphorylated (Thr410) and total PKCζ in lung nuclear protein by immunoblotting. Phosphorylated (Thr410) and total levels of PKCζ were significantly increased in lung nuclear proteins of WT mice exposed to CS and LPS (Fig. 1C). PKCζ and its phosphorylated form were not detected in lung nuclear protein of PKCζ−/− mice (Fig. 1C). Hence, PKCζ was activated and translocated into nucleus of mouse lung in response to both CS and LPS exposures.
FIGURE 1.
PKCζ was activated and translocated into nucleus in lungs of WT mice exposed to CS and LPS. WT and PKCζ-deficient mice were exposed to CS and LPS and were sacrificed at 2 and 24 h of post-last exposures. Protein levels of PKCζ and its phosphorylation on the Thr410 residue were analyzed by immunoblot in lung tissue (A) and BAL cell pellets (B) of whole cell lysates and in lung nuclear protein (C) of WT and PKCζ-deficient mice exposed to CS and LPS. PKCζ was activated and translocated into the nucleus in lungs and BAL cells of WT mice exposed to CS and LPS. There was no expression of PKCζ and its phosphorylated form in lungs and BAL cells of PKCζ-deficient mice. Blots are representative of three separate experiments. ***, p < 0.001, significant compared with respective air- or saline-exposed group.
Inflammatory Response in Lungs Is Decreased in PKCζ−/− Mice in Response to CS Exposure and LPS Aerosolization
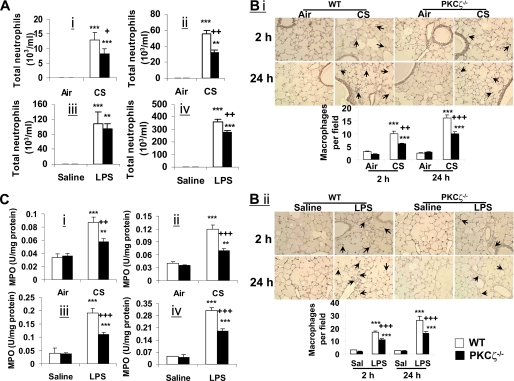
In light of activation of PKCζ by CS and LPS, and the pro-inflammatory effect of PKCζ (14–17), we hypothesized that CS- and LPS-induced lung inflammation is mediated by PKCζ activation. To test this hypothesis, PKCζ−/− and WT mice were exposed to CS, and influx of inflammatory cells into BAL fluid and lung interstitium was assessed by the stainings of Diff-Quik, hematoxylin and eosin, and immunohistochemistry (for macrophages). CS exposure resulted in a significant influx of neutrophils into BAL fluids of WT mice, which was significantly decreased in PKCζ−/− mice at both the 2- and 24-h time points (Fig. 2A, panels i and ii). However, the deficiency of PKCζ had no effect on the number of macrophages in BAL fluid of mice exposed to CS and LPS (data not shown). This may be due to the insensitivity of the influx of macrophages in BAL fluid to reflect lung inflammation under the present exposure protocol (3, 4). The staining of hematoxylin and eosin and immunohistochemistry showed that CS-mediated infiltration of inflammatory cells (e.g. macrophages and neutrophils) into the interstitium of lungs was also attenuated in PKCζ−/− mice exposed to CS as compared with WT mice (Fig. 2B, panel i, and supplemental Fig. S1A). Likewise, disruption of PKCζ decreased the influx of inflammatory cells into lungs in response to LPS aerosolization compared with that of WT mice (Fig. 2, A, panels iii and iv, and B, panel ii, and supplemental Fig. S1B). Furthermore, deficiency of PKCζ attenuated the activity of myeloperoxidase, a marker for neutrophilic inflammation (42), in mouse lungs in response to CS and LPS exposures (Fig. 2C). The levels of pro-inflammatory mediators, such as granulocyte-macrophage colony-stimulating factor, interferon-γ, IL-13, IL-17, IL-18, IL-1β, IL-6, KC, MCP-1, MIP-2, and tumor necrosis factor-α, were also significantly decreased in lungs of PKCζ-deficient mice compared with WT mice exposed to CS and LPS (supplemental Fig. S2, A–D). These results suggest that CS and LPS exposure-induced lung inflammation is dependent on PKCζ activation.
FIGURE 2.
Lung inflammation was decreased in PKCζ−/− mice in response to CS exposure and LPS aerosolization. Neutrophil influx in BAL fluid of mice was determined on cytospin-prepared slides stained with Diff-Quik (A). Immunohistochemical detection of macrophages was performed using rat anti-mouse Mac-3 antibody on mouse lung sections (B). Myeloperoxidase activity in lung tissue was determined spectrophotometrically (C). Number of neutrophils in BAL fluid (A) was decreased in PKCζ-deficient mice in response to CS exposure (panels i and ii) and LPS aerosolization (panels iii and iv) compared with respective WT mice at 2 h (panels i and iii) and 24 h (panels ii and iv) of post-last exposure. Deficiency of PKCζ decreased CS (panel i)- and LPS (panel ii)-induced macrophage influx into the lungs (B) at both the 2- and 24-h time points. PKCζ-deficient mice showed decreased myeloperoxidase activity in lung (C) at both 2-h (panels i and iii) and 24-h (panels ii and iv) time points as compared with WT mice exposed to CS (panels i and ii) and LPS (panels iii and iv). Data are shown as mean ± S.E. (n = 4 to 5 per group). **, p < 0.01; and ***, p < 0.001, significant compared with respective air- or saline-exposed groups; +, p < 0.05; ++, p < 0.01; and +++, p < 0.001, significant compared with respective CS- or LPS-exposed WT mice. Immunohistochemical pictures represent three separate experiments. Arrows indicate macrophages. Original magnification, ×200.
