Abstract
Detachment of non-malignant epithelial cells from the extracellular matrix causes their growth arrest and, ultimately, death. By contrast, cells composing carcinomas, cancers of epithelial origin, can survive and proliferate without being attached to the extracellular matrix. These properties of tumor cells represent hallmarks of malignant transformation and are critical for cancer progression. Previously we identified several mechanisms by which ras, a major oncogene, blocks detachment-induced apoptosis of intestinal epithelial cells, but mechanisms by which Ras promotes proliferation of those cells that remain viable following detachment are unknown. We show here that detachment of non-malignant intestinal epithelial cells promotes formation of autophagosomes, vacuole-like structures that mediate autophagy (a process of cellular self-cannibalization), and that oncogenic ras prevents this autophagosome formation. We also found that ras activates a GTPase RhoA, that RhoA promotes activation of a protease calpain, and that calpain triggers degradation of Beclin-1, a critical mediator of autophagy, in these cells. The reversal of the effect of ras on Beclin-1 (achieved by expression of exogenous Beclin-1) promoted autophagosome formation following cell detachment, significantly reduced the fraction of detached cells in the S phase of the cell cycle and their rate of proliferation without affecting their viability. Furthermore, RNA interference-induced Beclin-1 down-regulation in non-malignant intestinal epithelial cells prevented detachment-dependent reduction of the fraction of these cells in the S phase of the cell cycle. Thus, ras oncogene promotes proliferation of those malignant intestinal epithelial cells that remain viable following detachment via a distinct novel mechanism that involves Ras-induced down-regulation of Beclin-1.
Introduction
The ability of cells that form carcinomas, cancers of epithelial origins, to survive and proliferate in the absence of adhesion to the extracellular matrix (ECM)3 is thought to represent a critical prerequisite for carcinoma progression (1, 2). This concept is based on observations indicating that many types of normal epithelia grow in vivo as cellular monolayers in contact with a form of the ECM called basement membrane. Detachment from the ECM causes growth arrest (3) and apoptosis (4) of non-malignant epithelial cells (the latter type of death is referred to as anoikis (5)). By contrast, carcinomas typically represent three-dimensional disorganized multicellular masses in which the cells tend to be deprived of normal contacts with the basement membrane. It is known in this regard that carcinoma cells often secrete basement membrane degrading enzymes, and this allows tumors formed by these cells to invade adjacent tissues (6). Furthermore, at advanced stages of the disease cancer cells detach from the primary tumor and migrate toward ectopic locations, where they reattach and give rise to metastases (7, 8). Despite the fact that carcinoma cells are frequently deprived of normal contacts with the basement membrane during tumor progression, a significant fraction of the cells composing solid tumors maintain the ability to survive and proliferate even in the absence of adhesion to the ECM.
Numerous lines of evidence indicate that the ability of tumor cells to remain viable and capable of proliferation in the absence of adhesion to the ECM represents a critical requirement for the progression of solid tumors. First, the ability of such cells to survive and grow anchorage independently, in the absence of adhesion to the ECM as colonies in soft agar, has served as a “gold standard” for cell transformation for over three decades (9). As we and others have discovered, the ability to grow without being attached to the ECM is conferred to cancer cells by oncogenes, such as ras (4, 10), Epidermal Growth Factor Receptor (11, 12), and β-catenin (13, 14) or loss of tumor suppressor genes, such as PTEN (15). Furthermore, we and others found that treatment suppressing the ability of malignant cells to grow in an anchorage-independent manner blocks their in vivo tumorigenicity (10, 16–18) as well as their ability to metastasize (8, 16, 18–20). Finally, we found that spontaneous acquisition of the capacity for anchorage-independent growth by non-malignant epithelial cells is sufficient for the attainment of an overt tumorigenic phenotype by these cells (21).
Mutant ras, one of the most frequently occurring oncogenes in cancer (22), is well known to enable malignant cells to survive and grow without being attached to the ECM (10, 12, 17, 21, 23). Ras is a GTPase that is activated by receptor tyrosine kinases in response to various mitogenic signals (24). Once activated, Ras triggers numerous downstream pathways mediated by signaling molecules, such as Raf, Ral guanine nucleotide exchange factors, phosphoinositide 3-OH-kinase (PI3K), Rho, and others (24). Some of these events promote changes in the expression of genes that control proliferation, survival, and other critical cellular functions (25).
The mechanisms by which oncogenic Ras promotes growth of those cancer cells that have lost contact with the extracellular matrix are understood only in part. We found in this regard that Ras blocks anoikis of intestinal epithelial cells by multiple mechanisms that include Ras-induced down-regulation of the pro-apoptotic protein Bak (17) and up-regulation of the anti-apoptotic proteins Bcl-XL (10), cIAP2, and XIAP (12). The mechanisms by which Ras promotes proliferation of those cancer cells that remain viable following detachment from the ECM are not known.
One protein that was of high interest to us as a potential mediator of Ras-dependent anchorage-independent growth of cancer cells was Beclin-1, a critical regulator of a phenomenon called autophagy (26, 27). Autophagy is a process of self-cannibalization of cells via the lysosomal degradation pathway. Autophagy is mediated by vacuole-like structures called autophagosomes. Autophagosomes are known to engulf diverse cellular components and deliver them to lysosomes where these components are degraded by lysosomal enzymes. Autophagy is controlled by numerous Atg proteins, whose mechanisms of action are understood only in part (26, 27).
One of the reasons why we decided to study the role of mediators of autophagy, such as Beclin-1, a mammalian orthologue of yeast Atg6, in anchorage-independent growth of ras-transformed cells was that, Beclin-1 is known to act as a tumor suppressor, which, depending on the context, can kill cells (28) or cause their growth arrest without killing them. Moreover, beclin-1 gene is located at the tumor susceptibility locus 17q21 that is often deleted monoallelically in breast, ovarian, and prostate cancers (29). In addition, beclin-1 is a haploinsufficient tumor suppressor gene in mice, because loss of one beclin-1 allele in mice was shown to lead to a high incidence of lung and hepatocellular carcinomas, lymphomas, as well as mammary neoplastic lesions (30, 31). Also importantly, decreased Beclin-1 expression was found to be strongly associated with reduced survival rate of stage IIIB colon cancer patients (32). Furthermore, increased Beclin-1 expression was demonstrated to block anchorage-independent growth and in vivo tumorigenicity of human breast carcinoma cells (33). The role of autophagy as a mechanism of suppression of tumor growth is further supported by data indicating that the deletion in mice of other mediators of autophagy, such as Beclin-1 binding partner and agonist Bif-1, results in the development of spontaneous tumors in various mouse organs (34). Moreover, UVRAG, another agonist of Beclin-1 and a mediator of autophagy, is often monoallelically deleted in human colon cancer and blocks proliferation and tumorigenicity of human colon cancer cells (35).
We show in this study that oncogenic Ras promotes proliferation of malignant intestinal epithelial cells following their detachment from the ECM by down-regulating Beclin-1. This down-regulation is triggered by Ras-induced activation of a small GTPase RhoA, an established mediator of Ras signaling, by subsequent RhoA-dependent activation of calpain protease, and finally, by calpain-driven degradation of Beclin-1.
EXPERIMENTAL PROCEDURES
Cell Culture
The generation of the IEC clones expressing activated H-ras (4) and Bcl-XL (10) has been previously described (a clone of IEC-18 cells referred to in Ref. 10 as IEC-BclX-3 is referred to as IEC-BclX in this study). Expression of H-ras in MT-ras cells was induced by adding 100 μm ZnCl2 and 2 μm CdCl2 to cells. ras-tet-Beclin cells were generated as previously described (14). All IEC clones were cultured in α-minimum essential medium containing 5% fetal bovine serum, 10 μg/ml insulin, and 0.5% glucose. The HCT-116, Hkh-2, and Hke-3 cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. For suspension cultures cells were plated above a layer of 1% sea plaque-agarose polymerized in α-minimum essential medium or Dulbecco's modified Eagle's medium.
Expression Vectors
The expression vector carrying FLAG-tagged Beclin-1 was kindly provided by Dr. B. Levine, University of Texas Southwestern Medical Center, Dallas, TX. The GFP-LC3 expression vector was provided by Dr. T. Yoshimori, National Institute of Genetics, Mishima, Japan. The T-REX system (Invitrogen) was used to generate cells expressing tetracycline-inducible Beclin-1. FLAG-Beclin-1 cDNA was placed into the EcoRI site of the pcDNA4-TO vector (a component of the T-REX system).
