Abstract
Apoptosis plays important roles in host defense, including the elimination of virus-infected cells. The executioners of apoptosis are caspase family proteases. We report that vaccinia virus-encoded F1L protein, previously recognized as anti-apoptotic viral Bcl-2 family protein, is a caspase-9 inhibitor. F1L binds to and specifically inhibits caspase-9, the apical protease in the mitochondrial cell death pathway while failing to inhibit other caspases. In cells, F1L inhibits apoptosis and proteolytic processing of caspases induced by overexpression of caspase-9 but not caspase-8. An N-terminal region of F1L preceding the Bcl-2-like fold accounts for caspase-9 inhibition and significantly contributes to the anti-apoptotic activity of F1L. Viral F1L thus provides the first example of caspase inhibition by a Bcl-2 family member; it functions both as a suppressor of proapoptotic Bcl-2 family proteins and as an inhibitor of caspase-9, thereby neutralizing two sequential steps in the mitochondrial cell death pathway.
Keywords: Apoptosis, Caspase, Cell Death, Pox Viruses, Protein-Protein Interactions, Bcl-2, F1L, Apoptosis, Caspase, Host Defense
Introduction
Viruses have evolved multiple strategies to prevent or delay host anti-viral responses. Apoptosis is a host mechanism used to combat viral infections by eliminating virus-producing cells. By inhibiting apoptosis, viruses can ensure adequate time to replicate their genomes, as well as providing a possible mechanism for achieving chronic latent infections. Suppression of apoptosis can also contribute to escape from host immune surveillance, allowing virus-infected cells to withstand attack by cytolytic T-cells and thus continue viral production (1, 2).
Viruses have either evolved independently or usurped from host cells a diversity of anti-apoptotic strategies. Apoptosis is mediated by caspases, a family of intracellular cysteine proteases. Consequently, viruses often target either the caspases or their upstream cellular activators. For example, viral homologs of anti-apoptotic Bcl-2 family proteins have been identified in poxviruses, herpesviruses, adenoviruses, and other viruses. These proteins suppress a major pathway for caspase activation and cell death that involves mitochondria, which is often referred to as the “intrinsic” pathway (reviewed in Ref. 3). Multiple signals converge on mitochondria, including DNA damage, hypoxia, and oxidative stress, which modulate the expression or activity of various cellular Bcl-2 family proteins that associate with these organelles. Bcl-2 family proteins either block or cause the release of cytotoxic mitochondrial proteins including cytochrome c, which binds to and induces oligomerization of Apaf1, a central component of a caspase-9-activating complex known as the “apoptosome” (1, 2, 4). Activation of apoptosome-associated caspase-9 initiates a proteolytic cascade whereby activated caspase-9 cleaves and activates downstream executioner proteases such as procaspase-3 and -7, resulting in apoptotic cell demise. Viruses encode homologs of cellular anti-apoptotic Bcl-2 proteins to protect infected host cells by keeping the mitochondria intact, and several viral Bcl-2 protein homologs have been reported to date (1, 2).
In addition to the intrinsic (mitochondrial) pathway, tumor necrosis factor (TNF)3 family death receptors transduce apoptotic signals into cells, constituting the so-called “extrinsic” pathway (1, 2, 5). Upon binding TNF family cytokine ligands, these death receptors oligomerize in membranes and recruit caspase-binding adapter proteins, forming a “death-inducing signaling complex,” which activates caspase-8 and -10. To interfere with this pathway, viruses encode various antagonists including 1) vFLIPs, viral homologs of cellular FLIP (cFLIP), which bind procaspase-8 and-10 and inhibit their activation (1, 2, 6); 2) CrmA, a viral serpin that binds irreversibly to and inhibits caspase-8 and -10 (7, 8); and 3) soluble decoy receptors that compete with host TNF family receptors to bind cognate ligands (reviewed in Refs. 1, 9, and 10). Virus-encoded antagonists of caspases also include p35 of baculoviruses (a broad spectrum, irreversible inhibitor of caspases) and viral IAPs (inhibitor of apoptosis proteins) found in some insect and animal viruses, which inhibit certain caspases (1, 9–11).
Vaccinia virus represents a prototypical poxvirus, a family of viruses that have large DNA genomes encoding >250 genes (1, 9, 10). Poxviruses synthesize numerous gene products that inhibit cell death during infection and that interfere with host immune responses. For example, poxviruses produce the extrinsic pathway antagonists CrmA and vFLIP and soluble variants of the TNF family receptors such as Myx-MT-2 and TPV-2L (1, 2, 9, 10). Inhibitors of the intrinsic apoptotic pathway have also been identified in vaccinia virus, including the viral proteins F1L and N1L (1, 12, 13). Although the primary sequence of N1L lacks motifs indicative of Bcl-2 homologs, the three-dimensional structure of N1L has been shown to be very similar to Bcl-2, and the N1L protein possesses anti-apoptotic activity resembling that of Bcl-2 (1, 12). F1L shares less than 10% amino acid sequence similarity with NIL, and unlike N1L, it contains a C-terminal transmembrane domain that anchors it to mitochondrial membranes. However, F1L also has the same three-dimensional protein fold that characterizes Bcl-2 family proteins (1, 14), and it binds certain proapoptotic Bcl-2 family members (e.g. Bak and Bim), suppressing mitochondria-dependent apoptosis (1, 15–17).
Here we provide evidence that the vaccinia virus protein F1L is a direct antagonist of caspase-9, thus revealing an additional and complementary anti-apoptotic activity for this viral protein. Interestingly, unlike previously described viral anti-apoptotic proteins that either inhibit active caspases or prevent caspase activation, F1L possesses the ability to do both; it inhibits the activity of active caspase-9 and also inhibits the recruitment and activation of procaspase-9 by the apoptosome. F1L thus represents the first example of direct caspase inhibition by a member of the Bcl-2 family.
EXPERIMENTAL PROCEDURES
Materials
Cytochrome c (from bovine heart), dATP, and anti-α-tubulin antibody were purchased from Sigma. Rabbit anti-caspase-3 and anti-SMAC antibodies have been described (18, 19). Anti-caspase-7 antibody (clone 9494) was purchased from Cell Signaling Technology (Danvers, MA). Anti-Apaf-1 antibody (clone 94408) was purchased from R&D Systems, Inc. (Minneapolis, MN). Anti-caspase-9 antibody (clone 96-2-22) was purchased from Upstate Biotechnology (Charlottesville, VA). F1L antibody was a gift from Michael Way (Cancer Research UK, London Research Institute). The caspase peptidyl substrates were purchased from BIOMOL (Plymouth Meeting, PA). GST mouse monoclonal antibody was produced in our laboratory.
Plasmids
The gene encoding F1L from vaccinia virus strain Western Reserve was subcloned into pEGFP-C1 or pFlag-CMV2 for mammalian cell expression (full-length, residues 1–226) or into pGEX 4T1 vector for bacterial expression (F1LΔΤΜ; C-terminal truncated; residues 1–206, lacking the C-terminal transmembrane domain). F1L mutants C7A and M67P and double mutant C7A/M67P were made by using a one-step, PCR-based mutagenesis kit (QuikChange, Stratagene). Plasmids encoding mouse Bid, human Bcl-XL, viral N1L, human caspase-3, -7, -8, and -9, caspase-9ΔCARD (residues 138–416), caspase-C9–5A, human XIAP BIR1–3, and human full-length Apaf-1 and Apaf-1ΔC (residues 1–591) have been described (12, (19–24). Genes or cDNAs encoding SMAC (residues 56–239; pET-15b), p35, and CrmA were subcloned into pET-21b plasmid for expression in bacteria as His6 fusion proteins.
Protein Expression and Purification
Bid, Bcl-XL, Apaf-1ΔC-(1–591), SMAC, p35, CrmA, and caspase-2, -3, -7, -8, and -9 proteins were produced in bacteria as His6-tagged proteins and purified using nickel-chelated agarose beads following previously described methods (19, 25). GST-F1LΔTM, GST-F1LΔTM C7A, and GST-F1LΔTM M67P fusion proteins were expressed in bacteria and affinity-purified using glutathione-Sepharose followed by proteolytic removal of GST by thrombin digestion essentially as described (19, 26). Full-length Apaf-1 protein was expressed in Sf9 insect cells at 27 °C for 60 h and purified by fast protein liquid chromatography as described (22).