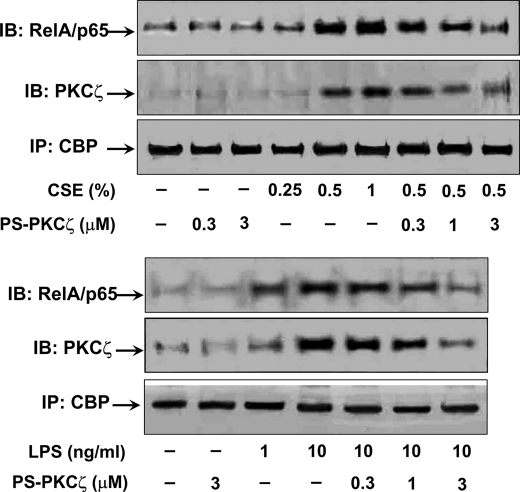
Inhibition of PKCζ-attenuated CSE-, Aldehyde-, and LPS-induced Pro-inflammatory Mediator Release and PKCζ Activation in Monocytes/Macrophages
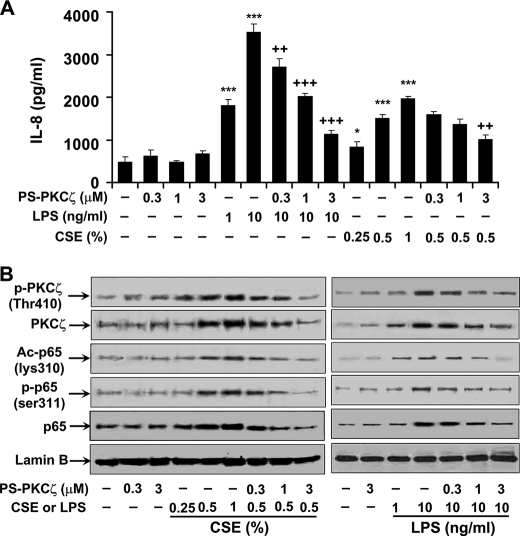
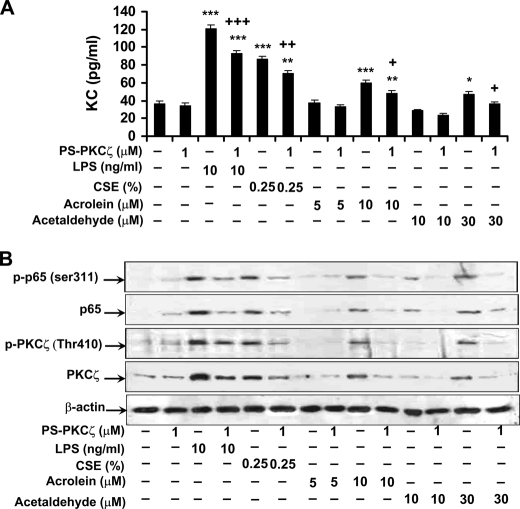
In the light of the above findings that disruption of PKCζ attenuated CS- and LPS-mediated lung inflammatory response, and both CS and LPS exposures activated PKCζ in BAL cells, which mainly consist of alveolar macrophages, we hypothesized that PKCζ in macrophages plays an important role in CS- and LPS-mediated lung inflammatory response. To test this hypothesis, MonoMac6 cells were pretreated with PS-PKCζ, a specific PKCζ inhibitor, for 2 h prior to incubation with CSE (0.25, 0.5, and 1%) or LPS (1 and 10 ng/ml) for 12 h. Pretreatment with PS-PKCζ (0.3-3 μm) significantly decreased CSE- and LPS-induced release of IL-8 from MonoMac6 cells (Fig. 3A). Furthermore, CSE- and LPS-mediated phosphorylation of PKCζ (Thr410) was attenuated in the nucleus of MonoMac6 cells, which were pretreated with PS-PKCζ (0.3–3 μm) (Fig. 3B). Interestingly, treatment with CSE, the reactive aldehydes, and LPS significantly induced KC release and PKCζ phosphorylation (Thr410) in mouse alveolar macrophages, which was decreased by the PKCζ inhibitor (Fig. 4, A and B). These results suggest that CS- and LPS-induced pro-inflammatory mediator release is mediated by activation of PKCζ, and aldehyde components are crucial for CS-induced PKCζ activation and inflammatory response in monocytes/macrophages.
FIGURE 3.
Inhibition of PKCζ-attenuated CSE- and LPS-induced IL-8 release and NF-κB activation in MonoMac6 cells. MonoMac6 cells were pretreated with PKCζ inhibitor, PS-PKCζ, for 2 h prior to CSE (0.25, 0.5, and 1%) or LPS (1 and 10 ng/ml) treatments for 12 h. Pretreatment with PS-PKCζ (0.3–3 μm) significantly decreased CSE- and LPS-induced release of IL-8 (A) and PKCζ activation (B) in MonoMac6 cells. CSE- and LPS-induced phosphorylated (Ser311), acetylated (Lys310), and total levels of RelA/p65 were also significantly reduced in the nucleus of MonoMac6 cells pretreated with PS-PKCζ (1 and 3 μm) (B). Data are shown as mean ± S.E. (n = 4–5 per group). Each lane represents results of three independent experiments. *, p < 0.05; ***, p < 0.001, significant compared with control group; ++, p < 0.01; +++, p < 0.001, significant as compared with respective CSE (0.5%)- or LPS (10 ng/ml)-treated group. p-PKCζ (Thr410), phosphorylated PKCζ on Thr410; Ac-p65 (lys310), acetylated RelA/p65 on Lys310; p-p65 (ser311), phosphorylated RelA/p65 on Ser311; p65, RelA/p65.
FIGURE 4.
PKCζ inhibitor pretreatment decreased KC release and RelA/p65 phosphorylation induced by CSE, LPS, acrolein, and acetaldehyde in primary mouse alveolar macrophages. Alveolar macrophages were isolated from C57BL/6J mice and were pretreated with PS-PKCζ for 2 h prior to CSE (0.25%), LPS (10 ng/ml), acrolein (5 and 10 μm), or acetaldehyde (10 and 30 μm) treatments for 12 h. Pretreatment with PS-PKCζ (1 μm) significantly decreased CSE-, LPS-, acrolein-, and acetaldehyde-induced KC release from mouse alveolar macrophages (A). Nuclear levels of phosphorylated RelA/p65 (Ser311) and PKCζ (Thr410) and total levels of RelA/p65 and PKCζ were decreased by PS-PKCζ (1 μm) pretreatment in primary mouse alveolar macrophages exposed to CSE, LPS, acrolein, and acetaldehyde (B). Data are shown as mean ± S.E. (n = 3–4 per group). Each lane represents results of three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001; significant compared with control groups; +, p < 0.01; ++, p < 0.01; +++, p < 0.001, significant as compared with respective CSE-, LPS-, acrolein (10 μm)- and acetaldehyde (30 μm)-treated groups. p-PKCζ (Thr410), phosphorylated PKCζ on Thr410; p-p65 (ser311), phosphorylated RelA/p65 on Ser311; p65, RelA/p65.
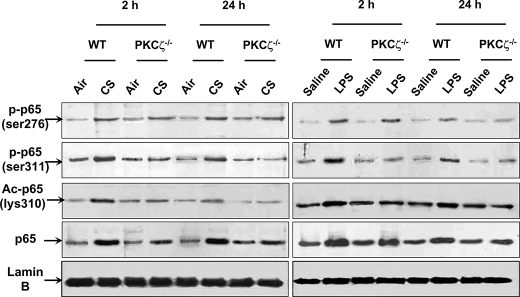
Phosphorylated, Acetylated, and Total Levels of RelA/p65 Are Decreased in Lung Nuclear Proteins of PKCζ−/− Mice Exposed to CS and LPS
NF-κB is shown to be regulated by PKCζ through the phosphorylation of RelA/p65 in embryo fibroblasts treated with tumor necrosis factor-α (19). However, it is not known whether PKCζ modulates NF-κB activation in lungs in response to CS exposure or LPS aerosolization. Hence, immunoblotting was performed to determine the phosphorylation and acetylation of RelA/p65 in lung nuclear proteins of WT and PKCζ−/− mice exposed to CS and LPS. Both CS and LPS exposures led to increased levels of phosphorylated (Ser311) and acetylated (Lys310) RelA/p65 in WT mice, which was attenuated in PKCζ−/− mice (Fig. 5). However, the phosphorylated levels of RelA/p65 at Ser276 were not altered in lungs of PKCζ−/− mice as compared with WT mice exposed to CS and LPS (Fig. 5). Interestingly, the total level of RelA/p65 was also decreased in lung nuclear proteins of PKCζ−/− mice compared with WT mice exposed to CS and LPS (Fig. 5). These results suggest that PKCζ activates NF-κB by translocating RelA/p65 into the nucleus as well as inducing phosphorylation and subsequent acetylation of RelA/p65 in response to CS and LPS exposures.
FIGURE 5.