Detection of Beclin-1 in Human Cancer Tissue by Immunohistochemistry
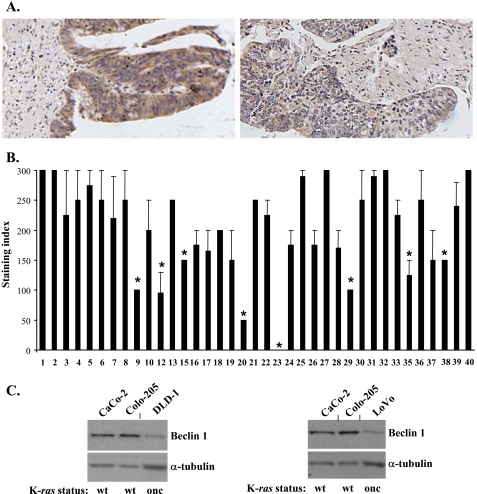
Tissue microarrays carrying patient-derived cancer tissue samples (Imgenex, San Diego, CA) were deparaffinized with xylene, rehydrated, and antigen-unmasked in 10 mm sodium citrate buffer (pH 6.0) at 95 °C. Arrays were further treated with 3% hydrogen peroxide followed by incubation with a rabbit polyclonal anti-Beclin-1 antibody at 1:100 dilution (Santa Cruz Biotechnology) and then with the secondary antibody by use of a Vectastain Elite ABC rabbit kit (Vector Laboratories, Inc.) according to the manufacturer's instructions. The binding of the anti-Beclin-1 antibody to Beclin-1 was visualized using 3,3′-diaminobenzidine substrate kit for peroxidase (Vector Laboratories, Inc.). Tissue sections were then counterstained with hematoxylin, dehydrated, and mounted. Negative controls were treated identically, but the primary antibody was omitted. Immunostaining of Beclin-1 was analyzed by light microscopy. A semi-quantitative scoring (H-score) system (36) was used to assess the levels of expression of Beclin-1 in these samples. The intensity of the specific staining was scored from 0 to 3 (0 for negative, 1 for weak, 2 for moderate, and 3 for strong staining). The percentage of epithelial cells showing a given staining intensity was estimated. The Beclin-1 staining index (H-score) was generated according to the formula H-score = 0xf0 + 1xf1 + 2xf2 + 3xf3, where 0, 1, 2, and 3 are staining intensity and f0 through f3 are fractions of the cells showing staining intensity from 0 to 3, respectively. The evaluation of all specimens was performed blindly.
Western Blot Analysis
Western blot analysis was performed as described elsewhere (12). The following antibodies were used in this study: anti-Beclin-1 (Santa Cruz Biotechnology), anti-α-tubulin (Upstate), anti-CDK4 (Santa Cruz Biotechnology), anti-β-actin (Sigma), anti-RhoA (Santa Cruz Biotechnology). When lanes were removed from Western blot images, and separate parts of an image were joined together, a short vertical black line was used to indicate where the image was cut.
Detection of Autophagic Vacuoles by Transmission Electron Microscopy
Cells were treated with 2.5% glutaraldehyde in 0.1 m sodium cacodylate (pH 7.4) for 2 h, and then with 1% osmium tetroxide in 0.1 m sodium cacodylate for 2 h. Cells were further washed with 50% acetone for 10 min, twice with 70% acetone for 10 min, twice with 90% acetone for 10 min, twice with 100% acetone for 10 min, and dried with 100% acetone in the presence of dehydrating beads. Samples were then embedded in Araldite using an Embed 812 (Epon-812) Kit. Ultrathin sections were produced by an LKB Huxley Ultramicrotome and stained with uranyl acetate and lead citrate. The samples were then examined by transmission electron microscopy by using a transmission electron microscope (JEOL JEM 1230) at 80 kV. Images were acquired using a Hamamatsu ORCA-HR digital camera.
Detection of Autophagic Vacuoles by Monitoring Cellular Distribution of GFP-LC3
Cells were transfected for 4 h with 1 μg of GFP-LC3 in Opti-MEM using Lipofectamine 2000. Cells were subsequently grown overnight in the growth medium that we normally use for their growth (see above) and plated either in monolayer or suspension. Cells were then harvested at indicated times, incubated for 15 min in 4% paraformaldehyde at room temperature, and washed twice with phosphate-buffered saline (PBS). GFP-LC3 distribution was subsequently monitored by fluorescence microscopy.
RNA Interference
All transfections with siRNAs were performed by using Lipofectamine 2000 (Invitrogen) as previously described (12). To block Beclin-1 expression in IEC-18 cells by Beclin-1-specific small interfering RNAs (siRNAs) cells were transfected with 100 nm of either control RNA or each of the respective Beclin-1-specific RNA for 72 h. In the case of RhoA-directed RNA interference ras-3 cells were subjected to 48-h transfections with 100 nm RhoA-specific siRNA-1 or RhoA-specific siRNA-2. The sequences of the sense strands of the RNAs used in this study were as follows: control RNA (siCONTROL non-targeting siRNA #1, Dharmacon), UAGCGACUAAACACAUCAAUU; Beclin-1 siRNA-1, GUACCGACUUGUUCCCUAUUU; Beclin-1 siRNA-2, GGAAAUCACUCGUAUCUGGUU; RhoA siRNA-1, CGACAGCCCUGAUAGUUUAUU; and RhoA siRNA-2, ACAGGAAGAUUAUGACCGUUU. All RNAs were from Dharmacon.
Analysis of Cellular Cell Cycle Profile by Flow Cytometry
Cells were harvested and washed with PBS and re-suspended in 0.5 ml of PBS, and 5 ml of cold ethanol was added drop by drop with continuous stirring. Samples were incubated at 4 °C for 30 min and washed with PBS twice. Cells were further re-suspended with 100 μl of propidium iodide (5 μg/ml). A FACSCalibur system (BD Biosciences) was used for the analysis of the cell cycle profile of the respective cells, and a ModiFit LT 3.0 software package was used to perform data analysis.
Calpain-dependent Cleavage of Beclin-1
3 × 105 tet-ras-Beclin cells were plated in monolayer culture for 48 h and treated with 800 ng/ml tetracycline for additional 24 h. Cells were rinsed with PBS twice. Cells were then lysed on the tissue culture dishes in a buffer containing 50 mm Tris HCl, pH 7.4, with 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, and a protease inhibitor mixture for 30 min with shaking. Cells were then scraped from the dishes, and centrifuged for 10 min at 12,000 × g. 500 μg of cell lysate was subsequently added to the anti-FLAG M2 affinity gel (Sigma) (the anti-FLAG M2 affinity gel was prepared by centrifugation of 120 μl of the gel and rinsing the gel-containing pellet twice with 0.5 ml of TBS). The lysate was incubated overnight at 4 °C. Samples were then centrifuged for 30 s at 6,000 × g, and supernatants were removed. The gel-containing pellet was washed with 0.5 ml of TBS three times, re-suspended in a buffer containing 20 mm HEPES, pH 7.5, 50 mm KCl, 2 mm MgCl2, 1 mm EGTA, 1 mm EDTA, 1 mm dithiothreitol, and 1 mm phenylmethylsulfonyl fluoride and divided into aliquots. Calpain-1 (1.5 μg, Calbiochem) was added to respective samples in the presence of 1 mm CaCl2. Reaction mixtures were then incubated for 30 min at 37 °C.
Cell Counting in Monolayer and Suspension
1 × 106 of tet-ras-Beclin cells were cultured for 17h and then for another 24 h in the presence of 8 ng/ml tetracycline. 50,000 cells were further plated in monolayer or suspension. Cells were harvested at various times, re-suspended in 5 ml of the medium that we normally use for their growth (see above), and counted.
Detection of Cell Death by Trypan Blue Staining
Cells were washed with PBS, incubated for 5 min at room temperature in the presence of 4% Trypan Blue, and then placed in a hemocytometer, and Trypan Blue-positive cells were counted.
Pulse-chase Assay
Cells were starved for 1 h in a Dulbecco's modified Eagle's medium-high glucose medium without l-methionine, l-cysteine, and l-glutamine (Sigma) supplemented with 5% dialyzed fetal bovine serum (Sigma), 1% penicillin/streptomycin/glutamine (Invitrogen), and 0.28% human insulin. The same medium containing 100 μCi/ml Redivue Pro-mix l-[35S] in vitro labeling mix (Amersham Biosciences) was then added to cells for 4 h and subsequently replaced with the same medium containing 2 mm l-cysteine and 2 mm l-methionine (Sigma). Cells were harvested at various times and lysed in a buffer containing 50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 0.25% sodium deoxycholate, 1% Nonidet P-40, and protease inhibitors (Roche Diagnostics). Beclin-1 was then immunoprecipitated with an anti-Beclin-1 antibody (Santa Cruz Biotechnology). The immunoprecipitates were resolved in 8% polyacrylamide gels. The intensity of the band corresponding to Beclin-1 was quantified by phosphorimaging and densitometry as described (12). When lanes were removed from the phosphorimages, and separate parts of an image were joined together, a short vertical black line was used to indicate where the image was cut.
RhoA Activity Assay
A Rho Activation Assay kit (Upstate) was used according to the manufacturer's instructions to detect activated RhoA.
Calpain Activity Assay
An InnoZymeTM Calpain 1/2 Activity Assay Kit (Calbiochem) was used according to the manufacturer's instructions for determining calpain activity. Detection of cell death by the Cell Death enzyme-linked immunosorbent assay, soft agar growth assay, and Northern blot assay were performed as previously published (10), and densitometry analysis was performed as described (12).
Statistical Analysis
One-sample Student's t test was used for the analysis of data in Fig. 1B. Two-sample Student's t test was used for the analysis of data in Figs. 3A, 3C, and 5C. Chi-square test for goodness-of-fit was used in all other cases. Dr. K. Ritchie, a consulting scientist at the Izaak Walton Killam Health Centre, Halifax, Nova Scotia, assisted us with statistical analysis of the data.
FIGURE 1.