Fluorescence Polarization Assays
Binding of SMAC peptide to F1LΔTM or XIAPs was measured by fluorescence polarization assays according to published procedures (27). Briefly, various concentrations of F1LΔTM or XIAPs were incubated in the dark in 384-well black plates (Greiner Bio-One) with 10–20 nm rhodamine-conjugated SMAC peptide (AVPIAQK-rhodamine) dissolved in water. Fluorescence polarization was measured using an Analyst TM AD assay detection system (LJL BioSystems, Sunnyvale, CA) in phosphate-buffered saline (PBS), pH 7.4. EC50 determinations were performed using GraphPad Prism software (GraphPad, Inc., San Diego, CA).
Caspase Activity Assays
Caspases were preincubated with F1LΔTM or other inhibitors for 10 min at 37 °C in 20 mm HEPES buffer, pH 7.2, and the reaction was performed in 100 μl of standard caspase assay buffer (50 mm HEPES, pH 7.4, 10% sucrose, 1 mm EDTA, 0.1% CHAPS, 100 mm NaCl, and 5 mm dithiothreitol) for 30 min at 37 °C. Caspase activity was measured by monitoring the cleavage of the fluorogenic tetrapeptide substrates Ac-DEVD-AFC (caspase-2, -3, and -7), Ac-IETD-AFC (caspase-8), and Ac-LEHD-AFC (caspase-9) (BIOMOL) at 100 μm. The generation of fluorogenic AFC (7-amino-4-trifluoromethylcoumarin) product was measured with a Molecular Devices fMAX fluorometer plate reader operating in kinetic mode for 30 min at 37 °C using excitation and emission wavelengths of 405 and 510 nm, respectively. Caspase-8 and -9 activities were also measured by monitoring the cleavage of their natural substrates, procaspase-3 and -7. The reactions were performed in caspase assay buffer at 37 °C for 60 min. The resulting samples were boiled and analyzed by SDS-PAGE and immunoblotting using anti-caspase-3 or -7 antibodies.
Enzyme Kinetics
Enzyme inhibition rates (ka) were determined. First, under pseudo-first order conditions, a constant amount of caspase-9 (activated by the apoptosome) was mixed with different concentrations of inhibitors and excess substrate. The amount of product formation (P) proceeds at an initial velocity (V) and is inhibited over time (t) at the following rate of inhibition (kobs): P = V/kobs × (1 − e−kobs × t) + C. Then, an apparent second order rate constant (k′) was determined by measuring the slope of various kobs values. The value of the second order rate constant (ka) was then calculated as ka = k′ × (1 + [S]/Km), where Km for caspase-9 in the assays was 0.6 mm (20, 23).
Caspase Cleavage Assays
Recombinant F1LΔTM (40 μm) or caspase-3 (C163A) (40 μm) was mixed with Apaf-1 (4 μm), cytochrome c (10 μm), procaspase-9 (4 μm), and dATP (200 μm) with or without benzyloxycarbonyl-VAD-fluoromethyl ketone (Z-VAD-fmk; 10 μm) in standard caspase assay buffer and incubated at 37 °C for 20 h. Samples were analyzed by SDS-PAGE and stained with Coomassie Blue.
Apoptosome Assembly
S-100 cell lysates from HeLa cells were obtained as reported previously (4) and activated by adding bovine cytochrome c (1 μm) and dATP (200 μm) for 20 min at 37 °C in reconstitution buffer (20 mm HEPES, pH 7.2, 2 mm MgCl2, and 10 mm KCl). For in vitro reconstituted apoptosomes, recombinant procaspase-9 (200 nm) was incubated with 200 μm dATP and with either recombinant Apaf-1ΔC in the absence of cytochrome c or full-length Apaf-1 (1 μm) together with cytochrome c for 10 min at 37 °C. Caspase-9 activity was measured by hydrolysis of the peptide substrate Ac-LEHD-AFC as described above.
Cell Culture, Transfection, and Apoptosis Assays
HEK293T, HeLa, or mouse embryonic fibroblast Apaf-1−/− cells were maintained in Dulbecco's modified Eagle's medium (Irvine Scientific, Irvine, CA) supplemented with 10% fetal bovine serum, 1 mm l-glutamine, and antibiotics. For transient transfection apoptosis assays, cells (5 × 105) in 6-well plates were co-transfected using Lipofectamine 2000 (Invitrogen) with 0.5 μg each of pEGFP-C1 (Clontech, Mountain View, CA) or pcDNA3-FLAG-caspase-9 or -8 together with various amounts of GFP-F1L or FLAG-F1L plasmids or F1L mutant M67P. For caspase assays, cell lysates prepared 20 h after transfection were normalized for protein content, and then 10-μg aliquots of cell lysates were incubated with 100 μm Ac-DEVD-AFC. Enzyme activity was determined by the loss of AFC fluorescence.
Apoptosis was assessed by staining cells with 4,6-diamidino-2-phenylindole (DAPI). Briefly, 20 h after transfection, both floating and adherent cells were collected, fixed with PBS containing 3.7% formaldehyde, and stained with 0.1 mg/ml DAPI in PBS. The percentages of apoptotic cells were determined by UV microscopy by counting GFP-positive cells having nuclear fragmentation and/or chromatin condensation. All assays were performed in triplicate.
In Vitro Protein Binding and Competition Assays
For binding assays, recombinant GST or GST-F1LΔTM proteins (1 μg) were incubated with 1 μg of active caspase-9 at 4 °C for 4 h in PBS together with 10 μl of glutathione-Sepharose 4B resin. For competition assays, GST-F1LΔTM was preincubated with caspase-9 at 4 °C for 4 h, the resin was spit into equal parts, and various concentrations of untagged recombinant F1L proteins was introduced to compete the binding at 4 °C for overnight. The resulting resin was washed three times using PBS buffer, and the associated proteins were boiled and analyzed by SDS-PAGE and immunoblotting using anti-caspase-9 or anti-GST antibodies.
Gel Filtration
A Superdex 200 HR 10/30 column (GE Healthcare) was equilibrated with buffer (20 mm HEPES, pH 7.4, and 100 mm NaCl). Then, 100-μl samples in 20 mm HEPES buffer were loaded onto the column, and 0.5-ml fractions were collected. Samples (100 μl) consisted of 1 μm caspase-9 alone or premixed with 5 μm F1LΔTM or with apoptosome components (5 μm Apaf-1, 5 μm cytochrome c, and 500 μm dATP) at 37 °C for 10 min prior to loading. Aliquots (30 μl) were analyzed by SDS-PAGE followed by immunoblotting using antibodies recognizing caspase-9, Apaf-1, or F1L.
RESULTS
F1L Inhibits Cytochrome c-induced Activation of Caspases
The viral caspase inhibitor CrmA is a well characterized anti-apoptotic protein of vaccinia virus. Nevertheless, vaccinia strains with CrmA gene deletions are still able to protect cells against numerous cell death signals, indicating that additional anti-apoptotic mechanisms are operative. A previous search for alternative mechanisms led to the discovery of F1L, a protein specific for the inhibition of the intrinsic cell death pathway (1, 13). F1L is anchored to mitochondria via its C-terminal hydrophobic tail. F1L inhibits apoptosis via binding to the proapoptotic Bcl-2 family proteins Bak and Bim (1, 15, 17).
We noticed that recombinant F1L protein, unlike Bcl-XL, inhibits caspase activation induced in cell extracts by cytochrome c (Fig. 1A). This is the first step downstream of mitochondria in the intrinsic pathway for apoptosis (reviewed in Refs. 1, 28, and 29).
FIGURE 1.
F1LΔTM inhibits cytochrome c-activated caspases in cell extracts. A and B, F1LΔTM (without GST tag), CrmA, N1L, p35, XIAP, and Bcl-XLΔTM (1 μm) proteins were preincubated with HeLa cell lysate (S100) for 10 min at 37 °C followed by the addition of cytochrome c (Cyt. c) and dATP to activate apoptosome (A) or 50 nm active caspase-8 (B) to activate procaspase-3/-7 in HEPES buffer for 20 min at 37 °C. Caspase-3/-7 activity was measured by hydrolysis of Ac-DEVD-AFC. C, F1LΔTM (2 μm without GST tag) was preincubated with HeLa cell lysate for 10 min at 37 °C followed by the addition of cytochrome c (500 nm) and dATP (200 μm) to activate apoptosome for 20 min at 37 °C. Alternatively, F1L (without GST tag) was added after the activation of apoptosome (indicated by an asterisk). Caspase-3 activity was measured by hydrolysis of Ac-DEVD-AFC (mean ± S.D.; n = 3). RFU, relative fluorescence units.