Phosphorylated, acetylated, and total levels of RelA/p65 were decreased in lung nuclear proteins of PKCζ−/− mice in response to CS and LPS exposure. Levels of phosphorylated (Ser276 and Ser311 residues), acetylated (Lys310), and total RelA/p65 in lung nuclear proteins were analyzed by immunoblotting. Lamin B was used as a nuclear marker, and no bands of α-tubulin were observed in nuclear fractions. Levels of phosphorylated RelA/p65 on Ser311 was decreased, whereas no alteration in phosphorylated RelA/p65 on Ser276 was observed in lungs of PKCζ-deficient mice as compared with WT mice exposed to CS and LPS at 2 and 24 h of post-last exposures. Knock-out of PKCζ decreased the levels of acetylated (Lys310) and total RelA/p65 in lung nuclear protein of mice exposed to CS and LPS. Each lane represents results of three independent experiments. p-p65 (ser276), phosphorylated RelA/p65 on Ser276; p-p65 (ser311), phosphorylated RelA/p65 on Ser311; Ac-p65 (lys310), acetylated RelA/p65 at Lys310; p65, RelA/p65.
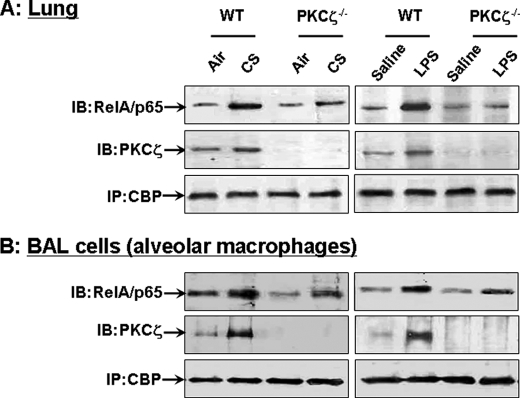
Increased Interaction of CBP with PKCζ in Response to CS and LPS Exposures in WT Mice and Disruption of PKCζ Decrease CS- and LPS-mediated Interaction of CBP with RelA/p65 in Lung Tissues and BAL Cells
Given the decreased phosphorylation of RelA/p65 (Ser311) in PKCζ−/− mice in response to CS and LPS, and enhanced binding of RelA/p65 with CBP upon RelA/p65 phosphorylation (20, 21), we proposed that PKCζ induces the interaction of RelA/p65 with CBP in response to CS and LPS exposures. Immunoprecipitation was performed to determine the interaction of CBP with RelA/p65 in lung tissues and BAL cells of WT and PKCζ−/− mice by CS and LPS. Both CS and LPS exposures increased the interaction of CBP with RelA/p65 in lung tissues and BAL cells of WT mice that was ameliorated in PKCζ−/− mice (Fig. 6, A and B). It is interesting to note that the interaction of CBP with PKCζ was also increased in lungs and BAL cells of WT mice in response to CS and LPS exposures (Fig. 6, A and B). These results suggest that PKCζ recruits the essential components of transcriptional machinery, such as CBP, and thereby controls the activation of NF-κB in lungs in response to CS and LPS exposures.
FIGURE 6.
Increased interaction of CBP with PKCζ in lungs and BAL cells of CS- and LPS-exposed WT mice, and disruption of PKCζ decreased the interaction of CBP with RelA/p65 in lungs and BAL cells of mice exposed to CS and LPS. The interaction of CBP with PKCζ and RelA/p65 in lungs (A) and BAL cells (B) was analyzed using immunoprecipitation (IP) assay. Both CS and LPS exposures increased the interaction of CBP with PKCζ in lungs and BAL cells of WT mice after 2 h post-last exposure. Disruption of PKCζ decreased the interaction of CBP with RelA/p65 induced by CS and LPS exposures in lungs and BAL cells at 2 h post-last exposure. Gel pictures shown are representative of at least three separate experiments. IB, immunoblot.
Pretreatment with PS-PKCζ Attenuates the Phosphorylation (Ser311) and Acetylation (Lys310) of RelA/p65 and the Interaction of CBP with PKCζ and RelA/p65 by CSE and LPS in Monocytes/Macrophages
In light of the increased interaction of CBP with PKCζ and RelA/p65 in mouse lungs and BAL cells in response to CS and LPS exposures, we determined whether inhibition of PKCζ attenuated these interactions in MonoMac6 cells in response to CSE and LPS treatments. As shown in Fig. 7, CSE- and LPS-induced interaction of CBP with PKCζ and RelA/p65 was significantly decreased in response to pretreatment with PS-PKCζ in MonoMac6 cells. Furthermore, PS-PKCζ (1 and 3 μm) significantly reduced CSE- and LPS-mediated the phosphorylation (Ser311) and acetylation (Lys310) of RelA/p65 in nuclear proteins of MonoMac6 cells (Fig. 3B). Similarly, the phosphorylation of RelA/p65 (Ser311) in primary mouse alveolar macrophages was induced by CSE/aldehydes and LPS, which was significantly decreased by PS-PKCζ (1 μm) (Fig. 4B). These data indicate that PKCζ functions as a master regulator and facilitates the assembly of RelA/p65 with coactivator CBP in transcriptional machinery, thereby increasing NF-κB-dependent inflammation in monocytes/macrophages by CS and LPS exposures.
FIGURE 7.
CSE and LPS induced the interaction of CBP with PKCζ and RelA/p65, which was decreased in MonoMac6 cells pretreated with PS-PKCζ. CSE- and LPS-induced interaction of CBP with PKCζ and RelA/p65 in MonoMac6 cells in the absence or presence of PS-PKCζ was assessed using immunoprecipitation (IP) assay. The interaction of CBP with PKCζ and RelA/p65 was increased in response to CSE (0.5 and 1%) and LPS (1 and 10 ng/ml) treatments for 12 h in MonoMac6 cells. Pretreatment of PS-PKCζ (1 and 3 μm for 2 h) attenuated CSE- and LPS-mediated interaction of CBP with PKCζ and RelA/p65 in MonoMac6 cells. Gel pictures shown are representative of at least three separate experiments. IB, immunoblot.
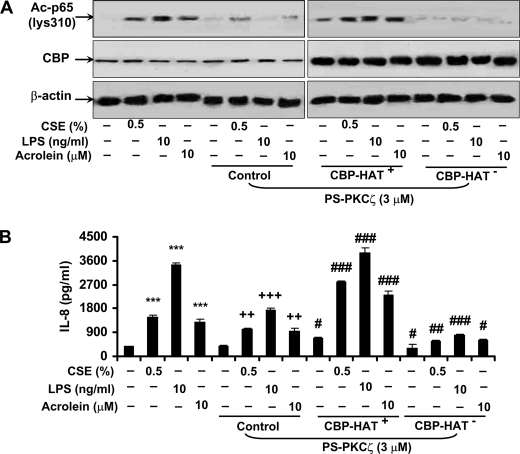
CBP Is Required for PKCζ-mediated RelA/p65 Acetylation and Pro-inflammatory Cytokine Release in MonoMac6 Cells in Response to CSE, Acrolein, and LPS Treatments
To further study whether CBP is required for the regulation of NF-κB activation and pro-inflammatory cytokine release by PKCζ in response to CSE, acrolein, and LPS, MonoMac6 cells were transfected with wild-type CBP mutant or CBP mutated with Leu1690/Cys1691, which lacked its HAT activity (20, 32). Pretreatment with PKCζ inhibitor attenuated the acetylation of RelA/p65 and IL-8 release induced by CSE, acrolein, and LPS in MonoMac6 cells (Fig. 3, A and B, and Fig. 8). This response to PKCζ inhibitor pretreatment was significantly ameliorated when MonoMac6 cells were transfected with WT CBP mutant and treated with CSE, acrolein, and LPS (Fig. 8, A and B). However, transfection of cells with CBP mutant without HAT activity further augmented the response to PKCζ inhibitor pretreatment in MonoMac6 cells by CSE, acrolein, and LPS exposures (Fig. 8, A and B). These results suggest that HAT activity of CBP is required for PKCζ-mediated NF-κB activation and inflammatory response.