The expression of Beclin-1 is reduced in a fraction of human colorectal cancers. Beclin-1 expression was analyzed by immunohistochemistry on the tissue microarray carrying patient-derived colorectal cancer tissue. A, representative images of colorectal cancers expressing high (left) and low (right) levels of Beclin-1. B, quantification of the Beclin-1 immunostaining intensity in human colorectal cancer samples. Cancer sample numbers shown on the figure are the same as on the original array. The intensity of staining was scored from 0 to 3 (0 for negative, 1 for weak, 2 for moderate, and 3 for strong staining). The staining index (H-score) was generated according to the formula H-score = 0xf0 + 1xf1 + 2xf2 + 3xf3, where 0, 1, 2, and 3 are staining intensity and f0 through f3 are the fractions of the cells showing staining intensity from 0 to 3, respectively. The data represent the average of two independent experiments (performed on two different serial sections) plus the S.D. Student's one-sample t test was used to measure the variance of Beclin-1 staining indexes among the samples derived from different patients and identify those tumors for which these indexes varied significantly from the mean value (204.59) observed for the whole population of tumor samples. The lowest index for samples that did not differ from the mean value within the 95% confidence interval was 179.39. Values that varied significantly (p < 0.0001) from the mean are indicated with an asterisk. These values ranged from 0 to 150. C, indicated human colon carcinoma-derived cell lines carrying wild type (wt) or oncogenic (onc) K-ras were assayed for Beclin-1 expression by Western blot. α-Tubulin was used as a loading control.
FIGURE 3.
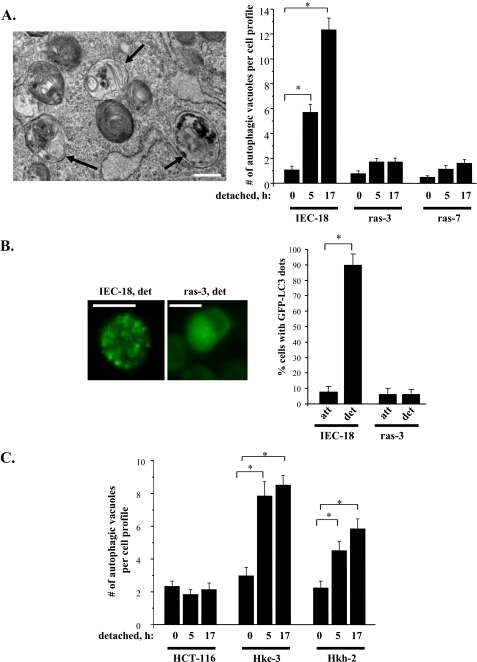
Oncogenic ras prevents detachment-induced formation of autophagic vacuoles in intestinal epithelial cells. A, left: representative electron micrographs (×20,000 magnification) of detached IEC-18 cells used for quantification in A (right). Arrows indicate the position of autophagic vacuoles. Scale bar, 500 nm. A, right: indicated cells were detached from the ECM for the indicated times, and the formation of autophagic vacuoles in these cells was assayed by transmission electron microscopy. The data represent the average of the number of autophagic vacuoles detected for 30 cell profiles per condition in case of IEC-18 and ras-3 cells and 15 cell profiles in case of ras-7 cells plus the S.E. This experiment was repeated twice with similar results. B, left: representative fluorescence microscopy images used for quantification in (B, right) of detached GFP-LC3-expressing IEC-18 and ras-3 cells. B, right: indicated cells were transiently transfected with GFP-fused LC3 (GFP-LC3), cultured attached to (att) or detached from (det) the ECM for 24 h, and the percentage of cells with punctuate distribution of GFP-LC3, characteristic of autophagosome-bound LC3, was determined by fluorescence microscopy. The data represent the average of two independent experiments plus the S.E. C, indicated cells were processed as in A. The data represent the average of the number of autophagic vacuoles detected for 30 cell profiles per condition plus the S.E. This experiment was repeated twice with similar results. *, p value < 0.05.
FIGURE 5.
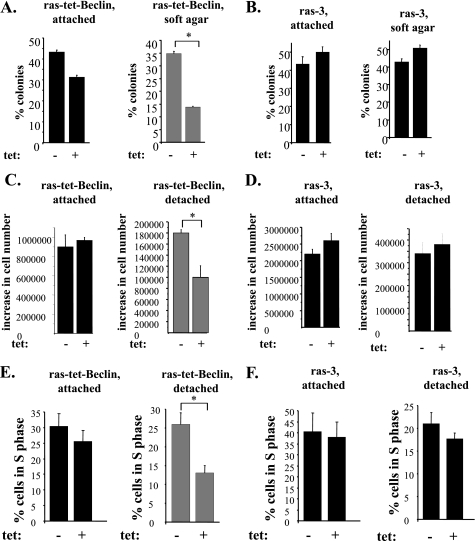
The reversal of ras-induced down-regulation of Beclin-1 blocks proliferation of detached intestinal epithelial cells. A and B, indicated cell lines were plated attached to the ECM or in soft agar in the absence (−) or in the presence (+) of 8 ng/ml tetracycline, cell colonies were allowed to form for 7–10 days and counted. The results are expressed as a percentage of cells initially plated. The data represent the average of the duplicates plus the S.D. This experiment was repeated twice with similar results. C and D, indicated cell lines were plated attached to or detached from the ECM for 96 h in the absence (−) or in the presence (+) of 8 ng/ml tetracycline, and cells were counted. The results are presented as the number of cells observed at the indicated time less the number of cells initially plated. The data represent the average of the triplicates plus the S.E. This experiment was repeated twice with similar results. E and F, indicated cell lines were plated attached to or detached from the ECM for 72 h in the absence (−) or in the presence (+) of 8 ng/ml tetracycline, and their cell cycle profile was analyzed by flow cytometry. The data in E represent the average of the duplicates plus the S.D. This experiment was repeated twice with similar results. The data in F represent the average of two independent experiments plus the S.D. *, indicates that the p value was <0.05.
Northern Blot Analysis
Northern blot analysis was performed as in Ref. 10. When lanes were removed from Northern blot images, and separate parts of an image were joined together, a short vertical black line was used to indicate where the image was cut.
RESULTS
Beclin-1 Expression Is Reduced in a Fraction of Colorectal Cancers
To begin to address the role of Beclin-1 in malignant transformation of intestinal epithelial cells we utilized a commercial tissue array to analyze Beclin-1 expression by immunohistochemistry in colorectal cancer tissue derived from 37 cancer patients. We used Student's one-sample t test to measure the variance of Beclin-1 staining indexes among the samples derived from different patients and identify those tumors for which these indexes varied significantly from the mean value (204.59) observed for the whole population of tumor samples. We found (Fig. 1, A and B) that staining indexes for 8 out of 37 samples (22%) were below the lowest staining index (179.39) that did not differ from the mean value within the 95% confidence interval. These eight indexes were significantly (p value < 0.0001) lower than the mean value and ranged from 0 to 150. We, therefore, concluded that expression of Beclin-1 is reduced in a fraction of colorectal cancers. Thus, it is possible that in the malignant cells composing at least some colorectal tumors molecular mechanisms are in place that are responsible for maintaining the expression of Beclin-1 at relatively low levels. Given that oncogenic mutations of K-ras often occur in colorectal cancer and that ras oncogene is thought to be a major factor in the progression of the disease (37, 38), we compared the levels of Beclin-1 expression between patient-derived colon carcinoma cells carrying oncogenic K-ras and those known to harbor the wild-type K-ras. We found that oncogenic-K-ras-carrying DLD-1 (38) and LoVo (39) colon cancer cell lines display significantly lower levels of Beclin-1 than the wild type-K-ras-harboring colon cancer cells CaCo-2 (40) and Colo-205 (40) (Fig. 1C).
Oncogenic ras Down-regulates Beclin-1 in Malignant Intestinal Epithelial Cells
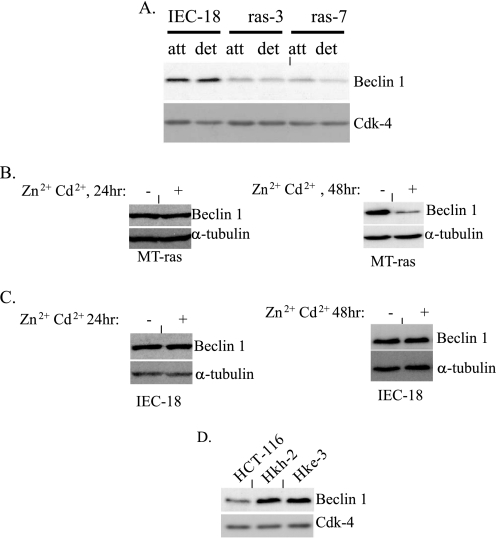
We further tested directly whether the presence of oncogenic ras in malignant intestinal epithelial cells can be responsible for maintaining low levels of Beclin-1 expression in these cells. To this end, we compared the expression of Beclin-1 between spontaneously immortalized non-malignant rat intestinal epithelial cells IEC-18 (these cells are not capable of anchorage-independent growth (4)) and two previously published highly transformed clones of IEC-18 cells ras-3 and ras-7 (4) stably expressing oncogenic H-ras. Both ras-3 and ras-7 cells can readily grow in an anchorage-independent manner (4). We found (Fig. 2A) that Ras significantly down-regulates Beclin-1 in these cells both when these cells are attached to and detached from the ECM. To confirm that the down-regulation of Beclin-1 is promoted by ras directly, we used a published clone of IEC-18 cells MT-ras that carries ectopic activated mutant of H-ras under the control of Zn2+- and Cd2+-inducible metallothionein promoter (41). We established previously that treatment of these cells with Zn2+ and Cd2+ results in changes in the expression of ras target genes as early as 24 h after the addition of the indicated metal ions (12) but that in some cases, depending on the nature of the target gene, it may take 48 h following the addition of ras inducers to observe alterations in the cellular levels of some of the respective gene products (12). We observed in this study that treatment of MT-ras cells with Zn2+ and Cd2+ for 24 h does not result in a significant reduction in the levels of Beclin-1 expression in these cells (Fig. 2B, left) and that a noticeable down-regulation of Beclin-1 in attached (Fig. 2B, right) and detached (not shown) cells occurs after 48 h of treatment of these cells with the indicated metal ions. By, contrast treatment of the parental IEC-18 cells with Zn2+ and Cd2+ either for 24 or 48 h did not result in the down-regulation of Beclin-1 (Fig. 2C).