Specifically, we found that F1LΔTM recombinant protein (amino acids 1–206; produced without its C-terminal transmembrane domain to aid solubility) at 1 μm potently suppressed cytochrome c-induced caspase activation in cytosolic extracts, whereas another anti-apoptotic vaccinia protein, N1L, did not. We also tested the activities of other recombinant anti-apoptotic proteins in cell extracts, including baculovirus p35, cowpox virus CrmA, human cellular XIAP, and Bcl-XL, as controls. The p35 protein is a broad spectrum caspase inhibitor, whereas XIAP inhibits several caspases within the cytochrome c-activated pathway, including the proximal protease caspase-9 and its downstream substrates, caspase-3 and -7 (reviewed in Refs. 1 and 11). As expected, 1 μm p35 or XIAP potently inhibited cytochrome c-induced caspase activity in cell extracts (Fig. 1A). CrmA inhibited caspases involved in the extrinsic pathway (e.g. caspase-8 and -10) but not the intrinsic pathway. Also as expected, CrmA had little effect on the activation of DEVD-cleaving effector caspases in cytochrome c-stimulated extracts. Similarly, Bcl-XLΔTM had no effect (Fig. 1A), consistent with prior studies demonstrating that the mechanism of anti-apoptotic Bcl-2 proteins resides upstream of cytochrome c (1, 30). These findings raised the possibility that F1L might have additional targets besides proapoptotic Bcl-2 family proteins that control mitochondrial membrane integrity.
To determine whether F1L is a broad spectrum inhibitor of caspases, we stimulated effector caspase activation in cell extracts by adding recombinant active caspase-8, thus mimicking the extrinsic pathway (1, 5). In contrast to cytochrome c stimulation, F1LΔTM failed to inhibit caspase-8-mediated induction of DEVD-cleaving effector caspase activity in cell extracts (Fig. 1B). As expected, CrmA was inhibitory in these assays, consistent with its ability to directly bind to, and irreversibly inhibit caspase-8 (1, 31). p35 and XIAP were also inhibitory, as anticipated, whereas Bcl-XL was not. Thus, our data demonstrate that F1L selectively inhibits caspase activation within the intrinsic pathway activated by cytochrome c but not in the extrinsic pathway activated by caspase-8. Moreover, the addition of F1LΔTM to S-100 extracts after (rather than before) stimulation with cytochrome c failed to suppress DEVDase activity (Fig. 2C). Thus, F1L does not directly suppress DEVD-cleaving caspases, but rather it interferes with cytochrome c-induced mechanisms leading to the activation of effector caspases.
FIGURE 2.
F1LΔTM inhibits apoptosome-mediated caspase-9 activation and apoptosome assembly. A, procaspase-9 was preincubated with F1LΔTM (lacking GST tag), N1L, XIAP, or Bcl-XLΔTM (1 μm) proteins for 10 min at 37 °C followed by the addition of cytochrome c (Cyt. c), dATP, and recombinant full-length Apaf-1 (1 μm) for 10 min at 37 °C. Caspase-9 activity was measured by hydrolysis of Ac-LEHD-AFC (mean ± S.D.; n = 3). B, procaspase-9 incubated with (+) or without (−) F1L (lacking GST tag) was then added to reactions containing Apaf-1 together with cytochrome c and dATP for 10 min. The samples were size-fractionated by S-200 gel-filtration chromatography, and the resulting fractions were analyzed by SDS-PAGE/immunoblotting (WB) using caspase-9, Apaf-1, or F1L antibodies. The fifth panel represents a chromatography of F1LΔTM alone, which migrated as an apparent dimer. Molecular mass standards are indicated in kDa. C, recombinant F1LΔTM, N1L, XIAP, Bid, Bcl-XLΔTM, or SMAC (10 μm) proteins were preincubated with Apaf-1ΔWD-40 recombinant protein (1 μm) for 10 min, and then procaspase-9 (100 nm) was introduced for 10 min. Caspase-9 activity was measured by hydrolysis of Ac-LEHD-AFC (mean ± S.D.; n = 3). D, various concentrations of F1L (μm) proteins (without GST tag) were preincubated with active caspase-9 (200 nm) preactivated by Apaf-1ΔWD-40 recombinant protein (1 μm) for 10 min. Caspase-9 activity was measured by hydrolysis of Ac-LEHD-AFC (mean ± S.D.; n = 3). RFU, relative fluorescence units.
F1L Inhibits Cytochrome c-induced, Apaf-1-dependent Activation of Caspase-9 and Apoptosome Assembly in Vitro
To investigate further the anti-apoptotic mechanism of F1L, we tested its activity against reconstituted apoptosomes, which form in vitro upon cytochrome c-induced oligomerization of Apaf-1, followed by recruitment and activation of procaspase-9 (1, 4). For these experiments, full-length Apaf-1 purified from insect cells was combined with recombinant procaspase-9, cytochrome c, and dATP (a necessary cofactor for Apaf-1 oligomerization). Caspase-9 activity was measured by hydrolysis of the fluorogenic tetrapeptide Ac-LEHD-AFC. We found that the preaddition of F1L at an equimolar ratio relative to Apaf-1 potently blocked cytochrome c-induced LEHDase activity (Fig. 2A). As expected, XIAP had a similar effect in this assay, whereas N1L and Bcl-XL showed no inhibitory activity.
Next, we used gel-filtration chromatography to investigate the effect of F1L on apoptosome assembly, analyzing column fractions by immunoblotting to unambiguously identify the protein species. Recombinant Apaf-1 and procaspase-9 were incubated with cytochrome c and dATP with or without F1LΔTM protein, and samples were analyzed by chromatography and immunoblotting for detection of Apaf-1 and caspase-9. In the absence of F1L, most of the Apaf-1 and caspase-9 migrated as a large (>670 kDa) complex, consistent with the formation of the apoptosome (1, 8). In contrast, when F1L was included, Apaf-1 and caspase-9 eluted from gel-filtration columns much earlier, indicating that F1L interferes with apoptosome assembly. Furthermore, in the absence of F1L, nearly all of the procaspase-9 was processed to its ∼35-kDa form, whereas in the presence of F1L, a significant fraction of the caspase-9 remained in its ∼50-kDa proform (Fig. 2B). We also noted that a significant portion of F1L protein co-eluted in fractions with caspase-9, consistent with an interaction of these proteins. In addition, we performed co-immunoprecipitation experiments to monitor the association of caspase-9 with Apaf-1 in the presence versus absence of F1LΔTM protein (supplemental Fig. S1). F1LΔTM reduced the association of caspase-9 with Apaf-1, whereas Bcl-XLΔTM had no effect.
Although Apaf-1 normally requires cytochrome c for activation, removal of its C-terminal WD40 repeats produces a constitutively active protein (Apaf-1ΔC; residues 1–591) that directly binds to and activates procaspase-9, presumably because the WD40 domains are normally engaged in an autoinhibitory interaction that is relieved by binding cytochrome c (1, 32). We therefore used Apaf-1ΔC to explore whether either cytochrome c or the WD40 repeats are required directly to mediate the inhibitory activities of F1L. When Apaf-1ΔC was combined with full-length procaspase-9, caspase-9 activity was induced (Fig. 2C). The preaddition of recombinant F1LΔTM protein at a 5-fold molar excess relative to Apaf-1 and caspase-9 significantly inhibited Apaf-1ΔC-induced caspase-9 activity (Fig. 2C). As a positive control, a fragment of XIAP (BIR1–3) that is known to inhibit apoptosome-induced caspase activation had a similar effect on caspase-9 activity. In contrast, several other proteins tested had no inhibitory activity; these included N1L, Bid, Bcl-XL, and SMAC (Fig. 2C). The suppression of Apaf-1ΔC-induced caspase-9 activity by F1L was dose-dependent, with half-maximal suppression occurring at approximately equimolar concentrations compared with apoptosome components (Fig. 2D).