FIGURE 8.
CBP was required for PKCζ-mediated RelA/p65 acetylation and IL-8 release in MonoMac6 cells in response to CSE, acrolein, and LPS treatments. MonoMac6 cells were transfected with WT CBP or CBP-HAT− mutants for 24 h before PKCζ inhibitor pretreatment and subsequent CSE, acrolein, and LPS exposures. HAT activity of CBP was required for PKCζ-mediated RelA/p65 acetylation (A) and IL-8 release (B) induced by CSE, acrolein, and LPS in MonoMac6 cells. Gel pictures shown are representative of at least three separate experiments. Data are shown as mean ± S.E. (n = 3–4 per group). ***, p < 0.001, significant compared with control groups; ++, p < 0.01; +++, p < 0.001, significant as compared with respective CSE-, LPS-, and acrolein-treated groups; #, p < 0.05; ##, p < 0.01; ###, p < 0.001, significant as compared with respective PS-PKCζ treated but without transfected groups.
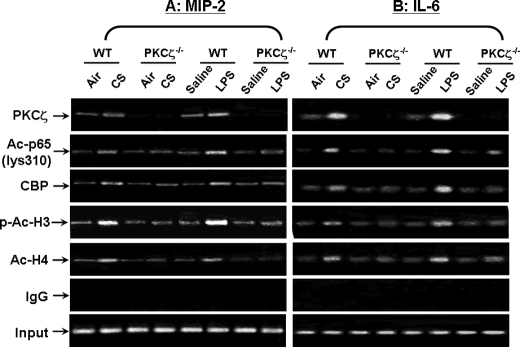
PKCζ Recruits CBP and RelA/p65 on Pro-inflammatory Gene Promoters in Mouse Lungs in Response to CS and LPS Exposures
It is known that both CS and LPS cause chromatin remodeling on the promoters of pro-inflammatory genes (1, 43, 44). Based on this, we hypothesized that CS and LPS induce PKCζ-mediated chromatin modifications, thereby promoting the binding of CBP and RelA/p65 on pro-inflammatory gene promoters in mouse lungs. ChIP assay was performed in lung homogenates using antibodies against PKCζ, acetylated RelA/p65 (Lys310), CBP, phosphorylated/acetylated (Ser10/Lys9) histone H3, and acetylated histone H4 so as to determine the chromatin remodeling on the promoters of mip-2 and il-6. We found that PKCζ was recruited on the promoters of mip-2 and il-6 in lungs of WT mice exposed to CS and LPS (Fig. 9, A and B). Interestingly, knock-out of PKCζ attenuated the recruitment of acetylated RelA/p65 (Lys310), CBP, phosphorylated/acetylated (Ser10/Lys9) histone H3, and acetylated histone H4 on the promoters of mip-2 and il-6 in lungs (Fig. 9, A and B). These results indicate that PKCζ increases the pro-inflammatory gene transcription in lungs by recruitment of RelA/p65 and transcriptional coactivator CBP, and thus increases histone H3 and H4 acetylation on the promoters of pro-inflammatory genes in response to CS and LPS exposure.
FIGURE 9.
PKCζ recruited CBP and RelA/p65 on pro-inflammatory gene promoters in mouse lungs in response to CS and LPS exposures. The recruitment of PKCζ, acetylated RelA/p65 (Lys310), CBP, phosphorylated/acetylated histone H3 (Ser10/Lys9), and acetylated histone H4 on the promoters mip-2 and il-6 in mouse lung was analyzed by ChIP assay. Lung homogenates were cross-linked and immunoprecipitated with relevant antibodies against the aforementioned proteins, and then chromatin modification on the promoter regions of mip-2 (A) and il-6 (B) was detected by PCR. Both CS and LPS exposures caused the recruitment of acetylated RelA/p65 (Lys310), CBP, phosphorylated/acetylated histone H3 (Ser10/Lys9), and acetylated histone H4 on the promoters mip-2 and il-6 in mouse lung, which was attenuated in PKCζ-deficient mice at 2 h of post-last exposures. Furthermore, PKCζ was also recruited on the promoters of mip-2 and il-6 in lungs of WT mice, but not in PKCζ deficient mice exposed to CS and LPS. IgG was used as a negative control. Gel pictures shown are representative of at least three separate experiments. Ac-p65 (lys310), acetylated RelA/p65 on Lys310; p-Ac-H3, phosphorylated/acetylated histone H3 at Ser10/Lys9; Ac-H4, acetylated histone H4.
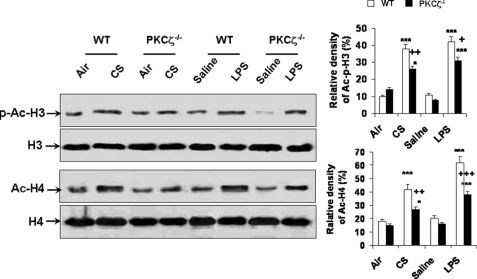
Disruption of PKCζ Attenuates the Phosphorylation/Acetylation of Histone H3 and Acetylation of Histone H4 in Mouse Lung Exposed to CS and LPS
To determine the effect of PKCζ on histone acetylation, immunoblotting was performed in acid-extracted histone proteins of WT and PKCζ−/− mice in response to CS and LPS exposures. Both CS and LPS exposures increased the phosphorylation on Ser10 and acetylation on Lys9 of histone H3 in lungs of WT mice, which was decreased in PKCζ−/− mice (Fig. 10). Similarly, deficiency of PKCζ also attenuated the acetylation of histone H4 in lungs in response to CS and LPS exposures (Fig. 10). These data suggest that both CS and LPS exposures-mediated histone modifications, such as phosphorylation and acetylation, are dependent on PKCζ activation.
FIGURE 10.
Disruption of PKCζ decreased the phosphorylation/acetylation of histone H3 and acetylation of histone H4 in mouse lung exposed to CS and LPS. Acid-extracted histone proteins in lungs of WT and PKCζ-deficient mice were used for immunoblotting against anti-phosphorylated/acetylated (Ser10/Lys9) histone H3 and acetylated histone H4. Levels of phosphorylated/acetylated (Ser10/Lys9) histone H3 and acetylated histone H4 were decreased in lungs of PKCζ−/− mice as compared with WT mice exposed to CS and LPS at 2 h of post-last exposure. Gel pictures shown are representative of at least three separate experiments. *, p < 0.05; ***, p < 0.001, significant compared with respective air- or saline-exposed groups; +, p < 0.05; ++, p < 0.01; +++, p < 0.001, significant compared with respective CS- and LPS-exposed WT mice. p-Ac-H3, phosphorylated/acetylated histone H3 on Ser10/Lys9; H3, histone H3; Ac-H4, acetylated histone H4; H4, histone H4.