FIGURE 2.
Oncogenic ras triggers down-regulation of Beclin-1 in intestinal epithelial cells. A, attached (att) and detached (det) IEC-18 cells and two independently derived H-ras-transformed clones of these cells ras-3 and ras-7 were assayed for the expression of Beclin-1 by Western blot. B, attached MT-ras cells were cultured in the absence (−) and in the presence (+) of 100 μm Zn2+ and 2 μm Cd2+ for 24 h (left) and 48 h (right) and assayed for the expression of Beclin-1 by Western blot. C, IEC-18 cells were processed as in B. D, attached human colorectal carcinoma cells HCT-116 and their K-ras knock-out derivatives Hkh-2 and Hke-3 were assayed for the expression of Beclin-1 by Western blot. The membranes in A and D were re-probed with a CDK-4 antibody, and the membranes in B and C were re-probed with an anti-α-tubulin as loading controls.
To test whether Ras can promote the down-regulation of Beclin-1 in human colon cancer cells, we used highly tumorigenic human colon carcinoma-derived cells HCT-116 that carry one copy of oncogenic form of K-ras and two poorly tumorigenic derivatives of these cells Hkh-2 and Hke-3, in which the mutant K-ras allele had been disrupted by homologous recombination (38). We found previously that both of the oncogenic K-ras knockout variants of HCT-116 cells are significantly less capable of growing in the absence of adhesion to the ECM than their parental oncogenic K-ras-carrying counterparts (21). We observed that ablation of oncogenic ras resulted in a noticeable up-regulation of Beclin-1 in the indicated cells both in the presence (Fig. 2D) and in the absence (not shown) of adhesion to the ECM. Collectively, our data indicate that ras oncogene down-regulates Beclin-1 in malignant intestinal epithelial cells.
Oncogenic ras Prevents Detachment-induced Formation of Autophagic Vacuoles in Malignant Intestinal Epithelial Cells
Given that ras down-regulates Beclin-1 in intestinal epithelial cells and that Beclin-1 is a major mediator of autophagy, we decided to examine how adhesion of intestinal epithelial cells to the ECM affects the formation of autophagic vacuoles in the absence and in the presence of oncogenic ras. To this end, we used transmission electron microscopy, a method that is frequently utilized to measure the formation of the indicated vacuoles (42). We found (Fig. 3A) that detachment of non-malignant intestinal epithelial cells IEC-18 from the ECM triggers a significant increase in the number of autophagic vacuoles per cell and that oncogenic ras blocks this increase.
To confirm these data by a complimentary method we used an autophagy marker LC3/Atg8, a protein that attaches to the inner autophagosomal membrane following the formation of an autophagic vacuole (43). Cellular re-distribution of GFP-fused LC3 (GFP-LC3) from diffuse cytoplasmic to punctate, typical of autophagosome-bound LC3, can be detected by fluorescence microscopy and is frequently utilized as an indicator of autophagosome formation (42). Similar to what was observed by electron microscopy, we found that detachment of IEC-18 cells promotes a strong increase in the number of autophagic vacuoles per cell and that ras prevents this increase (Fig. 3B). We further observed that detachment of the oncogenic ras knock-out cells Hkh-2 and Hke-3 resulted in a much more pronounced accumulation of autophagic vacuoles than that of the oncogenic ras-carrying colon carcinoma cells HCT-116 from which Hkh-2 and Hke-3 cells had been derived (Fig. 3C). Thus, oncogenic ras blocks the formation of autophagic vacuoles following detachment of intestinal epithelial cells from the ECM.
Ras-induced Down-regulation of Beclin-1 Is Required for the Ability of Malignant Intestinal Epithelial Cells to Proliferate following Detachment from the ECM
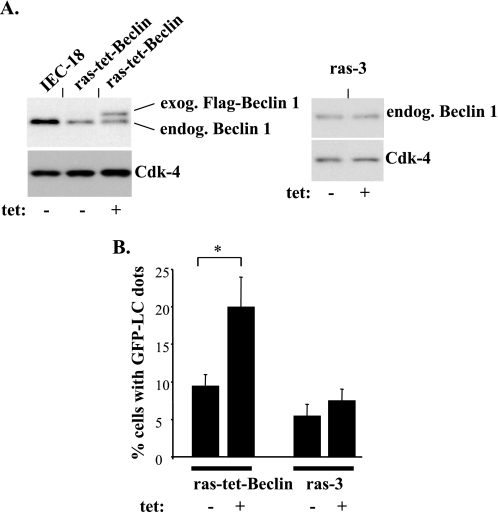
In an effort to test whether Ras-dependent down-regulation of Beclin-1 in malignant intestinal epithelial cells contributes to the ability of these cells to grow in the absence of adhesion to the ECM we decided to reverse this down-regulation. To this end, we generated a variant of ras-3 cells (ras-3 is a representative oncogenic ras-expressing clone of intestinal epithelial cells IEC-18, see Fig. 2) that we named ras-tet-Beclin, in which Beclin-1 could be expressed in a tetracycline-inducible manner (Fig. 4A). We induced the expression of Beclin-1 in these cells by using a tetracycline concentration at which the resulting total Beclin-1 levels in ras-tet-Beclin cells did not exceed those in the parental IEC-18 cells (Fig. 4A). Thus, the subsequently observed effects of ectopic Beclin-1 on the formation of autophagic vacuoles (Fig. 4B), and anchorage-independent growth (Fig. 5) of the Ras-transformed cells did not represent the consequence of abnormally high production of Beclin-1 by these cells but were rather the result of the presence of Beclin-1 in the indicated cells at levels that were close to physiological. We found that the reversal of ras-induced down-regulation of Beclin-1 triggered a significant increase in the formation of autophagic vacuoles following detachment of ras-tet-Beclin cells from the ECM (Fig. 4B).
FIGURE 4.
The reversal of ras-induced down-regulation of Beclin-1 promotes the formation of autophagic vacuoles following detachment of intestinal epithelial cells. A, indicated cell lines were assayed for Beclin-1 expression by using a Beclin-1-specific antibody by Western blot in the absence (−) or in the presence (+) of 8 ng/ml tetracycline. The positions of endogenous (endog.) Beclin-1 and exogenous (exog.) FLAG-tagged Beclin-1 on the gel are indicated. CDK-4 was used as a loading control. B, cells were cultured attached to detached from the ECM in the absence (−) or in the presence (+) of 8 ng/ml tetracycline for 48 h and analyzed for the presence of autophagic vacuoles as in Fig. 3B. Percentage of cells with punctuate GFP-LC3 distribution observed in attached cells was subtracted from that observed in detached cells as background. The data represent the average of the duplicates plus the S.D. This experiment was repeated twice with similar results. *, p value < 0.05.
We also established that the reversal of the effect of Ras on Beclin-1 resulted in a much more pronounced reduction of the ability of the ras-transformed cells to grow in the absence of adhesion to the ECM as colonies in soft agar than that observed in the monolayer culture (Fig. 5, A and B). In addition, the reversal of Ras-induced down-regulation of Beclin-1 caused a noticeable reduction of the rate of accumulation of the ras-transformed cells in suspension culture (Fig. 5, C and D). Furthermore, expression of exogenous Beclin-1 substantially reduced the fraction of detached ras-tet-Beclin cells in the S phase of the cell cycle (Fig. 5, E and F). This reduction was paralleled by an increase in the fraction of cells in the G1 phase of the cell cycle by the same percentage (data not shown). By contrast, exogenous Beclin-1 did not cause death of ras-tet-Beclin cells following their detachment from the ECM as judged by the lack of increase in cell permeability to a “vital” dye (not shown) or by lack of increase in the chromosomal DNA fragmentation (a hallmark of apoptosis) that we examined by using the Cell Death enzyme-linked immunosorbent assay (not shown) in response to the expression of ectopic Beclin-1. Thus, Ras-induced down-regulation of Beclin-1 is required for the ability of ras-transformed cells to proliferate following detachment from the ECM.