F1L Is a Direct and Selective Caspase-9 Inhibitor
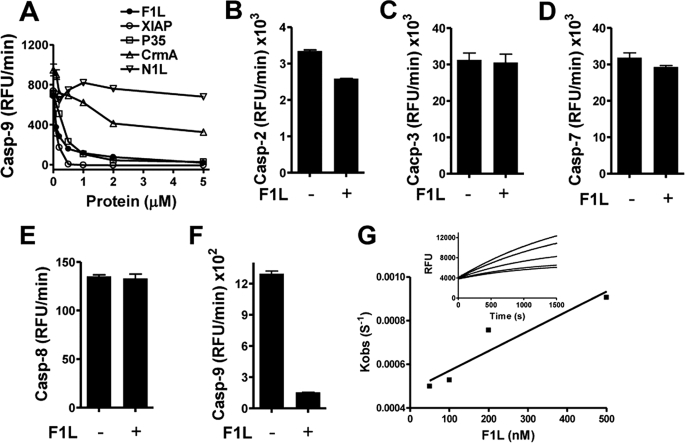
To further explore how F1L inhibits apoptosome-mediated caspase-9 activation, we compared the ability of F1LΔTM protein to inhibit caspase-9 activated by an alternative, Apaf-1-independent mechanism involving incubation with high concentrations of the kosmotropic salt sodium citrate as described previously (1, 33). F1LΔTM protein inhibited sodium citrate-activated caspase-9 in a dose-dependent manner in vitro (Fig. 3A), suggesting that F1L acts as a direct inhibitor of caspase-9 rather than an inhibitor of Apaf-1. Using the same assay, we found that F1LΔTM and XIAP (BIR1–3 domains), when compared with other caspase-9 inhibitors, displayed similar potencies, with IC50 values of 0.17 and 0.13 μm, respectively. Recombinant baculovirus p35 was slightly less potent, with an IC50 of 0.34 μm. However, the viral CrmA protein displayed much weaker inhibitory activity, whereas the control viral protein N1L had no effect (Fig. 3A).
FIGURE 3.
F1L selectively inhibits caspase-9 activity. A, various concentrations of F1LΔTM (without GST tag), XIAP BIR1–3 domain, p35, CrmA, or N1L (μm) proteins were preincubated with active caspase-9 (200 nm) preactivated by sodium citrate. Caspase-9 activity was measured by hydrolysis of Ac-LEHD-AFC (mean ± S.D.; n = 3). B–F, active caspase-2 (B), caspase-3 (C), caspase-7 (D), caspase-8 (E), and caspase-9 (F) were incubated with (+) or without (−) F1LΔTM (2 mm without GST tag) protein for 10 min before the addition of fluorogenic substrate peptides. Caspase activity was measured by hydrolysis of Ac-DEVD-AFC (caspase-2, -3, and -7), Ac-IETD-AFC (caspase-8), or Ac-LEHD-AFC (caspase-9) (mean ± S.D.; n = 3). RFU, relative fluorescence units. G, apoptosome-preactivated caspase-9 (100 nm) was mixed with various concentrations of F1LΔTM (without GST tag). Caspase activity was measured immediately by hydrolysis of Ac-LEHD-AFC at 37 °C for 30 min. The rate of inhibition (kobs) and the second order rate constant (ka) were calculated.
We next examined the specificity of the caspase inhibition by F1L. As expected, Fig. 3, B–F, shows that F1LΔTM strongly inhibited caspase-9 and slightly inhibited caspase-2, but it failed to inhibit caspase-3, -7, or -8 in vitro. Thus, F1L is a selective inhibitor of caspase-9. We also explored the ability of F1L to inhibit caspase-9 in the context of its physiological substrates by examining the proteolytic processing and activity of caspase-3 and -7 in vitro, which gave similar results (supplemental Fig. S2), indicating that F1L inhibits caspase-9-mediated cleavage and activation of its downstream physiological substrates.
The association rate (ka) for the interaction between F1L and caspase-9 (activated by apoptosome) was determined under pseudo-first order conditions. The process of caspase-9 inhibition was represented as a simple decay (kobs, decay rate). The kobs values obtained at different concentrations were plotted against the inhibitor concentrations. The slope of the line k′ and the Km were used to calculate the overall second order rate constant. The ka for F1LΔTM inhibition of caspase-9 was 0.91 × 103 m−1 s−1 (Fig. 3G).
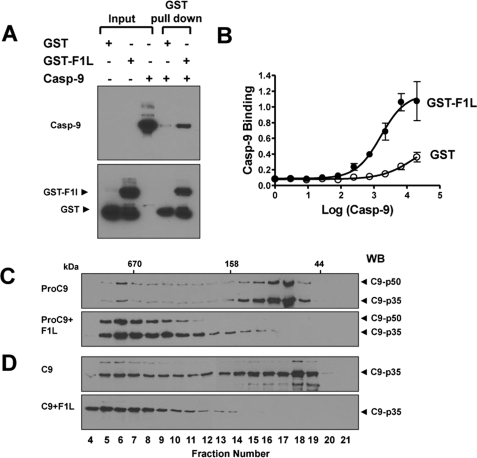
F1L Binds Caspase-9
To determine whether F1L binds caspase-9, we first performed GST pulldown assays using GST-F1LΔTM fusion protein immobilized on glutathione-Sepharose beads, which were incubated with recombinant citrate-activated caspase-9. We observed that GST-F1LΔTM, but not GST control protein, binds caspase-9 in vitro (Fig. 4A). These results were corroborated by an independent method whereby binding of activated caspase-9 to GST-F1LΔTM protein was assessed using an enzyme-linked immunosorbent assay in which GST or GST-F1LΔTM was adsorbed to microtiter plates and incubated with activated caspase-9, and binding was detected using anti-caspase-9 antibody (Fig. 4B). This method also showed that GST-F1L but not GST binds caspase-9 in vitro in a dose-dependent manner (demonstrating sigmoidal binding characteristics) (EC50 = 1.6 μm). Although an EC50 is provided here using citrate-activated caspase-9, we note that a formal determination of Kd for caspase-9 under physiological conditions is problematic because the native caspase-9 protein is known to require association with active Apaf-1 to assume an active conformation; thus, the preformed apoptosome (Apaf1·caspase-9·cytochrome c) is required, making a precise determination of protein concentration (1, 34) versus [free] problematic. Also, the term Kd implies an entirely reversible mechanism, a subject that requires further analysis.
FIGURE 4.
F1L binds caspase-9. A, recombinant GST or GST-F1LΔTM proteins (1 μg) were incubated with 1 μg of active caspase-9 (Casp-9) for 4 h together with 10 μl of glutathione-Sepharose 4B resin. The resin was washed three times, and associated proteins were analyzed by SDS-PAGE/immunoblotting using anti-caspase-9 or GST antibodies. B, caspase-9 binding to GST-F1L demonstrated by enzyme-linked immunosorbent assays. F1LΔTM (black symbols) or GST (white symbols) was incubated with various concentrations of caspase-9. C and D, procaspase-9 (C) or sodium citrate-activated caspase-9 (D) was incubated with (+) or without (−) GST-F1LΔTM and then analyzed by 2S00 gel-sieve chromatography. Eluted fractions were analyzed by SDS-PAGE/immunoblotting (WB) using caspase-9 antibody. Molecular mass standards are indicated in kDa. Note that bacterially expressed procaspase-9 contained both uncleaved p50 and cleaved p35 caspase-9 (top), which was reduced to cleaved p35 caspase-9 following activation with Sodium citrate. The addition of F1LΔTM shifted both unprocessed (p50) and processed (p35) caspase-9.
Finally, gel-filtration chromatography was used to investigate F1L binding to caspase-9. The elution of both unprocessed procaspase-9 and citrate-activated processed caspase-9 from gel-filtration columns was retarded by F1L (Fig. 4, C and D), consistent with high affinity binding of F1L to both the unprocessed and processed forms of caspase-9. Note that the addition of 5 μm F1LΔTM protein to 1 μm caspase-9 resulted in a nearly complete loss of monomeric caspase-9, with most of the caspase-9 protein migrating as a large protein complex upon binding F1LΔTM. The elution characteristics of the F1L·caspase-9 complex suggest the formation of a multimeric complex, which could be due to the ability of F1L and caspase-9 to each form homodimers, thus creating the opportunity for heterocomplexes of mixed stoichiometry.
The Caspase-9 Inhibitory Mechanism of F1L Differs from XIAP
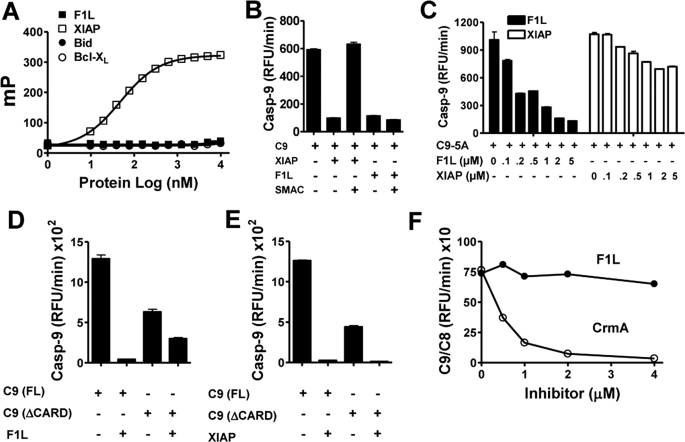
XIAP is a well characterized mammalian inhibitor of caspase-9, which binds with high affinity to the proteolytically processed form of caspase-9 but not unprocessed procaspase-9 (1, 35, 36). We therefore compared the caspase-9 inhibitory mechanisms of F1L and XIAP.