DISCUSSION
Cigarette smoke-induced oxidative stress, reactive aldehydes, and endotoxin present in tobacco smoke are important risk factors in the development of COPD/emphysema by increasing the release of pro-inflammatory mediators and recruiting inflammatory cells into the lungs (23–25, 45–51). Previous studies have shown that PKCζ is activated in lungs of patients with COPD, and PKCζ participates in inflammatory response through NF-κB activation (15, 16, 18, 19, 22). However, the role of endogenous PKCζ in lung inflammation via chromatin modifications on pro-inflammatory genes by CS exposure or LPS aerosolization or any other environmental agents is not known. We hypothesized that down-regulation of PKCζ would dampen lung inflammatory response to CS, its reactive aldehydes, and LPS exposure by attenuating chromatin modifications on pro-inflammatory gene promoters. Consistent with its pro-inflammatory effect of endogenous PKCζ seen in vitro and in vivo (14–17), the inflammatory response, such as inflammatory cell influx and pro-inflammatory mediator release, in lungs was attenuated in PKCζ−/− mice as compared with WT mice exposed to CS and LPS exposures suggesting that CS- and LPS-mediated lung inflammatory response is dependent on PKCζ activation. This is confirmed by our results showing that the activation of PKCζ, which is reflected by increased phosphorylation of PKCζ on Thr410, in WT mouse lung exposed to CS and LPS, and in monocytes/macrophages treated with CSE, LPS, and reactive aldehydes (i.e. acrolein and acetaldehyde). Similarly, the activation of PKCζ is also observed in lungs of patients with COPD, and in human adenocarcinoma A549 cells treated with nicotine (22, 52). The mechanism underlying the activation of PKCζ may be associated with activation of phosphoinositide 3-kinase and increased ceramide, which can be induced by CS and LPS (53–57).
Post-translational modifications of NF-κB, such as phosphorylation and acetylation, play an important role in regulation of its transcriptional activity (20, 21). It has been shown that PKCζ activates NF-κB by phosphorylating RelA/p65 at Ser311 (19). Hence, we determined whether reduced inflammatory response in PKCζ−/− mice is associated with decreased NF-κB activation in lungs in response to CS and LPS exposures. As expected, the levels of phosphorylated RelA/p65 on Ser311 were decreased in lungs of PKCζ-deficient mice as compared with WT mice exposed to CS and LPS. However, the phosphorylated levels of RelA/p65 on serine 276 were not altered in lungs of PKCζ-deficient mice compared with WT mice in response to CS and LPS. This is consistent with the previous observation where serine 311, but not serine 276, accounts for the phosphorylation of RelA/p65 by PKCζ, although the mechanism underlying these discrepancies is not clear (19). Furthermore, the levels of total RelA/p65 were also decreased in lung nuclear proteins of PKCζ-deficient mice as compared with WT mice exposed to CS and LPS implicating the involvement of PKCζ in activation/translocation of RelA/p65 into the nucleus. The mechanism might be associated with activation of IκB kinase β by PKCζ because the levels of phosphorylated IκB kinase β were decreased in PKCζ-deficient mice in response to CS and LPS exposures.3 This contention is in agreement with the previous studies showing that PKCζ was able to phosphorylate and activate IκB kinase β leading to NF-κB activation by inducing IκB degradation (17, 58–60). Nevertheless, these results suggest that PKCζ regulates CS- and LPS-mediated NF-κB activation by inducing the phosphorylation (Ser311) and translocation of RelA/p65, leading to increased transcription of pro-inflammatory genes in lungs, although certain pro-inflammatory cytokines (e.g. granulocyte-macrophage colony-stimulating factor and interferon-γ) determined in this study can also be transcriptionally regulated by other transcription factors, such as SP-1, AP-1, and nuclear factor of activated T-cells in addition to NF-κB (61–64).
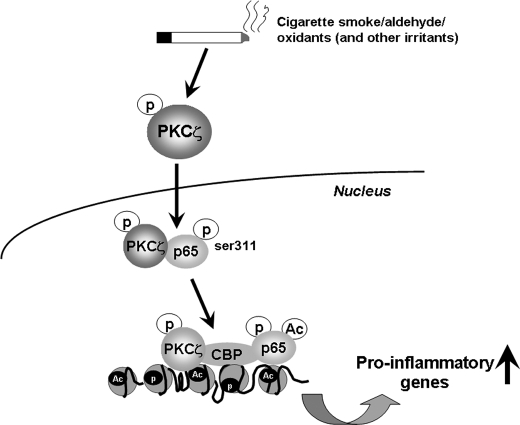
Phosphorylation of RelA/p65 (Ser311) is shown to enhance its interaction with CBP resulting in its acetylation, which is required for full activation of NF-κB (19, 65). Previous studies have shown that LPS increased the interaction of CBP with RelA/p65, and increased acetylation of RelA/p65 occurs in lungs in response to CS (3, 66). Most importantly, we show that the phosphorylation of RelA/p65 on Ser311 was regulated in lungs by PKCζ. In the light of these observations, we hypothesized that acetylation of RelA/p65 and its association with CBP was altered in lungs of PKCζ-deficient mice in response to CS and LPS exposures. As expected, disruption/inhibition of PKCζ attenuated the CS- and LPS-induced interaction of CBP with RelA/p65 and acetylation of RelA/p65 on Lys310 in lungs and MonoMac6 cells. This observation corroborates our previous study showing that the interaction of RelA/p65 with CBP is decreased in PKCζ-deficient embryonic fibroblasts treated with tumor necrosis factor-α (19). It is interesting to note that both CS and LPS exposures also increased the association of CBP with PKCζ in WT mouse lungs and MonoMac6 cells suggesting that PKCζ does translocate into the nucleus and forms a complex with CBP and RelA/p65. This is confirmed by our results showing the increased levels of phosphorylated and total PKCζ by CS and LPS in lung nuclear proteins of WT mice. Furthermore, HAT activity of CBP is required for PKCζ-mediated RelA/p65 acetylation and inflammatory response to CS, aldehyde, and LPS exposures. Therefore, we propose that PKCζ is a master regulator and facilitates the assembly of RelA/p65 with coactivator CBP in transcriptional machinery, thereby increasing acetylation of RelA/p65, subsequently enhancing the transcription of pro-inflammatory genes in lungs in response to CS and LPS exposures (Fig. 11).
FIGURE 11.
Summarized schematic model of the regulation of PKCζ in CS/aldehyde- and LPS-induced lung inflammatory response and chromatin remodeling. PKCζ was activated and translocated into the nucleus in response to CS and LPS exposures. Activated PKCζ interacted and phosphorylated RelA/p65 on Ser311, leading to increased NF-κB transactivation. Furthermore, PKCζ functions as a master regulator and facilitates the assembly of RelA/p65 with coactivator CBP in transcriptional machinery, thereby increasing acetylation of RelA/p65 and histones on the promoters of pro-inflammatory genes in response to CS and LPS exposures in lungs.