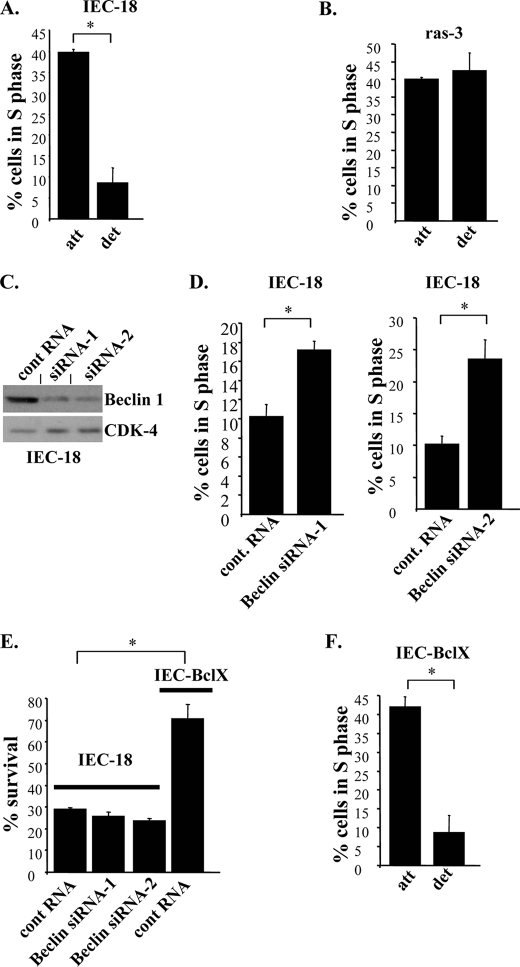
Loss of Beclin-1 by Non-malignant Intestinal Epithelial Cells Prevents Their Growth Arrest following Detachment from the ECM
We found that the fraction of the parental non-malignant intestinal epithelial cells IEC-18 (from which the ras-transformed cells were derived) in the S phase of the cell cycle is noticeably reduced following their detachment from the ECM (Fig. 6A). This was paralleled by an increase in the fraction of these cells in the G1 phase of the cell cycle by the same percentage (data not shown). By contrast, the ability of ras-transformed cells (when they were cultured under the conditions identical to those used in case of IEC-18 cells) to enter the S phase of the cell cycle did not depend on adhesion to the ECM (Fig. 6B). We further found that enforced down-regulation of Beclin-1 by RNA interference (Fig. 6C) significantly increased the fraction of detached IEC-18 cells in the S phase of the cell cycle (Fig. 6D). We and others found in the past that detachment of non-malignant epithelial cells, including IEC-18 cells, results not only in the loss of their ability to proliferate but also, ultimately, in a form of apoptotic death often referred to as anoikis (4, 17). In the case of IEC-18 cells this death is driven by several mechanisms, including detachment-induced down-regulation of the anti-apoptotic protein Bcl-XL (10). We observed in the present study in this regard that even though loss of Beclin-1 increases the fraction of IEC-18 cells in the S phase of the cell cycle (Fig. 6D), Beclin-1-deprived cells still succumb to anoikis (Fig. 6E). By contrast, in agreement with what we published before (10, 11), a representative clone of IEC-18 cells expressing exogenous Bcl-XL was much more anoikis-resistant than the parental IEC-18 cells (Fig. 6E) (the rescue of IEC-18 cells from detachment-induced apoptosis was previously published by us for two additional Bcl-XL-expressing clones of these cells (10, 11). Unlike the case with loss of Beclin-1, ectopic Bcl-XL was unable to prevent the reduction of the fraction of IEC-18 in the S phase of the cell cycle following detachment of these cells to the ECM (Fig. 6F).
FIGURE 6.
Enforced down-regulation of Beclin-1 increases the ability of detached non-malignant intestinal epithelial cells to enter the S phase of the cell cycle. IEC-18 (A), ras-3 (B), or IEC-Bcl-X cells (F) were cultured attached to or detached from the ECM for 24 h and analyzed for the cell cycle profile by flow cytometry. C, IEC-18 cells were transfected with a control non-targeting RNA (cont RNA) or Beclin-1-specific siRNA-1 (Beclin siRNA-1) or Beclin-1-specific siRNA-2 (Beclin siRNA-2) and analyzed for Beclin-1 expression by Western blot. CDK-4 was used as a loading control. D, cells processed as in C were analyzed as in A. E, IEC-18 or IEC-BclX cells transfected with the indicated RNAs as in C, were then plated in monolayer either immediately or after being cultured in suspension for 26 h and allowed to form colonies, which were counted 7–10 days later. Results are expressed as a percentage of the number of colonies obtained after culturing cells in monolayer immediately after transfection. The data in D represent the average of two independent experiments plus the S.D. The data in A, B, E, and F represent the average of the duplicates plus the S.D. All experiments were repeated twice with similar results. *, p value <0.05.
Thus, down-regulation of Beclin-1 can rescue non-malignant intestinal epithelial cells from detachment-induced growth arrest but not from detachment-induced apoptosis. By contrast, the reversal of the anoikis-inducing mechanism, such as that driven by detachment-induced down-regulation of Bcl-XL protects the indicated cells from anoikis but not from detachment-induced growth arrest. Not surprisingly, oncogenic ras, which is capable of both significantly reducing detachment-induced growth arrest of IEC-18 cells (Fig. 6B) and blocking their anoikis (17), down-regulates Beclin-1 and, at the same time, according to our previous studies, up-regulates Bcl-XL (10) (as well as triggers several other anti-anoikis mechanisms (12, 17, 23)) in the indicated cells. Conceivably, all of these mechanisms cooperate with each other to allow ras-transformed cells to survive and proliferate following detachment from the ECM.
Oncogenic Ras Reduces the Stability of Beclin-1 in Intestinal Epithelial Cells
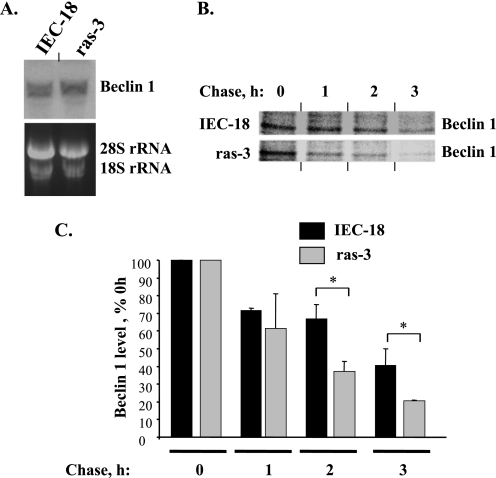
To investigate the mechanisms by which ras oncogene down-regulates Beclin-1 in intestinal epithelial cells we performed the Northern blot analysis of the levels of Beclin-1 mRNA in IEC-18 cells and their ras-transformed derivatives and found that, unlike Beclin-1 protein, the mRNA levels are not reduced by ras in the indicated cells (Fig. 7A). By contrast, according to the data derived from the pulse-chase assay, the stability of Beclin-1 protein was noticeably reduced by oncogenic ras (Fig. 7, B and C). Thus, Ras down-regulates Beclin-1 in intestinal epithelial cells by stimulating Beclin-1 degradation.
FIGURE 7.
Oncogenic ras reduces the stability of Beclin-1 in intestinal epithelial cells. A, attached IEC-18 and ras-3 cells were assayed for Beclin-1 expression by Northern blot. 18 S and 28 S ribosomal RNAs were used as loading controls. B, IEC-18 (top) and ras-3 (bottom) cells were cultured in the presence of 35S-labeled methionine and cysteine, which were then diluted with respective unlabeled amino acids, cells were collected at the indicated times, Beclin-1 was immunoprecipitated from the cells, and Beclin-1 levels were analyzed by electrophoresis in polyacrylamide gel followed by autoradiography. The contrast of the image corresponding to ras-3 cells was increased to illustrate the fact that the rate of reduction of Beclin-1 levels in ras-3 cells is higher than that in IEC-18 cells. C, cells were processed as in A, and Beclin-1 levels were quantified by phosphorimaging and densitometry. Beclin-1 level in each cell line at each time point is expressed as a percentage of that observed in the same cell line at 0-h chase. The data represent the average of the duplicates plus the S.D. This experiment was repeated twice with similar results. *, p value <0.05.