The BIR3 domain of XIAP binds and inhibits caspase-9, mediated in part by the interaction of a crevice on BIR3 with the first four amino acids of the unique N terminus of caspase-9 produced by cleavage at Asp315. The binding of processed caspase-9 to this crevice on BIR3 of XIAP is competitively inhibited by the mitochondrial protein SMAC, which similarly binds XIAP via a proteolytically processed N-terminal segment of four amino acids (1, 37). Thus, the tetrapeptide AVPI, corresponding to the N terminus of the mature SMAC protein, binds the BIR3 domain of XIAP, competitively displacing processed caspase-9. Unlike F1LΔTM, which inhibits recruitment of procaspase-9 to Apaf-1 (see Fig. 2B), XIAP reportedly does not interfere with caspase-9 association with Apaf-1 or with apoptosome assembly (1, 38, 39). Using fluorescence polarization assays, we further determined that F1L does not bind SMAC peptide, in contrast to the XIAP BIR3 domain, which displayed an apparent affinity of ∼50 nm (Fig. 5A). Moreover, unlike XIAP-BIR3, SMAC peptide does not neutralize the caspase-9 inhibitory activity of F1L (Fig. 5B). Thus, the mechanism by which F1L inhibits active caspase-9 presumably involves structural features distinct from those employed by XIAP.
FIGURE 5.
Studies of the mechanism of F1L-mediated inhibition of caspase-9. A, the fluorescence polarization assay method was used to monitor binding of rhodamine-labeled SMAC peptide to F1LΔTM (no GST tag). Various concentrations of F1LΔTM, XIAP BIR1–3 domain, Bid, or Bcl-XLΔTM were incubated with 20 mm rhodamine-conjugated SMAC peptide. Fluorescence polarization (millipolars (mP)) was measured after 10 min. XIAP BIR1–3 domain (0.5 μm) or F1LΔTM (no GST tag) (1 μm) proteins were preincubated with active caspase-9 (200 nm) with or without SMAC protein (2 μm) for 10 min. Caspase-9 activity was measured by hydrolysis of Ac-LEHD-AFC (mean ± S.D.; n = 3). B, XIAP BIR1–3 domain (0.5 μm) or F1LΔTM (1 μm) proteins were preincubated with active caspase-9 (200 nm) with or without SMAC protein (2 μm) for 10 min. Caspase-9 (Casp-9) activity was measured by hydrolysis of Ac-LEHD-AFC (mean ± S.D.; n = 3). C, various concentrations of F1LΔTM (lacking GST) or XIAP BIR1–3 domain (μm) proteins were preincubated with active caspase-9–5A (single chain) mutant (200 nm) for 10 min. Caspase-9 activity was measured by hydrolysis of Ac-LEHD-AFC (mean ± S.D.; n = 3). D and E, F1LΔTM (no GST tag) or XIAP (BIR1–3) proteins (2 μm) were preincubated with active full-length caspase-9 (activated by sodium citrate) or caspase-9 protein lacking the CARD for 10 min. Caspase-9 activity was measured by hydrolysis of Ac-LEHD-AFC (mean ± S.D.; n = 3). F, various concentrations of F1LΔTM (no GST tag) or CrmA proteins (μm) were preincubated with caspase-9/-8 protein (10 nm) for 10 min prior to the addition of substrate. Caspase-8 activity was measured by hydrolysis of Ac-IETD-AFC (mean ± S.D.; n = 3) RFU, relative fluorescence units.
We further explored the mechanism by which F1L inhibits caspase-9 using noncleavable mutants. Although caspase-9 is typically cleaved during the protease activation process, it has been shown that cleavage is not necessary for activation (1, 40, 41). C9–5A is a noncleavable mutant of caspase-9, in which Asp315, Asp330, Glu304, Asp305, and Glu306 have been mutated to alanine, but which nevertheless can be activated with Apaf-1 or sodium citrate (1, 23). We compared the ability of F1LΔTM protein and XIAP to inhibit the C9–5A mutant. Although XIAP-BIR3 failed to inhibit the activity of C9–5A, F1L demonstrated dose-dependent suppression, achieving approximately half-maximal inhibition at an approximately equimolar ratio, with IC50 ≈ 0.5 μm (Fig. 5C). Thus, cleavage of caspase-9, unlike XIAP, is not required for suppression by F1L.
To further examine the mechanism of F1L, we explored the role of the caspase recruitment domain (CARD). Capase-9 is the only CARD-containing caspase in which proteolytic cleavage does not remove the N-terminal prodomain where the CARD resides (1, 37). We therefore compared the inhibitory activity of F1L against activated full-length caspase-9 and truncated caspase-9 lacking the CARD. In assays in which caspase-9 activity was measured based on hydrolysis of Ac-LEHD-AFC, F1LΔTM protein showed a partial loss of its caspase-9 inhibitory activity against CARD-less caspase-9 compared with full-length caspase-9. In contrast, XIAP inhibited both forms of caspase-9 with similar potency (Fig. 5, D and E).
Next, to further investigate the role of the CARD, we tested F1L for inhibitory activity against a chimeric caspase containing the CARD of caspase-9 combined with the catalytic domain of caspase-8 (1, 21). F1L did not inhibit this chimeric caspase as measured by hydrolysis of the caspase-8 substrate Ac-IETD-AFC (Fig. 5E). In contrast, CrmA inhibited the chimeric caspase. We concluded, therefore, that the CARD makes important contributions to the inhibitory mechanism of F1L but that the catalytic domain of caspase-9 is also required. Also, F1L binds in vitro to both the full-length caspase-9 protein and caspase-9 lacking the CARD, but it does not bind the isolated CARD (supplemental Fig. S3).
Further studies of the mechanism of F1L-mediated inhibition of caspase-9 in vitro showed that F1L is not cleaved by caspase-9 (indicating that it is not a substrate of this protease) and that the inhibition is reversible (supplemental Figs. S4 and S5). Thus, the mechanism of caspase inhibition by F1L differs from that of CrmA, which is an irreversible inhibitor (1, 23).
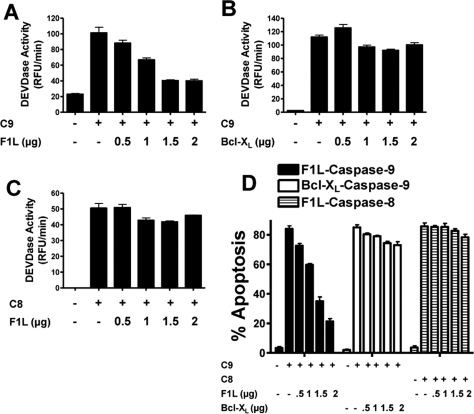
F1L Inhibits Caspase-9-induced Cell Death
Expression of F1L occurs rapidly after infection of host cells by vaccinia virus and has been shown to suppress apoptosis induced by stimuli that trigger the intrinsic pathway (20). We tested whether F1L could suppress caspase activation and apoptosis induced directly by caspase-9 overexpression in mammalian cells and compared this with caspase-8 overexpression. Accordingly, HEK293T cells were transfected with plasmids encoding FLAG-tagged caspase-9 or caspase-8 in combination with plasmids encoding F1L. After 1 day, caspase activity was either measured in cell lysates or proteolytic processing of caspases was evaluated by immunoblotting (Fig. 6, A–C). Alternatively, the percentage of apoptotic cells was determined by DAPI staining (Fig. 6D). When overexpressed in cells, caspase-9 undergoes autocleavage and is activated thereby leading to cell death, similar to other caspases (42), Co-transfection of plasmids encoding full-length F1L and full-length procaspase-9 resulted in concentration-dependent reductions in caspase activity and apoptosis (Fig. 6, A, B, and D). Proteolytic processing of caspase-9 in cells was also inhibited by F1L (supplemental Fig. S6). In contrast to F1L, co-expressing Bcl-XL with procaspase-9 did not suppress activation of effector caspases or apoptosis (Fig. 6, B and D), excluding an indirect mechanism for the F1L results related to its suppression of proapoptotic Bcl-2 family proteins.