CS and LPS can induce phosphorylation or acetylation of histones H3 and H4, and it is known that chromatin modification plays an important role in CS- and LPS-mediated transcription of pro-inflammatory genes (1, 43, 44, 66–68). Furthermore, it has been recently recognized that alterations in chromatin remodeling on pro-inflammatory gene promoters are the key mechanisms for underlying abnormal inflammation seen in lungs of patients with COPD. Interestingly, PKCζ is shown to regulate IL-6 expression by altering the acetylation of histone H4 on its promoter (69). Our data showed that the acetylation of RelA/p65 (Lys310), histone H3 (Lys9), and histone H4 on promoters of il-6 and mip-2 genes was decreased in lungs of PKCζ-deficient mice as compared with WT mice in response to CS and LPS exposures. This is associated with decreased recruitment of CBP, which is known to acetylate histone or non-histone proteins, on the promoters of pro-inflammatory genes in PKCζ−/− mice. Furthermore, the phosphorylation/acetylation of histone H3 (Ser10/Lys9) and acetylation of histone H4 were decreased in lungs of PKCζ-deficient mice exposed to CS and LPS. These results suggest that once PKCζ is activated, it forms a complex with CBP and RelA/p65 on the promoters of pro-inflammatory genes leading to acetylation of RelA/p65 and histones H3 and H4. Indeed, both CS and LPS exposures were able to recruit PKCζ on the promoters of il-6 and mip-2 genes in lungs of WT mice as detected by ChIP assay. Therefore, acetylated RelA/p65 interacts with PKCζ and CBP forming a coactivator complex on the promoters of pro-inflammatory genes, thereby leading to sustained transcription of pro-inflammatory mediators in lungs.
Inflammatory cells, such as macrophages, are the main orchestrators and amplifiers in chronic inflammatory and injurious responses in lungs of smokers and patients with COPD (70–73). Previous studies have shown that PKCζ is essential for activation and function of macrophages and neutrophils (16, 56, 74–76). In this study, we showed the enhanced activation of PKCζ in BAL cells, which is mainly composed of macrophages (neutrophils will migrate into BAL fluids in the context of lung inflammation), in WT mice exposed to CS and LPS. Importantly, the phosphorylation of PKCζ was also significantly increased by CSE and LPS treatments in MonoMac6 cells and primary mouse alveolar macrophages. Furthermore, inhibition of PKCζ by PS-PKCζ reduced CSE- and LPS-mediated release of pro-inflammatory cytokines from both MonoMac6 cells and mouse alveolar macrophages suggesting that PKCζ in macrophages plays an important role in CS- and LPS-induced lung inflammation. Interestingly, both acrolein and acetaldehyde, the reactive components of CS, significantly induced pro-inflammatory mediator release in primary mouse alveolar macrophages and MonoMac6 cells. This observation is corroborated by a previous report that shows that acrolein treatment increases IL-8 release in human alveolar macrophages (45). Importantly, these aldehyde-induced inflammatory responses were significantly decreased in the presence of the PKCζ inhibitor. Hence, it may be surmised that the reactive aldehydes play a critical role in CS-mediated sustained macrophage activation and lung inflammation through PKCζ signaling pathway. It is tempting to mention here that 80–85% aldehyde/acrolein is retained in the respiratory tract of animals exposed to CS at a dose of 400–600 mg/m3 total particulate matter according to the 1992 World Health Organization/International Programme on Chemical Safety report. One cigarette yields ∼500 μg of acrolein and ∼1000 μg of acetaldehyde depending on cigarette type and determination methods (31, 33–35). Hence, CS at a dose of ∼300 mg/m3 total particulate matter (corresponding to human consumption of 1–1.5 packs per day (28)) used in our study certainly relates to human exposure. Moreover, we used CSE (0.25–1%) to treat cells, which is equivalent to ∼10–40 μm aldehydes present in CS (31) and compares the in vivo exposure conditions and the elicited pro-inflammatory response. However, CSE differs from the gaseous phase of smoke, which makes it difficult to determine and compare which dose and exposure time are best to mimic the effects of CS in the lung. Moreover, individual components are unlikely to produce the same biological effects akin to whole CS to which humans are exposed. Nevertheless, our data obtained in lung in vivo and monocytes/macrophages in vitro suggest the importance of PKCζ in cigarette smoke/aldehydes-induced lung inflammation via its effects on pro-inflammatory gene promoters by chromatin modifications.
In conclusion, we have demonstrated a novel role of PKCζ in CS/aldehyde- and LPS-mediated inflammatory response and chromatin modifications in the lungs. Both CS and LPS exposures lead to activation and translocation of PKCζ into the nucleus where it forms a complex with CBP and RelA/p65 on the promoters of pro-inflammatory genes thereby resulting in sustained transcription of pro-inflammatory mediators in lungs, which is the hallmark of abnormal inflammation seen in lungs of patients with COPD (Fig. 11). These findings not only unravel the importance of PKCζ-mediated histone modifications in CS/aldehyde- and LPS-induced abnormal lung inflammation but also provide new insights into the molecular mechanisms underlying the pathogenesis of chronic lung diseases.
Acknowledgments
We thank Drs. Isaac K. Sundar, Sangwoon Chung, Gnanapragasam Arunachalam, Saravanan Rajendrasozhan, and Suzanne E. Cook for their technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-HL085613 and R01-HL097751 and Grant P30-ES01247 from the NIEHS Environmental Health Science Center.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
H. Yao and I. Rahman, unpublished observations.
- CS
- cigarette smoke
- BAL
- bronchoalveolar lavage
- CBP
- CREB-binding protein
- CREB
- cAMP-response element-binding protein
- ChIP
- chromatin immunoprecipitation
- COPD
- chronic obstructive pulmonary disease
- CSE
- cigarette smoke extract
- HAT
- histone acetyltransferase
- LPS
- lipopolysaccharide
- NF-κB
- nuclear factor-κB
- PKCζ
- protein kinase C ζ
- PS-PKCζ
- a myristoylated PKCζ pseudosubstrate peptide inhibitor
- WT
- wild type
- IL
- interleukin
- KC
- keratinocyte-derived chemokine.