Oncogenic Ras Promotes the Degradation of Beclin-1 in Intestinal Epithelial Cells Via a Calpain-dependent Mechanism
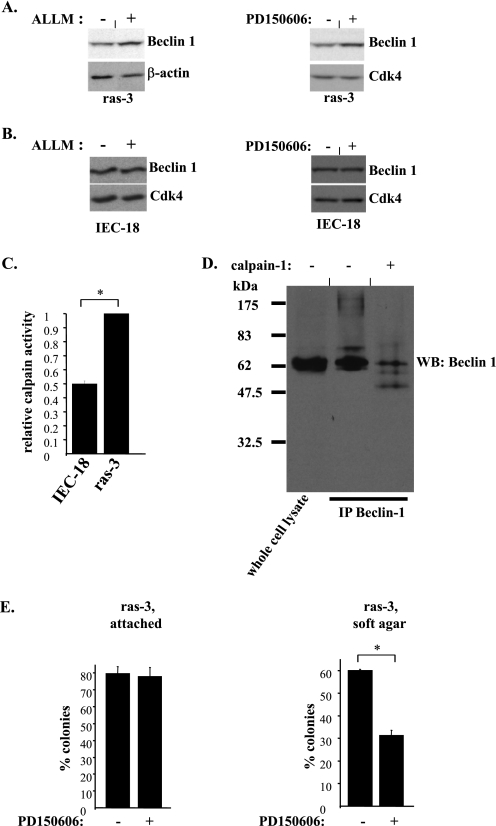
In an effort to identify the mechanisms by which oncogenic ras blocks the stability of Beclin-1 we found that the levels of Beclin-1 can not be increased in the ras-transformed cells by treatment with proteasomal inhibitors, such as MG132 and lactacystin (not shown). By contrast, treatment with a tetrapeptide ALLM, which is known to suppress proteolytic calpain activity (44), did reverse the effect of Ras on Beclin-1 (Fig. 8A, left), suggesting that calpain activity is required for Ras-dependent down-regulation of Beclin-1. (Calpains are Ca2+-dependent proteases that are thought to be involved in the control of proliferation, apoptosis, and cell migration. The mammalian calpain gene superfamily contains 16 known genes (45).) To confirm the possibility that ras down-regulates Beclin-1 in a calpain-dependent manner we treated the ras-transformed cells with PD150606, a small molecule calpain inhibitor that is structurally unrelated to ALLM (46). We found that, similar to what was observed with ALLM, treatment with PD150606 does result in the up-regulation of Beclin-1 in these cells (Fig. 8A, right). By contrast, treatment of the parental IEC-18 cells with ALLM and PD150606 did not cause the up-regulation of Beclin-1 in these cells (Fig. 8B). We also observed that calpain activity (determined on the basis of the degree of cleavage of a fluorigenic peptide that served as a model calpain substrate) is significantly higher in the lysates derived from the ras-transformed cells than that in the lysates derived form the parental IEC-18 cells (Fig. 8C). These data are consistent with the results obtained by others indicating that oncogenic K-ras increases calpain activity in Rat-1 fibroblasts (47). We further found that direct in vitro calpain treatment of Beclin-1 that was immunoprecipitated from tetracycline-treated ras-tet-Beclin cells (these cells were used as they represented a source of relatively large amounts of Beclin-1) results in Beclin-1 degradation (Fig. 8D). Moreover, we established that, similar to what was observed in the case of ras-transformed cells expressing exogenous Beclin-1 (Fig. 5A), exposure to PD150606 substantially blocked the ability of these cells to grow anchorage independently as colonies in soft agar (Fig. 8E). Collectively, these data indicate that ras oncogene promotes anchorage-independent growth of malignant intestinal epithelial cells, at least in part, by triggering calpain activation and subsequent calpain-induced degradation of Beclin-1 in these cells.
FIGURE 8.
Oncogenic ras down-regulates Beclin-1 in intestinal epithelial cells in a calpain-dependent manner. A, ras-3 cells were cultured attached to the ECM and treated with 10 μm ALLM (left) for 0 h (−) or 24 h (+) or 60 μm PD150606 (right) for 0 h (−) or 4 h (+) and analyzed for Beclin-1 expression by Western blot. CDK-4 was used as a loading control. Treatment with vehicle (DMSO) did not increase Beclin-1 expression in ras-3 cells under the conditions indicated above (not shown). B, IEC-18 cells were processed as in A. C, IEC-18 and ras-3 cells were cultured attached to the ECM for 24 h and assayed for calpain activity. Calpain activity observed in ras-3 cells was arbitrarily designated as 1.0. D, tet-ras-Beclin cells were treated with 800 ng/ml tetracycline for 24 h (to generate Beclin-1 in quantities sufficient for further analysis), Beclin-1 was immunoprecipitated from these cells, the immunoprecipitated material was not treated (−) or treated (+) with calpain-1 and analyzed for Beclin-1 expression by Western blot. E, ras-3 cells were analyzed for the ability to form colonies in monolayer culture (attached) or in soft agar (soft agar) as in Fig. 5A in the absence (−) and in the presence (+) of 60 μm PD150606. The data in C and E represent the average of the duplicates plus the S.D. These experiments were repeated twice with similar results. *, p value <0.05.
ras-induced Calpain Activation and Beclin-1 Down-regulation in Intestinal Epithelial Cells Requires the Presence of RhoA
We further decided to identify the mechanisms, by which oncogenic ras promotes calpain activation and Beclin-1 down-regulation, in intestinal epithelial cells. To this end, we treated the ras-transformed cells with PD98059 and LY294002, the small molecule inhibitors of two Ras-dependent signaling pathways that are mediated by protein kinases Mek and PI3K, respectively (the role of these two pathways in Ras signaling has so far been understood in more detail than that of other numerous effectors of Ras (24)). We found that neither inhibitor reversed the effect of Ras on Beclin-1 (not shown). We then reasoned that one well established and important mediator of Ras signaling, the activation of which by oncogenic Ras in malignant intestinal epithelial cells does not appear to require Mek or PI3K, is a small GTPase RhoA (25, 48). RhoA is known to be activated by oncogenic Ras via cell type-specific mechanisms. For example, in fibroblasts this activation requires the activity of Mek (49), whereas in the kidney epithelial cells it is the activity of PI3K that mediates Ras-dependent activation of RhoA (50). By contrast, in colon cancer cells, activation of RhoA by oncogenic Ras does not appear to require Mek or PI3K activity (48), and the mechanisms by which Ras activates RhoA in these cells remain to be understood.
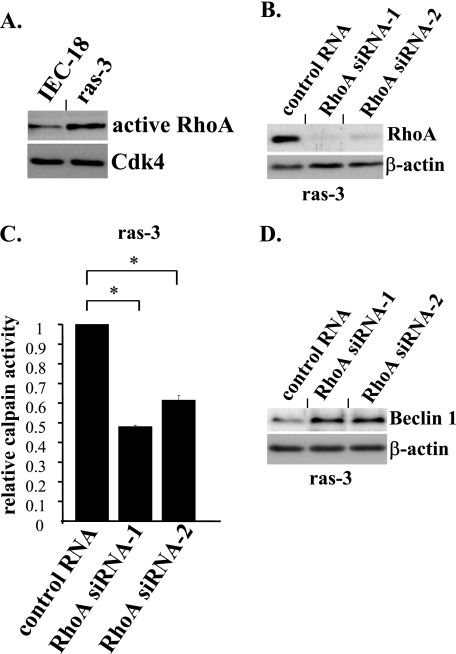
Importantly for this study: others observed (48) that RhoA activity is increased in colon carcinoma HCT-116 cells compared with their oncogenic K-ras knock-out derivatives Hkh-2 and Hke-3 used in this study (see Figs. 2D and 3C). We thus confirmed that the activity of RhoA is also increased in the ras-transformed IEC cells compared with that in the parental non-malignant intestinal epithelial cells IEC-18. To this end, we used an assay based on the ability of activated, GTP-bound RhoA to form a complex with the Rho-binding domain of the Rho binding partner Rhotekin (Fig. 9A). We further found that ablation of RhoA by RNA interference (Fig. 9B) in the ras-transformed cells results in a significant reduction of calpain activity (Fig. 9C) and an increase in Beclin-1 levels (Fig. 9D) in these cells. Thus, ras-induced calpain activation and subsequent Beclin-1 down-regulation in intestinal epithelial cells requires the presence of the Ras effector RhoA.
FIGURE 9.
Oncogenic ras triggers calpain activity and down-regulates Beclin-1 in intestinal epithelial cells in a RhoA-dependent manner. A, IEC-18 and ras-3 cells were cultured attached to the ECM for 24h, respective cell lysates were incubated with glutathione-agarose linked to the Rho binding domain of Rhotekin (Rho binding partner), and RhoA captured in this manner was detected by Western blot. B and D, ras-3 cells were transfected with a control non-targeting RNA (cont RNA) or RhoA-specific siRNA-1 (RhoA siRNA-1) or RhoA-specific siRNA-2 (RhoA siRNA-2) and analyzed for RhoA (B) or Belin-1 (D) expression by Western blot. β-Actin was used as a loading control. C, ras-3 cells were processed as in B and analyzed for calpain activity as in Fig. 8B. Calpain activity observed in ras-3 cells that were transfected with a control RNA was arbitrarily designated as 1.0. The data represent the average of the duplicates plus the S.D. This experiment was repeated twice with similar results. *, p value <0.05.
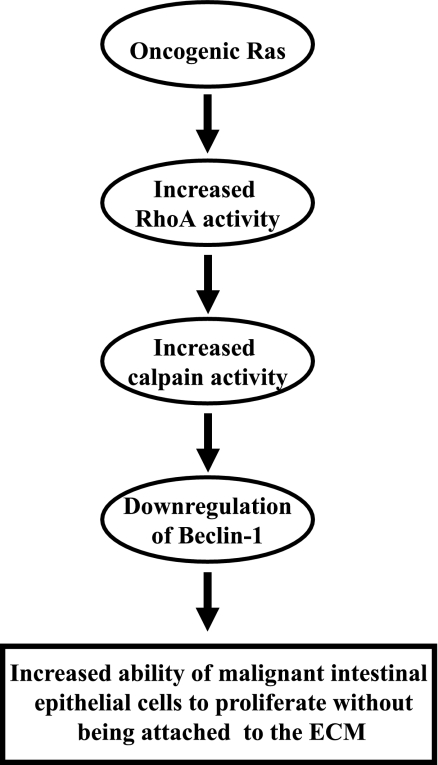
In summary, we have identified in this study a novel mechanism of Ras-induced anchorage-independent growth of malignant intestinal epithelial cells. This mechanism (schematically outlined in Fig. 10) is mediated by Ras-induced activation of RhoA, RhoA-dependent calpain activation, calpain-dependent degradation of Beclin-1, and subsequent increase in the ability of the ras-transformed cells to enter the S phase of the cell cycle and proliferate in the absence of adhesion to the ECM.