FIGURE 6.
F1L suppresses caspase-9-induced apoptosis. A–C, HEK293T cells were transfected with various amounts of plasmids (pcDNA3 alone (−)) encoding full-length FLAG-F1L or Bcl-XL (0–2.0 μg) with or without FLAG-caspase-9 (0.5 μg (A and B)) or caspase-8 (1 μg (C)) plasmid while maintaining total DNA constant at 3 μg by the addition of pcDNA3. Cell lysates were prepared 20 h after transfection, normalized for protein content (10 μg), and incubated with the caspase-3/-7 substrate Ac-DEVD-AFC. Enzyme activity was determined by the generation of fluorescent AFC product, and Vmax was calculated (mean ± S.D.; n = 3). D, HEK293T cells were transfected with various amounts of plasmids encoding GFP (−), GFP-F1L, or Bcl-XL (0–2.0 μg) with or without FLAG-caspase-9 (0.5 μg) or caspase-8 (1 μg) plasmid while maintaining total DNA constant at 3 μg by the addition of pcDNA3. At 20 h post-transfection, both floating and adherent cells were collected, fixed, and stained with 0.1 μg/ml DAPI. The percentages of apoptotic cells were determined by counting the GFP-positive cells having nuclear fragmentation and/or chromatin condensation (mean ± S.D.; n = 3). RFU, relative fluorescence units.
Interestingly, F1L lacking its C-terminal transmembrane domain was equally effective as full-length F1L in inhibiting apoptosis induced by either caspase-9 overexpression or by treatment with staurosporine (supplemental Fig. S7), an agonist of the mitochondria-dependent intrinsic pathway for cell death. In contrast to caspase-9, F1L failed to suppress apoptosis of cells transfected with caspase-8-producing plasmid (Fig. 6, C and D), demonstrating its specificity for caspase-9. F1L also failed to inhibit apoptosis induced by agonistic anti-Fas antibody (supplemental Fig. S8), an activator of caspase-8. Thus, the anti-apoptotic activity of F1L is specific for the intrinsic rather than extrinsic pathway.
Analysis of F1L Mutants That Fail to Bind Caspase-9 or Bak
To distinguish the role of caspase-9 binding from binding to proapoptotic Bcl-2 family proteins such as Bak in terms of the anti-apoptotic activity of the F1L protein, we compared the functions of alanine substitution mutants of F1L with differential loss of binding to either caspase-9 or Bak. We first determined by deletional analysis that the N-terminal region of F1L preceding the Bcl-2-like fold is required for caspase-9 inhibition (not shown). Through site-directed mutagenesis studies, we determined that converting the cysteine at position 7 to alanine in an N-terminal α-helix of F1L ablated binding to caspase-9 while having no effect of Bak binding (Fig. 7A), as determined by co-immunoprecipitation analysis (Fig. 7B). Conversely, the mutant M67P, previously reported to lack Bak binding activity (20), retained caspase-9 binding activity (Fig. 7B). The C7A mutant but not the M67P mutant of F1L also lacks in vitro caspase-9 inhibitory activity, as shown by experiments comparing the wild-type and mutant F1L proteins expressed as recombinant proteins (Fig. 7C). Moreover, adding recombinant Bid protein or synthetic Bak BH3 peptide to reactions did not interfere with the ability of F1LΔTM to suppress caspase-9 activity in vitro (supplemental Fig. S9). Thus, F1L binds and inhibits caspase-9 independent of its interactions with proapoptotic Bcl-2 family proteins.
FIGURE 7.
Analysis of F1L mutants. A, depiction of F1L and Bcl-XL proteins. The locations of some of the α-helices of F1L and Bcl-XL are shown as well as the C-terminal transmembrane (TM) domains. The regions required for caspase-9 and Bak/Bim/Bid binding are shown, illustrating the alanine substitutions that ablate protein interactions at C7A (caspase-9) and M67P (Bak). The numbers indicate amino acid residues that define the borders of the indicated structural elements. B, immunoprecipitation (IP) of caspase-9 or Bak with GFP-tagged wild-type and mutant F1L. HEK293T cells were co-transfected with GFP-F1L and FLAG-caspase-9 plasmids as indicated. After 24 h, cell lysates were prepared and analyzed by co-immunoprecipitation assay using anti-GFP antibody. Immunocomplexes (top) and cell lysates (bottom) were analyzed by SDS-PAGE/immunoblotting (WB) using antibodies specific for GFP, caspase-9, or Bak. Procaspase-9 (∼50 kDa) and the processed caspase-9 large subunit (Casp-9 LS) (∼35 kDa) are indicated. Selected molecular mass markers are indicated in kilodaltons. C, recombinant GST-tagged F1LΔTM or GST-F1LΔTM M67P (1 μm) was preincubated with Sodium citrate-activated caspase-9 (200 nm). Caspase-9 activity was measured by hydrolysis of Ac-LEHD-AFC (mean ± S.D.; n = 3). RFU, relative fluorescence units. D, mouse embryonic fibroblast Apaf-1−/− cells were transfected with various amounts of plasmids (pcDNA3 alone (−)) encoding GFP-F1L, F1L (C7A), or F1L (M67P) (0–2.0 g), with or without FLAG-procaspase-9 (0.5 μg) plasmid, while maintaining total DNA constant at 3 μg by the addition of pcDNA3. At 20 h post-transfection, both floating and adherent cells were collected, fixed, and stained with 0.1 μg/ml DAPI. The percentages of apoptotic cells were determined by counting the GFP-positive cells having nuclear fragmentation and/or chromatin condensation (mean ± S.D.; n = 3). E, HEK293T cells were transfected with various amounts of plasmid DNA encoding GFP (−)), GFP-F1L, GFP-F1L (C7A), or GFP-F1L (M67P) while maintaining total DNA constant at 3 μg by the addition of GFP plasmid. After 20 h, cells were treated with 0.1 μm staurosporine for 16 h. Both floating and adherent cells were collected, fixed, and stained with 0.1 μg/ml DAPI. The percentages of apoptotic cells (condensed chromatin and/or fragmented nucleus) among GFP-positive cells were determined by UV microscopy. Data represent the percent nonapoptotic GFP-positive cells (mean ± S.D.; n = 3).
In cell transfection experiments using Apaf-1−/− mouse embryonic fibroblasts, full-length F1L and full-length F1L M67P equally suppressed activation of effector caspases (not shown) and inhibited apoptosis induced by overexpression of procaspase-9, whereas F1LC7A was inactive (Fig. 7D). These experiments with the C7A mutant thus demonstrate that the ability of F1L to bind and suppress caspase-9 correlates with its ability to suppress caspase-9-induced apoptosis. The use of Apaf-1−/− cells excludes a role in these experiments for the cytochrome c molecule, the release of which from mitochondria is mediated by proapoptotic Bcl-2 family proteins. Moreover, F1L lacking its mitochondria-targeting C-terminal transmembrane domain was equally as effective as full-length F1L at blocking apoptosis induced by overexpression of caspase-9 (supplemental Fig. S7). Immunoblotting experiments showed equivalent levels of expression of the various F1L proteins (not shown). We concluded therefore that F1L is capable of inhibiting caspase-9 and suppressing caspase-9-induced apoptosis independently of its interactions with proapoptotic Bcl-2 family proteins.
To compare the roles of caspase-9 and Bak binding in F1L-mediated apoptosis suppression, we expressed the C7A (caspase-9-defective) and M67P (Bak-defective) in HEK293 cells and induced apoptosis by treating cells with staurosporine, an activator of the mitochondrial cell death pathway. Cells were transfected with various amounts of F1L-encoding plasmid DNA to compare potency. F1L and F1L M67P reduced the percentage of apoptotic cells with comparable efficiency, whereas F1L C7A showed significantly less anti-apoptotic activity (Fig. 7E). Thus, ablating the caspase-9-binding site on F1L reduces the anti-apoptotic activity of F1L, at least in some cellular contexts. A double mutant of F1L (C7A plus M67P) was completely inactive in terms of apoptosis suppression (supplemental Fig. S7).
DISCUSSION
The anti-apoptotic protein F1L specifically inhibits the intrinsic cell death pathway. It had previously been shown that F1L binds and regulates several proapoptotic Bcl-2 family proteins, including Bak and Bim (13, 15, 17, 20).