REFERENCES
- 1.Yang S. R., Valvo S., Yao H., Kode A., Rajendrasozhan S., Edirisinghe I., Caito S., Adenuga D., Henry R., Fromm G., Maggirwar S., Li J. D., Bulger M., Rahman I. (2008) Am. J. Respir. Cell Mol. Biol. 38, 689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang S. R., Wright J., Bauter M., Seweryniak K., Kode A., Rahman I. (2007) Am. J. Physiol. Lung Cell Mol. Physiol. 292, L567–L576 [DOI] [PubMed] [Google Scholar]
- 3.Yao H., Edirisinghe I., Rajendrasozhan S., Yang S. R., Caito S., Adenuga D., Rahman I. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 294, L1174–L1186 [DOI] [PubMed] [Google Scholar]
- 4.Yao H., Yang S. R., Edirisinghe I., Rajendrasozhan S., Caito S., Adenuga D., O'Reilly M. A., Rahman I. (2008) Am. J. Respir. Cell Mol. Biol. 39, 7–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogg J. C., Chu F., Utokaparch S., Woods R., Elliott W. M., Buzatu L., Cherniack R. M., Rogers R. M., Sciurba F. C., Coxson H. O., Paré P. D. (2004) N. Engl. J. Med. 350, 2645–2653 [DOI] [PubMed] [Google Scholar]
- 6.van der Strate B. W., Postma D. S., Brandsma C. A., Melgert B. N., Luinge M. A., Geerlings M., Hylkema M. N., van den Berg A., Timens W., Kerstjens H. A. (2006) Am. J. Respir. Crit. Care Med. 173, 751–758 [DOI] [PubMed] [Google Scholar]
- 7.Ito K., Charron C. E., Adcock I. M. (2007) Pharmacol. Ther. 116, 249–265 [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto Y., Verma U. N., Prajapati S., Kwak Y. T., Gaynor R. B. (2003) Nature 423, 655–659 [DOI] [PubMed] [Google Scholar]
- 9.Marwick J. A., Kirkham P. A., Stevenson C. S., Danahay H., Giddings J., Butler K., Donaldson K., Macnee W., Rahman I. (2004) Am. J. Respir. Cell Mol. Biol. 31, 633–642 [DOI] [PubMed] [Google Scholar]
- 10.Yang S. R., Yao H., Rajendrasozhan S., Chung S., Edirisinghe I., Valvo S., Fromm G., McCabe M. J., Jr., Sime P. J., Phipps R. P., Li J. D., Bulger M., Rahman I. (2009) Am. J. Respir. Cell Mol. Biol. 40, 147–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito K., Ito M., Elliott W. M., Cosio B., Caramori G., Kon O. M., Barczyk A., Hayashi S., Adcock I. M., Hogg J. C., Barnes P. J. (2005) N. Engl. J. Med. 352, 1967–1976 [DOI] [PubMed] [Google Scholar]
- 12.Szulakowski P., Crowther A. J., Jiménez L. A., Donaldson K., Mayer R., Leonard T. B., MacNee W., Drost E. M. (2006) Am. J. Respir. Crit. Care Med. 174, 41–50 [DOI] [PubMed] [Google Scholar]
- 13.LaVallie E. R., Chockalingam P. S., Collins-Racie L. A., Freeman B. A., Keohan C. C., Leitges M., Dorner A. J., Morris E. A., Majumdar M. K., Arai M. (2006) J. Biol. Chem. 281, 24124–24137 [DOI] [PubMed] [Google Scholar]
- 14.de Kozak Y., Omri B., Smith J. R., Naud M. C., Thillaye-Goldenberg B., Crisanti P. (2007) Am. J. Pathol. 170, 1241–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin P., Villares R., Rodriguez-Mascarenhas S., Zaballos A., Leitges M., Kovac J., Sizing I., Rennert P., Márquez G., Martínez-A C., Diaz-Meco M. T., Moscat J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 9866–9871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuschieri J., Umanskiy K., Solomkin J. (2004) J. Surg. Res. 121, 76–83 [DOI] [PubMed] [Google Scholar]
- 17.Savkovic S. D., Koutsouris A., Hecht G. (2003) Am. J. Physiol. Cell Physiol. 285, C512–C521 [DOI] [PubMed] [Google Scholar]
- 18.Leitges M., Sanz L., Martin P., Duran A., Braun U., García J. F., Camacho F., Diaz-Meco M. T., Rennert P. D., Moscat J. (2001) Mol. Cell 8, 771–780 [DOI] [PubMed] [Google Scholar]
- 19.Duran A., Diaz-Meco M. T., Moscat J. (2003) EMBO J. 22, 3910–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong H., May M. J., Jimi E., Ghosh S. (2002) Mol. Cell 9, 625–636 [DOI] [PubMed] [Google Scholar]
- 21.Chen L. F., Greene W. C. (2004) Nat. Rev. Mol. Cell Biol. 5, 392–401 [DOI] [PubMed] [Google Scholar]
- 22.Park J. W., Kim H. P., Lee S. J., Wang X., Wang Y., Ifedigbo E., Watkins S. C., Ohba M., Ryter S. W., Vyas Y. M., Choi A. M. (2008) J. Immunol. 180, 4668–4678 [DOI] [PubMed] [Google Scholar]
- 23.van Houwelingen A. H., Weathington N. M., Verweij V., Blalock J. E., Nijkamp F. P., Folkerts G. (2008) FASEB J. 22, 3403–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brass D. M., Hollingsworth J. W., Cinque M., Li Z., Potts E., Toloza E., Foster W. M., Schwartz D. A. (2008) Am. J. Respir. Cell Mol. Biol. 39, 584–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolk J., Rudolphus A., Davies P., Osinga D., Dijkman J. H., Agarwal L., Keenan K. P., Fletcher D., Kramps J. A. (1992) J. Pathol. 167, 349–356 [DOI] [PubMed] [Google Scholar]
- 26.Thatcher T. H., Maggirwar S. B., Baglole C. J., Lakatos H. F., Gasiewicz T. A., Phipps R. P., Sime P. J. (2007) Am. J. Pathol. 170, 855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thatcher T. H., McHugh N. A., Egan R. W., Chapman R. W., Hey J. A., Turner C. K., Redonnet M. R., Seweryniak K. E., Sime P. J., Phipps R. P. (2005) Am. J. Physiol. Lung Cell Mol. Physiol. 289, L322–L328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauderly J. L., Gigliotti A. P., Barr E. B., Bechtold W. E., Belinsky S. A., Hahn F. F., Hobbs C. A., March T. H., Seilkop S. K., Finch G. L. (2004) Toxicol. Sci. 81, 280–292 [DOI] [PubMed] [Google Scholar]
- 29.Foronjy R. F., Mirochnitchenko O., Propokenko O., Lemaitre V., Jia Y., Inouye M., Okada Y., D'Armiento J. M. (2006) Am. J. Respir. Crit. Care Med. 173, 623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao H., Edirisinghe I., Yang S. R., Rajendrasozhan S., Kode A., Caito S., Adenuga D., Rahman I. (2008) Am. J. Pathol. 172, 1222–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambert C., McCue J., Portas M., Ouyang Y., Li J., Rosano T. G., Lazis A., Freed B. M. (2005) J. Allergy Clin. Immunol. 116, 916–922 [DOI] [PubMed] [Google Scholar]
- 32.Korzus E., Torchia J., Rose D. W., Xu L., Kurokawa R., McInerney E. M., Mullen T. M., Glass C. K., Rosenfeld M. G. (1998) Science 279, 703–707 [DOI] [PubMed] [Google Scholar]
- 33.Carnevali S., Nakamura Y., Mio T., Liu X., Takigawa K., Romberger D. J., Spurzem J. R., Rennard S. I. (1998) Am. J. Physiol. 274, L591–L598 [DOI] [PubMed] [Google Scholar]
- 34.Fujioka K., Shibamoto T. (2006) Environ. Toxicol. 21, 47–54 [DOI] [PubMed] [Google Scholar]
- 35.Smith C. J., Hansch C. (2000) Food Chem. Toxicol. 38, 637–646 [DOI] [PubMed] [Google Scholar]