FIGURE 10.
Schematic representation of the mechanism by which oncogenic Ras promotes proliferation of malignant intestinal epithelial cells in the absence of adhesion to the ECM. Oncogenic Ras activates RhoA, which in turn increases cellular calpain activity. The latter event leads to the degradation of Beclin-1 and subsequent increase of the ability of oncogenic Ras-carrying malignant cells to proliferate without being attached to the ECM.
DISCUSSION
We have identified in this study a novel mechanism by which ras, a major oncogene, promotes anchorage-independent growth of malignant intestinal epithelial cells. This mechanism involves Ras-induced increase in activity of a small GTPase RhoA and subsequent RhoA-dependent calpain activation followed by calpain-driven degradation of Beclin-1. This down-regulation of Beclin-1, in turn, allows detached cancer cells to proliferate in the absence of adhesion to the ECM.
It is now known that to be able to grow in the absence of adhesion to the ECM (and this ability represents a critical prerequisite for cancer progression) malignant epithelial cells need to be able to survive following detachment (5) and then proliferate (3), two properties that their normal counterparts usually do not possess. Oncogenes, such as ras, can confer both of these properties to cancer cells (4), and several mechanisms by which Ras blocks detachment-induced apoptosis (anoikis) were identified by us (10, 12, 17, 23). By contrast, the mechanisms by which Ras allows cancer cells to proliferate in the absence of adhesion to the ECM have remained essentially unknown. In this study we for the first time demonstrated that Ras promotes proliferation of detached cancer cells by down-regulating Beclin-1, a critical component of cellular machinery that controls autophagy. Remarkably, the existence of this mechanism could not be predicted based on the current knowledge regarding the signaling pathways that link Ras with the cell cycle machinery. ras oncogene is well known to be able to deregulate the cell cycle progression by enhancing transcription of bona fide mediators of the cell cycle, such as cyclin D1 (41). However, our data demonstrate that Ras promotes anchorage-independent proliferation of malignant cells via a novel mechanism that involves the down-regulation of Beclin-1. Because autophagy represents a cellular mechanism of protein degradation (51), it is conceivable that Ras-dependent down-regulation of Beclin-1 ultimately prevents (or reduces the degree of) autophagic degradation of a protein (or proteins) that directly or indirectly control the cell cycle progression.
Our data indicating that Ras-induced down-regulation of Beclin-1 is required for Ras-dependent anchorage-independent growth of cancer cells well agree with numerous observations indicating that Beclin-1 can act as a tumor suppressor. It is known in this regard that beclin-1 gene is located at the tumor susceptibility locus 17q21 that is frequently lost monoallelically in various types of cancer (29). Furthermore, loss of one beclin-1 allele was demonstrated to often lead to the emergence of various tumors in mice (30, 31). In addition, loss of Beclin-1 binding partner and agonist Bif-1 was found to result in the development of spontaneous tumors in various mouse organs (34). Finally, UVRAG, another agonist of Beclin-1, tends to be monoallelically deleted in human colon cancer and, when overexpressed in human colon cancer cells, blocks their proliferation and tumorigenicity (35).
The role of Beclin-1 in cancer progression is not well understood. It was found in this regard that, depending on the circumstances, loss of Beclin-1 can increase cancer cell survival, facilitate proliferation of cancer cells without affecting their viability or, under certain conditions, even promote death of these cells (51). This study for the first time indicates that Beclin-1 represents a mediator of an important oncogenic mechanism that is driven by ras oncogene and allows malignant cells to proliferate in the absence of adhesion to the ECM. Our data are consistent with findings indicating that cells composing neoplastic lesions that spontaneously occur in mice lacking one beclin-1 allele possess increased ability to proliferate, whereas loss of the indicated allele does not affect the susceptibility of mouse cells to apoptosis (30). Likewise, it was found that the expression of exogenous Beclin-1 in breast carcinoma cells blocks their proliferation but does not kill them (33).
The fact that oncogenic ras-induced down-regulation of Beclin-1 promotes proliferation but not survival of detached cancer cells is particularly remarkable in light of observations indicating that, under certain circumstances (the nature of which remains to be understood), Beclin-1 is, in principle, capable of causing cell death (52, 53) and that the resistance of malignant cells to detachment-induced apoptosis (anoikis) represents a critical requirement for their ability to grow anchorage independently (4, 17). Thus, it appears that the role of Beclin-1 in Ras-dependent transformation of intestinal epithelial cells is highly specialized in that Beclin-1 is involved only in a particular aspect of the effect of Ras on malignant cells, namely, the ability of these cells to proliferate without being attached to the ECM.
Interestingly, we have found that treatments resulting in the increased expression of Beclin-1 in cancer cells promoted a much more pronounced degree of the formation of autophagic vacuoles in these cells as well as a much more noticeable inhibition of their proliferation when they were detached from the ECM compared with what was observed in the attached cells (see Figs. 4B and 5). Thus, it seems likely that a certain threshold level of Beclin-1 expression is necessary for the induction of autophagy and subsequent inhibition of cell growth triggered by signals that are induced by cell detachment. In agreement with this possibility, when levels of Beclin-1 in cells are reduced, for example as a result of the presence of oncogenic ras, signals that are triggered by detachment of these cells from the ECM appear to be by themselves insufficient for stimulating autophagosome formation and blocking the progression of cells through the cell cycle to the same degree as in the case of the cells that do not carry ras oncogene.
Our studies have identified a novel signaling pathway mediated by Ras-induced activation of RhoA, RhoA-dependent calpain activation, and subsequent calpain-triggered Beclin-1 degradation. Interestingly, the expression of RhoA (54) and that of m-calpain (55), one of 16 members of the family of calpains, was proposed to be often elevated in colon carcinoma, which is consistent with our observations suggesting that RhoA and calpains contribute to the progression of this disease. Identification of the mechanisms by which Ras activates RhoA and of those by which RhoA promotes calpain activation as well as establishing which of the members of the calpain family of proteases are involved in the effect of Ras on Beclin-1 represent the subject of our ongoing research.
In summary, our data indicate that the ability of ras oncogene-carrying malignant intestinal epithelial cells to grow anchorage independently requires Ras-induced down-regulation of Beclin-1. Thus, therapeutic treatment aimed at the elevation of Beclin-1 levels in colorectal cancer cells harboring oncogenic ras could serve as a novel approach for blocking the ability of Ras-driven tumors to grow in a three-dimensional manner.
Acknowledgments
We are grateful to Drs. B. Levine and T. Yoshimori for the materials provided to us and to Dr. K. Ritchie for assisting us with statistical analysis of the data.
This work was supported by a grant from the Canadian Cancer Society Research Institute (formerly the National Cancer Institute of Canada).
- ECM
- extracellular matrix
- PI3K
- phosphoinositide 3-OH-kinase
- GFP
- green fluorescent protein
- PBS
- phosphate-buffered saline
- siRNA
- small interference RNA
- Mek
- mitogen-activated protein kinase/extracellular signal-regulated kinase kinase.