Here, we have provided evidence that F1L has another activity as a selective inhibitor of capase-9, the apical protease in the intrinsic (mitochondrial) pathway. The inhibitory activity of F1L is comparable with that of other known caspase inhibitors, including viral p35 and CrmA, and cellular XIAP. In vitro, F1L also slightly inhibited caspase-2, the caspase most closely related to caspase-9, but not the extrinsic pathway apical protease caspase-8 or the downstream effector proteases, caspase-3 and -7. By suppressing caspase-9 activity, F1L inhibits the proteolytic processing and activation of its physiological substrates, procaspase-3 and -7, in vitro and also blocks caspase-9-induced apoptosis of cells. Thus, F1L interrupts the intrinsic pathway at two points: upstream of cytochrome c release through its actions on proapoptotic Bcl-2 family proteins, as reported previously, and downstream of cytochrome c release through its inhibition of caspase-9. Moreover, at least in some cellular contexts, such as staurosporine-induced apoptosis of HEK293T cells, the caspase-9 binding function of F1L appears to be more important than the Bak binding function.
Unlike other caspase inhibitors, F1L inhibited recruitment of procaspase-9 to Apaf-1 and appeared to bind the caspase-9 CARD in addition to its ability to inhibit caspase-9 activity directly. Most proteins that inhibit caspases either inhibit activation or inhibit the active protease but not both. For example, viral p35 and CrmA inhibit active caspases by serving as pseudo-substrates, becoming covalently bound to the active site cysteine (11, 20, 43). XIAP binds only the proteolytically processed forms of caspases, not the inactive zymogens (35, 36). In contrast, viral FLIPs prevent binding of procaspase-8 and -10 to their activating protein, FADD. Similarly, the cellular CARD-only proteins, Iceburg and COP, bind procaspase-1 via interactions with the N-terminal CARD-containing prodomains of the zymogen forms of these proteases, blocking activation but failing to suppress once the proteases have become activated (6, 44, 45). Our studies suggest that both the N-terminal CARD of caspase-9 and the catalytic domain make contributions to the inhibitory mechanisms of F1L, raising the possibility that F1L interacts cooperatively with both regions in caspase-9 or that the CARD is required for establishing the conformational states of caspase-9 that are optimally inhibited by F1L. We observed however that F1L does not bind to the isolated CARD, which argues in favor of the protein conformation model; however, a two-site interaction model, where the catalytic domain drives cooperative interactions with the CARD, cannot be excluded. Importantly, among the caspases, only caspase-9 retains its N-terminal prodomain where the CARD resides following activation and proteolytic processing (37), thus making the CARD relevant to both zymogen and processed (active) protease. A determination of the three-dimensional structure of F1L in complex with full-length caspase-9 would therefore be insightful.
Unlike the findings in CrmA and p35, which are irreversible inhibitors, our studies suggest that F1L-mediated inhibition of caspase-9 is reversible, which is analogous to the mechanism used by XIAP. However, XIAP binding and inhibition require proteolytic processing of caspase-9, whereas F1L does not. Also, SMAC peptides block XIAP-mediated inhibition of casapse-9 but have no effect on F1L. Thus, XIAP and F1L suppress caspase-9 by different mechanisms.
F1L is, for the most part, anchored in the mitochondrial membrane via a C-terminal transmembrane domain, with the extramembrane portion oriented toward the cytosol, similar to cellular members of the Bcl-2 family. How membrane anchoring impacts the role of F1L as a caspase-9 inhibitor was not explored in detail here, although we did demonstrate that F1LΔTM is equally as effective as full-length F1L in inhibiting apoptosis induced by overexpression of caspase-9 or by treatment with staurosporine. It is interesting to note however that some reports have demonstrated procaspase-9 association with mitochondria in certain tissues and cell lines (46–48). Other studies have suggested that some caspases, including caspase-9, tend to localize to mitochondria after activation (49, 50). Thus, by F1L being placed at the surface of mitochondria, it may be suitably located to interact with caspase-9 in the context of cytochrome c-mediated activation of the protease.
The notion that Bcl-2 family proteins can interact with two or more classes of cellular target proteins is not without precedent, thus making it entirely plausible that F1L inhibits proapoptotic Bcl-2 family proteins such as Bim and Bak while also inhibiting caspase-9. For example, although cellular Bcl-2 has a central function related to its binding to the BH3 domains of proapoptotic Bcl-2 family proteins, it also interacts with a variety of cellular proteins, including inositol triphosphate receptors, Bax inhibitor-1, bifunctional apoptosis regulator, and Bap31 in membranes of the endoplasmic reticulum (reviewed in Refs. 20 and 51), and with the autophagy protein Beclin (52) as well as also directly binding and suppressing cytosolic NLR family proteins such as NALP1 (NLRP1) (53). It will be interesting therefore to explore whether F1L has additional unrecognized cellular targets analogous to cellular Bcl-2. Nevertheless, our data have provided the first demonstration of direct caspase inhibition by a Bcl-2 family protein, a theme that may eventually be found repeated among other viral or cellular members of this protein family. Moreover, we mapped the caspase-9-binding domain in F1L to an N-terminal α-helix preceding the Bcl-2-like fold, thus suggesting that this viral Bcl-2 homolog acquired an additional functional domain relative to its cellular counterparts. Computer-assisted searches of genomic data bases failed to reveal a clear cellular analog of either the full-length F1L protein or its N-terminal caspase-9-binding domain.
Most strains of vaccinia and variola virus (59 of 60) possess genes encoding both F1L and CrmA. As such, vaccinia virus is armed with anti-apoptotic tools for suppressing the apical caspases in both the intrinsic and extrinsic pathways for apoptosis. Genetic analysis of vaccinia virus has demonstrated that ablation of F1L leads to apoptosis of infected host cells, indicating that F1L is crucial for blocking vaccinia virus-induced cell death (10, 20). Moreover, it seems likely that F1L is a dual inhibitor that both neutralizes proapoptotic Bcl-2 family proteins, blocking the intrinsic pathway upstream of cytochrome c release (15, 20), and inhibits caspase-9, thus also arresting the pathway downstream of cytochrome c release. In contrast, the related vaccinia virus gene product, N1L, neutralizes proapoptotic Bcl-2 family proteins but does not inhibit caspase-9. Therefore, these poxviruses are armed with both complementary and partially redundant weapons for suppressing host cell apoptosis, presumably affording them with the ability to maintain survival of their infected hosts for purposes of viral production.
Acknowledgments
We thank Christina Pop, Jean-Bernard Denault, Fiona Scott, Brendan Eckelman, Michael Way, and Mika Aoyagi for providing reagents and useful suggestions and Prof. Ziwei Huang and Arnold Satterthwait for providing some peptides. We thank Melanie Hanaii and Tessa Siegfried for manuscript preparation.
This work was supported, in whole or in part, by National Institutes of Health Grant P01AI055789.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S9.
- TNF
- tumor necrosis factor
- SMAC
- second mitochondria-derived activator of caspase
- XIAP
- X-linked inhibitor of apoptosis protein
- GST
- glutathione S-transferase
- PBS
- phosphate-buffered saline
- AFC
- 7-amino-4-trifluoromethylcoumarin
- DAPI
- 4,6-diamidino-2-phenylindole
- GFP
- green fluorescent protein
- CARD
- caspase recruitment domain
- HEK
- human embryonic kidney
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid.