- 36.Bulger M., Schübeler D., Bender M. A., Hamilton J., Farrell C. M., Hardison R. C., Groudine M. (2003) Mol. Cell. Biol. 23, 5234–5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou M. M., Hou W., Johnson J., Graham L. K., Lee M. H., Chen C. S., Newton A. C., Schaffhausen B. S., Toker A. (1998) Curr. Biol. 8, 1069–1077 [DOI] [PubMed] [Google Scholar]
- 38.Marchisio M., Santavenere E., Paludi M., Gaspari A. R., Lanuti P., Bascelli A., Ercolino E., Di Baldassarre A., Miscia S. (2005) J. Cell. Physiol. 205, 32–36 [DOI] [PubMed] [Google Scholar]
- 39.Neri L. M., Martelli A. M., Borgatti P., Colamussi M. L., Marchisio M., Capitani S. (1999) FASEB J. 13, 2299–2310 [PubMed] [Google Scholar]
- 40.Calcerrada M. C., Miguel B. G., Martín L., Catalán R. E., Martínez A. M. (2002) FEBS Lett. 514, 361–365 [DOI] [PubMed] [Google Scholar]
- 41.Martelli A. M., Evangelisti C., Nyakern M., Manzoli F. A. (2006) Biochim. Biophys. Acta 1761, 542–551 [DOI] [PubMed] [Google Scholar]
- 42.Birrell M. A., McCluskie K., Wong S., Donnelly L. E., Barnes P. J., Belvisi M. G. (2005) FASEB J. 19, 840–841 [DOI] [PubMed] [Google Scholar]
- 43.Garrett S., Fitzgerald M. C., Sullivan K. E. (2008) Inflammation 31, 235–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan C., Li L., McCall C. E., Yoza B. K. (2005) J. Immunol. 175, 461–468 [DOI] [PubMed] [Google Scholar]
- 45.Facchinetti F., Amadei F., Geppetti P., Tarantini F., Di Serio C., Dragotto A., Gigli P. M., Catinella S., Civelli M., Patacchini R. (2007) Am. J. Respir. Cell Mol. Biol. 37, 617–623 [DOI] [PubMed] [Google Scholar]
- 46.Motz G. T., Eppert B. L., Sun G., Wesselkamper S. C., Linke M. J., Deka R., Borchers M. T. (2008) J. Immunol. 181, 8036–8043 [DOI] [PubMed] [Google Scholar]
- 47.Doz E., Noulin N., Boichot E., Guénon I., Fick L., Le Bert M., Lagente V., Ryffel B., Schnyder B., Quesniaux V. F., Couillin I. (2008) J. Immunol. 180, 1169–1178 [DOI] [PubMed] [Google Scholar]
- 48.Sethi J. M., Rochester C. L. (2000) Clin. Chest Med. 21, 67–86, viii [DOI] [PubMed] [Google Scholar]
- 49.Wright J. L., Cosio M., Churg A. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 295, L1–L15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maeno T., Houghton A. M., Quintero P. A., Grumelli S., Owen C. A., Shapiro S. D. (2007) J. Immunol. 178, 8090–8096 [DOI] [PubMed] [Google Scholar]
- 51.Corradi M., Rubinstein I., Andreoli R., Manini P., Caglieri A., Poli D., Alinovi R., Mutti A. (2003) Am. J. Respir. Crit. Care Med. 167, 1380–1386 [DOI] [PubMed] [Google Scholar]
- 52.Xin M., Gao F., May W. S., Flagg T., Deng X. (2007) J. Biol. Chem. 282, 21268–21277 [DOI] [PubMed] [Google Scholar]
- 53.Syed D. N., Afaq F., Kweon M. H., Hadi N., Bhatia N., Spiegelman V. S., Mukhtar H. (2007) Oncogene 26, 673–682 [DOI] [PubMed] [Google Scholar]
- 54.Monick M. M., Carter A. B., Gudmundsson G., Mallampalli R., Powers L. S., Hunninghake G. W. (1999) J. Immunol. 162, 3005–3012 [PubMed] [Google Scholar]
- 55.Petrache I., Medler T. R., Richter A. T., Kamocki K., Chukwueke U., Zhen L., Gu Y., Adamowicz J., Schweitzer K. S., Hubbard W. C., Berdyshev E. V., Lungarella G., Tuder R. M. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 295, L44–L53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monick M. M., Carter A. B., Flaherty D. M., Peterson M. W., Hunninghake G. W. (2000) J. Immunol. 165, 4632–4639 [DOI] [PubMed] [Google Scholar]
- 57.Fox T. E., Houck K. L., O'Neill S. M., Nagarajan M., Stover T. C., Pomianowski P. T., Unal O., Yun J. K., Naides S. J., Kester M. (2007) J. Biol. Chem. 282, 12450–12457 [DOI] [PubMed] [Google Scholar]
- 58.Kishore N., Sommers C., Mathialagan S., Guzova J., Yao M., Hauser S., Huynh K., Bonar S., Mielke C., Albee L., Weier R., Graneto M., Hanau C., Perry T., Tripp C. S. (2003) J. Biol. Chem. 278, 32861–32871 [DOI] [PubMed] [Google Scholar]
- 59.Dallot E., Méhats C., Oger S., Leroy M. J., Breuiller-Fouché M. (2005) Biochimie 87, 513–521 [DOI] [PubMed] [Google Scholar]
- 60.Lallena M. J., Diaz-Meco M. T., Bren G., Payá C. V., Moscat J. (1999) Mol. Cell. Biol. 19, 2180–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samten B., Townsend J. C., Weis S. E., Bhoumik A., Klucar P., Shams H., Barnes P. F. (2008) J. Immunol. 181, 2056–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cockerill P. N., Osborne C. S., Bert A. G., Grotto R. J. (1996) Cell Growth Differ. 7, 917–922 [PubMed] [Google Scholar]
- 63.Koch A., Giembycz M., Ito K., Lim S., Jazrawi E., Barnes P. J., Adcock I., Erdmann E., Chung K. F. (2004) Am. J. Respir. Cell Mol. Biol. 30, 342–349 [DOI] [PubMed] [Google Scholar]
- 64.Najjar I., Baran-Marszak F., Le Clorennec C., Laguillier C., Schischmanoff O., Youlyouz-Marfak I., Schlee M., Bornkamm G. W., Raphaël M., Feuillard J., Fagard R. (2005) J. Virol. 79, 4936–4943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen L. F., Mu Y., Greene W. C. (2002) EMBO J. 21, 6539–6548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haller D., Holt L., Kim S. C., Schwabe R. F., Sartor R. B., Jobin C. (2003) J. Biol. Chem. 278, 23851–23860 [DOI] [PubMed] [Google Scholar]
- 67.Garrean S., Gao X. P., Brovkovych V., Shimizu J., Zhao Y. Y., Vogel S. M., Malik A. B. (2006) J. Immunol. 177, 4853–4860 [DOI] [PubMed] [Google Scholar]
- 68.Engdahl R., Monroy M. A., Daly J. M. (2007) Biochem. Biophys. Res. Commun. 359, 88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galvez A. S., Duran A., Linares J. F., Pathrose P., Castilla E. A., Abu-Baker S., Leitges M., Diaz-Meco M. T., Moscat J. (2009) Mol. Cell. Biol. 29, 104–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barnes P. J. (2004) COPD 1, 59–70 [DOI] [PubMed] [Google Scholar]
- 71.Shapiro S. D. (1999) Am. J. Respir. Crit. Care Med. 160, S29–S32 [DOI] [PubMed] [Google Scholar]
- 72.Lapperre T. S., Willems L. N., Timens W., Rabe K. F., Hiemstra P. S., Postma D. S., Sterk P. J. (2007) Chest 131, 53–59 [DOI] [PubMed] [Google Scholar]
- 73.Shapiro S. D., Goldstein N. M., Houghton A. M., Kobayashi D. K., Kelley D., Belaaouaj A. (2003) Am. J. Pathol. 163, 2329–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chakrabarti S., Patel K. D. (2008) Microcirculation 15, 555–567 [DOI] [PubMed] [Google Scholar]
- 75.Guo H., Ma Y., Zhang B., Sun B., Niu R., Ying G., Zhang N. (2009) J. Leukocyte Biol. 85, 911–918 [DOI] [PubMed] [Google Scholar]
- 76.Huang X., Chen L. Y., Doerner A. M., Pan W. W., Smith L., Huang S., Papadimos T. J., Pan Z. K. (2009) J. Immunol. 182, 5810–5815 [DOI] [PubMed] [Google Scholar]