REFERENCES
- 1.Jacks T., Weinberg R. A. (2002) Cell 111, 923–925 [DOI] [PubMed] [Google Scholar]
- 2.Frisch S. M., Screaton R. A. (2001) Curr. Opin. Cell Biol. 13, 555–562 [DOI] [PubMed] [Google Scholar]
- 3.Benaud C. M., Dickson R. B. (2001) Oncogene 20, 4554–4567 [DOI] [PubMed] [Google Scholar]
- 4.Rak J., Mitsuhashi Y., Erdos V., Huang S. N., Filmus J., Kerbel R. S. (1995) J. Cell Biol. 131, 1587–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frisch S. M., Francis H. (1994) J. Cell Biol. 124, 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ljubimov A. V., Bartek J., Couchman J. R., Kapuller L. L., Veselov V. V., Kovarik J., Perevoshchikov A. G., Krutovskikh V. A. (1992) Int. J. Cancer 50, 562–566 [DOI] [PubMed] [Google Scholar]
- 7.Douma S., Van Laar T., Zevenhoven J., Meuwissen R., Van Garderen E., Peeper D. S. (2004) Nature 430, 1034–1039 [DOI] [PubMed] [Google Scholar]
- 8.Berezovskaya O., Schimmer A. D., Glinskii A. B., Pinilla C., Hoffman R. M., Reed J. C., Glinsky G. V. (2005) Cancer Res. 65, 2378–2386 [DOI] [PubMed] [Google Scholar]
- 9.Freedman V. H., Shin S. I. (1974) Cell 3, 355–359 [DOI] [PubMed] [Google Scholar]
- 10.Rosen K., Rak J., Leung T., Dean N. M., Kerbel R. S., Filmus J. (2000) J. Cell Biol. 149, 447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen K., Coll M. L., Li A., Filmus J. (2001) J. Biol. Chem. 276, 37273–37279 [DOI] [PubMed] [Google Scholar]
- 12.Liu Z., Li H., Derouet M., Filmus J., LaCasse E. C., Korneluk R. G., Kerbel R. S., Rosen K. V. (2005) J. Biol. Chem. 280, 37383–37392 [DOI] [PubMed] [Google Scholar]
- 13.Orford K., Orford C. C., Byers S. W. (1999) J. Cell Biol. 146, 855–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H., Ray G., Yoo B. H., Erdogan M., Rosen K. V. (2009) J. Biol. Chem. 284, 2012–2022 [DOI] [PubMed] [Google Scholar]
- 15.Lu Y., Lin Y. Z., LaPushin R., Cuevas B., Fang X., Yu S. X., Davies M. A., Khan H., Furui T., Mao M., Zinner R., Hung M. C., Steck P., Siminovitch K., Mills G. B. (1999) Oncogene 18, 7034–7045 [DOI] [PubMed] [Google Scholar]
- 16.Duxbury M. S., Ito H., Zinner M. J., Ashley S. W., Whang E. E. (2004) Oncogene 23, 1448–1456 [DOI] [PubMed] [Google Scholar]
- 17.Rosen K., Rak J., Jin J., Kerbel R. S., Newman M. J., Filmus J. (1998) Curr. Biol. 8, 1331–1334 [DOI] [PubMed] [Google Scholar]
- 18.Scotlandi K., Maini C., Manara M. C., Benini S., Serra M., Cerisano V., Strammiello R., Baldini N., Lollini P. L., Nanni P., Nicoletti G., Picci P. (2002) Cancer Gene Ther. 9, 296–307 [DOI] [PubMed] [Google Scholar]
- 19.Duxbury M. S., Ito H., Zinner M. J., Ashley S. W., Whang E. E. (2004) Oncogene 23, 465–473 [DOI] [PubMed] [Google Scholar]
- 20.Jiang K., Sun J., Cheng J., Djeu J. Y., Wei S., Sebti S. (2004) Mol. Cell. Biol. 24, 5565–5576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derouet M., Wu X., May L., Hoon Yoo. B., Sasazuki T., Shirasawa S., Rak J., Rosen K. V. (2007) Neoplasia 9, 536–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox A. D., Der C. J. (2003) Oncogene 22, 8999–9006 [DOI] [PubMed] [Google Scholar]
- 23.Liu Z., Li H., Derouet M., Berezkin A., Sasazuki T., Shirasawa S., Rosen K. (2006) J. Biol. Chem. 281, 14738–14747 [DOI] [PubMed] [Google Scholar]
- 24.Shields J. M., Pruitt K., McFall A., Shaub A., Der C. J. (2000) Trends Cell Biol. 10, 147–154 [DOI] [PubMed] [Google Scholar]
- 25.Khosravi-Far R., Campbell S., Rossman K. L., Der C. J. (1998) Adv. Cancer Res. 72, 57–107 [DOI] [PubMed] [Google Scholar]
- 26.Tsujimoto Y., Shimizu S. (2005) Cell Death. Differ. 12, Suppl. 2, 1528–1534 [DOI] [PubMed] [Google Scholar]
- 27.Kroemer G., Jäättelä M. (2005) Nat. Rev. Cancer 5, 886–897 [DOI] [PubMed] [Google Scholar]
- 28.Crighton D., Wilkinson S., O'Prey J., Syed N., Smith P., Harrison P. R., Gasco M., Garrone O., Crook T., Ryan K. M. (2006) Cell 126, 121–134 [DOI] [PubMed] [Google Scholar]
- 29.Aita V. M., Liang X. H., Murty V. V., Pincus D. L., Yu W., Cayanis E., Kalachikov S., Gilliam T. C., Levine B. (1999) Genomics 59, 59–65 [DOI] [PubMed] [Google Scholar]
- 30.Qu X., Yu J., Bhagat G., Furuya N., Hibshoosh H., Troxel A., Rosen J., Eskelinen E. L., Mizushima N., Ohsumi Y., Cattoretti G., Levine B. (2003) J. Clin. Invest. 112, 1809–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yue Z., Jin S., Yang C., Levine A. J., Heintz N. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 15077–15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B. X., Li C. Y., Peng R. Q., Wu X. J., Wang H. Y., Wan D. S., Zhu X. F., Zhang X. S. (2009) Autophagy 5, 303–306 [DOI] [PubMed] [Google Scholar]
- 33.Liang X. H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H., Levine B. (1999) Nature 402, 672–676 [DOI] [PubMed] [Google Scholar]
- 34.Takahashi Y., Coppola D., Matsushita N., Cualing H. D., Sun M., Sato Y., Liang C., Jung J. U., Cheng J. Q., Mulé J. J., Pledger W. J., Wang H. G. (2007) Nat. Cell Biol. 9, 1142–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang C., Feng P., Ku B., Dotan I., Canaani D., Oh B. H., Jung J. U. (2006) Nat. Cell Biol. 8, 688–699 [DOI] [PubMed] [Google Scholar]
- 36.McCarty K. S., Jr., Szabo E., Flowers J. L., Cox E. B., Leight G. S., Miller L., Konrath J., Soper J. T., Budwit D. A., Creasman W. T. (1986) Cancer Res. 46, 4244s–4248s [PubMed] [Google Scholar]
- 37.Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. (1987) Nature 327, 293–297 [DOI] [PubMed] [Google Scholar]
- 38.Shirasawa S., Furuse M., Yokoyama N., Sasazuki T. (1993) Science 260, 85–88 [DOI] [PubMed] [Google Scholar]
- 39.Edkins S., O'Meara S., Parker A., Stevens C., Reis M., Jones S., Greenman C., Davies H., Dalgliesh G., Forbes S., Hunter C., Smith R., Stephens P., Goldstraw P., Nicholson A., Chan T. L., Velculescu V. E., Yuen S. T., Leung S. Y., Stratton M. R., Futreal P. A. (2006) Cancer Biol. Ther. 5, 928–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brink M., de Goeij A. F., Weijenberg M. P., Roemen G. M., Lentjes M. H., Pachen M. M., Smits K. M., de Bruïne A. P., Goldbohm R. A., van den Brandt P. A. (2003) Carcinogenesis 24, 703–710 [DOI] [PubMed] [Google Scholar]
- 41.Filmus J., Robles A. I., Shi W., Wong M. J., Colombo L. L., Conti C. J. (1994) Oncogene 9, 3627–3633 [PubMed] [Google Scholar]
- 42.Pattingre S., Tassa A., Qu X., Garuti R., Liang X. H., Mizushima N., Packer M., Schneider M. D., Levine B. (2005) Cell 122, 927–939 [DOI] [PubMed] [Google Scholar]
- 43.Eskelinen E. L. (2005) Autophagy 1, 1–10 [DOI] [PubMed] [Google Scholar]
- 44.Sasaki T., Kishi M., Saito M., Tanaka T., Higuchi N., Kominami E., Katunuma N., Murachi T. (1990) J. Enzyme Inhib. 3, 195–201 [DOI] [PubMed] [Google Scholar]
- 45.Goll D. E., Thompson V. F., Li H., Wei W., Cong J. (2003) Physiol. Rev. 83, 731–801 [DOI] [PubMed] [Google Scholar]
- 46.Wang K. K., Nath R., Posner A., Raser K. J., Buroker-Kilgore M., Hajimohammadreza I., Probert A. W., Jr., Marcoux F. W., Ye Q., Takano E., Hatanaka M., Maki M., Caner H., Collins J. L., Fergus A., Lee K. S., Lunney E. A., Hays S. J., Yuen P. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 6687–6692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carragher N. O., Fonseca B. D., Frame M. C. (2004) Neoplasia 6, 53–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollock C. B., Shirasawa S., Sasazuki T., Kolch W., Dhillon A. S. (2005) Cancer Res. 65, 1244–1250 [DOI] [PubMed] [Google Scholar]
- 49.Chen J. C., Zhuang S., Nguyen T. H., Boss G. R., Pilz R. B. (2003) J. Biol. Chem. 278, 2807–2818 [DOI] [PubMed] [Google Scholar]
- 50.Watnick R. S., Cheng Y. N., Rangarajan A., Ince T. A., Weinberg R. A. (2003) Cancer Cell 3, 219–231 [DOI] [PubMed] [Google Scholar]
- 51.Levine B., Yuan J. (2005) J. Clin. Investig. 115, 2679–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Høyer-Hansen M., Bastholm L., Mathiasen I. S., Elling F., Jäättelä M. (2005) Cell Death. Differ. 12, 1297–1309 [DOI] [PubMed] [Google Scholar]
- 53.Shimizu S., Kanaseki T., Mizushima N., Mizuta T., Arakawa-Kobayashi S., Thompson C. B., Tsujimoto Y. (2004) Nat. Cell Biol. 6, 1221–1228 [DOI] [PubMed] [Google Scholar]
- 54.Fritz G., Just I., Kaina B. (1999) Int. J. Cancer 81, 682–687 [DOI] [PubMed] [Google Scholar]
- 55.Lakshmikuttyamma A., Selvakumar P., Kanthan R., Kanthan S. C., Sharma R. K. (2004) Cancer Epidemiol. Biomarkers Prev. 13, 1604–1609 [PubMed] [Google Scholar]