REFERENCES
- 1.Boya P., Pauleau A. L., Poncet D., Gonzalez-Polo R. A., Zamzami N., Kroemer G. (2004) Biochim. Biophys. Acta 1659, 178–189 [DOI] [PubMed] [Google Scholar]
- 2.Cuconati A., White E. (2002) Genes Dev. 16, 2465–2478 [DOI] [PubMed] [Google Scholar]
- 3.Danial N. N., Korsmeyer S. J. (2004) Cell 116, 205–219 [DOI] [PubMed] [Google Scholar]
- 4.Li P., Nijhawan D., Budihardjo I., Srinivasula S. M., Ahmad M., Alnemri E. S., Wang X. (1997) Cell 91, 479–489 [DOI] [PubMed] [Google Scholar]
- 5.Peter M. E., Krammer P. H. (2003) Cell Death Differ. 10, 26–35 [DOI] [PubMed] [Google Scholar]
- 6.Thome M., Schneider P., Hofmann K., Fickenscher H., Meinl E., Neipel F., Mattmann C., Burns K., Bodmer J. L., Schröter M., Scaffidi C., Krammer P. H., Peter M. E., Tschopp J. (1997) Nature 386, 517–521 [DOI] [PubMed] [Google Scholar]
- 7.Ray C. A., Black R. A., Kronheim S. R., Greenstreet T. A., Sleath P. R., Salvesen G. S., Pickup D. J. (1992) Cell 69, 597–604 [DOI] [PubMed] [Google Scholar]
- 8.Cain K., Bratton S. B., Langlais C., Walker G., Brown D. G., Sun X. M., Cohen G. M. (2000) J. Biol. Chem. 275, 6067–6070 [DOI] [PubMed] [Google Scholar]
- 9.Everett H., McFadden G. (2002) Curr. Opin. Microbiol. 5, 395–402 [DOI] [PubMed] [Google Scholar]
- 10.Taylor J. M., Barry M. (2006) Virology 344, 139–150 [DOI] [PubMed] [Google Scholar]
- 11.Stennicke H. R., Ryan C. A., Salvesen G. S. (2002) Trends Biochem. Sci. 27, 94–101 [DOI] [PubMed] [Google Scholar]
- 12.Aoyagi M., Zhai D., Jin C., Aleshin A. E., Stec B., Reed J. C., Liddington R. C. (2007) Protein Sci. 16, 118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasilenko S. T., Stewart T. L., Meyers A. F., Barry M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14345–14350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kvansakul M., Yang H., Fairlie W. D., Czabotar P. E., Fischer S. F., Perugini M. A., Huang D. C., Colman P. M. (2008) Cell Death Differ. 15, 1564–1571 [DOI] [PubMed] [Google Scholar]
- 15.Wasilenko S. T., Banadyga L., Bond D., Barry M. (2005) J. Virol. 79, 14031–14043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer S. F., Ludwig H., Holzapfel J., Kvansakul M., Chen L., Huang D. C., Sutter G., Knese M., Häcker G. (2006) Cell Death Differ. 13, 109–118 [DOI] [PubMed] [Google Scholar]
- 17.Taylor J. M., Quilty D., Banadyga L., Barry M. (2006) J. Biol. Chem. 281, 39728–39739 [DOI] [PubMed] [Google Scholar]
- 18.Krajewska M., Wang H. G., Krajewski S., Zapata J. M., Shabaik A., Gascoyne R., Reed J. C. (1997) Cancer Res. 57, 1605–1613 [PubMed] [Google Scholar]
- 19.Zhai D., Luciano F., Zhu X., Guo B., Satterthwait A. C., Reed J. C. (2005) J. Biol. Chem. 280, 15815–15824 [DOI] [PubMed] [Google Scholar]
- 20.Postigo A., Cross J. R., Downward J., Way M. (2006) Cell Death Differ. 13, 1651–1662 [DOI] [PubMed] [Google Scholar]
- 21.Pop C., Timmer J., Sperandio S., Salvesen G. S. (2006) Mol. Cell 22, 269–275 [DOI] [PubMed] [Google Scholar]
- 22.Kim H. E., Du F., Fang M., Wang X. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 17545–17550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renatus M., Stennicke H. R., Scott F. L., Liddington R. C., Salvesen G. S. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 14250–14255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deveraux Q. L., Welsh K., Reed J. C. (2000) Methods Enzymol. 322, 154–161 [DOI] [PubMed] [Google Scholar]
- 25.Stennicke H. R., Salvesen G. S. (1999) Methods 17, 313–319 [DOI] [PubMed] [Google Scholar]
- 26.Zhai D., Jin C., Satterthwait A. C., Reed J. C. (2006) Cell Death Differ. 13, 1419–1421 [DOI] [PubMed] [Google Scholar]
- 27.Zhai D., Ke N., Zhang H., Ladror U., Joseph M., Eichinger A., Godzik A., Ng S. C., Reed J. C. (2003) Biochem. J. 376, 229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riedl S. J., Salvesen G. S. (2007) Nat. Rev. Mol. Cell Biol. 8, 405–413 [DOI] [PubMed] [Google Scholar]
- 29.Bao Q., Shi Y. (2007) Cell Death Differ. 14, 56–65 [DOI] [PubMed] [Google Scholar]
- 30.Haraguchi M., Torii S., Matsuzawa S., Xie Z., Kitada S., Krajewski S., Yoshida H., Mak T. W., Reed J. C. (2000) J. Exp. Med. 191, 1709–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Q., Snipas S., Orth K., Muzio M., Dixit V. M., Salvesen G. S. (1997) J. Biol. Chem. 272, 7797–7800 [DOI] [PubMed] [Google Scholar]
- 32.Riedl S. J., Li W., Chao Y., Schwarzenbacher R., Shi Y. (2005) Nature 434, 926–933 [DOI] [PubMed] [Google Scholar]
- 33.Boatright K. M., Renatus M., Scott F. L., Sperandio S., Shin H., Pedersen I. M., Ricci J. E., Edris W. A., Sutherlin D. P., Green D. R., Salvesen G. S. (2003) Mol. Cell 11, 529–541 [DOI] [PubMed] [Google Scholar]
- 34.Chen L., Willis S. N., Wei A., Smith B. J., Fletcher J. I., Hinds M. G., Colman P. M., Day C. L., Adams J. M., Huang D. C. (2005) Mol. Cell 17, 393–403 [DOI] [PubMed] [Google Scholar]
- 35.Srinivasula S. M., Hegde R., Saleh A., Datta P., Shiozaki E., Chai J., Lee R. A., Robbins P. D., Fernandes-Alnemri T., Shi Y., Alnemri E. S. (2001) Nature 410, 112–116 [DOI] [PubMed] [Google Scholar]
- 36.Shiozaki E. N., Chai J., Rigotti D. J., Riedl S. J., Li P., Srinivasula S. M., Alnemri E. S., Fairman R., Shi Y. (2003) Mol. Cell 11, 519–527 [DOI] [PubMed] [Google Scholar]
- 37.Shi Y. (2004) Protein Sci. 13, 1979–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bratton S. B., Walker G., Srinivasula S. M., Sun X. M., Butterworth M., Alnemri E. S., Cohen G. M. (2001) EMBO J. 20, 998–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill M. M., Adrain C., Duriez P. J., Creagh E. M., Martin S. J. (2004) EMBO J. 23, 2134–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stennicke H. R., Deveraux Q. L., Humke E. W., Reed J. C., Dixit V. M., Salvesen G. S. (1999) J. Biol. Chem. 274, 8359–8362 [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez J., Lazebnik Y. (1999) Genes Dev. 13, 3179–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boatright K. M., Salvesen G. S. (2003) Curr. Opin. Cell Biol. 15, 725–731 [DOI] [PubMed] [Google Scholar]
- 43.Xu G., Cirilli M., Huang Y., Rich R. L., Myszka D. G., Wu H. (2001) Nature 410, 494–497 [DOI] [PubMed] [Google Scholar]
- 44.Humke E. W., Shriver S. K., Starovasnik M. A., Fairbrother W. J., Dixit V. M. (2000) Cell 103, 99–111 [DOI] [PubMed] [Google Scholar]
- 45.Lee S. H., Stehlik C., Reed J. C. (2001) J. Biol. Chem. 276, 34495–34500 [DOI] [PubMed] [Google Scholar]
- 46.Krajewski S., Krajewska M., Ellerby L. M., Welsh K., Xie Z., Deveraux Q. L., Salvesen G. S., Bredesen D. E., Rosenthal R. E., Fiskum G., Reed J. C. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 5752–5757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Susin S. A., Lorenzo H. K., Zamzami N., Marzo I., Brenner C., Larochette N., Prévost M. C., Alzari P. M., Kroemer G. (1999) J. Exp. Med. 189, 381–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costantini P., Bruey J. M., Castedo M., Métivier D., Loeffler M., Susin S. A., Ravagnan L., Zamzami N., Garrido C., Kroemer G. (2002) Cell Death Differ. 9, 82–88 [DOI] [PubMed] [Google Scholar]
- 49.Chandra D., Tang D. G. (2003) J. Biol. Chem. 278, 17408–17420 [DOI] [PubMed] [Google Scholar]
- 50.Chandra D., Choy G., Deng X., Bhatia B., Daniel P., Tang D. G. (2004) Mol. Cell. Biol. 24, 6592–6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim I., Xu W., Reed J. C. (2008) Nat. Rev. Drug Discov. 7, 1013–1030 [DOI] [PubMed] [Google Scholar]
- 52.Levine B., Kroemer G. (2008) Cell 132, 27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bruey J. M., Bruey-Sedano N., Luciano F., Zhai D., Balpai R., Xu C., Kress C. L., Bailly-Maitre B., Li X., Osterman A., Matsuzawa S., Terskikh A. V., Faustin B., Reed J. C. (2007) Cell 129, 45–56 [DOI] [PubMed] [Google Scholar]